Published online Nov 15, 2025. doi: 10.4251/wjgo.v17.i11.111171
Revised: August 27, 2025
Accepted: September 28, 2025
Published online: November 15, 2025
Processing time: 142 Days and 20.3 Hours
Chemotherapy is an essential treatment for colorectal cancer (CRC) patients after surgery, but many patients do not benefit from chemotherapy because tumour heterogeneity results in varied responses.
To study the effectiveness of in vitro chemosensitivity tests adenosine tripho
Between January 2015 to December 2021, a total of 1549 CRC patients underwent surgery and in vitro chemosensitivity testing using ATP-TCA. A subset of 405 patients who met the survival assessment criteria were followed to collect data on overall survival (OS) and disease-free survival (DFS). Cox regression analysis revealed independent prognostic factors that affect OS and DFS for those re
Tumour heterogeneity and resistance to multiple drugs were observed in 1549 patients. The sensitivity to 5-fluorouracil (5-FU) combined with L-OPH was tested among 1474 of these patients, yielding a sensitivity rate of 11.9%. ATP-TCA results were identified as an independent prognostic factor for DFS [P = 0.002, hazard ratio (95% confidence interval): 4.98 (1.81-13.72)] in patients with resectable CRC. Compared with drug-resistant patients, sensitive CRC patients treated with 5-FU and L-OPH had significantly prolonged DFS (P = 0.027). Further Kaplan-Meier analysis indicated that ATP-TCA sensitivity was significantly associated with improved OS (P = 0.048) and DFS (P = 0.003) in patients with stage III CRC.
The response of CRC patients to the combination regimen of 5-FU and L-OPH is heterogeneous. This study confirmed that the ATP-TCA is a valuable tool for predicting clinical outcomes, such as DFS, in patients with resectable CRC receiving chemotherapy. Although further validation with multicentre data is still necessary, these findings support that the ATP-TCA may function as a guiding tool for personalized chemotherapy administration, thereby optimizing treatment opportunities for patients.
Core Tip: In this study, retrospectively evaluated the clinical application of adenosine triphosphate-based tumour chemotherapy sensitivity test (ATP-TCA) and revealed that it is an independent prognostic factor for disease-free survival in patients with resectable colorectal cancer. Additionally, an ATP-TCA-sensitive chemotherapy regimen was shown to significantly improve overall survival and disease-free survival in colorectal patients in stage III. Although the necessity for further validation, this study provides evidence supporting the extension of ATP-TCA assays to combination chemotherapy regimens for colorectal cancer while providing a theoretical basis and perspective for future advancements in personalized medicine.
- Citation: Li SJ, Lu YX, Zheng FY, Bian YC, Miao LY, Huang CR. Tumour chemotherapy sensitivity test may predict clinical outcomes in colorectal cancer patients receiving oxaliplatin and fluoropyrimidine-based regimens. World J Gastrointest Oncol 2025; 17(11): 111171
- URL: https://www.wjgnet.com/1948-5204/full/v17/i11/111171.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i11.111171
Colorectal cancer (CRC) is a prevalent and highly lethal malignancy worldwide that results in an estimated 903859 deaths reported in 2022 according to the International Agency for Research on Cancer, making it the second leading cause of cancer-related mortality globally[1]. Among them, China has significantly higher CRC morbidity than other countries, accounting for approximately 26.7% of the global incidence[2]. Currently, the primary therapeutic modalities for treating CRC include surgical intervention, postoperative adjuvant chemotherapy, radiotherapy, targeted therapy, immunotherapy and other approaches[3-6]. Patients with pathological tumour-node-metastasis stage II or III disease typically need postoperative adjuvant chemotherapy[3,7]. Postoperative adjuvant chemotherapy has the potential to effectively control residual tumour cells, thereby reducing the risk of recurrence and metastasis, prolonging survival, and enhancing quality of life. However, owing to tumour heterogeneity and the development of drug resistance, not all CRC patients can benefit from first-line drug regimens based on oxaliplatin (L-OPH) and fluoropyrimidine agents [such as 5-fluorouracil (5-FU), capecitabine, and deoxy-fluorouridine] for postoperative adjuvant chemotherapy in CRC patients[8-10]. Relying solely on clinical experience without accounting for individual differences may result in a lack of insight, underscoring the utmost importance of tailoring chemotherapy regimens to suit each patient’s unique needs[11]. Although techniques such as genetic testing and programmed death ligand-1 assessment enable more accurate drug response prediction, their clinical utility is limited by a subsequent lack of specific treatment options. Additionally, a high percentage of tumour patients have no detectable mutations and thus cannot benefit from corresponding targeted therapies. The adenosine triphosphate-based tumour chemotherapy sensitivity test (ATP-TCA) is a viable method for assessing individual chemosensitivity by quantifying the in vitro drug inhibition rate of tumour cell growth. The clinical application value of in vitro tumour cell chemosensitivity assays in guiding individualized treatment for patients with lung cancer[12], gastric cancer[13], and leukaemia[14] has been well established by our research group. However, the heterogeneity of the response of CRC patients to combination drug regimens based on L-OPH and fluoropyrimidine, as well as the correlation between in vitro chemosensitivity and clinical outcomes, remains unclear. The aim of the present study was to examine the associations between ATP-TCA outcomes and clinical outcomes in patients diagnosed with CRC.
Patients with histologically confirmed CRC who underwent surgical treatment and ATP-TCAs at our hospital between January 1, 2015 to December 31, 2021 were enrolled in this study. The inclusion criteria for survival assessment were as follows: (1) Patients who underwent radical colorectal resection; (2) Patients with histologically confirmed high-risk stage II or III CRC according to the American Joint Committee on Cancer/Union for International Cancer Control staging guidelines for CRC (8th edition); (3) Patients who received at least one cycle of chemotherapy with L-OPH and fluoro
Ethical approval for this study was obtained from the Research Ethics Board of the First Affiliated Hospital of Soochow University (No. 2023178). All procedures involving human participants were conducted in accordance with the ethical standards set by institutional and national research committees as well as the principles outlined in the 1964 Declaration of Helsinki and its subsequent amendments.
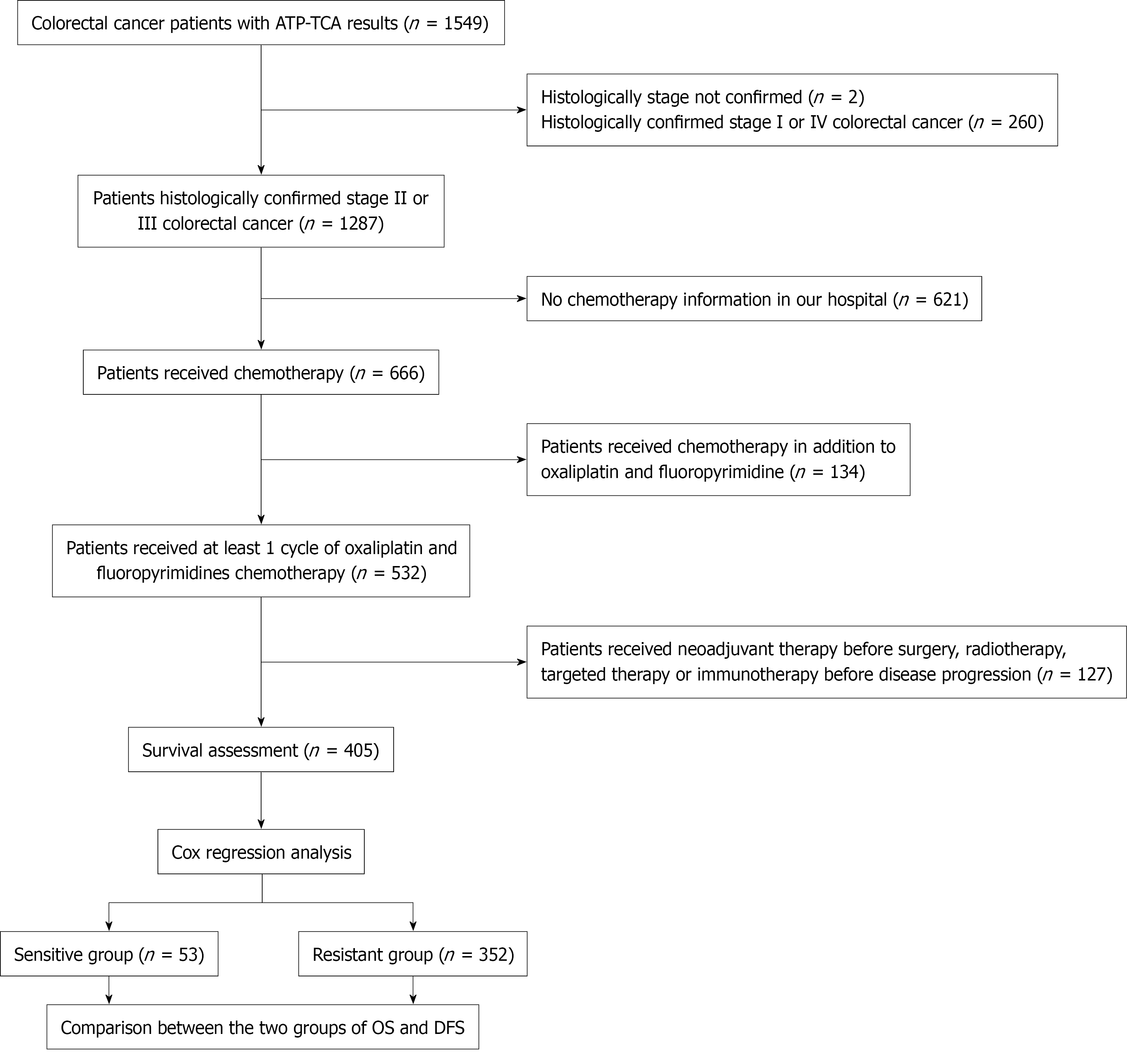
The correlation between the ATP-TCA results of the combination drug regimen consisting of 5-FU + L-OHP was retrospectively analysed in patients who met the survival assessment criteria, with a focus on clinical prognosis in these patients. The study design is illustrated in Figure 1.
The chemosensitivity of surgically resected CRC specimens to three single chemotherapy regimens [L-OHP, 5-FU, and irinotecan (SN-38)] or two combined chemotherapy regimens (5-FU + L-OHP and 5-FU + SN-38) was assessed in vitro using the ATP-TCA method. The tumour samples were collected from each patient and placed in sterile containers containing tissue preservation solution. Within 24 hours, adipose and necrotic tissues were aseptically removed from the sample, and 1 cm3 of tumour tissue was extracted for chemosensitivity detection using the ATP-TCA. In brief, the tumour samples were dissected into small fragments, dissociated into cell suspensions using trypsin, and subsequently filtered through a 100 μm mesh sieve. After cell counting and viability assessment, the cells were evenly distributed into 96-well plates (1 × 104 per well to 3 × 104 cells per well) and subjected to the selected chemotherapy regimen. In accordance with the instructions provided with the kit, each chemical drug was diluted to five different concentrations (12.5%, 25%, 50%, 100%, and 200% of the peak plasma concentrations corresponding to conventional clinical doses). Each individual test drug concentration (TDC) was assessed in duplicate wells (X), while untreated cells (M0) and blank complete assay medium without cells (MI) served as controls. The cells were incubated at 37 °C under a CO2 atmosphere of 5% and a humidity exceeding 95% for 5-7 days. The cells were subsequently lysed, and adenosine triphosphate levels were quantified using a microplate luminometer (Berthold Detection Systems, Germany) through a luciferin-luciferase luminescence reaction. The inhibition rate was calculated as follows: Inhibition rate (%) = [1 - (X - MI)/(M0 - MI)] × 100%. M0 served as the control for 100% tumour cell viability. X and MI represent the fluorescence intensities of the different wells as described above. Dose inhibition rate curves were generated, and the IC90 and IC50 values, which represent the concentrations at which 90% and 50% inhibition occurred, respectively, were determined using SPSS software.
The recurrence rate and mortality rate of patients eligible for survival assessment were obtained via medical records inquiry and regular follow-up until March 2023. Overall survival (OS) and disease-free survival (DFS) were measured from the date of surgery to the date of death or disease progression, with the last follow-up time serving as the endpoint for survival assessment in cases where such events did not transpire (censored data).
The in vitro chemosensitivity results were classified in accordance with the reagent instructions and established studies[12-15] as follows: (1) Strong sensitivity, with an IC90 ≤ 100% TDC and an IC50 ≤ 25% TDC; (2) Partial sensitivity, with an IC90 > 100% TDC and an IC50 ≤ 25% TDC; (3) Weak sensitivity, with an IC90 ≤ 100% TDC and an IC50 > 25% TDC; and (4) Resistance, with an IC90 > 100% TDC and an IC50 > 25% TDC. Patients eligible for survival assessment exhibiting strong in vitro chemosensitivity or partial sensitivity were assigned to the sensitive group, whereas those demonstrating weak sensitivity or resistance to chemotherapy were allocated to the resistant group.
The data were randomly separated into training and validation sets at a 7:3 ratio. Univariate and multivariate Cox regression analyses were performed on the training set to identify independent prognostic factors, with the validation set used for internal validation. Factors with P < 0.1 according to the univariate analysis were included in the multivariate model. The clinical predictive model was validated via calibration curves, receiver operating characteristic curves based on the area under the curve (AUC), and decision curve analysis, this analysis is visually presented as a nomogram. Survival curves were generated using the Kaplan-Meier method, and the log-rank test was used to compare OS and DFS between the drug-resistant group and the drug-sensitive group. Statistical significance was defined as P < 0.05. The data were analysed using GraphPad Prism 9 software and R version 4.3.3. Descriptive statistics were employed to characterize the patients, whereas nonparametric tests and Fisher’s exact test were used for group comparisons.
The study included a total of 1549 patients diagnosed with CRC, and the characteristics of these patients are presented in Table 1.
| Characteristics | Value |
| Sex | |
| Male | 909 (58.68) |
| Female | 640 (41.32) |
| Age, years, mean ± SD | 64.01 ± 11.62 |
| Histology | |
| Adenocarcinoma | 1539 (99.35) |
| Neuroendocrine carcinoma | 5 (0.32) |
| Melanoma | 2 (0.13) |
| Small cell carcinoma | 1 (0.06) |
| Squamous carcinoma | 1 (0.06) |
| Adenosquamous carcinoma | 1 (0.06) |
| Sampling position | |
| Colon | 884 (57.07) |
| Rectum | 571 (36.86) |
| Colon + rectum | 94 (6.07) |
| Stage | |
| I | 198 (12.78) |
| II | 594 (38.35) |
| III | 693 (44.74) |
| IV | 62 (4.00) |
| Not confirmed | 2 (0.13) |
The ATP-TCA results of the 1549 tumour samples demonstrated the heterogeneity of chemosensitivity in CRC (Table 2). A total of 1474 patients were screened for combined chemotherapy regimens consisting of 5-FU + L-OHP and 5-FU + SN-38, whereas 75 patients underwent testing for single-drug regimens involving 5-FU, L-OHP, and SN-38 in vitro. The combination chemotherapy regimens exhibited sensitivities of 11.9% and 18.0% for 5-FU + L-OHP and 5-FU + SN-38, respectively. The sensitivities of the single-drug chemotherapy regimens were 5.3%, 5.3%, and 16.0% for the drugs 5-FU, L-OHP, and SN-38, respectively. These findings suggest that combination therapy results in greater sensitivity than monotherapy with either drug does.
| Drugs | n | Level of chemosensitivity | Sensitivity rate (%) | |||
| Strong | Partial | Weak | Resistant | |||
| 5-FU + L-OHP | 1474 | 22 | 153 | 1 | 1298 | 11.9 |
| 5-FU + SN-38 | 1474 | 48 | 217 | 1 | 1208 | 18.0 |
| 5-FU | 75 | 1 | 3 | 0 | 71 | 5.3 |
| L-OHP | 75 | 1 | 3 | 0 | 71 | 5.3 |
| SN-38 | 75 | 2 | 10 | 0 | 63 | 16.0 |
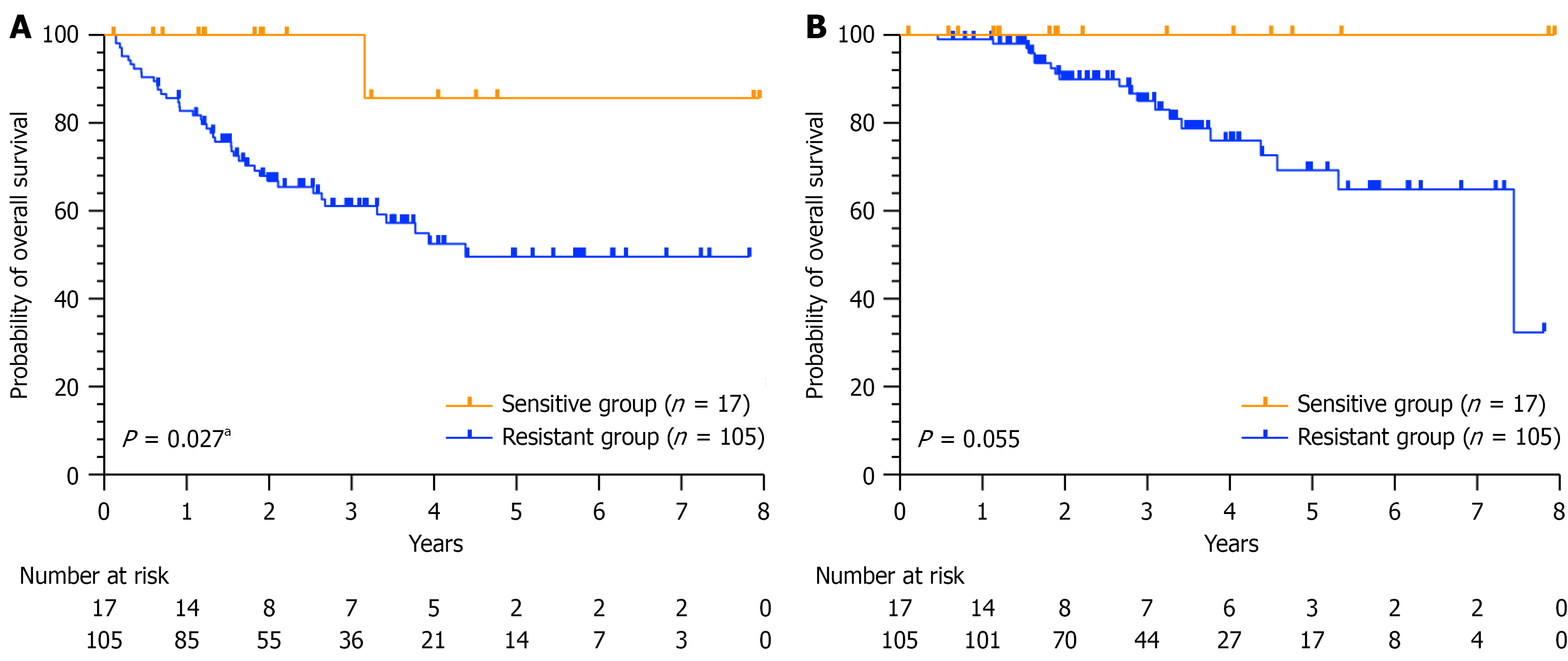
Among the 1549 patients who underwent the ATP-TCA, survival data were available for 405 patients according to the eligibility criteria. Among these patients, 53 presented in vitro sensitivity to 5-FU + L-OHP, whereas the remaining 352 presented resistance. The median follow-up period from the time of surgery was 2.78 years (1.70-4.42 years) as of March 2023, on the basis of the available follow-up data. Disease progression was observed in 135 patients (33.33%), among whom 54 patients (13.33%) died from the disease. A total of 405 patients were randomly allocated into the training and validation sets, with no significant differences between them (Supplementary Table 1). Table 3 presents the clinical data of CRC patients who in the training set, categorized into a sensitive group and a drug-resistant group. No significant differences were observed between the two groups in terms of demographic characteristics such as sex, histology, sampling location or stage. However, the patients in the sensitive group were significantly older than those in the resistant group were (P = 0.032).
| Characteristics | Sensitive group (n = 36) | Resistant group (n = 247) | P value |
| Sex | |||
| Male | 20 (55.56) | 141 (57.09) | 0.863 |
| Female | 16 (44.44) | 106 (42.91) | |
| Age, years | |||
| mean ± SD | 63.44 ± 8.71 | 59.33 ± 10.96 | 0.032a |
| Histology | |||
| Adenocarcinoma | 53 (100.00) | 352 (100.00) | - |
| Sampling location | |||
| Colon | 20 (55.56) | 169 (68.42) | 0.126 |
| Rectum | 16 (44.44) | 78 (31.58) | |
| Stage | |||
| II | 13 (36.11) | 85 (34.41) | 0.841 |
| III | 23 (63.89) | 162 (65.59) |
Through both univariate and multivariate regression analyses in the training set, three and four covariates were respectively identified as potential independent prognostic factors for OS (Table 4) and DFS (Table 5), respectively. Further internal validation was performed. Kaplan-Meier analysis of the validation set revealed that DFS was sig
| Variables | Univariate analysis | Multivariate analysis | ||
| P value | HR (95%CI) | P value | HR (95%CI) | |
| Age, years | < 0.001c | 1.08 (1.04-1.13) | < 0.001c | 1.10 (1.05-1.14) |
| Sex | ||||
| Male | 0.365 | 1.00 (reference) | - | - |
| Female | 0.72 (0.36-1.46) | - | ||
| Sampling position | ||||
| Colon | 0.671 | 1.00 (reference) | - | - |
| Rectum | 0.85 (0.42-1.76) | - | ||
| Stage | ||||
| II | 0.017a | 1.00 (reference) | 0.019a | 1.00 (reference) |
| III | 3.17 (1.23-8.19) | 3.13 (1.21-8.10) | ||
| ATP-TCA | ||||
| Sensitive | 0.100 | 1.00 (reference) | 0.048a | 1.00 (reference) |
| Resistant | 5.31 (0.73-38.85) | 7.48 (1.02-55.16) | ||
| Variables | Univariate analysis | Multivariate analysis | ||
| P value | HR (95%CI) | P value | HR (95%CI) | |
| Age, years | 0.012a | 1.03 (1.01-1.05) | 0.003b | 1.04 (1.01-1.06) |
| Sex | ||||
| Male | 0.700 | 1.00 (reference) | - | - |
| Female | 0.92 (0.61-1.40) | - | ||
| Sampling position | ||||
| Colon | 0.071 | 1.00 (reference) | 0.030a | 1.00 (reference) |
| Rectum | 1.47 (0.97-2.22) | 1.59 (1.05-2.41) | ||
| Stage | ||||
| II | < 0.001c | 1.00 (reference) | < 0.001c | 1.00 (reference) |
| III | 2.77 (1.64-4.69) | 2.76 (1.63-4.68) | ||
| ATP-TCA | ||||
| Sensitive | 0.008b | 1.00 (reference) | 0.002b | 1.00 (reference) |
| Resistant | 3.92 (1.44-10.68) | 4.98 (1.81-13.72) | ||
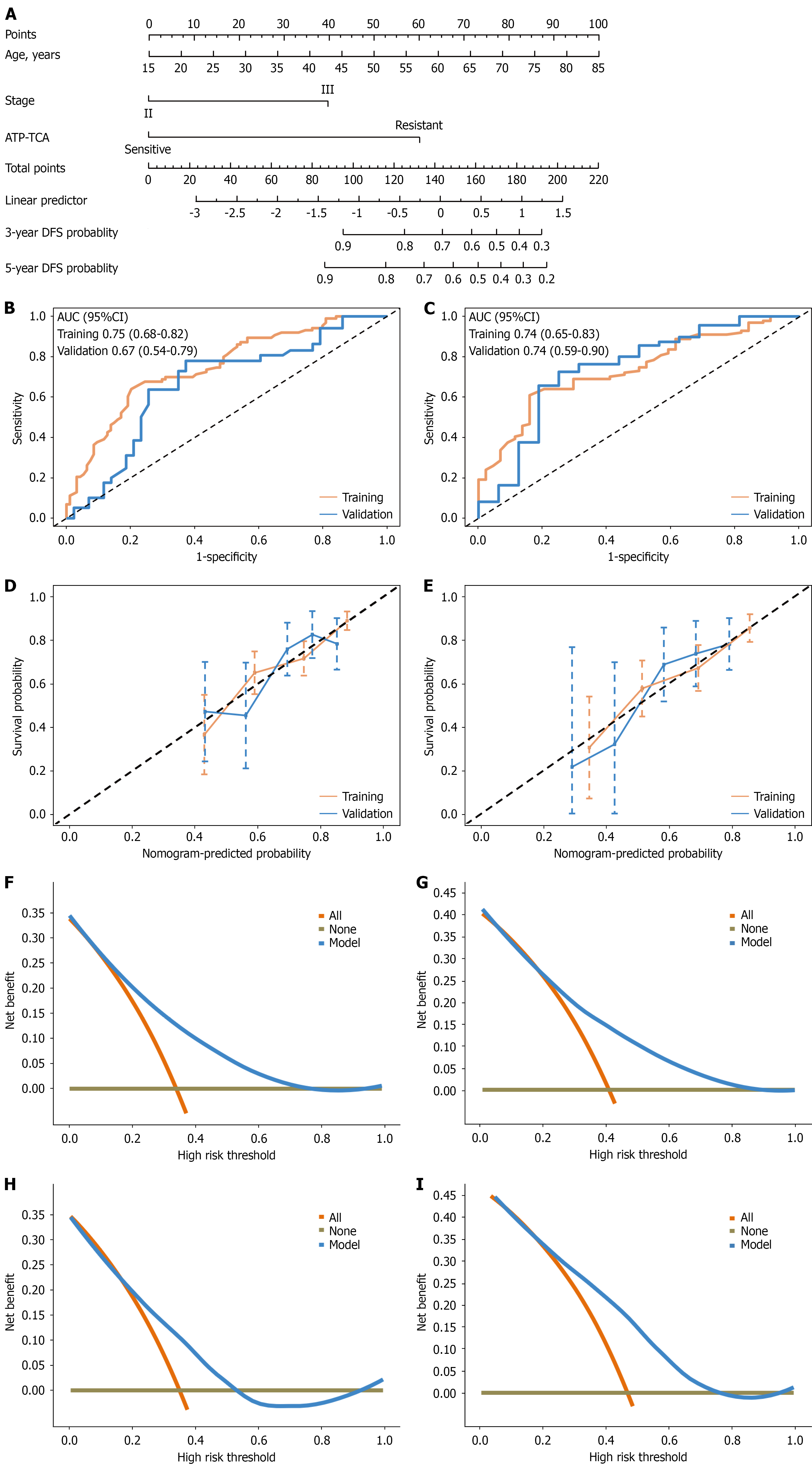
The clinical prediction model for DFS is visually represented as a nomogram in Figure 3A. The C-indexes for the training and validation sets were 0.68 [95% confidence interval (CI): 0.62-0.74] and 0.67 (95%CI: 0.59-0.75), respectively. The receiver operating characteristic curve analysis demonstrated that the AUC values for predicting 3-year DFS were 0.75 (95%CI: 0.68-0.82) in the training set and 0.67 (95%CI: 0.54-0.79) in the validation set in Figure 3B. Similarly, the AUC values for 5-year DFS were 0.74 (95%CI: 0.65-0.83) and 0.74 (95%CI: 0.59-0.90) in the training and validation sets, respectively (Figure 3C). The calibration curves for both the 3-year and the 5-year DFS rates indicated a high degree of consistency between the predicted outcomes and the actual observed results (Figure 3D and E). Furthermore, decision curve analysis demonstrated that this model provides significant net benefits across a wide range of threshold probabilities (Figure 3F-I).
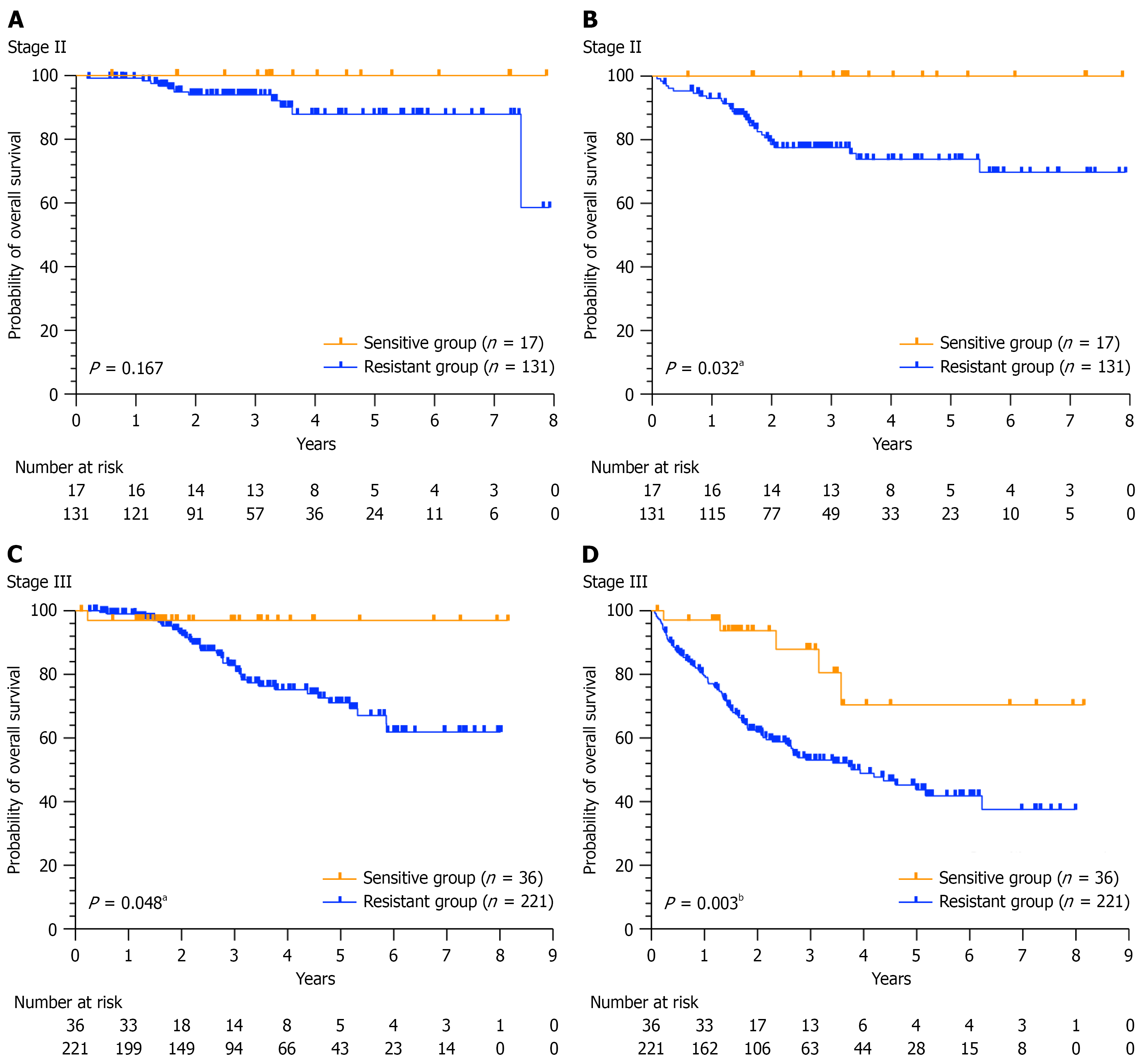
To further investigate this topic, we performed a stratified analysis based on tumour stage. However, owing to the limited sample size, a stratified clinical prediction model was not developed, instead, only Kaplan-Meier survival curve analysis was conducted. Among the 148 patients diagnosed with high-risk stage II disease, OS and DFS were longer among those in the sensitive group than among those in the drug-resistant group (P = 0.167, P = 0.032). Notably, a statistically significant difference was observed in the DFS (P < 0.05, Figure 4A and B). In a cohort of 257 patients diagnosed with stage III disease, the OS and DFS rates in the sensitive group were significantly greater than those in the resistant group (P = 0.048 and P = 0.003, respectively, Figure 4C and D).
Approximately one-third of all global CRC cases occur in China, and this proportion is increasing[2,16]. In clinical practice, the ATP-TCA assay is frequently utilized for individualized chemotherapy regimens in cancer patients. Previous studies have demonstrated that the ATP-TCA can effectively identify the heterogeneity of responses to specific chemo
The sensitivity of patients to the 5-FU + L-OHP regimen was 11.9%, which differs from that in previous reports. This difference may be due to factors such as patient population diversity, variations in inclusion and exclusion criteria, and different thresholds for defining sensitivity vs resistance. Cox regression analysis confirmed that the ATP-TCA ratio serves as an independent prognostic factor for DFS [P = 0.002, hazard ratio (95%CI): 4.98 (1.81-13.72)] in patients with CRC. Furthermore, clinical prediction models for predicting DFS were successfully developed and validated, thereby increasing enhancing the precision of clinical treatment. In patients whose tumours were predicted to be sensitive to 5-FU + L-OHP, chemotherapy significantly increased DFS (P = 0.027). Although Cox regression analysis in the training set suggested that ATP-TCA results could serve as an independent prognostic factor of OS in CRC patients, Kaplan-Meier analysis in the validation set indicated only a trend towards improved OS in the sensitive group compared with the resistant group (P = 0.055), which did not reach statistical significance. The observed effect may be explained by periodic follow-up examinations, allowing for early intervention upon the detection of recurrence. This prompt initiation of treatment likely contributed to the extension of OS. Additionally, Kaplan-Meier analysis was performed separately for patients in stages II and III. The results indicated that in stage II, the DFS of the sensitive group was significantly longer than that of the resistant group (P = 0.032). Although OS tended to increase, this increase did not reach statistical significance (P = 0.167). In stage III patients, both OS (P = 0.048) and DFS (P = 0.003) were significantly longer in the sensitive group than in the resistant group. The results of this study suggest that in vitro ATP-TCA results could predict the benefits of adjuvant chemotherapy in patients with CRC.
Research by Hur et al[20] revealed that for patients with unresectable colorectal liver metastasis, ATP-TCA yielded positive short-term treatment responses to L-OPH and a fluoropyrimidine-based regimen and improved resectability. Kim et al[21] reported that stage III CRC patients who were eligible for surgery and classified as L-OPH and a fluo
This study is subject to sample selection bias. To focus on the effects of chemotherapy, we excluded all patients who had received neoadjuvant (13), targeted (16), radiotherapy (33) or immunotherapy (87). Some patients received multiple treatments from the aforementioned options concurrently. Of the 532 patients screened, 127 were excluded based on this criterion, representing 23% of the screening population. Although this exclusion ensured internal consistency, it significantly limits the generalizability of our findings. Therefore, these conclusions are applicable only to patients receiving chemotherapy, which somewhat limits the clinical application of the ATP-TCA. Given the retrospective design and considerable loss to follow-up, the median follow-up period was 2.78 years. Consequently, the generalizability of our conclusions is restricted to the midterm prognosis of CRC patients. To address these limitations, we will continue to collect a larger cohort, extend the follow-up duration, and seek multicentre collaborations to validate our findings.
In conclusion, in this study, the clinical application of the ATP-TCA was retrospectively evaluated, revealing that this assay is an independent prognostic factor for DFS in patients with resectable CRC. Additionally, an ATP-TCA-sensitive chemotherapy regimen was shown to significantly improve OS and DFS in stage III CRC patients. Despite the need for further validation, this study provides evidence supporting the extension of the ATP-TCA to combination chemotherapy regimens for CRC while providing a theoretical basis and perspective for future advancements in personalized medicine.
The authors would like to thank the staff that assisted with the study in the First Affiliated Hospital of Soochow University.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12644] [Article Influence: 6322.0] [Reference Citation Analysis (6)] |
| 2. | Zheng RS, Chen R, Han BF, Wang SM, Li L, Sun KX, Zeng HM, Wei WW, He J. [Cancer incidence and mortality in China, 2022]. Zhonghua Zhong Liu Za Zhi. 2024;46:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 244] [Reference Citation Analysis (0)] |
| 3. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2351] [Article Influence: 195.9] [Reference Citation Analysis (2)] |
| 4. | Tjader NP, Toland AE. Immunotherapy for colorectal cancer: insight from inherited genetics. Trends Cancer. 2024;10:444-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Skelton WP 4th, Franke AJ, Iqbal A, George TJ. Comprehensive literature review of randomized clinical trials examining novel treatment advances in patients with colon cancer. J Gastrointest Oncol. 2020;11:790-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Sebag-Montefiore D, Cervantes A, Rodel C. Preoperative Treatment of Locally Advanced Rectal Cancer. N Engl J Med. 2023;389:1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Simillis C, Singh HKSI, Afxentiou T, Mills S, Warren OJ, Smith JJ, Riddle P, Adamina M, Cunningham D, Tekkis PP. Postoperative chemotherapy improves survival in patients with resected high-risk Stage II colorectal cancer: results of a systematic review and meta-analysis. Colorectal Dis. 2020;22:1231-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Van der Jeught K, Xu HC, Li YJ, Lu XB, Ji G. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol. 2018;24:3834-3848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 448] [Cited by in RCA: 437] [Article Influence: 54.6] [Reference Citation Analysis (5)] |
| 9. | Gmeiner WH, Okechukwu CC. Review of 5-FU resistance mechanisms in colorectal cancer: clinical significance of attenuated on-target effects. Cancer Drug Resist. 2023;6:257-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 10. | Zeng K, Li W, Wang Y, Zhang Z, Zhang L, Zhang W, Xing Y, Zhou C. Inhibition of CDK1 Overcomes Oxaliplatin Resistance by Regulating ACSL4-mediated Ferroptosis in Colorectal Cancer. Adv Sci (Weinh). 2023;10:e2301088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 100] [Reference Citation Analysis (0)] |
| 11. | Yang L, Yang J, Kleppe A, Danielsen HE, Kerr DJ. Personalizing adjuvant therapy for patients with colorectal cancer. Nat Rev Clin Oncol. 2024;21:67-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 68] [Reference Citation Analysis (0)] |
| 12. | Chen Z, Zhang S, Ma S, Li C, Xu C, Shen Y, Zhao J, Miao L. Evaluation of the in vitro Chemosensitivity and Correlation with Clinical Outcomes in Lung Cancer using the ATP-TCA. Anticancer Agents Med Chem. 2018;18:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Bian Y, Huang M, Ma S, Liu L, Xia F, Chen Z, Yu D, Huang C, Miao L. Adenosine triphosphate-based tumor chemosensitivity assay may predict the clinical outcomes of gastric cancer patients receiving taxane-based post-operative adjuvant chemotherapy. Chin Med J (Engl). 2022;135:1383-1385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Xia F, Ma S, Bian Y, Yu D, Ma W, Miao M, Huang C, Miao L. A retrospective study of the correlation of in vitro chemosensitivity using ATP-TCA with patient clinical outcomes in acute myeloid leukemia. Cancer Chemother Pharmacol. 2020;85:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Fehm T, Zwirner M, Wallwiener D, Seeger H, Neubauer H. Antitumor activity of zoledronic acid in primary breast cancer cells determined by the ATP tumor chemosensitivity assay. BMC Cancer. 2012;12:308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Wang W, Yin P, Liu YN, Liu JM, Wang LJ, Qi JL, You JL, Lin L, Meng SD, Wang FX, Zhou MG. Mortality and years of life lost of colorectal cancer in China, 2005-2020: findings from the national mortality surveillance system. Chin Med J (Engl). 2021;134:1933-1940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Whitehouse PA, Knight LA, Di Nicolantonio F, Mercer SJ, Sharma S, Cree IA; Portsmouth Colorectal Cancer Multidisciplinary Team. Heterogeneity of chemosensitivity of colorectal adenocarcinoma determined by a modified ex vivo ATP-tumor chemosensitivity assay (ATP-TCA). Anticancer Drugs. 2003;14:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Huh JW, Park YA, Lee KY, Sohn SK. Heterogeneity of adenosine triphosphate-based chemotherapy response assay in colorectal cancer--secondary publication. Yonsei Med J. 2009;50:697-703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Yuan Y, Wang X, Chen G, Wang Y, Sheng W, Li X, Zhou A, Zhang Z, Li G, Cai S, Xu R, Li J, Zhang S. Updates in version 2019 of CSCO guidelines for colorectal cancer from version 2018. Chin J Cancer Res. 2019;31:423-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Hur H, Kim NK, Kim HG, Min BS, Lee KY, Shin SJ, Cheon JH, Choi SH. Adenosine triphosphate-based chemotherapy response assay-guided chemotherapy in unresectable colorectal liver metastasis. Br J Cancer. 2012;106:53-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Kim CD, Kim SH, Jung SH, Kim JH. Clinical value of an adenosine triphosphate-based chemotherapy response assay in resectable stage III colorectal cancer. Ann Surg Treat Res. 2019;97:93-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/