Published online Nov 15, 2025. doi: 10.4251/wjgo.v17.i11.110840
Revised: July 23, 2025
Accepted: September 24, 2025
Published online: November 15, 2025
Processing time: 149 Days and 21.8 Hours
Liver cancer poses a significant public health threat. The difference between disease patterns and national policies is crucial to elucidating factors influencing hepatocellular carcinoma (HCC) incidence.
To investigate the secular trend and disease pattern of liver cancer in Taiwan, Poland, and Belgium.
This population-based cohort study presents the incidence, period, and cohort effects in HCC incidence between 2000 and 2019 in Taiwan, Poland, and Flanders, Belgium. Data on HCC were obtained from cancer registry data from Taiwan, Poland, and regional data from Belgium. Age-standardized incidence rates (ASIRs), annual per
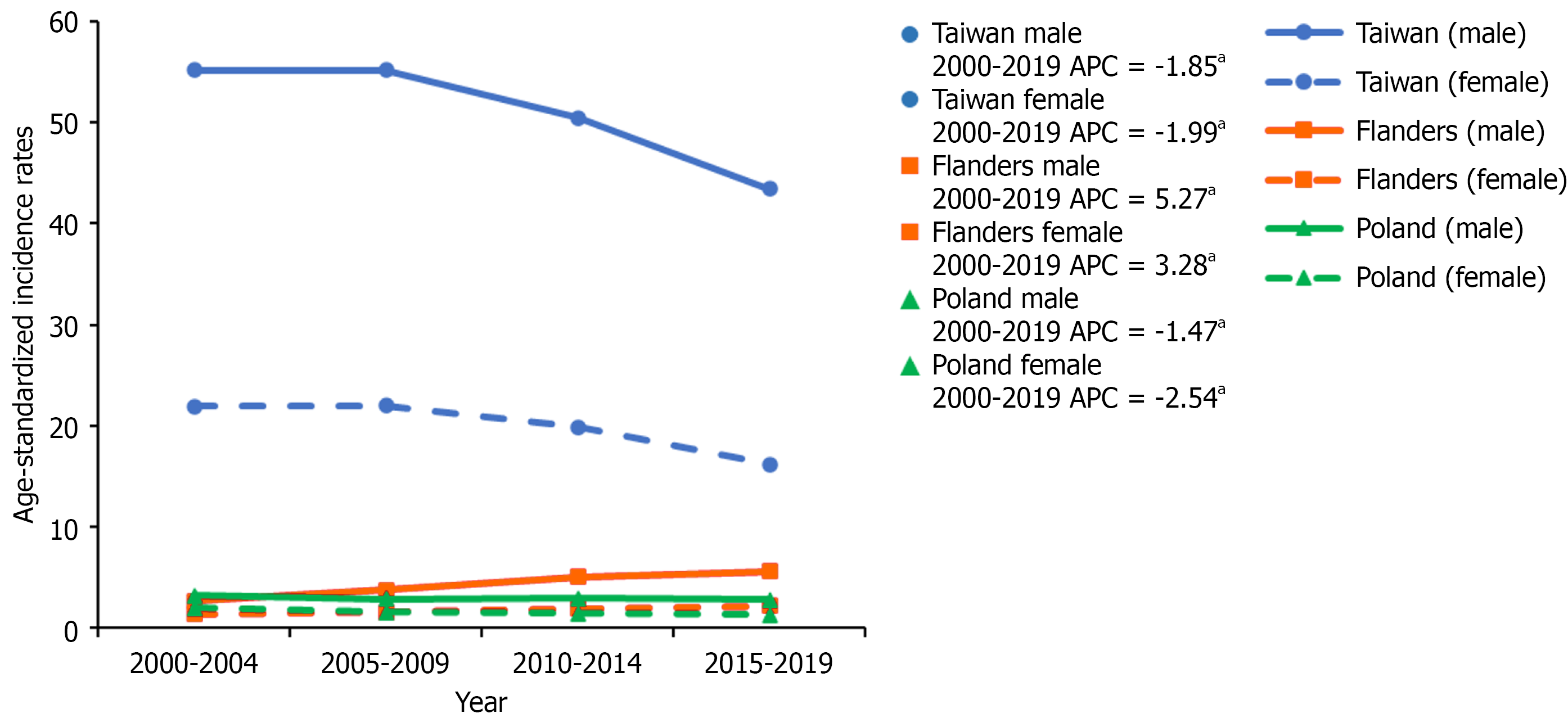
Taiwan’s ASIR decreased from 2000 to 2019 (males: 55.17 to 43.42, females: 21.91 to 16.20, per 100000). In Poland, ASIR declined from 2000 to 2019 (males: 3.21 to 2.77, females: 1.95 to 1.32, per 100000). However, Flanders experienced an increase in ASIR from 2000 to 2019 (males: 2.66 to 5.63, females: 1.40 to 2.20, per 100000). In Taiwan, the cohort effect rate ratio increased from 1915 to 1935 (males: 1.02 to 1.36, females: 1.04 to 1.54) and decreased from 1935 to 1989 (males: 1.36 to 0.22, females: 1.54 to 0.20). In Poland, rate ratios consistently decreased (males: 1.75 to 0.25, females: 3.46 to 0.26). Flanders exhibited an increase in both males (0.14 to 2.52, 1915 to 1975) and females (0.53 to 3.66, 1915 to 1989).
Taiwan and Poland’s declining ASIR may be due to effective hepatitis B virus immunization and viral hepatitis therapy. Flanders’ persistent increase may be tied to higher HCC risk in high hepatitis C virus risk populations.
Core Tip: Due to differences in etiological factors and nationwide policies, limited evidence exists regarding the prevention outcomes of hepatocellular carcinoma (HCC) in different countries. This retrospective cohort study presented the incidence, period, and cohort effects in HCC incidence between 2000 and 2019 in Taiwan, Poland, and Flanders, Belgium. Decreasing cohort effect in Taiwan and Poland resulted from effective hepatitis B virus immunization and viral hepatitis therapy. The increasing cohort effect in Flanders, Belgium suggests that further measures are needed to prevent the increasing risk of the hepatitis C virus.
- Citation: Huang ZZ, Żmudka K, Ruggiano V, Hsu WL, Liu J, Chiang CJ, Chen YC, Wang V. Secular trend in universal hepatocellular carcinoma prevention: Taiwan, Poland, and Belgium experience. World J Gastrointest Oncol 2025; 17(11): 110840
- URL: https://www.wjgnet.com/1948-5204/full/v17/i11/110840.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i11.110840
Primary liver cancer is the seventh most common type of cancer and the second leading cause of cancer-related deaths[1]. Hepatocellular carcinoma (HCC) is the major type of primary liver cancer. Depending on the population, the risk factors for HCC vary considerably. However, HCC incidence is mostly attributed to chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, metabolic dysfunction, and alcohol consumption[2]. Viral hepatitis is the major risk factor for HCC. Generally speaking, HBV infection is the predominant type across East Asian countries, whereas in European countries, the primary concern is HCV infection[2]. In previous decades, national vaccination programs[3-5], screening programs[6,7], hospital-transmission control[6], and novel antiviral therapies[7-9] were implemented. Such programs resulted in different rates of reduction in viral hepatitis, and thus, a later reduction in HCC incidence.
In this study, we analyzed the change in HCC incidence between 2000 and 2019 in Taiwan, Poland, and the Flanders region of Belgium, and discussed the major risk factors contributing to observed changes and trends. The discrepancies between HCC incidence and major etiological factors across Taiwan, Poland, and Flanders will be the central purpose of this article.
We conducted a study of HCC cases diagnosed from 2000 to 2019 using data from the Taiwan cancer registry[10], the Polish national cancer registry[11], and the Belgian cancer registry[12], respectively. Each cancer registry is a nationwide population-based database and provides critical cancer data. The completeness of the Taiwan cancer registry was 97.7% with an overall 91.3 microscopically verified percentage (MV) (%) in 2011[13]. The completeness of the Polish national cancer registry was 91% with a 66% mortality-to-incidence ratio in 2010[14]. The completeness of the Belgian cancer registry for Flanders for invasive tumors, except for non-melanoma skin cancer, was 95% for the clinical network and 91% for the pathological network in 2019[15]. HCC was coded using the third edition of the International Classification of Diseases for Oncology for all three cancer registries. The third edition of the International Classification of Diseases for Oncology morphology code for HCC is No. C22.
We calculated age-specific incidence rates for various age groups (30-34, 35-39, 40-44, …, 80-84, 85-89) and age-standardized incidence rate (ASIR), per 100000 people. ASIR was computed using the World Health Organization (WHO) 2000 standard population with age-truncated population ≥ 30 and ≤ 89 years. We divided a 20-year time period and birth cohort into 5-year intervals and calculated annual percentage changes and 95% confidence intervals (CIs) with the Joinpoint regression program (version 5.0)[16].
To investigate the temporal trends in HCC incidence, we applied an age-period-cohort (APC) analysis using the National Cancer Institute web-based APC tool[17]. The tool estimates longitudinal age-specific rates, period effects, and cohort rate ratios (CRRs) based on a Poisson log-linear model. HCC incidence data were stratified by 5-year age groups (30-34 to 85-89 years), calendar periods (2000-2004 to 2015-2019), and corresponding birth cohorts (1915-1919 to 1985-1989). Although the tool computes the estimates internally, the underlying R code is available for transparency and reproducibility. In this model, age, period, and cohort effects are expressed as incidence rate ratios. The expected incidence rate for individuals born in year c and followed up at age a is estimated using the following equation: (1) Longitudinal age (a|c0): The longitudinal age-specific rate at age a based on a reference cohort c0; (2) CRR (c|c0): The CRR comparing birth cohort c to the reference cohort; and (3) Period deviation (c + a): The period deviation at calendar year
In Taiwan, the ASIR from 2000-2004 to 2015-2019 significantly decreased for both males and females (55.17 to 43.42 and 21.91 to 16.20, per 100000, respectively). The APC was -1.85 (95%CI: -3.30 to -0.49) in males and -1.99 (95%CI: -3.92 to
| Country/region | HCC case number | Population (≥ 30 years and ≤ 89 years) in 1000 | ASIR of HCC (≥ 30 years and ≤ 89 years) per 100000 | |||||
| 2000 | 2019 | 2000-2004 | 2005-2009 | 2010-2014 | 2015-2019 | |||
| Taiwan | Male | 153427 | 11390 | 11705 | 55.17 | 55.20 | 50.42 | 43.42 |
| Female | 65370 | 10113 | 11897 | 21.91 | 21.95 | 19.89 | 16.20 | |
| Poland | Male | 16696 | 18537 | 18373 | 3.21 | 2.89 | 2.91 | 2.77 |
| Female | 13126 | 19716 | 19584 | 1.95 | 1.60 | 1.42 | 1.32 | |
| Flanders | Male | 4689 | 3050 | 3294 | 2.66 | 3.76 | 5.03 | 5.63 |
| Female | 2237 | 3081 | 3295 | 1.40 | 1.57 | 1.92 | 2.20 | |
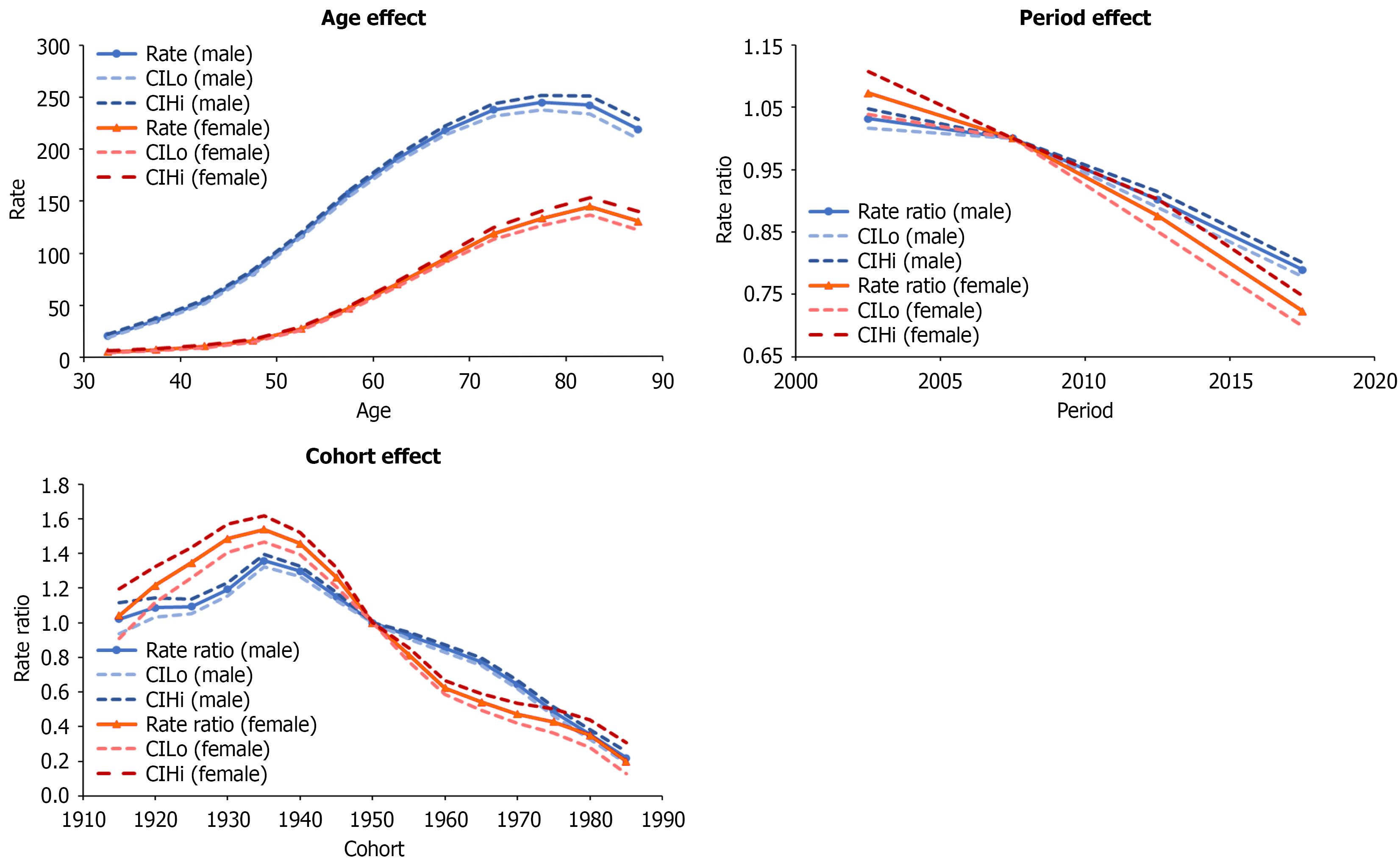
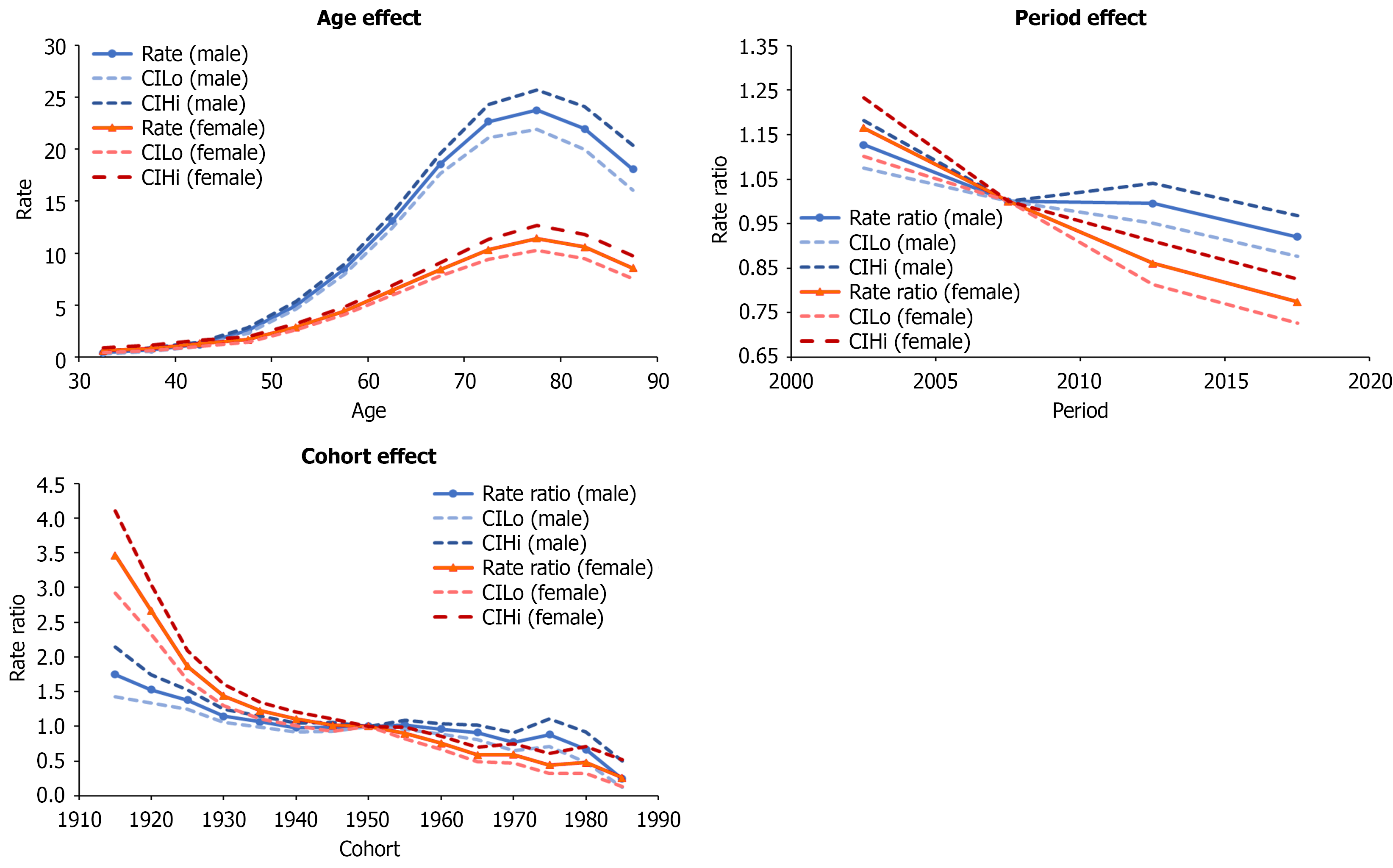
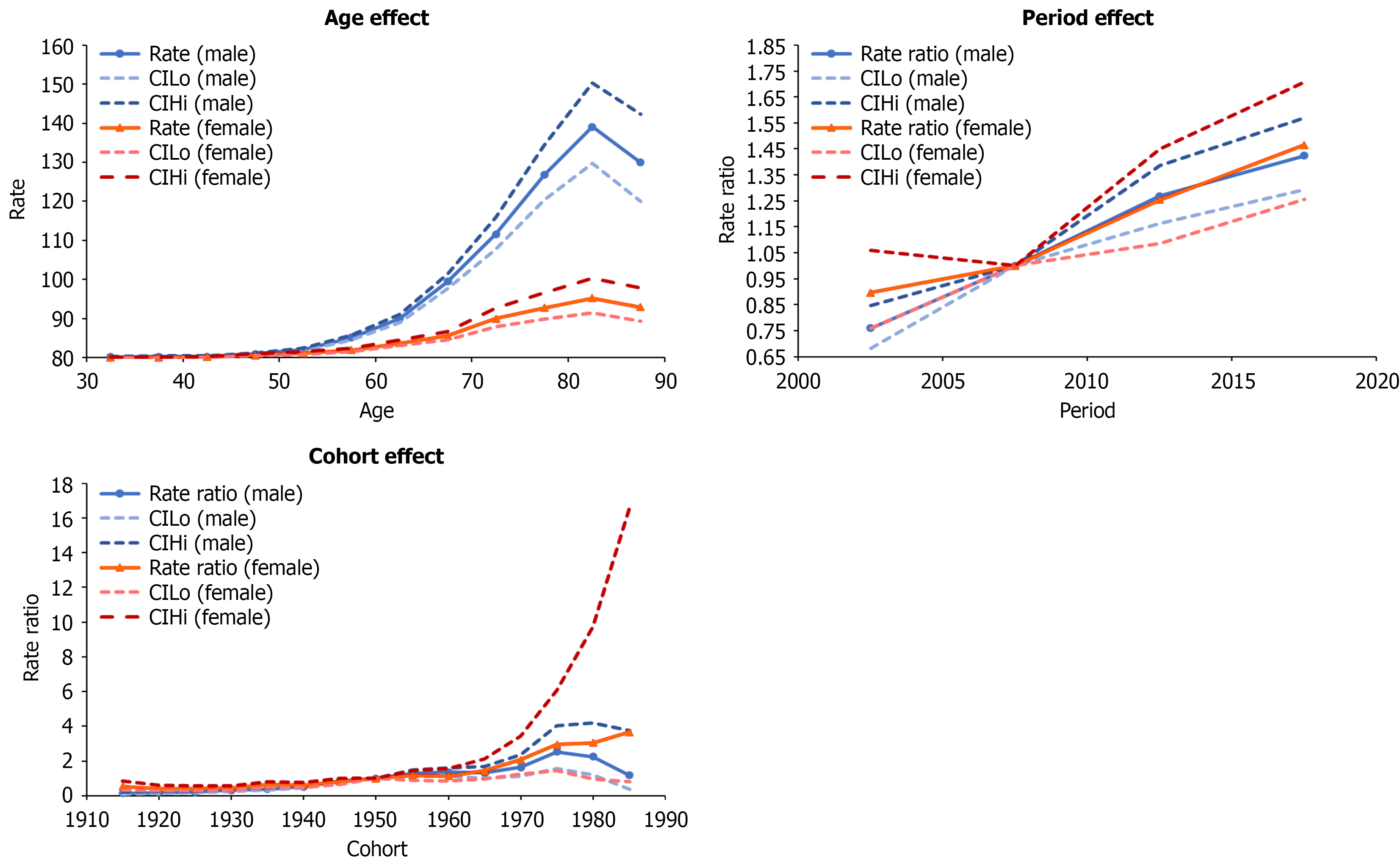
Age, period, and cohort effects are presented in (Figures 2, 3, and 4) for Taiwanese, Polish, and Flemish data, respectively. Age effects showed similar trends in all three countries, showing increasing incidence with increasing age, and then slightly decreasing in the oldest age group. The highest age effects in Taiwan were 243.99 in males aged 75-79 and 144.04 in females aged 80-84. In Poland, they were 23.73 and 11.42 in males and females aged 75-79. In Flanders, they were 118.08 and 30.46 in males and females aged 80-84. The rate ratios of the period effects from 2000 to 2019 decreased for both males and females in Taiwan (1.03 to 0.79 and 1.07 to 0.72, respectively) and Poland (1.13 to 0.92 and 1.17 to 0.77, respectively), but increased for both males and females in Flanders (0.76 to 1.42 and 0.90 to 1.47, respectively).
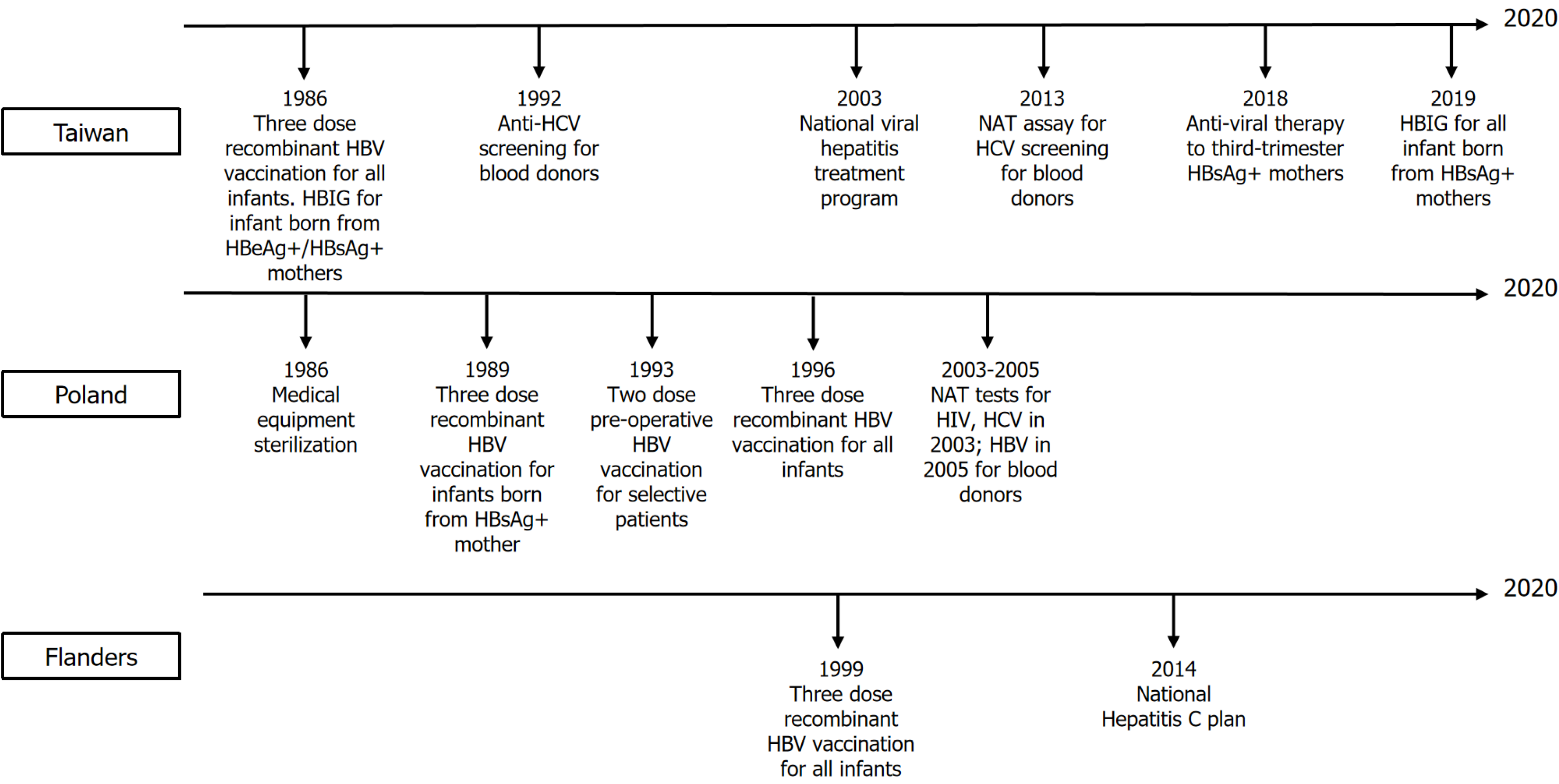
The rate ratios of the cohort effects in Taiwan increased during 1915-1935 (1.02 to 1.36 in males and 1.04 to 1.54 in females), but continually decreased from 1935 to 1989 (1.36 to 0.22 in males and 1.54 to 0.20 in females). In Poland, the rate ratios of the cohort effects continually decreased from 1915 to 1989 (1.75 to 0.25 in males and 3.46 to 0.26 in females). In Flanders, the rate ratios of the cohort effects for males increased from 0.14 to 2.52 for 1915 to 1975, and then decreased from 2.52 to 1.18 for 1975 to 1989. In females, rate ratios increased from 0.53 to 3.66 for 1915 to 1989. National or regional intervention of viral hepatitis or HCC in Taiwan, Poland, and Flanders is shown in Figure 5.
The ASIR and age-specific incidence rates indicated that both Taiwan and Poland had similar decreasing trends by period and by sex; however, Flemish data showed consistently increasing trends by sex and by period. For both Taiwan and Poland, this observation can be traced back to several major factors. Long-term HBV immunization programs with high coverage rates resulted in decreasing trends of HCC prevalence through individual and herd immunity in the vaccinated cohort[3,4,18]. The national viral hepatitis therapy program launched in Taiwan in 2003 may have contributed to reducing HCC risk[7]. Hospital transmission control in Poland, such as sterilizing medical equipment or blood donor screening, may be associated with decreasing HCC prevalence[6]. In Flanders, the reasons for increasing HCC risk could be due to higher HCV prevalence in certain populations, especially injection drug users (IDUs) and patients with human immunodeficiency virus (HIV)[9].
In Taiwan and Poland, HBV and HCV infection are the main risk factors for HCC[19,20], with HBV infection being the majority in Taiwan and HCV being a more prominent risk factor for HCC in Poland[21]. In Taiwan, the 30-34 age group (1985-1989) had the lowest cohort effect. The decrease may be attributed to the HBV vaccination policy starting in 1984 for infants of HBV-infected mothers, which expanded to all infants in 1986. Moreover, hepatitis B immune globulin (HBIG) was provided to infants of hepatitis B e antigen-positive/hepatitis B surface antigen-positive (HBsAg+) mothers since 1986 and to all HBsAg+ mothers since 2019. Seroepidemiological data collected between 1984 and 2014 demonstrated that the seropositive rates of HBsAg and antibody to hepatitis B core antigen (anti-HBc) were significantly lower among individuals in the vaccinated cohort than those in the unvaccinated cohort[22]. Another 20-year follow-up study conducted from 1983 to 2004 pointed out that the universal three-dose recombinant HBV vaccination policy significantly reduced HCC incidence rates in the vaccinated cohort[3]. However, highly infectious mothers, especially hepatitis B e antigen+/HBsAg+, were still an important risk factor for HBV vaccine failure[23]. As a result, starting in 2018, anti-viral therapy, such as tenofovir and telbivudine, was given to highly infectious mothers in the third trimester to prevent mother-to-infant transmission, reducing the HBV DNA positivity rate by 25.3% in HBIG+ infants compared with HBIG- infants[24].
In Taiwan, for the unvaccinated population, cohort effects increased from 1915 to 1935, and then decreased. This decreasing trend may be attributed to several reasons listed below. First, the national viral hepatitis therapy program was launched in 2003. Based on the community-based Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-HBV/HCV study, 15%-25% of patients with chronic hepatitis B or chronic hepatitis C eligible for reimbursed treatment were covered by 2011[25,26]. There was a 22% reduction in chronic liver diseases (LDs) and cirrhosis-associated mortality, a 24% reduction in HCC mortality, and a 14% reduction in HCC incidence in 2008-2011, compared with the 4-year period before the program launch (2000-2003)[7]. As a result, the antiviral therapy is considered effective to prevent the occurrence of end-stage LDs (ESLDs) and HCC. Second, the disposable syringes and improvement of medical equipment sterilization since the 1980s. Third, universal anti-HCV serologic screening of blood donors was also implemented in 1992 and further enhanced by the introduction of highly sensitive nucleic acid test (NAT) assays in 2013. Lastly, several HCV screening programs were also launched since 2011[27]. A nationwide seroepidemiological study demonstrated that the overall crude prevalence of anti-HCV decreased from 15.5 to 4.5 per 1000 blood donors between 1999 and 2017[28]. Overall, as the HBV incidence decreased throughout the period, HCV incidence also decreased. Liver transplantation (LT) was also an important management for HCC or ESLD. However, due to strict patient selection criteria and a shortage of organs, most patients with ESLD or HCC were not able to undergo LT. Although overall 1-year and 3-year survival rates in patients with LT were 86% to 98% and 61% to 96%[29-31], respectively, a previous study showed that only 3017 LT cases were performed from 2003 to 2012[32], which is far less than patients with HCC. As a result, transarterial embolism or surgical resection remain an important therapy for HCC curation or survival im
In Poland, the cohort effect for the 30-34 age group (1985-1989) was also lower than any previous cohort. Infants of HBsAg+ mothers were vaccinated since 1989, and this policy expanded to all newborns in 1996[4]. However, in contrast to Taiwan, where most HBV cases resulted from highly infectious mothers and incomplete immunization[18], Polish HBV cases mostly resulted from hospital transmission[6]. From 2010 to 2014, 74% of acute HBV cases were attributed to hospital transmission or outpatient medical procedures, and the overall hospitalization population attributable risk was 25.7% (95%CI: 20.3%-31.1%)[6]. Due to the low prevalence of HBV infection and horizontal transmission being the major transmission route, a two-dose preoperative vaccination policy for selective surgical patients was implemented in 1993-1997.
In Poland, for those without vaccination coverage, cohort effects consistently decreased during the period from 1915 to 1989. Although there is no nationwide antiviral therapy program, policies for hospital-transmission control[6] and HBV vaccination in high-risk groups[4] in the past may have contributed to the reduced HCC incidence. This is supported by data showing that HBV incidence dropped from approximately 45 to under 15 cases per 100000 by 1997 following the introduction of medical sterilization protocols and later targeted vaccination programs[33]. Programs to control hospital-related infections included improving medical equipment sterilization and early vaccination programs for health care workers[6,34]. Moreover, NAT screening tests in blood donors for both HCV and HIV began in 2003, and NAT screening tests for HBsAg and anti-HBc began in 2005 with a two-decade decrease in the prevalence of HBsAg among donors[35]. As a result, such improvements might have contributed to the decreasing trends in the non-vaccinated cohort. Lastly, data for the LTs in Poland during the 2001-2017 period showed that 3332 primary LTs were performed, of whom patients with HCV infection accounted for 581 cases, whereas those with only HBV accounted for 185 cases[36]. More recent data showed that the total number of LTs in 2022 was 362[37]. Similar to the Taiwanese experience, the number of patients who annually undergo LTs in Poland is still limited.
In the Flanders region of Belgium, cohort effects showed an overall increasing trend after 1915. In Belgium, HCV infection is the major risk factor for HCC[38]. In 2015, the HCV RNA seropositivity rates among IDU, people living with HIV (PLHIV), and men who have sex with men (MSM) with HIV were 25.0%, 6.3%, and 18.4%, respectively, which was higher than the 0.1% in the general population[9]. Moreover, IDU, PLHIV, and MSM with HIV accounted for 18.3%, 8.7%, and 8.5% (overall 35.5%) of patients with HCV, respectively, which are the three most important risk factors for HCV transmission in Belgium[9]. The prevalence of IDUs increased from 2.9% to 3.5% from 2000 to 2010 and continues to increase in recent years[39]. Subnational surveillance data also revealed that about 30% of undiagnosed HIV patients lived in Antwerp, the largest city in Flanders, where MSM, particularly those born abroad, account for the most cases, showing much higher rates than other regions in Belgium[40]. These factors might contribute to the increasing cohort effect in Flanders.
WHO’s Global Hepatitis Strategy aims to eliminate hepatitis by 2030 – a 90% reduction in incidence and 65% reduction in mortality. In 2014, Taiwan’s high vaccination coverage rate led to significantly lower HBsAg and anti-HBc prevalence rates in children under 30 years (0.5% and 4.5%, respectively) compared to older individuals (30-50 years) born before the vaccination program (HBsAg: 6.7%; anti-HBc: 44.1%)[22]. Since 2018, anti-viral therapy for highly infectious mothers in their third trimester has further bolstered prevention efforts against vertical transmission[24], providing strong evidence of HBV infection prevention success. The Taiwanese government’s 2025 short-term goals are to maintain HBV vaccination coverage rate above 98% and raise coverage rate for the national viral hepatitis screening program to 70%[41]. Despite this, studies indicate a rising HCV infection risk in certain groups like homosexual, IDU, and HIV patients[42]. Therefore, goals include precision screening and expanding oral anti-HCV drug coverage to 40% enhancing health education for IDUs, aiming for a 50% reduction in viral hepatitis[41].
In Poland, the HBV vaccination coverage rate was 90.9% in 2018[43], which is still less than the 2020 WHO target. Thus, a vaccine coverage rate over 95% will be the future target[43]. For HCV treatment, due to the availability of highly effective and safe direct-acting antivirals (DAAs)[8], HCV infection is highly controlled, with an average 0.4% HCV RNA positivity in recent years. Moreover, the efficacy of DAAs improved from 50% during 2009-2013 to around 97% during 2016-2018. It is predicted to reach 99% efficacy and reduce the HCV RNA positivity rate to 0.3% by 2030[8].
In Flanders, the overall HBV and HCV prevalence was low, whereas the IDU, PLHIV, and MSM with HIV had a significantly higher HCV risk than the general population. Instead of providing nationwide antiviral therapy, focusing on reducing transmission and providing antiviral therapy to high-risk groups would be more important. With the DAA drug, a ≥ 95% cure rate, achieving HCV elimination[44,45]. However, unlimited access to DAA started late in 2019[46]. For the HCV treatment program, 67% of PLHIV and migrants and 73% of IDUs will be left untreated by 2030[47] to reach the WHO Global Hepatitis Strategy. A Belgian “HCV plan” was developed in 2014 in response to the WHO’s objectives, and included developing a national HCV screening program, expanded access to HCV medication, and epidemiological follow-up studies[48]. However, the effect of the program is still under observation.
As the prevalence of hepatitis B and C continues to decline following widespread antiviral and vaccination programs[49], non-viral LD such as alcoholic LD (ALD) and metabolic-associated fatty LD (MAFLD) are becoming relatively more prominent contributors to HCC. While these conditions may not confer significant cancer risk during early stages, their progression to advanced fibrosis or cirrhosis is a well-established pathway to HCC development[50]. Given this epidemiologic shift, long-term surveillance is essential to monitor the evolving role of MAFLD and ALD in hepatocarcinogenesis. Preventive strategies should prioritize modifiable risk factor control through lifestyle interventions, including weight management, dietary improvement, physical activity promotion, and reduction of harmful alcohol use.
In this study, we analyzed long-term trends of HCC in Taiwan, Poland, and Flanders from 2000 to 2019, and discussed differences in trends and risk factors. Our study observed age groups from 30 to ≥ 85 over 20 years. We also compared public health policies across countries and suggested improvements to meet the WHO’s 2030 Global Hepatitis Strategy. The limitations of our study include the use of three cancer registry databases, with potential underrepresentation of HCC incidence in Poland due to discrepancies with National Health Fund Institution reports, and data from only a single region in Belgium. However, the changes in overall HCC incidence trends and the explainable differences in etiology are of particular importance in this study. Understanding the future impact of metabolic syndrome on liver cancer necessitates extended research efforts for conclusive findings.
To achieve the WHO’s 2030 goals of eliminating HBV and HCV, urgent and concerted international efforts are essential, with tailored strategies for various regions or countries. Increasing diagnosis and treatment coverage through robust public health policies is crucial. Sustainable financing and innovation are also pivotal in transforming the global response to hepatitis, facilitating the development of vaccines, diagnostics, and treatments.
The authors are grateful to Professor Jerzy Jaroszewicz for his invaluable suggestions.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56575] [Article Influence: 7071.9] [Reference Citation Analysis (134)] |
| 2. | Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15 Suppl 4:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 362] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 3. | Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, Chu HC, Wu TC, Yang SS, Kuo HS, Chen DS; Taiwan Hepatoma Study Group. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101:1348-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 435] [Article Influence: 25.6] [Reference Citation Analysis (1)] |
| 4. | Galimska J. The expanded programme on immunization calendar in Poland. Vaccine. 2000;18 Suppl 1:S41-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Theeten H, Hutse V, Hoppenbrouwers K, Beutels P, VAN Damme P. Universal hepatitis B vaccination in Belgium: impact on serological markers 3 and 7 years after implementation. Epidemiol Infect. 2014;142:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Stępień M, Zakrzewska K, Rosińska M. Significant proportion of acute hepatitis B in Poland in 2010-2014 attributed to hospital transmission: combining surveillance and public registries data. BMC Infect Dis. 2018;18:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Chiang CJ, Yang YW, Chen JD, You SL, Yang HI, Lee MH, Lai MS, Chen CJ. Significant reduction in end-stage liver diseases burden through the national viral hepatitis therapy program in Taiwan. Hepatology. 2015;61:1154-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Flisiak R, Zarębska-Michaluk D. Perspectives of hepatitis C virus (HCV) elimination in Poland. Clin Exp Hepatol. 2019;5:210-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Busschots D, Ho E, Blach S, Nevens F, Razavi H, Van Damme B, Vanwolleghem T, Robaeys G. Ten years countdown to hepatitis C elimination in Belgium: a mathematical modeling approach. BMC Infect Dis. 2022;22:397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Taiwan Cancer Registry. [cited 19 January 2024]. Available from: https://twcr.tw/?page_id=1843&lang=en. |
| 11. | Polish National Cancer Registry. [cited 19 January 2024]. Available from: https://onkologia.org.pl/pl/publikacje. |
| 12. | Belgian Cancer Registry. [cited 19 January 2024]. Available from: https://kankerregister.org/default.aspx?lang=EN. |
| 13. | Chiang CJ, You SL, Chen CJ, Yang YW, Lo WC, Lai MS. Quality assessment and improvement of nationwide cancer registration system in Taiwan: a review. Jpn J Clin Oncol. 2015;45:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 244] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 14. | Wojcieszak PZ, Poletajew S, Rutkowski D, Radziszewski P. The incidence of renal cancer in Polish National Cancer Registry: is there any epidemiological data we can rely on? Cent European J Urol. 2014;67:253-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Rosskamp M, De Schutter H, Henau K, Nackaerts K, Van Meerbeeck JP, Praet M, Van Eycken L. Assessing the completeness and correctness of the registration of malignant mesothelioma in Belgium. Lung Cancer. 2018;122:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | National Cancer Institute. Joinpoint Regression Program, Version 5.0. [cited 19 January 2024]. Available from: https://surveillance.cancer.gov/joinpoint/. |
| 17. | Rosenberg PS, Check DP, Anderson WF. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2014;23:2296-2302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 570] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 18. | Chang MH, You SL, Chen CJ, Liu CJ, Lai MW, Wu TC, Wu SF, Lee CM, Yang SS, Chu HC, Wang TE, Chen BW, Chuang WL, Soon MS, Lin CY, Chiou ST, Kuo HS, Chen DS; Taiwan Hepatoma Study Group. Long-term Effects of Hepatitis B Immunization of Infants in Preventing Liver Cancer. Gastroenterology. 2016;151:472-480.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 19. | Chang IC, Huang SF, Chen PJ, Chen CL, Chen CL, Wu CC, Tsai CC, Lee PH, Chen MF, Lee CM, Yu HC, Lo GH, Yeh CT, Hong CC, Eng HL, Wang J, Tseng HH, Hsiao CH, Wu HI, Yen TC, Liaw YF. The Hepatitis Viral Status in Patients With Hepatocellular Carcinoma: a Study of 3843 Patients From Taiwan Liver Cancer Network. Medicine (Baltimore). 2016;95:e3284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Łapiński TW, Tarasik A, Januszkiewicz M, Flisiak R. Clinical aspects and treatment of hepatocellular carcinoma in north-eastern Poland. Clin Exp Hepatol. 2021;7:79-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Wasiak D, Pliszczyński J, Łągiewska B, Jonas M, Panczyk M, Małkowski P, Lisik W, Kosieradzki M. The epidemiology of hepatocellular cancer in Poland. Clin Exp Hepatol. 2018;4:13-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Ni YH, Chang MH, Jan CF, Hsu HY, Chen HL, Wu JF, Chen DS. Continuing Decrease in Hepatitis B Virus Infection 30 Years After Initiation of Infant Vaccination Program in Taiwan. Clin Gastroenterol Hepatol. 2016;14:1324-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Chien YC, Jan CF, Chiang CJ, Kuo HS, You SL, Chen CJ. Incomplete hepatitis B immunization, maternal carrier status, and increased risk of liver diseases: a 20-year cohort study of 3.8 million vaccinees. Hepatology. 2014;60:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Chen HL, Lee CN, Chang CH, Ni YH, Shyu MK, Chen SM, Hu JJ, Lin HH, Zhao LL, Mu SC, Lai MW, Lee CL, Lin HM, Tsai MS, Hsu JJ, Chen DS, Chan KA, Chang MH; Taiwan Study Group for the Prevention of Mother-to-Infant Transmission of HBV (PreMIT Study); Taiwan Study Group for the Prevention of Mother-to-Infant Transmission of HBV PreMIT Study. Efficacy of maternal tenofovir disoproxil fumarate in interrupting mother-to-infant transmission of hepatitis B virus. Hepatology. 2015;62:375-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 199] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 25. | Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, Iloeje UH, Su J, Hsiao CK, Wang LY, You SL, Lu SN, Chen CJ; Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer in HBV (REVEAL–HBV) Study Group. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology. 2011;141:1240-1248, 1248.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 26. | Lee MH, Yang HI, Lu SN, Jen CL, Yeh SH, Liu CJ, Chen PJ, You SL, Wang LY, Chen WJ, Chen CJ. Hepatitis C virus seromarkers and subsequent risk of hepatocellular carcinoma: long-term predictors from a community-based cohort study. J Clin Oncol. 2010;28:4587-4593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 27. | Chien RN, Lu SN, Hui-Min Wu G, Yang WW, Pwu RF, Liu CL, Cheng KP, Chen SC, Chen CJ. Policy and Strategy for Hepatitis C Virus Elimination at the National Level: Experience in Taiwan. J Infect Dis. 2023;228:S180-S188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 28. | Chen YY, Chen CL, Chen JW, Hsu NT, Wei ST, Hou SM, Lu SN, Chen PJ. Secular Trends and Geographic Maps of Hepatitis C Virus Infection among 4 Million Blood Donors in Taiwan from 1999 to 2017. Hepatol Commun. 2020;4:1193-1205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Chen CL, Concejero A, Wang CC, Wang SH, Lin CC, Liu YW, Yong CC, Yang CH, Lin TS, Chiang YC, Jawan B, Huang TL, Cheng YF, Eng HL. Living donor liver transplantation for biliary atresia: a single-center experience with first 100 cases. Am J Transplant. 2006;6:2672-2679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Angelico R, Grimaldi C, Saffioti MC, Castellano A, Spada M. Hepatocellular carcinoma in children: hepatic resection and liver transplantation. Transl Gastroenterol Hepatol. 2018;3:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Lin NC, Hsia CY, Loong CC, Liu CS, Tsai HL, Lui WY, Wu CW. Liver transplantation at a small-volume procedure center--preliminary results from Taipei Veterans General Hospital. J Chin Med Assoc. 2008;71:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Pillai VG, Chen CL. Living donor liver transplantation in Taiwan-challenges beyond surgery. Hepatobiliary Surg Nutr. 2016;5:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 33. | Magdzik WW. Hepatitis B epidemiology in Poland, Central and Eastern Europe and the newly independent states. Vaccine. 2000;18 Suppl 1:S13-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Ganczak M, Korzen M, Jurewicz A, Szych Z. A cross-sectional sero-survey on preoperative HBV vaccination policy in Poland. BMC Infect Dis. 2017;17:515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Brojer E, Grabarczyk P, Liszewski G, Mikulska M, Allain JP, Letowska M; Polish Blood Transfusion Service Viral Study Group. Characterization of HBV DNA+/HBsAg- blood donors in Poland identified by triplex NAT. Hepatology. 2006;44:1666-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Koczkodaj P, Straś W, Czerwiński J, Małkowski P, Panczyk M, Gotlib J. Evolution of Indications for Liver Transplantation (LTx) in the Years 2001-2017 in Poland. Ann Transplant. 2019;24:312-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Czerwiński J, Pszenny A, Antoszkiewicz K, Danek T, Górski Ł, Hermanowicz M, Łęczycka A, Malanowski P, Parulski A, Szemis Ł, Ziaja J, Kamiński A. Current Data on Organ Donation and Transplantation in Poland: Poltransplant Activity 2017 to 2022. Transplant Proc. 2024;56:758-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 38. | Ito T, Nguyen MH. Perspectives on the Underlying Etiology of HCC and Its Effects on Treatment Outcomes. J Hepatocell Carcinoma. 2023;10:413-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 39. | Bollaerts K, Aerts M, Sasse A. Improved benchmark-multiplier method to estimate the prevalence of ever-injecting drug use in Belgium, 2000-10. Arch Public Health. 2013;71:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Marty L, Van Beckhoven D, Ost C, Deblonde J, Costagliola D, Sasse A, Supervie V; HERMETIC Study Group. Estimates of the HIV undiagnosed population in Belgium reveals higher prevalence for MSM with foreign nationality and for geographic areas hosting big cities. J Int AIDS Soc. 2019;22:e25371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Burki T. Eliminating hepatitis C. Lancet Infect Dis. 2019;19:246-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Lo YC, Tsai MS, Sun HY, Hung CC, Chuang JH. National Trend and Characteristics of Acute Hepatitis C among HIV-Infected Individuals: A Matched Case-Control Study-Taiwan, 2001-2014. PLoS One. 2015;10:e0139687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Wiktor A, Stępień M. Hepatitis B in Poland in 2018. Przegl Epidemiol. 2020;74:196-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Marshall AD, Cunningham EB, Nielsen S, Aghemo A, Alho H, Backmund M, Bruggmann P, Dalgard O, Seguin-Devaux C, Flisiak R, Foster GR, Gheorghe L, Goldberg D, Goulis I, Hickman M, Hoffmann P, Jancorienė L, Jarcuska P, Kåberg M, Kostrikis LG, Makara M, Maimets M, Marinho RT, Matičič M, Norris S, Ólafsson S, Øvrehus A, Pawlotsky JM, Pocock J, Robaeys G, Roncero C, Simonova M, Sperl J, Tait M, Tolmane I, Tomaselli S, van der Valk M, Vince A, Dore GJ, Lazarus JV, Grebely J; International Network on Hepatitis in Substance Users (INHSU). Restrictions for reimbursement of interferon-free direct-acting antiviral drugs for HCV infection in Europe. Lancet Gastroenterol Hepatol. 2018;3:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 45. | Marshall AD, Pawlotsky JM, Lazarus JV, Aghemo A, Dore GJ, Grebely J. The removal of DAA restrictions in Europe - One step closer to eliminating HCV as a major public health threat. J Hepatol. 2018;69:1188-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 46. | Busschots D, Kremer C, Bielen R, Koc ÖM, Heyens L, Dercon E, Verrando R, Windelinckx T, Maertens G, Bourgeois S, Hens N, Matheï C, Robaeys G. Identification and treatment of viral hepatitis C in persons who use drugs: a prospective, multicenter outreach study in Flanders, Belgium. Harm Reduct J. 2021;18:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Busschots D, Toghanian S, Bielen R, Salomonsson S, Koc ÖM, Hendrickx G, Jadoul M, Nevens F, Sokal E, Brixko C, Peerlinck K, Apers L, Robaeys G, Lazarus JV. Eliminating viral hepatitis C in Belgium: the micro-elimination approach. BMC Infect Dis. 2020;20:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Papatheodoridis GV, Hatzakis A, Cholongitas E, Baptista-Leite R, Baskozos I, Chhatwal J, Colombo M, Cortez-Pinto H, Craxi A, Goldberg D, Gore C, Kautz A, Lazarus JV, Mendão L, Peck-Radosavljevic M, Razavi H, Schatz E, Tözün N, van Damme P, Wedemeyer H, Yazdanpanah Y, Zuure F, Manns MP. Hepatitis C: The beginning of the end-key elements for successful European and national strategies to eliminate HCV in Europe. J Viral Hepat. 2018;25 Suppl 1:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 49. | Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1927] [Cited by in RCA: 1936] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 50. | Wong VW, Ekstedt M, Wong GL, Hagström H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023;79:842-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 436] [Article Influence: 145.3] [Reference Citation Analysis (0)] |