Published online Nov 15, 2025. doi: 10.4251/wjgo.v17.i11.110735
Revised: June 29, 2025
Accepted: October 9, 2025
Published online: November 15, 2025
Processing time: 154 Days and 2.8 Hours
Hepatocellular carcinoma (HCC) remains one of the commonest cancers world
Core Tip: Recurrent hepatocellular carcinoma (HCC) is indeed an increasing concern, especially as the global incidence of chronic liver diseases continues to rise. The management of recurrent HCC remains complex, particularly after liver transplantation or hepatic resection, as there is no universally agreed-upon approach. Several treatment modalities are available as locoregional and systemic therapies, and each comes with its own set of advantages and challenges. As recurrent HCC becomes more prevalent, developing a clear, evidence-based treatment approach will be crucial. Further research and prospective randomized trials are essential to determine the best management strategies, establish guidelines, and improve long-term patient outcomes in this challenging clinical scenario.
- Citation: Elsayed MOK. Treatment of recurrent hepatocellular carcinoma: The current standards and future perspectives. World J Gastrointest Oncol 2025; 17(11): 110735
- URL: https://www.wjgnet.com/1948-5204/full/v17/i11/110735.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i11.110735
Hepatocellular carcinoma (HCC) is among the fastest-growing malignant diseases globally[1]. HCC is highly aggressive, with a 5-year survival rate below 20% and recurrence rates reaching up to 88%[2]. Regardless of the underlying cause or initial treatment approach, HCC frequently recurs, emphasizing the need for diligent post-operative monitoring[3]. When recurrence occurs, it is crucial to reassess the disease’s progression and reevaluate treatment strategies. However, staging frameworks for recurrent HCC (RHCC) are less well-established compared to those for primary HCC[4]. RHCC differs from primary HCC due to the reduced residual liver size and diminished liver function often associated with previous interventions[5]. For patients with resectable RHCC, repeat hepatectomy (RH) is considered the treatment of choice. Unfortunately, RH is not feasible for many patients due to factors such as tumor location, size, advanced cirrhosis, or portal hypertension[6].
In cases where RH is not an option, locoregional therapies such as radiofrequency ablation (RFA), transcatheter arterial chemoembolization (TACE), and percutaneous ethanol injection (PEI) have been employed with curative intent[7]. More recently, salvage liver transplantation (SLT) has emerged as a potential curative treatment for intrahepatic recurrence of HCC. However, its application is limited by a shortage of cadaveric donors and the restricted availability of suitable living donors[8]. This review aims to explore the various treatment options available for managing RHCC.
The management of RHCC differs from that of primary HCC in several key aspects.
RHCC generally exhibits more aggressive behavior and is associated with a poorer prognosis. Two mechanisms of recurrence are identified, each with distinct clinicopathological characteristics: Intrahepatic metastasis and multicentric occurrence[9].
When resection is considered as a treatment option, the future liver remnant is typically smaller for RHCC compared to primary HCC, making surgery more challenging[10].
Liver function tends to be more impaired in patients with RHCC due to the cumulative effects of previous treatments and disease progression. While treatment strategies for RHCC often follow similar principles as those for primary HCC, it is essential to perform a comprehensive assessment before selecting the optimal treatment modality. This evaluation should consider factors such as the size, location, and number of recurrent lesions, and whether the recurrence is confined to the liver (intrahepatic) or involves extrahepatic sites. Additionally, patient-specific factors, including age, sex, psychological condition, prior surgical details (such as the site of surgery and involvement of major blood vessels), and baseline liver function, must be carefully evaluated.
Given the variability in recurrence patterns and available treatment options, RHCC management should be individualized. A multidisciplinary team approach is crucial, involving hepatologists, transplant and hepatobiliary surgeons, medical oncologists, interventional radiologists, and palliative care specialists. This collaborative “tumor board” app
Treatment options for RHCC can be classified into: Surgical treatment: Including both surgical resection (SR) and liver transplantation (LT). Locoregional treatment: (1) Ablative therapies [RFA, microwave ablation (MWA)]; (2) Endovascular or catheter-based therapies [transarterial bland embolization, TACE, transarterial radioembolization (TARE) and hepatic arterial infusion (HAI) chemotherapy]; (3) External beam radiation therapy (EBRT) and stereotactic body radiation therapy (SBRT); (4) Systemic treatment; and (5) Combined modalities.
In this part we will discuss both SR and LT.
A small percentage (10%-30%) of patients with RHCC present with isolated extrahepatic or hepatic metastases that are considered suitable for surgical removal. For patients unfit for resection but with solitary recurrences, curative treatment using thermal ablation is an alternative option. However, it is important to note that the high recurrence rates often associated with HCC may limit the effectiveness of resection or ablation, necessitating repeated interventions[12].
In a French study by Fernandez-Sevilla et al[13], 22 out of 70 patients with RHCC underwent SR (2 with intrahepatic recurrences and 20 with extrahepatic recurrences). Their survival outcomes were significantly better compared to those who did not undergo resection (35 months vs 15 months, P < 0.001). The study concluded that HCC recurrence following LT is generally linked to poor prognosis, but resection significantly improves survival and should be considered when feasible. RH has been shown to be safe, with complication rates similar to those of the initial surgery[14]. However, the risk of further recurrence remains, particularly in patients with multinodular cirrhosis in the remaining liver tissue[15].
The largest Eastern study, conducted by Zou et al[16], analyzed 635 patients who underwent a second resection for RHCC. The median overall survival (mOS) was 54.8 months, and the 1-, 3-, and 5-year overall survival (OS) rates were 96.9%, 74.8%, and 47.8%, respectively. Post-recurrence survival rates for the same periods were 75.8%, 45.7%, and 37.6%, respectively. Over the past decade, laparoscopic liver resection (LLR) has gained wider adoption. A recent meta-analysis highlighted that while 90-day mortality rates were similar between LLR and open resection for RHCC, LLR offered advantages such as reduced in-hospital complications, less blood loss, and shorter hospital stays[17]. Further research suggests that repeat LLR is both feasible and effective for RHCC, demonstrating promising short-term outcomes[18]. Although laparoscopic repeat LLR is associated with prolonged operation times compared to open repeat liver resection (LR), it results in shorter hospital stays and comparable perioperative outcomes[19].
A meta-analysis involving 767 patients (334 undergoing repeat laparoscopic hepatectomy and 433 undergoing repeat open hepatectomy) found that laparoscopic procedures were associated with less intraoperative blood loss, fewer major complications, shorter hospital stays, and higher rates of R0 resections[20]. For RHCC, patients with intrahepatic-only recurrences demonstrated better prognoses compared to those with extrahepatic or combined intra- and extrahepatic recurrences. Additionally, patients who experienced recurrence within six months of the initial resection had worse survival outcomes[21].
Theoretically, LT is the optimal treatment for HCC within the Milan criteria because it allows for both the complete removal of the tumor and the treatment of underlying liver cirrhosis. However, routinely recommending LT for RHCC is considered logistically unfeasible due to the scarcity of donor organs and prolonged waiting periods[22]. The primary benefit of LT following the first hepatic recurrence is the treatment of cirrhosis, which reduces the likelihood of further recurrences. For patients with RHCC meeting the Milan criteria, SLT can be considered if the patient’s age and comor
In 2000, Majno et al[24] were the first to describe SLT for RHCC in carefully selected patients. Their findings indicated that OS and disease-free survival (DFS) following SLT were comparable to those achieved with primary LT (PLT). A meta-analysis by Zhu et al[25] of 14 studies conducted between 2000 and 2012 further confirmed that SLT and PLT have similar mortality rates. The pooled mortality rate across 10 studies was 6.34%, with no significant statistical differences between SLT and PLT.
SLT remains a life-saving option for patients with intrahepatic recurrences or declining liver function following primary hepatic resection[26]. Moreover, SLT has demonstrated superior outcomes compared to curative locoregional therapies in seven retrospective studies. A meta-analysis found that, compared to RH alone, SLT yielded significantly better 3- and 5-year DFS, as well as higher 5-year OS[27]. However, the limited availability of donor organs continues to pose a challenge to the feasibility of SLT.
For patients with RHCC following hepatectomy, SLT is associated with poorer OS and recurrence-free survival (RFS), along with a higher risk of recurrence and mortality, compared to PLT - especially for patients within the Milan criteria[28]. On the other hand, another study reported no significant differences in 5-year recurrence risk or actuarial survival between patients undergoing PLT and those receiving SLT for HCC recurrence following initial treatments such as LR or RFA[29].
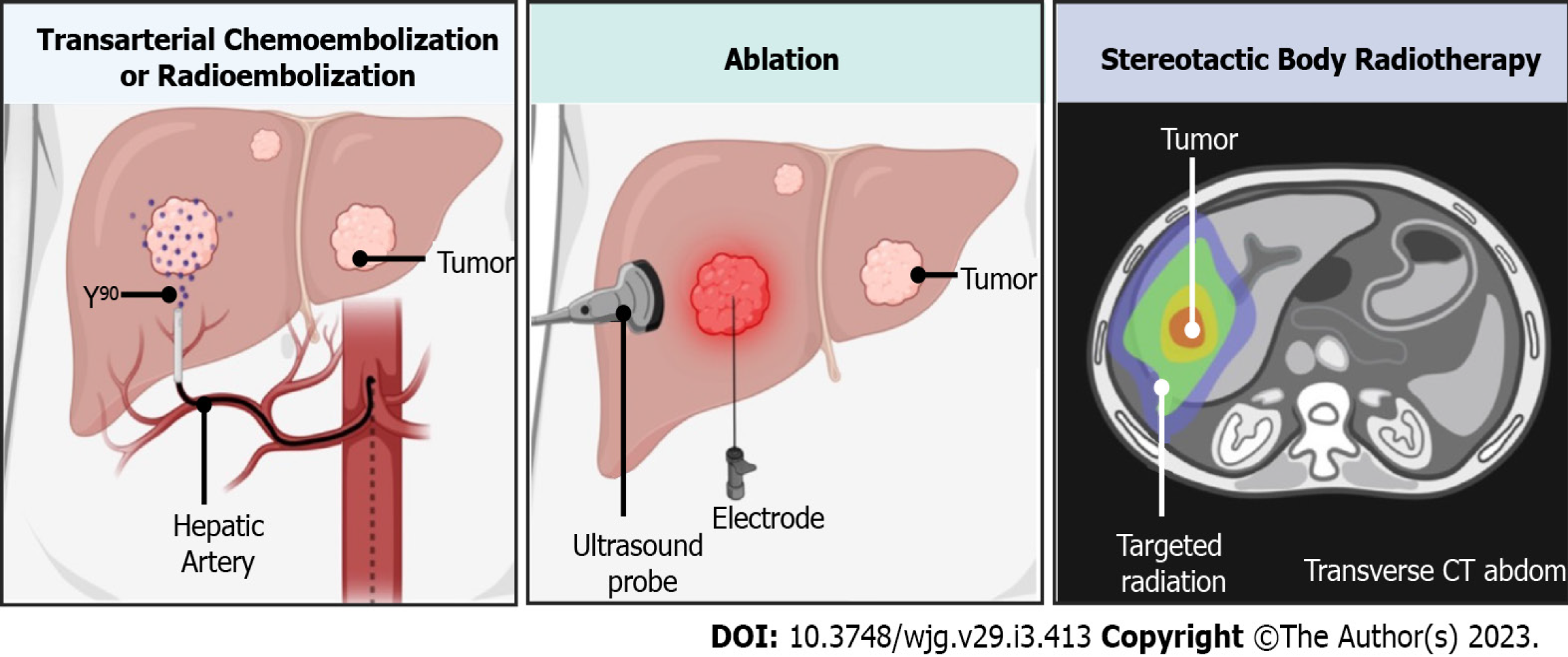
Locoregional therapies encompass a range of minimally invasive, liver-targeted treatments. These therapies include ablative techniques, endovascular or catheter-based approaches, and SBRT. Depending on the clinical scenario, these methods can be applied as either curative interventions or neoadjuvant therapies for HCC, as illustrated in Figure 1[29].
RFA: RFA, which can be performed via percutaneous or open approaches, is considered a safe procedure and, in selected patients, as effective as SR in achieving long-term survival. Sun et al[30] reported that for small RHCCs following SR, RFA provided OS and DFS rates comparable to repeat SR, with the added benefit of a shorter hospital stay. Similarly, a meta-analysis by Gavriilidis et al[31] showed no significant differences in 5-year OS and DFS between RFA and RH for RHCC, although morbidity was significantly lower with RFA (2% vs 17%). However, a systematic review by Thomasset et al[32] analyzing 18 studies concluded that while RFA for RHCC is associated with a very low complication rate, it is less effective than RH and should therefore be reserved for patients unable to undergo surgical resection. A case report highlighted the efficacy of percutaneous RFA, achieving 2-year DFS in a 65-year-old patient with a solitary RHCC in a grafted liver[33]. In a retrospective study, Huang et al[34] compared 15 patients with post-LT HCC recurrence treated surgically to 11 patients treated with RFA. No significant difference was observed in 5-year OS (35% vs 28%, P = 0.88), although the RFA group showed worse DFS, which was not statistically significant (16% vs 0%; P = 0.75). For RHCC after liver resection, multiprobe stereotactic RFA demonstrated low morbidity, with 1-, 3-, and 5-year OS rates of 94.0%, 70.2%, and 53.3%, respectively, and DFS rates of 52.6%, 19.7%, and 15.8%, respectively[35]. RFA has proven particularly beneficial and effective for intrahepatic RHCC, especially following LT in the absence of extrahepatic metastases. For these cases, the 1-, 3-, and 5-year OS rates were 68.5%, 40.3%, and 40.3%, respectively[36]. While RFA is associated with lower RFS compared to LT, it is considered a superior therapy for intrahepatic HCC recurrence due to its minimal invasiveness, precision, and reproducibility[37].
MWA: MWA offers several advantages over RFA, including shorter ablation times, larger ablation zones, higher intra-tumoral temperatures, and more effective coagulative necrosis[38]. Recent studies have demonstrated the efficacy, safety, and suitability of MWA as a treatment for RHCC following curative SR. MWA is particularly beneficial as a parenchyma-sparing therapy, especially for patients with reduced liver volume due to previous surgical interventions. It provides long OS and progression-free survival (PFS) without significant complications or extended hospitalization and should be considered a viable option for managing RHCC after successful initial resection[39].
MWA has also shown promise in treating HCC recurrence following[40]. Zhai et al[41] evaluated the safety and effectiveness of MWA in a cohort of 11 LT recipients with intrahepatic RHCC. The procedure was well tolerated, with only three cases of tumor progression reported within 1-7 months post-treatment. However, the 2-year survival rate was 15.3%, and the mean survival time was 17.3 months, highlighting the need for further investigation into its long-term outcomes in this context.
TACE: The liver receives a dual blood supply, with approximately two-thirds of the blood flow originating from the portal vein and the remaining one-third from the hepatic artery. Transarterial embolization works by selectively occluding tumor-supplying vessels via the hepatic artery, inducing tumor ischemia and necrosis. TACE adds regional chemotherapy to embolizing microparticles for enhanced therapeutic effects[42]. The efficacy of conventional TACE in treating RHCC after living donor LT was assessed by Ko et al[43]. Among 28 patients, a tumor size reduction of ≥ 25% was achieved in 67.9% (19/28). However, intrahepatic or extrahepatic metastases were observed in 92.9% of patients within the first six months after TACE. Two recent meta-analyses highlighted the benefits of adjuvant TACE, demon
TARE: TARE, also known as selective internal radiation therapy, is an emerging treatment modality that offers effective disease control with a favorable safety profile. This technique involves the intra-arterial administration of therapeutic radiation doses to liver tumors using yttrium-90-loaded resin or glass microspheres[50]. TARE can be applied using three primary techniques: Radiation lobectomy, radiation segmentectomy, and the modified radiation lobectomy approach[51]. A 2016 meta-analysis by Lobo et al[52] found that TARE has OS and complication rates similar to TACE, but the prospe
HAI: HAI is a commonly used alternative to systemic chemotherapy, as it delivers chemotherapeutic agents directly to the tumor-feeding vessels while minimizing systemic toxic side effects through the liver’s first-pass effect[61]. A recent phase-three study compared HAI using FOLFOX (a combination of fluorouracil, leucovorin, and oxaliplatin) to TACE for large, unresectable HCC without macrovascular invasion. The results demonstrated a significantly improved OS with HAI (mOS: 13.3 months) compared to TACE (mOS: 10.8 months). Infusions were administered once every three weeks for up to six courses[62]. In another study, the combination of sorafenib with FOLFOX HAI was compared to sorafenib alone. The combination of sorafenib with FOLFOX HAI group exhibited a tolerable safety profile and significantly improved efficacy, with a mOS of 13.37 months vs 7.13 months for sorafenib alone[63]. Additionally, a randomized controlled trial evaluated sorafenib combined with HAI vs sorafenib alone in patients with HCC and major portal vein tumor thrombus. The combination therapy showed superior outcomes, including improved mOS, a higher objective response rate, and longer median PFS[64]. Despite these promising results, the survival benefit of combining sorafenib with HAI has shown variability across studies, highlighting the need for further research to better define its role in treating advanced HCC.
The two main types of radiation therapy commonly used are EBRT and SBRT. In EBRT, high doses of radiation are directed at the tumor tissue (liver tumor), while lower doses are delivered to the surrounding normal liver tissue. SBRT is a more advanced form of EBRT, designed to deliver a higher ablative dose to the target tissue (liver tumor) without significantly increasing the dose to the surrounding healthy liver tissue[65]. Several studies have demonstrated the effectiveness of SBRT in treating patients with unresectable, locally advanced, or RHCC[66-68]. In patients with preserved liver function, repeated SBRT for RHCC has shown good tumor ablation results, with an acceptable safety profile and OS comparable to other ablative therapies aimed at curative treatment[69]. The American Society of Radiation Oncology recommends SBRT as a treatment option for HCC[70]. SBRT has demonstrated reasonable benefits in terms of tumor control and survival, with 3-year disease control rates ranging from 68% to 97%, and 3-year survival rates from 39% to 84%. It is particularly useful in cases where RFA is not feasible or in HCC recurrence following RFA or TACE[71].
Sorafenib: Sorafenib, a multitarget tyrosine kinase inhibitor (TKI), was the first drug approved for treating HCC patients. It is most commonly used as adjuvant therapy in patients with resected HCC and as a frontline systemic treatment for those with RHCC after LT[72]. The landmark SHARP trial established sorafenib as the first Food and Drug Administration-approved systemic therapy for advanced HCC. The trial demonstrated that sorafenib significantly improved mOS compared to a placebo (mOS: 10.7 months vs 7.9 months; hazard ratio = 0.69; 95% confidence interval: 0.55-0.87; P < 0.001)[63]. Multiple studies have shown that sorafenib, as a representative molecular targeted therapy, extends survival for patients with RHCC after LT[73-76].
Lenvatinib: After a decade, another targeted therapy, lenvatinib, was assessed for use as a first-line treatment in patients with unresectable HCC. Lenvatinib’s approval was based on the results of the phase III randomized noninferiority trial REFLECT, which compared lenvatinib to sorafenib. In the study, patients treated with lenvatinib had a mOS of 13.6 months, while those treated with sorafenib had a mOS of 12.3 months. Additionally, median PFS was significantly longer in the lenvatinib group (7.4 months) compared to the sorafenib group (3.7 months)[77].
Cabozantinib, a TKI targeting vascular endothelial growth factor receptor 2, is an effective and safe monotherapy used in third-line systemic treatment for advanced HCC[78]. As a multi-kinase inhibitor, cabozantinib is considered a promising option for advanced HCC patients who have developed tolerance to sorafenib[79]. The phase III RESORCE trial (regorafenib after sorafenib in patients with HCC) evaluated regorafenib vs placebo in 573 patients (379 receiving regorafenib, 194 receiving placebo) with HCC (Child-Pugh A) whose disease had progressed after sorafenib treatment. Based on the findings, regorafenib was approved in the United States, European Union, and Japan for patients with HCC previously treated with sorafenib[80]. The following drugs are licensed for use in the second-line setting: Regorafenib, nivolumab/ipilimumab, pembrolizumab, cabozantinib, and ramucirumab[81].
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that target and block key immune checkpoint proteins such as programmed cell death protein-1 (PD-1), programmed cell death ligand-1 (PD-L1), and cytotoxic T lymphocyte-associated antigen-4. This blockade enhances T-cell function and boosts the immune response against tumor cells[82]. Atezolizumab is an ICI that targets PD-L1, while bevacizumab is an antibody against vascular endothelial growth factor A. In a phase Ib trial, the combination of atezolizumab and bevacizumab showed safety and promising preliminary activity in patients with advanced HCC[83]. Subsequently, an open-label, phase 3 trial comparing atezolizumab-bevacizumab to sorafenib in patients with previously untreated advanced HCC demonstrated superior outcomes. The study, which included 336 patients in the atezolizumab-bevacizumab group and 165 patients in the sorafenib group, found that the combination therapy resulted in better OS and PFS in patients with unresectable HCC[84].
Recently, a combination therapy strategy has been used to achieve better outcome than single modality of treatment. Different combination modalities have been used to treat HCC with favourable outcomes.
RFA combined with TACE has been shown to provide the best survival outcomes for patients who are not candidates for RH. This combined treatment demonstrates a survival benefit, particularly in patients with RHCC and 2-3 tumors[85]. In comparison to SR, the combination of TACE and RFA offers comparable one- and three-year OS rates, as well as a one-year RFS rate. However, the three-year RFS rate is significantly lower in the TACE-RFA group compared to SR. On the other hand, the TACE-RFA approach is associated with a lower risk of major complications compared to SR[86].
Choi et al[87] reported positive perioperative results and long-term survival in a cohort of 53 patients who underwent combined hepatectomy and RFA for multifocal HCC. This approach demonstrated promising outcomes for patients with multiple tumors. SR combined with intraoperative RFA has been shown to provide better OS and PFS compared to TACE for patients with intermediate-stage (Barcelona Clinic Liver Cancer B) HCC[88]. Furthermore, Zhou et al[89] demon
According to Ryu et al[90], for selected patients with multifocal HCC, a combination of hepatectomy and MWA offers good long-term results with minimal complication rates. This combination approach is considered safe and practical, especially for individuals with multifocal HCC. For patients who are otherwise ineligible for hepatectomy, the combi
The combination of TACE and PEI has been shown to improve survival compared to TACE alone. A study revealed a statistically significant improvement in survival for HCC patients at Okuda stage I. Additionally, the side effects of this combination therapy were minimal, and the treatment did not substantially prolong the duration of hospitalization[91]. This suggests that combining TACE and PEI could be an effective and well-tolerated treatment option for HCC patients in the early stages, offering improved survival outcomes without significant additional risks or extended recovery times.
The combination of TACE with sorafenib has shown significantly better outcomes than TACE alone in patients with HCC. This combination therapy is associated with prolonged PFS, improved OS, and a significantly higher tumor response rate. These findings suggest that combining TACE with sorafenib not only enhances the effectiveness of treatment but also provides a controllable safety profile, demonstrating its potential as a promising therapeutic approach for managing HCC[92].
The combination of atezolizumab and cabozantinib was tested in the COSMIC-312 phase III randomized trial. This trial showed an improvement in median PFS, increasing from 4.2 months in the sorafenib group to 6.8 months in the combination group (hazard ratio = 0.63; 99% confidence interval: 0.44-0.91, P = 0.0012). However, despite this improvement in PFS, the study did not show a significant benefit in OS at the interim analysis[93]. The HIMALAYA trial evaluated the combination of anti-PD-L1 durvalumab and anti-cytotoxic T lymphocyte-associated antigen-4 tre
Many trials are under investigations to find out an effective second-line agents. The anti-PD-1 monoclonal antibody tislelizumab demonstrated efficacy in the treatment of advanced HCC in the RATIONALE-301 trial. In this noninferiority study, 674 patients were randomized to receive either 200 mg of tislelizumab every three weeks or 400 mg of sorafenib twice daily. The final analysis revealed that patients treated with tislelizumab had a mOS of 15.9 months, compared to 14.1 months in the sorafenib group[97]. In the second-line setting, additional ICIs are under investigation. One such combination involves apatinib and the anti-PD-1 antibody camrelizumab, which is being tested in patients with advanced HCC, gastric cancer, or esophagogastric junction cancer[98]. Camrelizumab (administered at 3 mg/kg intravenously every two to three weeks) was evaluated in a multicenter, open-label, randomized, phase II trial in China for patients with advanced HCC who had progressed on or were intolerant to prior systemic therapy[99]. Another anti-PD-1 mono
In addition to ICIs, cytokine-induced killing (CIK) cell-based immunotherapy has emerged as a promising adjuvant therapy for HCC. Several clinical trials have indicated that CIK cell therapy improves RFS in HCC patients who undergo SR[101,102]. A meta-analysis of 22 studies involving 3756 HCC patients who received dendritic cell-based vaccines and/or CIK-based adoptive therapy after interventional treatments showed prolonged OS at 6 months, 1 year, 3 years, and 5 years. The therapy also reduced mortality and recurrence at 1, 2, and 3 years, but not at 5 years[103].
Moreover, immunological studies have highlighted promising results, including the generation of circulating multiclonal neoantigen-specific T-cell responses, activation of neoantigen-specific immunity, and an upregulated immune stimulatory signature. In 70% of patients, these responses were linked to improved DFS. Notably, 71.4% of these patients remained relapse-free for 2 years following curative treatment. However, evidence of immune evasion was also noted in recurrent tumors compared to primary tumors, suggesting that immunological therapy may lead to immunoediting and resistance[104].
Artificial intelligence (AI) has emerged as a potential alternative biomarker for predicting cancer recurrence following treatment, showing promising performance when compared to traditional clinical and pathological indicators. By leveraging computer technology, AI can extract advanced, detailed information from medical imaging modalities such as computed tomography[105], magnetic resonance imaging[106], and ultrasound[107], incorporating techniques like radiomics and deep learning[108].
A recent study conducted in Australia and Hong Kong employed AI to create and evaluate a high-performing predictive model for HCC recurrence after curative SR. The researchers concluded that applying AI to extensive clinical, biochemical, and tumor-related data allowed for accurate identification of risk factors linked to HCC recurrence following surgery[109]. A systematic review and meta-analysis highlighted the broad applicability of AI in predicting recurrence following a single first-line treatment for liver cancer, yielding favorable outcomes. These findings suggest that AI holds significant potential for clinical implementation in forecasting liver cancer recurrence[110].
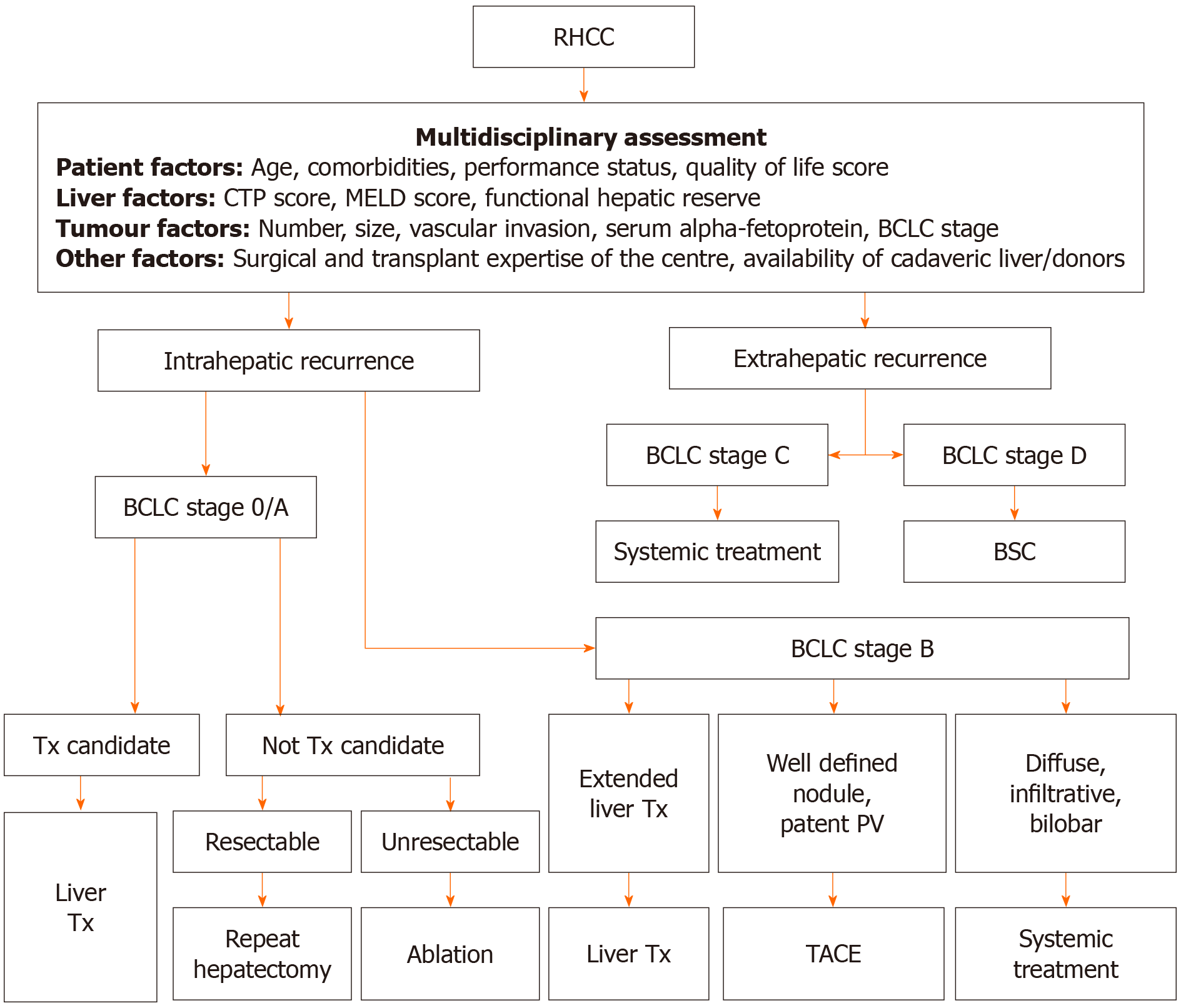
In view of this systematic review and after reviewing all the available treatment options, I have proposed an algorithm for the treatment of RHCC. This algorithm will help those who need to make a decision in choosing the modality of treatment for RHCC (Figure 2).
The treatment of RHCC following LT or hepatic resection is indeed a challenging aspect of HCC management. The lack of international guidelines for RHCC treatment underscores the complexity of these cases, as treatment strategies are highly individualized based on a variety of factors such as hepatic reserve, tumor staging, and previous treatments. For patients with RHCC following resection, RH is the most commonly considered approach, provided the patient maintains adequate liver function and there is no significant underlying liver disease. However, for patients who are ineligible for reoperation or for those with recurrence after LT, SLT is emerging as a promising option, offering new hope for those with RHCC post-transplant.
Locoregional therapies such as TACE, RFA, and selective internal radiation therapy remain important treatment options for patients who are not candidates for resection or transplantation. The use of combination therapy - combining different treatment modalities - has shown promise in improving therapeutic efficacy, reducing complications, and potentially prolonging survival. By combining surgery, ablation techniques, or locoregional therapies with systemic treatments like sorafenib, lenvatinib, or ICIs, clinicians aim to optimize the treatment approach for RHCC. Although ongoing and completed clinical trials are beginning to expand systemic therapy options for advanced HCC and specific patient populations, there remains a need for more rigorous prospective randomized controlled studies. These studies are essential to develop evidence-based guidelines for managing RHCC, refine therapeutic strategies, and optimize outcomes for this challenging clinical scenario. In summary, although treatment options for RHCC are constantly advancing, a personalized approach remains key to obtaining the best results. The use of new therapies and combination methods, together with continuous clinical research, holds promise for better management of RHCC going forward.
| 1. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1283] [Article Influence: 183.3] [Reference Citation Analysis (3)] |
| 2. | Brar G, Greten TF, Graubard BI, McNeel TS, Petrick JL, McGlynn KA, Altekruse SF. Hepatocellular Carcinoma Survival by Etiology: A SEER-Medicare Database Analysis. Hepatol Commun. 2020;4:1541-1551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 3. | Tsilimigras DI, Bagante F, Moris D, Hyer JM, Sahara K, Paredes AZ, Mehta R, Ratti F, Marques HP, Soubrane O, Lam V, Poultsides GA, Popescu I, Alexandrescu S, Martel G, Workneh A, Guglielmi A, Hugh T, Aldrighetti L, Endo I, Pawlik TM. Recurrence Patterns and Outcomes after Resection of Hepatocellular Carcinoma within and beyond the Barcelona Clinic Liver Cancer Criteria. Ann Surg Oncol. 2020;27:2321-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 4. | Vitale A, Farinati F, Finotti M, Di Renzo C, Brancaccio G, Piscaglia F, Cabibbo G, Caturelli E, Missale G, Marra F, Sacco R, Giannini EG, Trevisani F, Cillo U; Associazione Italiana Per Lo Studio Del Fegato Aisf Hcc Special Interest Group; Italian Liver Cancer Ita Li Ca Study Group. Overview of Prognostic Systems for Hepatocellular Carcinoma and ITA.LI.CA External Validation of MESH and CNLC Classifications. Cancers (Basel). 2021;13:1673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Marasco G, Colecchia A, Colli A, Ravaioli F, Casazza G, Bacchi Reggiani ML, Cucchetti A, Cescon M, Festi D. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J Hepatol. 2019;70:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 6. | Mise Y, Hasegawa K, Shindoh J, Ishizawa T, Aoki T, Sakamoto Y, Sugawara Y, Makuuchi M, Kokudo N. The Feasibility of Third or More Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma. Ann Surg. 2015;262:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Wang HL, Mo DC, Zhong JH, Ma L, Wu FX, Xiang BD, Li LQ. Systematic review of treatment strategy for recurrent hepatocellular carcinoma: Salvage liver transplantation or curative locoregional therapy. Medicine (Baltimore). 2019;98:e14498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Wu L, Hu A, Tam N, Zhang J, Lin M, Guo Z, He X. Salvage liver transplantation for patients with recurrent hepatocellular carcinoma after curative resection. PLoS One. 2012;7:e41820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Hao S, Fan P, Chen S, Tu C, Wan C. Distinct Recurrence Risk Factors for Intrahepatic Metastasis and Multicenter Occurrence After Surgery in Patients with Hepatocellular Carcinoma. J Gastrointest Surg. 2017;21:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Tan HL, Goh BKP. Management of recurrent hepatocellular carcinoma after resection. Hepatobiliary Surg Nutr. 2020;9:780-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Agarwal PD, Phillips P, Hillman L, Lucey MR, Lee F, Mezrich JD, Said A. Multidisciplinary Management of Hepatocellular Carcinoma Improves Access to Therapy and Patient Survival. J Clin Gastroenterol. 2017;51:845-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Valdivieso A, Bustamante J, Gastaca M, Uriarte JG, Ventoso A, Ruiz P, Fernandez JR, Pijoan I, Testillano M, Suarez MJ, Montejo M, Ortiz de Urbina J. Management of hepatocellular carcinoma recurrence after liver transplantation. Transplant Proc. 2010;42:660-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Fernandez-Sevilla E, Allard MA, Selten J, Golse N, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Adam R. Recurrence of hepatocellular carcinoma after liver transplantation: Is there a place for resection? Liver Transpl. 2017;23:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Wu CC, Cheng SB, Yeh DC, Wang J, P'eng FK. Second and third hepatectomies for recurrent hepatocellular carcinoma are justified. Br J Surg. 2009;96:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 720] [Article Influence: 65.5] [Reference Citation Analysis (1)] |
| 16. | Zou Q, Li J, Wu D, Yan Z, Wan X, Wang K, Shi L, Lau WY, Wu M, Shen F. Nomograms for Pre-operative and Post-operative Prediction of Long-Term Survival of Patients Who Underwent Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma. Ann Surg Oncol. 2016;23:2618-2626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Cai W, Liu Z, Xiao Y, Zhang W, Tang D, Cheng B, Li Q. Comparison of clinical outcomes of laparoscopic versus open surgery for recurrent hepatocellular carcinoma: a meta-analysis. Surg Endosc. 2019;33:3550-3557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Onoe T, Yamaguchi M, Irei T, Ishiyama K, Sudo T, Hadano N, Kojima M, Kubota H, Ide R, Tazawa H, Shimizu W, Suzuki T, Shimizu Y, Hinoi T, Tashiro H. Feasibility and efficacy of repeat laparoscopic liver resection for recurrent hepatocellular carcinoma. Surg Endosc. 2020;34:4574-4581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Goh BKP, Syn N, Teo JY, Guo YX, Lee SY, Cheow PC, Chow PKH, Ooi LLPJ, Chung AYF, Chan CY. Perioperative Outcomes of Laparoscopic Repeat Liver Resection for Recurrent HCC: Comparison with Open Repeat Liver Resection for Recurrent HCC and Laparoscopic Resection for Primary HCC. World J Surg. 2019;43:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Liang Y, Lin C, Zhang B, Cao J, Chen M, Shen J, Feng X, Xiao G, Pan L, Chen K, Maher H, Cai X. Perioperative outcomes comparing laparoscopic with open repeat liver resection for post-hepatectomy recurrent liver cancer: A systematic review and meta-analysis. Int J Surg. 2020;79:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Chen ZH, Zhang XP, Feng JK, Li LQ, Zhang F, Hu YR, Zhong CQ, Wang K, Chai ZT, Wei XB, Shi J, Guo WX, Wu MC, Lau WY, Cheng SQ. Patterns, treatments, and prognosis of tumor recurrence after resection for hepatocellular carcinoma with microvascular invasion: a multicenter study from China. HPB (Oxford). 2022;24:1063-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Ning Chong CC, San Lai PB. Treatment Strategy for Recurrent Hepatocellular Carcinoma. In: Liver Tumors. Croatia: IntechOpen, 2012: 121-144. [DOI] [Full Text] |
| 23. | Lacaze L, Scotté M. Surgical treatment of intra hepatic recurrence of hepatocellular carcinoma. World J Hepatol. 2015;7:1755-1760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 24. | Majno PE, Sarasin FP, Mentha G, Hadengue A. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology. 2000;31:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 264] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 25. | Zhu Y, Dong J, Wang WL, Li MX, Lu Y. Short- and long-term outcomes after salvage liver transplantation versus primary liver transplantation for hepatocellular carcinoma: a meta-analysis. Transplant Proc. 2013;45:3329-3342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Sala M, Fuster J, Llovet JM, Navasa M, Solé M, Varela M, Pons F, Rimola A, García-Valdecasas JC, Brú C, Bruix J; Barcelona Clinic Liver Cancer (BCLC) Group. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transpl. 2004;10:1294-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 235] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 27. | Shan Y, Huang L, Xia Q. Salvage Liver Transplantation Leads to Poorer Outcome in Hepatocellular Carcinoma Compared with Primary Liver Transplantation. Sci Rep. 2017;7:44652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Muaddi H, Al-Adra DP, Beecroft R, Ghanekar A, Moulton CA, Doyle A, Selzner M, Wei A, McGilvray ID, Gallinger S, Grant DR, Cattral MS, Greig PD, Kachura J, Cleary SP, Sapisochin G. Liver Transplantation is Equally Effective as a Salvage Therapy for Patients with Hepatocellular Carcinoma Recurrence Following Radiofrequency Ablation or Liver Resection with Curative Intent. Ann Surg Oncol. 2018;25:991-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Criss CR, Makary MS. Salvage locoregional therapies for recurrent hepatocellular carcinoma. World J Gastroenterol. 2023;29:413-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (8)] |
| 30. | Sun WC, Chen IS, Liang HL, Tsai CC, Chen YC, Wang BW, Lin HS, Chan HH, Hsu PI, Tsai WL, Cheng JS. Comparison of repeated surgical resection and radiofrequency ablation for small recurrent hepatocellular carcinoma after primary resection. Oncotarget. 2017;8:104571-104581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Gavriilidis P, Askari A, Azoulay D. Survival following redo hepatectomy vs radiofrequency ablation for recurrent hepatocellular carcinoma: a systematic review and meta-analysis. HPB (Oxford). 2017;19:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Thomasset SC, Dennison AR, Garcea G. Ablation for recurrent hepatocellular carcinoma: a systematic review of clinical efficacy and prognostic factors. World J Surg. 2015;39:1150-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Ho CK, Chapman WC, Brown DB. Radiofrequency ablation of recurrent hepatocellular carcinoma in a patient after liver transplantation: two-year follow-up. J Vasc Interv Radiol. 2007;18:1451-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Huang J, Yan L, Wu H, Yang J, Liao M, Zeng Y. Is radiofrequency ablation applicable for recurrent hepatocellular carcinoma after liver transplantation? J Surg Res. 2016;200:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Schullian P, Laimer G, Putzer D, Levy E, Braunwarth E, Stättner S, Bale R. Stereotactic radiofrequency ablation as first-line treatment of recurrent HCC following hepatic resection. Eur J Surg Oncol. 2020;46:1503-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Liu B, Huang G, Xie X, Zhao Q, Su L, Liu M, Li X, Long J, Kuang M, Xie X. Feasibility and outcomes of percutaneous radiofrequency ablation for intrahepatic recurrent hepatocellular carcinoma after liver transplantation: a single-center experience. Int J Hyperthermia. 2020;37:1202-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Chen X, Chen Y, Li Q, Ma D, Shen B, Peng C. Radiofrequency ablation versus surgical resection for intrahepatic hepatocellular carcinoma recurrence: a meta-analysis. J Surg Res. 2015;195:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Groeschl RT, Pilgrim CH, Hanna EM, Simo KA, Swan RZ, Sindram D, Martinie JB, Iannitti DA, Bloomston M, Schmidt C, Khabiri H, Shirley LA, Martin RC, Tsai S, Turaga KK, Christians KK, Rilling WS, Gamblin TC. Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg. 2014;259:1195-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 39. | Adwan H, Hammann L, Vogl TJ. Microwave Ablation of Recurrent Hepatocellular Carcinoma after Curative Surgical Resection. J Clin Med. 2023;12:2560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Gringeri E, Boetto R, Bassi D, D'Amico FE, Polacco M, Romano M, Neri D, Feltracco P, Zanus G, Cillo U. Laparoscopic microwave thermal ablation for late recurrence of local hepatocellular carcinoma after liver transplant: case report. Prog Transplant. 2014;24:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Zhai H, Liang P, Yu XL, Cheng Z, Han ZY, Liu F, Yu J. Microwave ablation in treating intrahepatic recurrence of hepatocellular carcinoma after liver transplantation: An analysis of 11 cases. Int J Hyperthermia. 2015;31:863-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Makary MS, Khandpur U, Cloyd JM, Mumtaz K, Dowell JD. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers (Basel). 2020;12:1914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 43. | Ko HK, Ko GY, Yoon HK, Sung KB. Tumor response to transcatheter arterial chemoembolization in recurrent hepatocellular carcinoma after living donor liver transplantation. Korean J Radiol. 2007;8:320-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Yang J, Liang H, Hu K, Xiong Z, Cao M, Zhong Z, Yao Z, Deng M. The effects of several postoperative adjuvant therapies for hepatocellular carcinoma patients with microvascular invasion after curative resection: a systematic review and meta-analysis. Cancer Cell Int. 2021;21:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 45. | Chen W, Ma T, Zhang J, Zhang X, Chen W, Shen Y, Bai X, Liang T. A systematic review and meta-analysis of adjuvant transarterial chemoembolization after curative resection for patients with hepatocellular carcinoma. HPB (Oxford). 2020;22:795-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 46. | Wang K, Liu G, Li J, Yan Z, Xia Y, Wan X, Ji Y, Lau WY, Wu M, Shen F. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Eur J Surg Oncol. 2015;41:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Tampaki M, Papatheodoridis GV, Cholongitas E. Intrahepatic recurrence of hepatocellular carcinoma after resection: an update. Clin J Gastroenterol. 2021;14:699-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 48. | Zhang X, Li C, Wen T, Yan L, Li B, Yang J, Wang W, Xu M, Lu W, Jiang L. Appropriate treatment strategies for intrahepatic recurrence after curative resection of hepatocellular carcinoma initially within the Milan criteria: according to the recurrence pattern. Eur J Gastroenterol Hepatol. 2015;27:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Kim SS, Kang TW, Song KD, Cho SK, Lee MW, Rhim H, Sinn DH, Jung SH. Radiofrequency ablation and transarterial chemoembolisation as first-line treatment for recurrent hepatocellular carcinoma or isolated intrahepatic recurrent hepatocellular carcinoma in transplanted livers. Clin Radiol. 2017;72:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Sidali S, Trépo E, Sutter O, Nault JC. New concepts in the treatment of hepatocellular carcinoma. United European Gastroenterol J. 2022;10:765-774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 51. | Miller FH, Lopes Vendrami C, Gabr A, Horowitz JM, Kelahan LC, Riaz A, Salem R, Lewandowski RJ. Evolution of Radioembolization in Treatment of Hepatocellular Carcinoma: A Pictorial Review. Radiographics. 2021;41:1802-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 52. | Lobo L, Yakoub D, Picado O, Ripat C, Pendola F, Sharma R, ElTawil R, Kwon D, Venkat S, Portelance L, Yechieli R. Unresectable Hepatocellular Carcinoma: Radioembolization Versus Chemoembolization: A Systematic Review and Meta-analysis. Cardiovasc Intervent Radiol. 2016;39:1580-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 53. | Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, Mulcahy MF, Baker T, Abecassis M, Miller FH, Yaghmai V, Sato K, Desai K, Thornburg B, Benson AB, Rademaker A, Ganger D, Kulik L, Lewandowski RJ. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;151:1155-1163.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 515] [Article Influence: 51.5] [Reference Citation Analysis (30)] |
| 54. | Sangro B, Maini CL, Ettorre GM, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI, Salvatori R, Giampalma E, Geatti O, Wilhelm K, Hoffmann RT, Izzo F, Iñarrairaegui M, Urigo C, Cappelli A, Vit A, Ahmadzadehfar H, Jakobs TF, Sciuto R, Pizzi G, Lastoria S; European Network on Radioembolization with Yttrium-90 resin microspheres (ENRY). Radioembolisation in patients with hepatocellular carcinoma that have previously received liver-directed therapies. Eur J Nucl Med Mol Imaging. 2018;45:1721-1730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Ali R, Riaz A, Gabr A, Abouchaleh N, Mora R, Al Asadi A, Caicedo JC, Abecassis M, Katariya N, Maddur H, Kulik L, Lewandowski RJ, Salem R. Clinical outcomes of Y90 radioembolization for recurrent hepatocellular carcinoma following curative resection. Eur J Nucl Med Mol Imaging. 2017;44:2195-2202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Salem R, Gabr A, Riaz A, Mora R, Ali R, Abecassis M, Hickey R, Kulik L, Ganger D, Flamm S, Atassi R, Atassi B, Sato K, Benson AB, Mulcahy MF, Abouchaleh N, Asadi AA, Desai K, Thornburg B, Vouche M, Habib A, Caicedo J, Miller FH, Yaghmai V, Kallini JR, Mouli S, Lewandowski RJ. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology. 2018;68:1429-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 57. | Hilgard P, Hamami M, Fouly AE, Scherag A, Müller S, Ertle J, Heusner T, Cicinnati VR, Paul A, Bockisch A, Gerken G, Antoch G. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 353] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 58. | Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, Sibert A, Bouattour M, Lebtahi R, Allaham W, Barraud H, Laurent V, Mathias E, Bronowicki JP, Tasu JP, Perdrisot R, Silvain C, Gerolami R, Mundler O, Seitz JF, Vidal V, Aubé C, Oberti F, Couturier O, Brenot-Rossi I, Raoul JL, Sarran A, Costentin C, Itti E, Luciani A, Adam R, Lewin M, Samuel D, Ronot M, Dinut A, Castera L, Chatellier G; SARAH Trial Group. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 614] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 59. | Fidelman N, Kerlan RK Jr. Transarterial Chemoembolization and (90)Y Radioembolization for Hepatocellular Carcinoma: Review of Current Applications Beyond Intermediate-Stage Disease. AJR Am J Roentgenol. 2015;205:742-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Parikh ND, Waljee AK, Singal AG. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transpl. 2015;21:1142-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 61. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10529] [Article Influence: 584.9] [Reference Citation Analysis (9)] |
| 62. | Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, Wei W, Zhang YJ, Guo Y, Guo RP, Chen MS, Shi M. Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Transarterial Chemoembolization for Large Hepatocellular Carcinoma: A Randomized Phase III Trial. J Clin Oncol. 2022;40:150-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 283] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 63. | He M, Li Q, Zou R, Shen J, Fang W, Tan G, Zhou Y, Wu X, Xu L, Wei W, Le Y, Zhou Z, Zhao M, Guo Y, Guo R, Chen M, Shi M. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma With Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol. 2019;5:953-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 415] [Article Influence: 69.2] [Reference Citation Analysis (1)] |
| 64. | Zheng K, Zhu X, Fu S, Cao G, Li WQ, Xu L, Chen H, Wu D, Yang R, Wang K, Liu W, Wang H, Bao Q, Liu M, Hao C, Shen L, Xing B, Wang X. Sorafenib Plus Hepatic Arterial Infusion Chemotherapy versus Sorafenib for Hepatocellular Carcinoma with Major Portal Vein Tumor Thrombosis: A Randomized Trial. Radiology. 2022;303:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 65. | Hoffe SE, Finkelstein SE, Russell MS, Shridhar R. Nonsurgical options for hepatocellular carcinoma: evolving role of external beam radiotherapy. Cancer Control. 2010;17:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Huang WY, Jen YM, Lee MS, Chang LP, Chen CM, Ko KH, Lin KT, Lin JC, Chao HL, Lin CS, Su YF, Fan CY, Chang YW. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 67. | Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, Bae SH, Jung DH, Kim KB, Lee DH, Han CJ, Kim J, Park SC, Kim YH. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118:5424-5431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 254] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 68. | Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, Dinniwell RE, Kassam Z, Ringash J, Cummings B, Sykes J, Sherman M, Knox JJ, Dawson LA. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 609] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 69. | Kimura T, Takeda A, Tsurugai Y, Kawano R, Doi Y, Oku Y, Hioki K, Miura H, Nagata Y. A Multi-Institutional Retrospective Study of Repeated Stereotactic Body Radiation Therapy for Intrahepatic Recurrent Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys. 2020;108:1265-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 70. | Apisarnthanarax S, Barry A, Cao M, Czito B, DeMatteo R, Drinane M, Hallemeier CL, Koay EJ, Lasley F, Meyer J, Owen D, Pursley J, Schaub SK, Smith G, Venepalli NK, Zibari G, Cardenes H. External Beam Radiation Therapy for Primary Liver Cancers: An ASTRO Clinical Practice Guideline. Pract Radiat Oncol. 2022;12:28-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 149] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 71. | Mathew AS, Dawson LA. Current Understanding of Ablative Radiation Therapy in Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:575-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 72. | Sun KX, Cao SS, Shi FH, Guan Y, Tang M, Zhao MN, Jian YF, Cui B, Li ZY, Wang JW, Yu F, Ding Y. First-line treatments for advanced hepatocellular carcinoma: a network meta-analysis and cost-effectiveness analysis in China and the United States. Therap Adv Gastroenterol. 2022;15:17562848221140662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 73. | Yoon DH, Ryoo BY, Ryu MH, Lee SG, Hwang S, Suh DJ, Lee HC, Kim TW, Ahn CS, Kim KH, Moon DB, Kang YK. Sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Jpn J Clin Oncol. 2010;40:768-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 74. | Li BCW, Chiu J, Shing K, Kwok GGW, Tang V, Leung R, Ma KW, She WH, Tsang J, Chan A, Cheung TT, Lo CM, Yau T. The Outcomes of Systemic Treatment in Recurrent Hepatocellular Carcinomas Following Liver Transplants. Adv Ther. 2021;38:3900-3910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Sposito C, Mariani L, Germini A, Flores Reyes M, Bongini M, Grossi G, Bhoori S, Mazzaferro V. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013;59:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 76. | Martin RC 2nd, Bruenderman E, Cohn A, Piperdi B, Miksad R, Geschwind JF, Goldenberg A, Sanyal A, Zigmont E, Babajanyan S, Foreman P, Mantry P, McGuire B, Gholam P. Sorafenib use for recurrent hepatocellular cancer after resection or transplantation: Observations from a US regional analysis of the GIDEON registry. Am J Surg. 2017;213:688-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4110] [Article Influence: 513.8] [Reference Citation Analysis (5)] |
| 78. | Ho WJ, Zhu Q, Durham J, Popovic A, Xavier S, Leatherman J, Mohan A, Mo G, Zhang S, Gross N, Charmsaz S, Lin D, Quong D, Wilt B, Kamel IR, Weiss M, Philosophe B, Burkhart R, Burns WR, Shubert C, Ejaz A, He J, Deshpande A, Danilova L, Stein-O'Brien G, Sugar EA, Laheru DA, Anders RA, Fertig EJ, Jaffee EM, Yarchoan M. Neoadjuvant Cabozantinib and Nivolumab Converts Locally Advanced HCC into Resectable Disease with Enhanced Antitumor Immunity. Nat Cancer. 2021;2:891-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 275] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 79. | Azhie A, Grant RC, Herman M, Wang L, Knox JJ, Bhat M. Phase II clinical trial of cabozantinib for the treatment of recurrent hepatocellular carcinoma after liver transplantation. Future Oncol. 2022;18:2173-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2839] [Article Influence: 315.4] [Reference Citation Analysis (1)] |
| 81. | Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1313] [Article Influence: 187.6] [Reference Citation Analysis (1)] |
| 82. | Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2655] [Cited by in RCA: 3352] [Article Influence: 304.7] [Reference Citation Analysis (0)] |
| 83. | Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, Hack SP, Spahn J, Liu B, Abdullah H, Wang Y, He AR, Lee KH; GO30140 investigators. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21:808-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 437] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 84. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 5304] [Article Influence: 884.0] [Reference Citation Analysis (29)] |
| 85. | Zhang B, Wan J, Xu X, Li Y, Lv T, Yang J. Radiofrequency Ablation, TACE and Combined Treatment for Multiple Recurrent HCC Patients Within the Milan Criteria. 2021 Preprint. Available from: rs.3.rs-379856/v1. [DOI] [Full Text] |
| 86. | Guo W, He X, Li Z, Li Y. Combination of Transarterial Chemoembolization (TACE) and Radiofrequency Ablation (RFA) vs. Surgical Resection (SR) on Survival Outcome of Early Hepatocellular Carcinoma: A Meta-Analysis. Hepatogastroenterology. 2015;62:710-714. [PubMed] |
| 87. | Choi D, Lim HK, Joh JW, Kim SJ, Kim MJ, Rhim H, Kim YS, Yoo BC, Paik SW, Park CK. Combined hepatectomy and radiofrequency ablation for multifocal hepatocellular carcinomas: long-term follow-up results and prognostic factors. Ann Surg Oncol. 2007;14:3510-3518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Kim GH, Kim JH, Ko HK, Chu HH, Kim SH, Shin JH, Gwon DI, Ko GY, Yoon HK, Kim KH, Shim JH, Kim N. Surgical Resection plus Intraoperative Radiofrequency Ablation versus Chemoembolization for the Treatment of Intermediate-Stage (BCLC B) Hepatocellular Carcinoma with Preserved Liver Function: A Propensity Score-Matched Analysis. Cancers (Basel). 2022;14:2440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Zhou C, Peng Y, Zhou K, Zhang L, Zhang X, Yu L, Hu J, Chen F, Qiu S, Zhou J, Fan J, Ren Z, Wang Z. Surgical resection plus radiofrequency ablation for the treatment of multifocal hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2019;8:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 90. | Ryu T, Takami Y, Wada Y, Sasaki S, Imamura H, Ureshino H, Saitsu H. Combined hepatectomy and microwave ablation for multifocal hepatocellular carcinoma: Long-term outcomes and prognostic factors. Asian J Surg. 2021;44:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | Becker G, Soezgen T, Olschewski M, Laubenberger J, Blum HE, Allgaier HP. Combined TACE and PEI for palliative treatment of unresectable hepatocellular carcinoma. World J Gastroenterol. 2005;11:6104-6109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 92. | Zou X, Fan W, Xue M, Li J. Evaluation of the Benefits of TACE Combined with Sorafenib for Hepatocellular Carcinoma Based on Untreatable TACE (unTACEable) Progression. Cancer Manag Res. 2021;13:4013-4029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 93. | Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, Chan SL, Melkadze T, Sukeepaisarnjaroen W, Breder V, Verset G, Gane E, Borbath I, Rangel JDG, Ryoo BY, Makharadze T, Merle P, Benzaghou F, Banerjee K, Hazra S, Fawcett J, Yau T. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:995-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 399] [Article Influence: 99.8] [Reference Citation Analysis (1)] |
| 94. | Abou-Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang Y, Dao TV, De Toni EN, Rimassa L, Breder VV, Vasilyev A, Heurgue A, Tam V, Mody K, Thungappa SC, He P, Negro A, Sangro B. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40:379. [RCA] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 95. | Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38:2960-2970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 921] [Article Influence: 153.5] [Reference Citation Analysis (0)] |
| 96. | Finn R, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, Xu R, Edeline J, Ryoo B, Ren Z, Cheng A, Galle P, Kaneko S, Kumada H, Wang A, Mody K, Dubrovsky L, Siegel A, Llovet J. LBA34 Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2022;33:S1401. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 151] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 97. | Qin S, Finn RS, Kudo M, Meyer T, Vogel A, Ducreux M, Macarulla TM, Tomasello G, Boisserie F, Hou J, Li X, Song J, Zhu AX. RATIONALE 301 study: tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol. 2019;15:1811-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 98. | Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, Zhang G, Zhao C, Zhang Y, Chen C, Wang Y, Yi X, Hu Z, Zou J, Wang Q. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clin Cancer Res. 2019;25:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 376] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 99. | Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, Bai Y, Yang L, Zhu H, Fang W, Lin X, Chen X, Li E, Wang L, Chen C, Zou J. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 455] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 100. | Pishvaian M, Weiss G, Falchook G, Yee N, Gil-Martin M, Shahda S, Moreno V, Brana I, Crittenden M, Formenti S, Al-Rajabi R, Papadopoulos K, Stankevich E, Feng M, Li J, Mathias M, Kroog G, Lowy I, Fury M. Cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with advanced or metastatic hepatocellular carcinoma (HCC): Data from an expansion cohort in a phase I study. Ann Oncol. 2018;29:viii410. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 101. | Weng DS, Zhou J, Zhou QM, Zhao M, Wang QJ, Huang LX, Li YQ, Chen SP, Wu PH, Xia JC. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunother. 2008;31:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 102. | Cassese G, Han HS, Lee B, Lee HW, Cho JY, Panaro F, Troisi RI. Immunotherapy for hepatocellular carcinoma: A promising therapeutic option for advanced disease. World J Hepatol. 2022;14:1862-1874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 103. | Cao J, Kong FH, Liu X, Wang XB. Immunotherapy with dendritic cells and cytokine-induced killer cells for hepatocellular carcinoma: A meta-analysis. World J Gastroenterol. 2019;25:3649-3663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 104. | Peng S, Chen S, Hu W, Mei J, Zeng X, Su T, Wang W, Chen Z, Xiao H, Zhou Q, Li B, Xie Y, Hu H, He M, Han Y, Tang L, Ma Y, Li X, Zhou X, Dai Z, Liu Z, Tan J, Xu L, Li S, Shen S, Li D, Lai J, Peng B, Peng Z, Kuang M. Combination Neoantigen-Based Dendritic Cell Vaccination and Adoptive T-Cell Transfer Induces Antitumor Responses Against Recurrence of Hepatocellular Carcinoma. Cancer Immunol Res. 2022;10:728-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 105. | Ansari MY, Abdalla A, Ansari MY, Ansari MI, Malluhi B, Mohanty S, Mishra S, Singh SS, Abinahed J, Al-Ansari A, Balakrishnan S, Dakua SP. Practical utility of liver segmentation methods in clinical surgeries and interventions. BMC Med Imaging. 2022;22:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 106. | Rai P, Ansari MY, Warfa M, Al-Hamar H, Abinahed J, Barah A, Dakua SP, Balakrishnan S. Efficacy of fusion imaging for immediate post-ablation assessment of malignant liver neoplasms: A systematic review. Cancer Med. 2023;12:14225-14251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 107. | Ansari MY, Qaraqe M, Righetti R, Serpedin E, Qaraqe K. Unveiling the future of breast cancer assessment: a critical review on generative adversarial networks in elastography ultrasound. Front Oncol. 2023;13:1282536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 108. | Han Z, Jian M, Wang G. ConvUNeXt: An efficient convolution neural network for medical image segmentation. Knowledge-based Syst. 2022;253:109512. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 109. | Zandavi SM, Kim C, Goodwin T, Thilakanathan C, Bostanara M, Akon AC, Al Mouiee D, Barisic S, Majeed A, Kemp W, Chu F, Smith M, Collins K, Wong VW, Wong GL, Behary J, Roberts SK, Ng KKC, Vafaee F, Zekry A. AI-powered prediction of HCC recurrence after surgical resection: Personalised intervention opportunities using patient-specific risk factors. Liver Int. 2024;44:2724-2737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 110. | Wu L, Lai Q, Li S, Wu S, Li Y, Huang J, Zeng Q, Wei D. Artificial intelligence in predicting recurrence after first-line treatment of liver cancer: a systematic review and meta-analysis. BMC Med Imaging. 2024;24:263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/