Published online Nov 15, 2025. doi: 10.4251/wjgo.v17.i11.110715
Revised: August 6, 2025
Accepted: October 9, 2025
Published online: November 15, 2025
Processing time: 154 Days and 12.5 Hours
Early esophageal neuroendocrine carcinoma (E-NEC) is a rare but aggressive malignancy with poorly understood endoscopic features. Despite advancements in multi-model endoscopy, including white light endoscopy, magnifying end
To characterize early E-NEC using multi-model endoscopy and identify diag
Clinical data of four patients with esophageal submucosal lesions identified by gastroscopy and pathologically diagnosed as E-NEC in the Department of Gastroenterology, The First Affiliated Hospital of Zhejiang University School of Medicine between January 2020 and August 2024 were retrospectively analyzed, and their manifestations under multi-model endoscopy were observed. Grayscale values of ultrasound images in three patients with E-NEC and eight with esophageal leiomyoma were calculated using Image J software and compared using the Mann-Whitney U test.
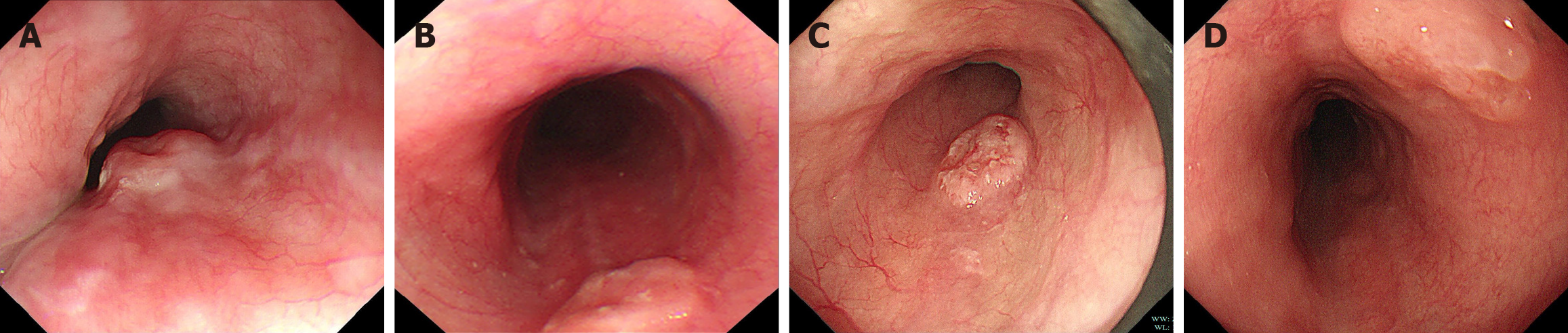
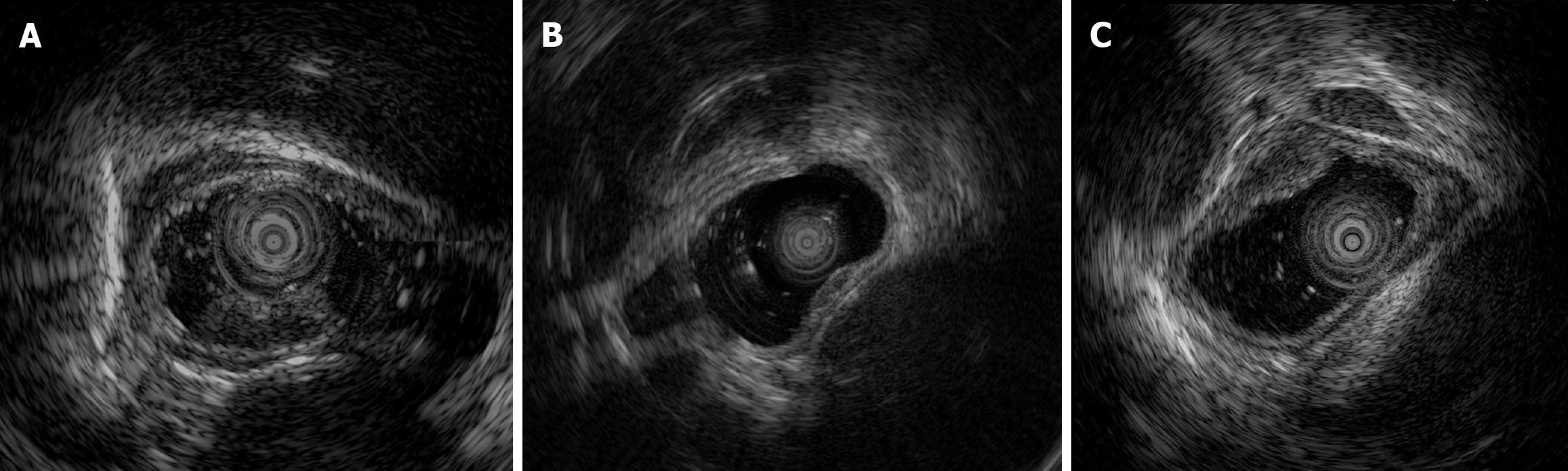
Among the four patients with early E-NEC, two were males and two were females, with ages ranging from 60-70 years. White light endoscopy revealed three patients with mild local ulceration on the lesion surface. NBI images were available in two patients, revealing the intraepithelial papillary capillary loop of B1-B2 and B2 types. EUS was performed in three patients, all showing solitary hypoechoic lesions originating from the muscularis mu
Endoscopic features of early E-NEC differ from those of esophageal leiomyoma, including NBI imaging, lymph node metastasis, and a notably higher echo intensity on EUS.
Core Tip: This was a retrospective observational study that investigated the endoscopic characteristics of early esophageal neuroendocrine carcinoma (E-NEC) and how they differed from those of other esophageal submucosal tumors. This study analyzed four patients with early E-NEC using multi-model endoscopy and revealed key diagnostic differences from esophageal leiomyomas, including higher echogenicity on endoscopic ultrasonography, irregular microvascular patterns on narrow-band imaging, and lymph node enlargement. These findings highlight the potential of combined endoscopic modalities to improve the early detection and diagnostic accuracy of E-NEC.
- Citation: Jin T, Zhou YW, Sun PS, Huang Y, Gao JG, Jin X. Unraveling the characteristics of early esophageal neuroendocrine carcinoma using multi-model endoscopy: A retrospective study of serial cases. World J Gastrointest Oncol 2025; 17(11): 110715
- URL: https://www.wjgnet.com/1948-5204/full/v17/i11/110715.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i11.110715
Neuroendocrine neoplasms (NENs) are a rare heterogeneous group of epithelial tumors that originate from peptidergic neurons and neuroendocrine cells, and are characterized by neuroendocrine differentiation. NENs include well-differentiated neuroendocrine tumors (NETs), poorly differentiated neuroendocrine carcinomas (NECs), and mixed neuroendocrine-non-NENs[1], which exhibit a wide range of biological behaviors, from indolent, slow-growing lesions to highly aggressive and metastatic malignancies[2]. NENs have the capacity for whole-body distribution, with gastroenteropancreatic NENs comprising approximately 60%-75% of cases, followed by those in the lungs and mediastinum[3]. Eso
The tumor-node-metastasis staging system of E-NEC mirrors that of esophageal squamous cell carcinoma (ESCC)[11,12]. Early E-NEC is defined as lesions limited to the mucosal or submucosal layer (stages T1a and T1b), with or without nodal metastasis. Clinically, we found that early E-NEC often presents with absent or non-specific symptoms, such as mild dysphagia or retrosternal discomfort, which are easily overlooked or mistaken for benign esophageal conditions such as leiomyoma. Conventional imaging and tumor markers frequently fail to detect early lesions, and endoscopic biopsy results may be inconclusive due to submucosal tumor growth and the overlying normal epithelium[13]. As a result, E-NEC is frequently diagnosed at an advanced stage (31%-90% of cases) when regional lymph node or distant metastases have already occurred, contributing to its poor prognosis[2,13,14]. Studies have reported a median survival of approximately 11 months and 5-year survival rate of < 10%[14]. Given these challenges, early detection using endoscopy is essential for improving patient outcomes.
Conventional white light endoscopy (WLE) uses broad-spectrum white light to visualize the gastrointestinal mucosa, and is currently the standard modality for initial screening and surveillance because of its high-resolution, real-time imaging capabilities for anatomical structures[15]. However, WLE has limited sensitivity for detecting premalignant and early-stage flat malignant lesions such as intraepithelial neoplasia and early cancer. Early E-NECs, which typically present as subtle mucosal or submucosal lesions, may therefore be missed on standard WLE. These diagnostic limitations can be addressed by integrating advanced techniques such as narrow-band imaging (NBI) and endoscopic ultrasound (EUS). NBI utilizes filtered blue (415 nm) and green (540 nm) lights to enhance visualization of the microvascular architecture[16]. By exploiting the absorption characteristics of hemoglobin, NBI improves contrast, highlights abnormal vascular and mucosal patterns, aids in the differentiation of benign and malignant lesions, and enhances the detection of early neoplasia.
Early E-NEC typically appears as a hypoechoic lesion originating from the mucosa, submucosa, or muscularis propria[17]. Although EUS is recommended by the European Society of Gastrointestinal Endoscopy as an optimal tool for characterizing subepithelial lesions because of its ability to assess lesion location, size, layer of origin, echogenicity, morphology, and surrounding structures[18], it cannot reliably differentiate all subepithelial lesion types when used independently. Early E-NEC often appears similar to esophageal leiomyoma on EUS, leading to misdiagnosis, poor prognosis, and delayed treatment[2]. Therefore, this study aimed to investigate the characteristics of early E-NEC using multimodal endoscopic imaging techniques, including WLE, NBI, and EUS, and to compare these findings, especially the gray value of a lesion under EUS, with those of esophageal leiomyoma to enhance diagnostic accuracy and reduce diagnostic pitfalls. Additionally, this article reviews the literature on recent advances in the diagnosis, treatment, and pathogenesis of early E-NEC.
We retrospectively reviewed patients with submucosal esophageal lesions detected by gastroscopy at the Department of Gastroenterology, The First Affiliated Hospital of Zhejiang University School of Medicine between January 2020 and August 2024. Within the review period, four patients diagnosed with NEC using diagnostic endoscopic submucosal dissection (ESD) or surgical resection were included. Patients’ clinical profiles, laboratory and imaging findings, endoscopic observations, and pathological results were collected. This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine.
Image analysis software Image J was used to calculate the gray values of the images of EUS. Clinical parameters of patients were expressed as mean ± SD of continuous variables and comparisons between groups were presented using the Mann-Whitney U test. Statistical analysis was performed using SPSS version 26.0 statistical software. Statistical significance was considered at P < 0.05.
For review of the literature, database search was performed in PubMed, with the following search algorithm, [“neuroendocrine carcinoma” (All Fields) OR “NEC” (All Fields)] AND [“oesophagus” (All Fields) OR “esophagus” (All Fields) OR esophageal (All Fields)]. A literature search was conducted for articles published up to the year 2025. Studies focusing on early E-NEC were included, whereas reviews without full texts were excluded.
Clinical characteristics of the four patients with early E-NEC are summarized in Table 1. Age of the patients ranged between 60-70 years (mean 66 years; median 67 years). Presenting symptoms included dysphagia in one patient, recurrent retrosternal discomfort in two patients, and no symptoms in one patient. Tumor marker levels were within normal limits in all the patients. Contrast-enhanced computed tomography (CT) showed mild wall thickening in the mid-esophagus in one patient, while no significant abnormalities were detected in the remaining three patients.
| Case | Sex | Age (year) | Presentation | Examination | Location (cm) | Size (cm) |
| 1 | Male | 60 | No symptoms | Tumor markers were normal; contrast-enhanced CT showed no definite mass | 30 | 0.94 × 0.36 |
| 2 | Male | 64 | Retrosternal discomfort | Tumor markers were normal; contrast-enhanced CT showed no definite mass | 27 | 1.3 × 0.9 |
| 3 | Female | 70 | Recurrent retrosternal discomfort with abdominal distension and chest tightness | Tumor markers were normal; contrast-enhanced CT revealed mild mucosal enhancement in the mid-esophagus and a small nodule in the anterior mediastinum | 28 | 1.2 × 0.7 |
| 4 | Female | 70 | Dysphagia | Tumor markers were normal; contrast-enhanced CT showed no definite mass | 30 | 0.5 × 0.6 |
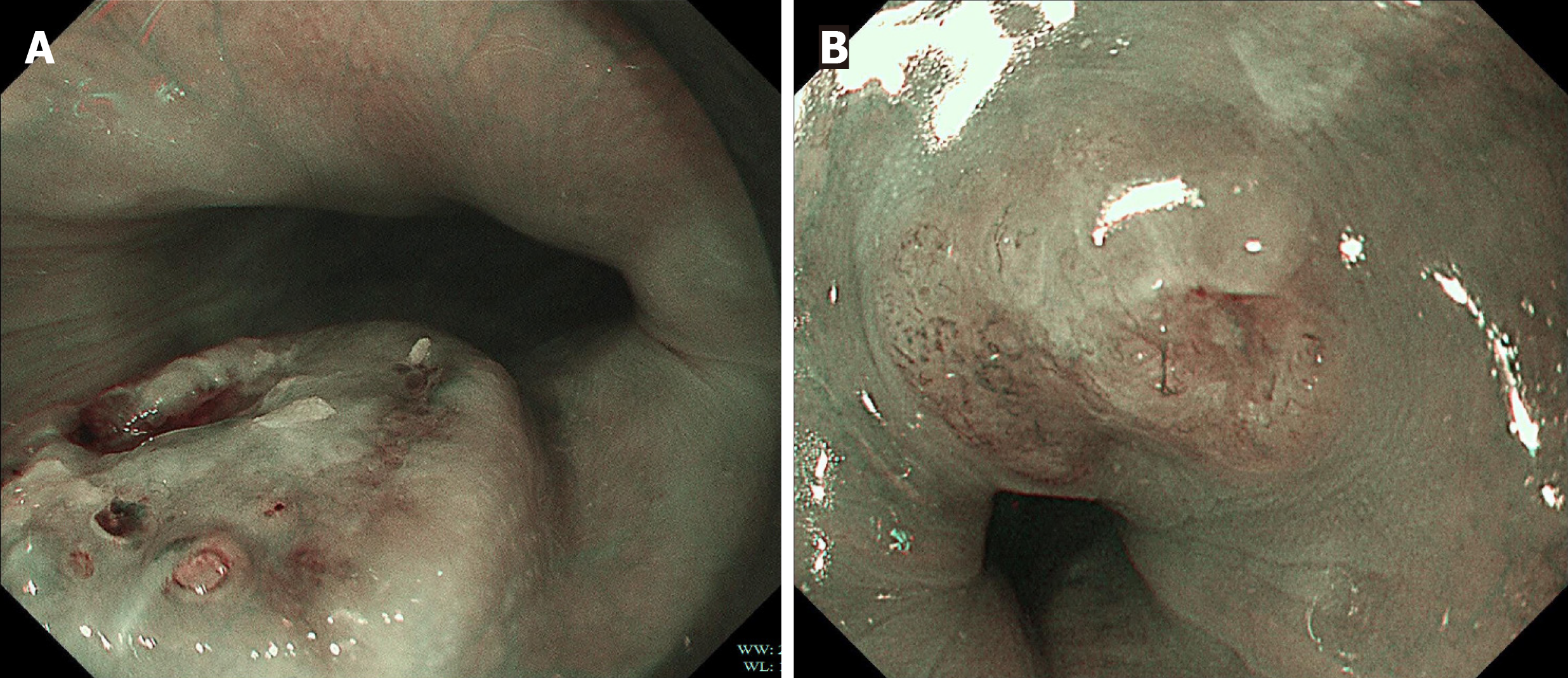
Under WLE, three of the four patients with early E-NEC exhibited localized mild ulceration on the lesion surface, while one presented with a smooth-surfaced polypoid lesion (Figure 1). The lesions were located at 27 cm, 28 cm, 30 cm, and 30 cm from the incisors and involved the mid to lower esophagus. The lesion sizes were between 0.5 cm × 0.6 cm and 1.2 cm × 1.1 cm. NBI was performed in two patients (Figure 2), which revealed irregular or dilated abnormal mic
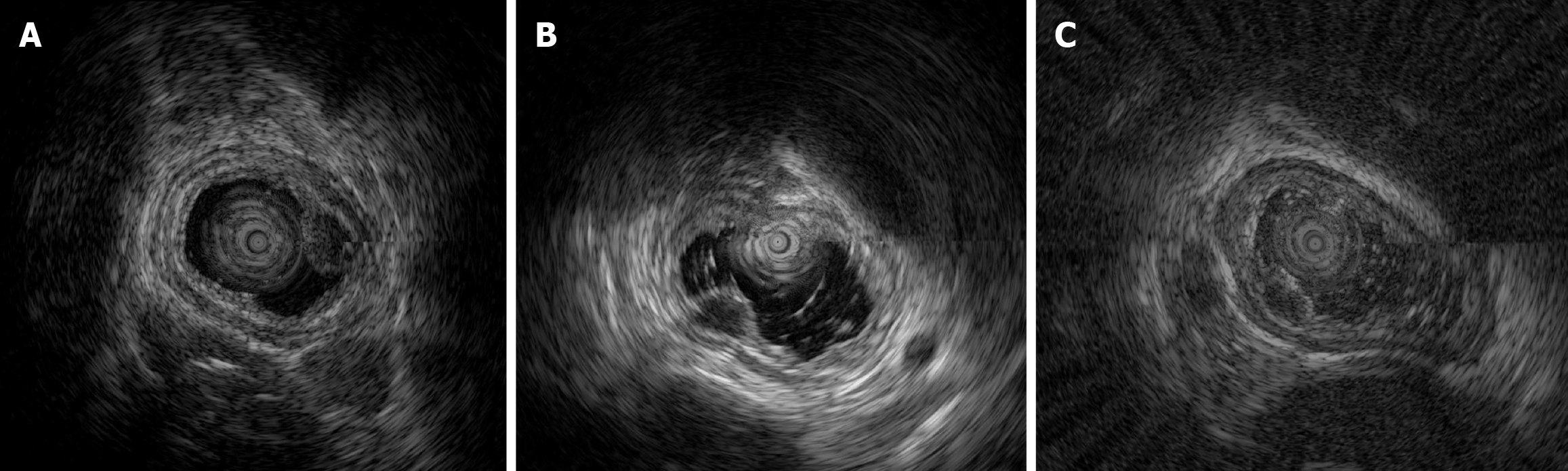
EUS was routinely performed according to well-acknowledged procedures using an Olympus EU-ME2 (Olympus Corporation, Tokyo, Japan) and mini probe with a frequency of 12 MHz. Among the patients with early E-NEC, three underwent EUS (Figure 3), which revealed solitary hypoechoic lesions originating from the muscularis mucosae or submucosa with homogeneous internal echogenicity, clear borders, normal peripheral esophageal hierarchical architecture, and intact muscularis mucosae and epithelial membranes. In one patient, multiple enlarged lymph nodes were observed outside the esophageal wall. The average grayscale value of EUS images for early E-NEC (n = 3) was significantly higher than that of esophageal leiomyoma [n = 8, 63.79 ± 1.42 vs 49.44 ± 11.57, P = 0.01, Cohen’s d = 1.402; 95% confidence intervals (CI): -0.2718 to 3.077], indicating that early E-NEC lesions exhibited higher echogenicity than esophageal leiomyomas (Figure 4).
E-NEC is an extremely rare malignancy characterized by rapid progression, high metastatic potential, and poor prognosis[20,21], and is influenced by tumor stage, grade, and distant metastases[6]. A multicenter retrospective study in Taiwan reported a median survival of 8.2 months (range: 0.2-104 months) among 38 patients with E-NEC[22]. A 10-year database-based retrospective cohort study further demonstrated that E-NEC carries a higher risk of metastasis [62.20% vs (58.72% and 46.66%)] and worse prognosis than ESCC and adenocarcinoma[9]. E-NEC predominantly affects individuals > 60 years of age, with a male-to-female ratio of (4-6):1, and is most commonly located in the middle and lower esophagus, particularly in the lower segment[9].
In this study, we found that early E-NEC was frequently misdiagnosed as esophageal leiomyoma in clinical practice. Esophageal leiomyoma, a common benign tumor of the esophagus, typically presents as a slow-growing, solitary, sub
Clinically, patients with early E-NEC are often asymptomatic or present with non-specific gastrointestinal symptoms similar to those of esophageal leiomyoma, such as dysphagia, retrosternal discomfort, etc.[10]. This lack of distinctive clinical features complicates the early diagnosis of E-NEC. Additionally, routine examinations, including tumor markers, typically present within normal limits, and contrast-enhanced CT shows no abnormalities, further mimicking the presentation of esophageal leiomyoma. Furthermore, E-NEC exhibits submucosal growth with normal epithelium, typically overlying the lesion[17], often resulting in negative or non-specific biopsy findings such as esophageal papillomatous hyperplasia[13]. These factors contribute to pathological diagnostic challenges and may delay accurate identification and timely disease management.
However, E-NEC is characterized by rapid progression and high metastatic potential. In this study, one patient initially presented with a 1.0 cm submucosal elevation at 30 cm from the incisors on conventional gastroscopy, with central depression, smooth mobility, and intact overlying mucosa. EUS suggested a submucosal lesion, likely a leiomyoma. Tumor markers were unremarkable, and contrast-enhanced CT showed no mass. Biopsy revealed squamous epithelial papillary hyperplasia, leading to a presumptive diagnosis of leiomyoma and follow-up recommendations. Six months later, repeat endoscopy revealed a large ulcerative lesion. Biopsy revealed small-cell E-NEC, and subsequent surgery confirmed lymph node metastasis. A retrospective review noted enlarged periesophageal lymph nodes on the initial EUS, which was overlooked. This case highlights the non-specific clinical features and auxiliary findings of early E-NECs and their endoscopic resemblance to leiomyomas. Diagnosis via biopsy can be challenging, and attention should be paid to extramural lymphadenopathy. These findings emphasize the importance of better characterization of endoscopic features of early E-NEC.
Therefore, three additional patients who were pathologically diagnosed with E-NEC at The First Affiliated Hospital of Zhejiang University School of Medicine were included in this study. Their clinical data and endoscopic findings were retrospectively analyzed. Notably, none of the patients were definitively diagnosed with E-NEC during initial gas
Additionally, early E-NEC typically exhibits a dense and irregular microvascular network with twisted and dilated vessels, which appears under NBI as prominent brownish or tan vascular patterns in contrast to the adjacent normal tissue[24]. In contrast, esophageal leiomyomas generally show lower vascular density and more regular vascular stru
Furthermore, E-NEC is associated with a high rate of lymph node metastasis, whereas esophageal leiomyomas are benign and rarely metastasize[11,25]. Thus, enlarged periesophageal lymph nodes on EUS should raise a suspicion of E-NEC. Although this study identified several distinguishing features between E-NEC and esophageal leiomyoma, including differences in EUS echogenicity and NBI images, as well as lymph node metastasis, there are some limitations that should be considered. First, as E-NECs are extremely rare, we included only four patients in our study, which limited the ability to derive more significant statistical interpretations. Although the observed effect size (Cohen’s d = 1.402) indicated a large difference between the two groups, the 95%CI of the effect size included 0, which may raise concerns regarding the robustness of the effect. This finding should also be interpreted in the context of a small sample size, which is known to inflate uncertainty in estimating effect sizes and widen CI, especially in studies with unequal group sizes. Importantly, despite the wide interval, the intergroup difference remained statistically significant (P = 0.01), suggesting that the observed effect was unlikely to be due to random chance. We believe that the inclusion of 0 in the CI reflects limited statistical power rather than the absence of a true effect. Thus, future clinical studies with larger sample sizes are needed to validate these observations and more precisely determine the true magnitude of the observed effect.
Second, our study employed offline analysis using Image J software to compare echogenicity between E-NECs and esophageal leiomyomas. Although this method allows quantitative assessment of gray-scale values, it has several limitations that constrain its clinical applicability. First, because this post-processing approach requires exporting and manually processing images, it lacks real-time interaction with the operator, thereby limiting its utility in real-time diagnosis and intraoperative decision-making. Second, the lack of standardization in region of interest selection and filtering parameters, which often depend on operator experience, results in poor reproducibility and limits cross-center validation.
To address the aforementioned limitations, recent advances have extended artificial intelligence (AI)-integrated endoscopic systems beyond in vitro image analysis for classification to real-time detection of tissue characterization in early esophageal neoplasia[26]. Convolutional neural networks based on WLE and NBI have achieved high accuracy in detecting superficial ESCC, with a reported sensitivity and specificity of 84% and 73%, respectively[27]. Knabe et al[28] developed an EUS-AI system using a deep convolutional neural network for esophageal adenocarcinoma, demonstrating that AI could accurately identify mucosal (T1a) and submucosal (T1b) carcinomas, with high accuracy in differentiating between the two. This architecture can be adapted to differentiate early E-NECs from esophageal leiomyomas. Prospective clinical studies should account for the rarity of E-NECs. Few-shot learning may help overcome data scarcity. Furthermore, integrating multimodal inputs, such as WLE, NBI, and EUS lymph node metastasis with AI-based grayscale analysis, may enhance the accuracy of early E-NEC diagnosis.
For the literature review, 15 studies identified by the database search were eligible and included (Table 2)[13,24,29-41]. Multi-model endoscopy, which is typically used for the detection of early E-NEC, including WLE, NBI, and EUS, is summarized in Table 2. WLE remains the initial screening tool; however, its sensitivity for early flat and subepithelial lesions is limited. NBI has become increasingly valuable because it offers enhanced visualization of microvascular patterns and mucosal architecture. E-NECs often present with irregular IPCLs, which can be classified using the Japan Esophageal Society system to differentiate malignancies from benign lesions[19]. Additionally, EUS plays a critical role in assessing lesion depth and origin, particularly in submucosal tumors. Hypoechoic masses originating from the muscularis mucosa or submucosa with possible lymphadenopathy, raise the suspicion of E-NEC. Further quantitative analysis of EUS images, such as grayscale value comparison, may better distinguish E-NECs from benign subepithelial tumors such as leiomyomas.
| Case | Ref. | Age | Sex | Location | Presentation | NBI | EUS | Stage | Pathology | Treatment | Survival |
| 1 | [13] | 77 | M | Middle | No symptoms | Type B1, type R | The lesion was localized in the mucosa | T1aN0M0 | NEC, SCC | ESD, surgery, CTx | No sign of disease recurrence 12 months after surgery |
| 2 | [24] | 80 | M | Middle/28 cm | No symptoms | Type B3, type R | A hypoechoic mass invading the third hyperechoic layer (submucosal layer) | cT1bN0M0/SM2 | NEC, SCC | ESD, CTx, RTx | 3 months lymph node metastasis; 6 months multiple liver metastases; 8 months died |
| 3 | [29] | 51 | M | 25 cm | Dysphagia | NA | NA | T1aN0M0 | NEC, squamous cell HGIN | ESD | No recurrence or metastasis 18 months |
| 4 | [30] | 75 | F | Middle | NA | Type B1 | A hypoechoic lesion located in the mucosal layer and partially within the superficial submucosal layer | pT1aN0M0 | NEC, SCC | ESD, CTx | 60 months without any recurrence |
| 5 | [31] | 49 | F | 35 cm | Intermittent epigastric soreness and heartburn | NA | A 44 mm × 3.3 mm, well demarcated, homogenous, hypoechoic, round mass lesion within the mucosal layer, and the submucosal layer beneath the lesion was observed to be intact | T1aN0M0 | NEC | EMR | 2 months alive |
| 6 | [32] | 55 | F | Upper | Intermittent mild dysphagia | NA | Showed that the bulged lesion was highly echoic and homogeneous, originating from the muscularis mucosa | SM2/T1aN0M0 | NEC | ESD, CTx | Remained disease-free during a 2-year follow-up |
| 7 | [33] | 65 | M | 27 cm | Retrosternal discomfort | NA | Characterized the lesion as an ovoid hypoechoic mass originating from the muscularis mucosae, displaying clear margins and homogeneous echogenicity | T2N1M0 | NEC | ESD | Developed systemic metastases after 16 months |
| 8 | [34] | 54 | M | Lower | Dysphagia, dizziness, and melena, GERD | NA | NA | NA | NEC | CTx, surgery | 20 months alive |
| 9 | [35] | 62 | M | Distal | Epigastric pain for 2 months; obvious difficulty in swallowing accompanied by chest pain 14 months later | NA | A low echo nodule in the muscularis mucosa, with un-uniform internal echo and a clear boundary. The nodule appeared to have a capsule and the submucosa was smooth and continuous | NA | NEC | CTx, RTx | Developed after 14 months, died of brain metastases 17 months later |
| [35] | 68 | F | NA | Epigastric pain for 1 month | NA | A hypoechoic mass in the muscularis mucosa invading the submucosal layer, and the submucosal layer was thinner than normal but still continuous | T1bN0M0 | NEC | ESD, surgery | 9 months alive | |
| 10 | [36] | 79 | M | Esophagogastric junction | No symptoms | NA | NA | T1a | NEC, EC | ESD, surgery | 4 years alive |
| 11 | [37] | 63 | M | 37 cm | No symptoms | NA | NA | cT1aSM1 | NEC, SCC, EC | ESD | 15 months alive |
| 12 | [38] | 55 | M | Middle | NA | Type V1, type V2, type V3 | NA | SM2 | NEC, SCC | ESD, CTx | 55 months alive |
| 13 | [39] | 61 | M | Middle | Epigastralgia provoked by swallowing | NA | There was thickening of the second layer, but the SM layer was not infiltrated, and it was judged to be a lesion in the mucosa | SM1 | NEC | ESD, CTx, surgery | Lymph node metastasis 14 months later; lung metastases 17 months later; died 25 months later |
| 14 | [40] | 70 | M | Lower/33 cm | Discomfort when swallowing, weight loss | Type 3, type 4IB | The lesion was depicted as a hypoechoic area in layer 2; the third layer was preserved and the depth of the lesion was considered to be intramucosal | pT1b (SM: 200 μm) | NEC, SCC | ESD, CTx | Paracardial lymph node swelling 5 months later; died 22 months later |
| 15 | [41] | 63 | M | 33 cm | A loss of appetite | NA | A 7 mm hypoechoic nodule on the mucosal layer with slight invasion to the submucosal layer | T1bN0M0 | NEC | EMR | 18 months alive |
Histopathological confirmation of E-NEC remains essential, with biopsies showing small round cells with scant cytoplasm, high mitotic activity, and necrosis. Immunohistochemistry is indispensable for the definitive diagnosis of E-NEC, with positive markers, such as chromogranin A, synaptophysin, and cluster of differentiation 56, along with high Ki-67 proliferation indices, confirming the neuroendocrine nature and grading of a tumor[6]. However, owing to sam
Treatments and prognoses are listed in Table 2. Among the 16 patients reviewed, four underwent surgery, in which three received additional surgical treatment after postoperative pathological confirmation of E-NEC by ESD, and one patient underwent surgery after chemotherapy. All the patients achieved favorable outcomes. Nine patients underwent ESD without subsequent surgery, including three who underwent ESD alone, two who achieved good prognoses, and one who developed systemic metastases 16 months later. The remaining six patients received adjuvant chemotherapy or radiotherapy, resulting in mixed outcomes. Three patients had favorable responses, while three experienced disease progression and ultimately died. Specifically, one patient developed lymph node metastasis at 3 months, liver metastases at 6 months, and died at 8 months. Another patient underwent thoracotomy with esophagectomy and lymphadenectomy for lymph node metastasis at 14 months, developed lung and bone metastases at 17 months, and died at 25 months. The third patient developed paracardial lymph node swelling at 5 months and died at 22 months. Two patients underwent endoscopic mucosal resection with good outcomes. One patient who received chemoradiotherapy alone had a poor prognosis. No consensus on standardized treatment strategy for early E-NEC has been reached at present, although various treatment modalities, including endoscopic resection, surgery, chemotherapy, radiotherapy, biological therapy, and targeted therapy, have been described[51-54]. Traditionally, surgical resection has been the cornerstone of E-NEC treatment, particularly due to its aggressive nature and high propensity for early metastasis. Esophagectomy with regional lymphadenectomy is considered the standard approach for localized or resectable disease, providing comprehensive pathological assessment and regional control[11]. However, surgery is associated with significant morbidity, particularly in older adult patients and those with comorbidities, which limits its applicability in certain clinical contexts. In recent years, advances in endoscopic techniques have led to an increased interest in less invasive options such as ESD[29,33,35], especially for early E-NEC confined to the mucosa or superficial submucosa without lymphovascular invasion. ESD allows for en bloc resection with precise histological evaluation and organ preservation; however, it is limited to early lesions without nodal involvement. Therefore, individualized treatment plan is essential, with consideration of tumor stage, depth of invasion, histological grade, patient comorbidities, and preferences. A multidisciplinary approach is crucial for optimizing outcomes and appropriate integration of endoscopic, surgical, and systemic therapies[20,55].
The pathogenesis and molecular characteristics of E-NEC remain unclear, but are hypothesized to involve the transformation of pluripotent stem cells, neuroendocrine precursor cells, or malignant cells from various histologic subtypes in the esophageal epithelium[14,56,57]. Genomic studies have shown that genetic alterations commonly associated with NETs, such as tumor protein p53 and RB1 mutations, also play a role in E-NEC pathogenesis[58-60]. Mutations in notch receptor 1, FAT atypical cadherin 1, ARID3A, and nuclear factor erythroid 2-related factor 2 occur more frequently in E-NECs than in NECs from other gastrointestinal locations[61]. Significant somatic copy number variations have been investigated in E-ENC, including deletions at 13q14 (harboring RB1) and 3p12-14 (harboring fragile histidine triad and roundabout guidance receptor 1), and amplifications of cyclin E1 and MYC[60]. Additionally, epigenetic regulation of the p63 gene has been demonstrated to play an important role in the transdifferentiation of ESCC into NEC[62]. Commonly mutated signaling pathways in E-NEC include the Wnt-β-catenin, Notch, and ERBB pathways[61]. For example, low activity of the Notch signaling pathway was observed in E-NECs, which is characterized by downregulation of Notch receptors and upregulation of Notch antagonists[60].
From transcriptomic landscape, E-NEC shares similar gene expression with small cell lung cancer, but differs from ESCC and esophageal adenocarcinoma. The upregulated genes were involved in DNA replication, cell cycle, and neuroendocrine differentiation, whereas the downregulated genes were associated with cell adhesion. From an immune perspective, E-NECs exhibit a T cell-excluded phenotype, with insufficient T cell infiltration observed in most cases. A study revealed that cluster of differentiation 8 T cells failed to infiltrate a tumor parenchyma, instead, they aggregated in the surrounding stroma[60].
Early E-NEC and esophageal leiomyoma share similar clinical symptoms and endoscopic manifestations, often leading to missed or delayed diagnoses. However, the findings of the present study suggest that multimodal endoscopic imaging is a promising tool for differentiation. Suspicion of E-NEC should be increased in cases showing similar or slightly increased echogenicity on EUS, irregular microvascular patterns on NBI, or enlarged periesophageal lymph nodes. Such cases require close monitoring and proactive management. Further studies with larger cohorts are needed to validate these findings and enhance diagnostic accuracy in clinical practice.
We thank Farhin Shaheed Kalyani (School of Medicine, Zhejiang University) for kindly supporting this study.
| 1. | Chauhan A, Chan K, Halfdanarson TR, Bellizzi AM, Rindi G, O'Toole D, Ge PS, Jain D, Dasari A, Anaya DA, Bergsland E, Mittra E, Wei AC, Hope TA, Kendi AT, Thomas SM, Flem S, Brierley J, Asare EA, Washington K, Shi C. Critical updates in neuroendocrine tumors: Version 9 American Joint Committee on Cancer staging system for gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin. 2024;74:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 2. | Giannetta E, Guarnotta V, Rota F, de Cicco F, Grillo F, Colao A, Faggiano A; NIKE. A rare rarity: Neuroendocrine tumor of the esophagus. Crit Rev Oncol Hematol. 2019;137:92-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Koizumi T, Otsuki K, Tanaka Y, Kanda S. Epidemiology of neuroendocrine neoplasmas in Japan: based on analysis of hospital-based cancer registry data, 2009 - 2015. BMC Endocr Disord. 2022;22:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 4. | Ilett EE, Langer SW, Olsen IH, Federspiel B, Kjær A, Knigge U. Neuroendocrine Carcinomas of the Gastroenteropancreatic System: A Comprehensive Review. Diagnostics (Basel). 2015;5:119-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2665] [Article Influence: 296.1] [Reference Citation Analysis (5)] |
| 6. | Mastracci L, Rindi G, Grillo F, Solcia E, Campora M, Fassan M, Parente P, Vanoli A, La Rosa S. Neuroendocrine neoplasms of the esophagus and stomach. Pathologica. 2021;113:5-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Tustumi F, Takeda FR, Uema RH, Pereira GL, Sallum RA, Cecconello I. Primary neuroendocrine neoplasm of the esophagus - Report of 14 cases from a single institute and review of the literature. Arq Gastroenterol. 2017;54:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Huang Q, Wu H, Nie L, Shi J, Lebenthal A, Chen J, Sun Q, Yang J, Huang L, Ye Q. Primary high-grade neuroendocrine carcinoma of the esophagus: a clinicopathologic and immunohistochemical study of 42 resection cases. Am J Surg Pathol. 2013;37:467-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Cai W, Ge W, Yuan Y, Ding K, Tan Y, Wu D, Hu H. A 10-year Population-based Study of the Differences between NECs and Carcinomas of the Esophagus in Terms of Clinicopathology and Survival. J Cancer. 2019;10:1520-1527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Awada H, Hajj Ali A, Bakhshwin A, Daw H. High-grade large cell neuroendocrine carcinoma of the esophagus: a case report and review of the literature. J Med Case Rep. 2023;17:144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 11. | Deng HY, Ni PZ, Wang YC, Wang WP, Chen LQ. Neuroendocrine carcinoma of the esophagus: clinical characteristics and prognostic evaluation of 49 cases with surgical resection. J Thorac Dis. 2016;8:1250-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Zhang X, Ma L, Bao H, Zhang J, Wang Z, Gong P. Clinical, pathological and prognostic characteristics of gastroenteropancreatic neuroendocrine neoplasms in China: a retrospective study. BMC Endocr Disord. 2014;14:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Fujihara S, Kobayashi M, Nishi M, Yachida T, Yoshitake A, Deguchi A, Muraoka A, Kobara H, Masaki T. Composite neuroendocrine carcinoma and squamous cell carcinoma with regional lymph node metastasis: a case report. J Med Case Rep. 2018;12:227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Lu XJ, Luo JD, Ling Y, Kong YZ, Feng LL, Zhou J, Wang F. Management of small cell carcinoma of esophagus in China. J Gastrointest Surg. 2013;17:1181-1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Hoffman A, Manner H, Rey JW, Kiesslich R. A guide to multimodal endoscopy imaging for gastrointestinal malignancy - an early indicator. Nat Rev Gastroenterol Hepatol. 2017;14:421-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 16. | Chiam KH, Shin SH, Choi KC, Leiria F, Militz M, Singh R. Current Status of Mucosal Imaging with Narrow-Band Imaging in the Esophagus. Gut Liver. 2021;15:492-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Geng ZH, Liu YL, Xu C, Zhu Y, Zhou PH, Cai MY. Neuroendocrine carcinomas of the esophagus: clinicopathological and immunohistochemical features of 43 cases. Clin Cancer Bull. 2023;2:5. [DOI] [Full Text] |
| 18. | Deprez PH, Moons LMG, OʼToole D, Gincul R, Seicean A, Pimentel-Nunes P, Fernández-Esparrach G, Polkowski M, Vieth M, Borbath I, Moreels TG, Nieveen van Dijkum E, Blay JY, van Hooft JE. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:412-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 243] [Article Influence: 60.8] [Reference Citation Analysis (1)] |
| 19. | Oyama T, Inoue H, Arima M, Momma K, Omori T, Ishihara R, Hirasawa D, Takeuchi M, Tomori A, Goda K. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus. 2017;14:105-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 243] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 20. | Ji A, Jin R, Zhang R, Li H. Primary small cell carcinoma of the esophagus: progression in the last decade. Ann Transl Med. 2020;8:502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Xu L, Li Y, Liu X, Sun H, Zhang R, Zhang J, Zheng Y, Wang Z, Liu S, Chen X. Treatment Strategies and Prognostic Factors of Limited-Stage Primary Small Cell Carcinoma of the Esophagus. J Thorac Oncol. 2017;12:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Wu IC, Chu YY, Wang YK, Tsai CL, Lin JC, Kuo CH, Shih HY, Chung CS, Hu ML, Sun WC, Wang JP, Wang HP. Clinicopathological features and outcome of esophageal neuroendocrine tumor: A retrospective multicenter survey by the digestive endoscopy society of Taiwan. J Formos Med Assoc. 2021;120:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Ha C, Regan J, Cetindag IB, Ali A, Mellinger JD. Benign esophageal tumors. Surg Clin North Am. 2015;95:491-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Tanaka Y, Hirata D, Fujii S, Kusaka T, Shibuya S. Early-stage Neuroendocrine Carcinoma of the Esophagus Observed with Annual Endoscopy for Three Years. Intern Med. 2019;58:1727-1731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Egashira A, Morita M, Kumagai R, Taguchi KI, Ueda M, Yamaguchi S, Yamamoto M, Minami K, Ikeda Y, Toh Y. Neuroendocrine carcinoma of the esophagus: Clinicopathological and immunohistochemical features of 14 cases. PLoS One. 2017;12:e0173501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 26. | Huang LM, Yang WJ, Huang ZY, Tang CW, Li J. Artificial intelligence technique in detection of early esophageal cancer. World J Gastroenterol. 2020;26:5959-5969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Tokai Y, Yoshio T, Aoyama K, Horie Y, Yoshimizu S, Horiuchi Y, Ishiyama A, Tsuchida T, Hirasawa T, Sakakibara Y, Yamada T, Yamaguchi S, Fujisaki J, Tada T. Application of artificial intelligence using convolutional neural networks in determining the invasion depth of esophageal squamous cell carcinoma. Esophagus. 2020;17:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (2)] |
| 28. | Knabe M, Welsch L, Blasberg T, Müller E, Heilani M, Bergen C, Herrmann E, May A. Artificial intelligence-assisted staging in Barrett's carcinoma. Endoscopy. 2022;54:1191-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 29. | He MJ, Liu XY, Chen TY, Zhou PH, Zhang YQ. Endoscopic submucosal dissection for an early-stage neuroendocrine carcinoma composited with squamous cell dysplasia. Endoscopy. 2022;54:E573-E575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Ishikawa-Kakiya Y, Nagami Y, Fujiwara Y. Intramucosal esophageal neuroendocrine carcinoma treated with endoscopic submucosal dissection and chemotherapy. Dig Endosc. 2019;31:466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Lim CS, Park SJ, Park MI, Moon W, Kim HH, Lee JS, Kim BJ, Ku DY. Successful endoscopic mucosal resection of a low esophageal carcinoid tumor. Clin Endosc. 2013;46:576-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Tang N, Feng Z. Endoscopic submucosal dissection combined with adjuvant chemotherapy for early-stage neuroendocrine carcinoma of the esophagus: A case report. World J Clin Cases. 2022;10:3164-3169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 33. | Yan J, Xiao X. Case report: A case of esophageal small cell carcinoma misdiagnosed as leiomyoma. Front Med (Lausanne). 2024;11:1489207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Haag A, Jayakrishnan T, Shah D, Sandhu A, Monga D. First Reported Case of Localized Extra-pulmonary Small Cell Carcinoma (EPSC) of the Esophagus Treated With Triple Therapy. Anticancer Res. 2020;40:5919-5923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Er LM, Ding Y, Sun XF, Ma WQ, Yuan L, Zheng XL, An NN, Wu ML. Endoscopic diagnosis of early-stage primary esophageal small cell carcinoma: Report of two cases. World J Clin Cases. 2021;9:2562-2568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 36. | Kinoshita T, Ishikawa S, Inaba T, Sakakihara I, Izumikawa K, Takahashi S, Yamamoto K, Tanaka S, Wato M, Nakamura S, Yao T. Neuroendocrine carcinoma arising from Barrett's esophageal adenocarcinoma: a case report. Clin J Gastroenterol. 2020;13:1028-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Fukui H, Dohi O, Miyazaki H, Yasuda T, Yoshida T, Ishida T, Doi T, Hirose R, Inoue K, Harusato A, Yoshida N, Uchiyama K, Ishikawa T, Takagi T, Konishi H, Morinaga Y, Itoh Y. A case of endoscopic submucosal dissection for neuroendocrine carcinoma of the esophagus with invasion to the muscularis mucosae. Clin J Gastroenterol. 2022;15:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Watanabe K, Hikichi T, Sato M, Nakamura J, Takagi T, Suzuki R, Sugimoto M, Waragai Y, Kikuchi H, Konno N, Watanabe H, Obara K, Ohira H. A case of endocrine cell carcinoma combined with squamous cell carcinoma of the esophagus resected by endoscopic submucosal dissection. Fukushima J Med Sci. 2014;60:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Kobayashi K, Aoki T, Nishioka K, Takachi K, Komori T, Hatano H, Yoshida K, Hayashi E. A Case of Esophageal Endocrine Cell Carcinoma That Recurred in the Lymph Nodes Around the Thoracic Aorta After ESD. Gastroenterol Endosc. 2011;53:28-34. [DOI] [Full Text] |
| 40. | Tokunaga N, Itaba S, Nakamura K, Yamada M, Okamoto R, Aso A, Igarashi H, Akiho H, Ito T, Takayanagi R, Goto A. [A case of lymph node metastasis from esophageal small-cell-type endocrine cell carcinoma diagnosed by endoscopic ultrasound-guided fine-needle aspiration]. Nihon Shokakibyo Gakkai Zasshi. 2012;109:1360-1366. [PubMed] |
| 41. | Nata T, Fujiya M, Tanabe H, Ueno N, Konno Y, Ishikawa C, Inaba Y, Ito T, Sato R, Moriichi K, Okamoto K, Maemoto A, Mizukami Y, Watari J, Ashida T, Kohgo Y. A case of small cell carcinoma of the oesophagus treated with endoscopic mucosal resection who remained in clinical remission for 18 months: its endoscopic features with specific light spectra. BMJ Case Rep. 2009;2009:bcr06.2009.2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 42. | Cannizzaro R, Maiero S, Fornasarig M, Canzonieri V, Magris R, Guarnieri G, Urbani M, Buonadonna A, Baresic T, Spessotto P. Probe-based confocal laser endomicroscopy (pCLE) is a suitable method for extrapulmonary high grade neuroendocrine rectal carcinoma (HGNEC) evaluation. Onco Targets Ther. 2019;12:4577-4583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Pilonis ND, Januszewicz W, di Pietro M. Confocal laser endomicroscopy in gastro-intestinal endoscopy: technical aspects and clinical applications. Transl Gastroenterol Hepatol. 2022;7:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 44. | Yuan XL, Liu W, Lin YX, Deng QY, Gao YP, Wan L, Zhang B, Zhang T, Zhang WH, Bi XG, Yang GD, Zhu BH, Zhang F, Qin XB, Pan F, Zeng XH, Chaudhry H, Pang MY, Yang J, Zhang JY, Hu B. Effect of an artificial intelligence-assisted system on endoscopic diagnosis of superficial oesophageal squamous cell carcinoma and precancerous lesions: a multicentre, tandem, double-blind, randomised controlled trial. Lancet Gastroenterol Hepatol. 2024;9:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 45. | Luo D, Kuang F, Du J, Zhou M, Liu X, Luo X, Tang Y, Li B, Su S. Artificial Intelligence-Assisted Endoscopic Diagnosis of Early Upper Gastrointestinal Cancer: A Systematic Review and Meta-Analysis. Front Oncol. 2022;12:855175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 46. | Gerard L, Patte C, Chardon L, Hervieu V, Payen L, Allio M, Marx C, Clermidy H, Durand A, Mehlen P, Bollard J, Poncet G, Roche C, Gibert B, Walter T. Neuropilin 2 and soluble neuropilin 2 in neuroendocrine neoplasms. Endocr Relat Cancer. 2024;31:e240052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 47. | La Salvia A, Fanciulli G. Progastrin-Releasing Peptide As a Diagnostic Biomarker of Pulmonary and Non-Pulmonary Neuroendocrine Neoplasms. Endocr Res. 2024;49:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 48. | Mosele F, Remon J, Mateo J, Westphalen CB, Barlesi F, Lolkema MP, Normanno N, Scarpa A, Robson M, Meric-Bernstam F, Wagle N, Stenzinger A, Bonastre J, Bayle A, Michiels S, Bièche I, Rouleau E, Jezdic S, Douillard JY, Reis-Filho JS, Dienstmann R, André F. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2020;31:1491-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 827] [Article Influence: 137.8] [Reference Citation Analysis (5)] |
| 49. | Liu M, Li N, Tang H, Chen L, Liu X, Wang Y, Lin Y, Luo Y, Wei S, Wen W, Chen M, Wang J, Zhang N, Chen J. The Mutational, Prognostic, and Therapeutic Landscape of Neuroendocrine Neoplasms. Oncologist. 2023;28:e723-e736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 50. | Chen Y, Cui X, Wang D, Xia G, Xing M, Cheng L, Sheng L, Du X. Molecular Characterization and Prognostication of Large Cell Neuroendocrine Carcinoma and Large Cell Carcinoma. Front Oncol. 2021;11:664397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Purwar P, Jiwnani S, Karimundackal G, Pramesh CS. Management of esophageal small cell carcinoma. Ann Thorac Surg. 2015;99:1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Xie MR, Xu SB, Sun XH, Ke L, Mei XY, Liu CQ, Ma DC. Role of surgery in the management and prognosis of limited-stage small cell carcinoma of the esophagus. Dis Esophagus. 2015;28:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Zou B, Li T, Zhou Q, Ma D, Chen Y, Huang M, Peng F, Xu Y, Zhu J, Ding Z, Zhou L, Wang J, Ren L, Yu M, Gong Y, Li Y, Chen L, Lu Y. Adjuvant Therapeutic Modalities in Primary Small Cell Carcinoma of Esophagus Patients: A Retrospective Cohort Study of Multicenter Clinical Outcomes. Medicine (Baltimore). 2016;95:e3507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | Wong AT, Shao M, Rineer J, Osborn V, Schwartz D, Schreiber D. Treatment and survival outcomes of small cell carcinoma of the esophagus: an analysis of the National Cancer Data Base. Dis Esophagus. 2017;30:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Vos B, Rozema T, Miller RC, Hendlisz A, Van Laethem JL, Khanfir K, Weber DC, El Nakadi I, Van Houtte P. Small cell carcinoma of the esophagus: a multicentre Rare Cancer Network study. Dis Esophagus. 2011;24:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 56. | Wu Z, Ma JY, Yang JJ, Zhao YF, Zhang SF. Primary small cell carcinoma of esophagus: report of 9 cases and review of literature. World J Gastroenterol. 2004;10:3680-3682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Zhu Y, Qiu B, Liu H, Li Q, Xiao W, Hu Y, Liu M. Primary small cell carcinoma of the esophagus: review of 64 cases from a single institution. Dis Esophagus. 2014;27:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 58. | Wang F, Liu DB, Zhao Q, Chen G, Liu XM, Wang YN, Su H, Qin YR, He YF, Zou QF, Liu YH, Lin YE, Liu ZX, Bei JX, Xu RH. The genomic landscape of small cell carcinoma of the esophagus. Cell Res. 2018;28:771-774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 59. | Ishida H, Kasajima A, Kamei T, Miura T, Oka N, Yazdani S, Ozawa Y, Fujishima F, Sakurada A, Nakamura Y, Tanaka Y, Kurosumi M, Ishikawa Y, Okada Y, Ohuchi N, Sasano H. SOX2 and Rb1 in esophageal small-cell carcinoma: their possible involvement in pathogenesis. Mod Pathol. 2017;30:660-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 60. | Li R, Yang Z, Shao F, Cheng H, Wen Y, Sun S, Guo W, Li Z, Zhang F, Xue L, Bi N, Wang J, Sun Y, Li Y, Tan F, Xue Q, Gao S, Shi S, Gao Y, He J. Multi-omics profiling of primary small cell carcinoma of the esophagus reveals RB1 disruption and additional molecular subtypes. Nat Commun. 2021;12:3785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 61. | Wu H, Yu Z, Liu Y, Guo L, Teng L, Guo L, Liang L, Wang J, Gao J, Li R, Yang L, Nie X, Su D, Liang Z. Genomic characterization reveals distinct mutation landscapes and therapeutic implications in neuroendocrine carcinomas of the gastrointestinal tract. Cancer Commun (Lond). 2022;42:1367-1386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 62. | Zhang Y, Karagiannis D, Liu H, Lin M, Fang Y, Jiang M, Chen X, Suresh S, Huang H, She J, Shi F, Liu J, Luo D, Angel JC, Lin G, Yang P, El-Rifai W, Zaika A, Oro AE, Liu K, Rustgi AK, Wang TC, Lu C, Que J. Epigenetic regulation of p63 blocks squamous-to-neuroendocrine transdifferentiation in esophageal development and malignancy. Sci Adv. 2024;10:eadq0479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/