Published online Nov 15, 2025. doi: 10.4251/wjgo.v17.i11.109375
Revised: September 5, 2025
Accepted: October 13, 2025
Published online: November 15, 2025
Processing time: 107 Days and 17 Hours
Gastric cancer is a major global health issue, and the perioperative period critic
To compare the effects of sevoflurane inhalation anesthesia and propofol total in
This single-center retrospective cohort study included 204 patients who underw
Baseline demographic and clinical characteristics were similar between groups. No significant differences were observed in intraoperative indicators or most 30-day postoperative outcomes, including length of stay, emergency department visits, and readmission rates. The propofol group showed elevated mean VAS pain score at 24 hours postoperatively, but no differences were found at other time points. The propofol group also had significantly higher postoperative nausea incidence and transiently higher systolic/diastolic blood pressure and heart rate at the time of incision than the sevoflurane group. No significant differences were seen in overall rates or severity of postoperative complications, intraoperative adverse events, or in overall survival and progression-free survival.
In patients undergoing radical gastrectomy for gastric cancer, sevoflurane and propofol anesthesia demonstrated similar profiles regarding intraoperative safety, postoperative complications, adverse events, postoperative pain, and long-term survival. The selection of anesthesia can be personalized without significantly affecting perioperative or oncologic outcomes.
Core Tip: This study compares sevoflurane inhalation anesthesia with propofol total intravenous anesthesia in patients undergoing radical gastrectomy for gastric cancer. Both anesthetic techniques showed equivalent intraoperative safety and postoperative outcomes, including complications, pain scores, and long-term survival. Importantly, the propofol group exhibited higher postoperative nausea incidence and transiently elevated blood pressure and heart rate during surgery. These findings suggest that the choice of anesthetic can be individualized based on patient-specific factors without compromising perioperative or oncologic outcomes.
- Citation: Wang Z, Cheng JW, Yu KY. Short-term and long-term effects of sevoflurane inhalation vs propofol total intravenous anesthesia in gastrectomy for gastric cancer. World J Gastrointest Oncol 2025; 17(11): 109375
- URL: https://www.wjgnet.com/1948-5204/full/v17/i11/109375.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i11.109375
Gastric cancer remains a major health burden worldwide, ranking as the fifth most prevalent malignancy and the fourth leading cause of cancer-related mortality globally[1]. Despite advances in diagnosis and multimodal therapy, the prognosis for gastric cancer remains poor, particularly in regions with high incidence[2]. Radical gastrectomy is the primary approach for curative treatment, often complemented by perioperative chemotherapy or chemoradiotherapy[3]. As surgical and oncologic techniques evolve, optimizing perioperative care, including anesthesia management, has gained attention for its potential influence on short-term recovery and long-term oncologic outcomes[4].
The perioperative period represents a unique window during which surgical stress, inflammatory responses, and immunosuppression may contribute to cancer recurrence and metastasis[5]. Accumulating evidence suggests that the choice of anesthetic agent and the mode of anesthesia maintenance may influence these physiological processes, thereby affecting immediate postoperative complications, tumor biology, and the long-term survival of patients with cancer[6]. Two commonly employed anesthetic techniques in major abdominal oncology surgery are volatile inhalational anesth
Sevoflurane is a widely used volatile anesthetic recognized for its rapid onset and recovery characteristics, cardiovascular stability, and minimal airway irritation[8]. Recent experimental studies indicate that sevoflurane may exert immunosuppressive and immunoprotective effects via the modulation of cytokine release, oxidative stress, and neuroendocrine pathways[9]. However, some preclinical research suggests that volatile anesthetics may inhibit natural killer (NK) cell activity and increase hypoxia-inducible factor-1α (HIF-1α) expression, potentially promoting tumor growth and metastasis in certain cancers[10].
By contrast, propofol-based TIVA has been proposed to exert favorable immunomodulatory and antitumor effects through several mechanisms. Propofol, a phenolic compound, is known for its rapid onset and offset, making it ideal for precise control of anesthetic depth. Propofol has been shown to reduce the release of pro-inflammatory cytokines and inhibit cyclooxygenase activity, which can mitigate the inflammatory response and reduce prostaglandin E2 synthesis. These properties make propofol the preferred choice for minimizing perioperative immune suppression and enhancing recovery in patients with cancer[11]. Propofol has also demonstrated potent antioxidant properties and may preserve perioperative cellular immune competence, particularly in the context of surgical stress[12]. These observations have led to speculation that propofol TIVA may be associated with improved postoperative recovery and enhanced oncologic outcomes compared with volatile anesthetics[12].
Despite these insights, robust clinical evidence remains inconclusive regarding the superiority of any single anesthetic technique for major cancer surgery[13]. Previous prospective and retrospective studies[14-16] have yielded conflicting results, with some reporting marginal improvements in recurrence-free or overall survival (OS) with propofol-based anesthesia, while others found no difference between volatile and intravenous techniques. Additionally, many studies[17,18] are limited by heterogeneous patient populations, variations in surgical procedure, and inconsistent perioperative management. In patients undergoing gastrectomy for gastric cancer—a population at high risk of perioperative morbidity and disease recurrence—the influence of anesthetic choice on immediate and long-term outcomes remains underexplored.
Furthermore, the intraoperative hemodynamic profile, depth of anesthesia, and adverse event rates are critical concerns in high-risk oncologic patients[17]. Achieving optimal anesthesia strikes a balance between providing adequate surgical conditions and minimizing perioperative complications such as hemodynamic instability, postoperative nausea and vomiting (PONV), pain, and infection, all of which can possibly delay recovery and reduce the quality of life[18]. Rigorous intraoperative monitoring practices, including Bispectral Index (BIS) guidance and multimodal physiological surveillance, may mitigate some of the inherent risks associated with different anesthetic modalities[18].
To address these knowledge gaps, the present study was designed to compare the short-term and long-term effects of sevoflurane inhalation anesthesia vs propofol TIVA on perioperative physiological indicators, postoperative recovery, and oncologic outcomes in a well-characterized cohort of patients undergoing radical gastrectomy for gastric cancer. We used standardized protocols for perioperative management and comprehensive follow-up to examine survival outcomes and the nuanced perioperative physiological and clinical parameters that may guide optimal practices in anesthesia for oncologic surgery.
This study is a single-center retrospective cohort analysis of 204 patients who underwent radical gastrectomy for gastric cancer at Yantaishan Hospital between February 2019 to December 2022. This study has been approved by the Ethics Committee of Yantaishan Hospital. Given the retrospective design of the study, informed consent was waived.
Inclusion criteria: (1) Age ≥ 18 years; (2) Meeting the diagnostic criteria for gastric cancer; (3) American Society of Anesthesiologists (ASA) physical status classification I-III; (4) Undergoing radical gastrectomy; (5) Receiving general anesthesia via sevoflurane inhalation or propofol TIVA; and (6) Eastern Cooperative Oncology Group (ECOG) perfor
Exclusion criteria: (1) The use of sedatives or analgesics within 2 weeks before surgery; (2) The presence of other malignancies; (3) Conversion of the anesthetic method during surgery; (4) Incomplete case records; (5) The presence of spinal deformity or spinal disease; (6) The presence of severe pulmonary, hepatic, or renal dysfunction; and (7) Contraindications to epidural or intrathecal anesthesia.
Patients were divided into two groups based on the anesthetic used during surgery: The sevoflurane group (n = 103) and the propofol group (n = 101).
All patients adhered to a standardized preoperative fasting protocol that required abstaining from solid food for 6-8 hours and clear liquids for at least 2 hours prior to surgery. Anesthesia was induced via intravenous administration of midazolam (0.05-0.15 mg/kg), sufentanil (0.3 μg/kg), and propofol (1-2.5 mg/kg). Anesthesia maintenance was stratified by group assignment: In the sevoflurane group, patients received 3% sevoflurane inhalation via face mask (oxygen/air mixture with a flow rate of 2 L/minutes). The concentration of 3% sevoflurane was chosen because it can provide adequate anesthesia depth, maintain cardiovascular stability, and minimize airway irritation. This concentration is within the clinically recommended range for maintaining stable anesthetic depth during major abdominal surgeries[19]. End-tidal carbon dioxide was maintained at 35 ± 5 mmHg through ventilatory adjustments, and pure oxygen ventilation was initiated 10 minutes before the end of surgery. In the propofol group, propofol [3 mg/(kg·hour)] was administered continuously via target-controlled infusion to maintain anesthesia. This infusion rate was selected to achieve a steady-state plasma concentration of propofol, thereby ensuring adequate anesthesia depth, minimizing hemodynamic disturbances, and promoting rapid postoperative recovery[20]. In both groups, remifentanil was continuously infused at a rate of 0.1-0.2 μg/(kg·minute) to optimize intraoperative analgesia.
Intraoperative physiological monitoring was conducted using a multimodal approach. The depth of anesthesia was monitored by applying a BIS sensor on the forehead. The BIS is a processed electroencephalogram (EEG) parameter that quantifies the hypnotic effect of anesthetics by analyzing EEG signals and providing a numerical value between 0 and 100, where 100 represents full wakefulness and 0 indicates no brain activity. We aimed to maintain BIS values within 40-60, which is considered optimal for ensuring adequate anesthetic depth without excessive suppression of consciousness. Continuous invasive arterial blood pressure was measured via 20G radial artery catheterization, with systolic, diastolic, and heart rate parameters recorded. Regional cerebral oxygen saturation (SrO2) was monitored by using a cerebral oximeter. Additionally, urinary output was recorded using an indwelling urinary catheter, and intraoperative blood loss was measured precisely using quantitative suction devices. Key time points, including anesthesia and surgical durations, were documented in real time and verified by two members of the anesthesia team.
Postoperative analgesia was standardized using a patient-controlled intravenous analgesia (PCA) pump, configured with either fentanyl (3 μg/mL) or sufentanil (0.5 μg/mL). The background infusion rate was set at 2 mL/hours, with a bolus dose of 0.5 mL and a lock-out interval of 15 minutes. Data collection during intraoperative and postoperative periods followed standardized procedures to ensure the reliability and reproducibility of study findings. Detailed information regarding all instruments and medications is provided in Supplementary Table 1.
This study employed standardized data collection methods to systematically evaluate patients' baseline characteristics and clinical outcomes. All baseline clinical data [including age, gender, body mass index (BMI), and disease diagnosis staging] were based on the final preoperative diagnosis. Surgical-related metrics (total anesthesia time and total operation time) were recorded in real time by the anesthesiologist and surgical nurse team at the end of the surgery to ensure data accuracy. Inpatient data were precisely collected using the electronic medical record system, including admission/discharge dates (used to calculate the actual length of hospital stay) and discharge status (with particular notation of in-hospital deaths).
Postoperative follow-up employed a multi-modal system, including weekly telephone follow-ups (for the first month), standardized text message reminders, and outpatient review record checks. Follow-up frequency was set at regular intervals from 1 month to 3 months post-surgery until patient death or the study cutoff date (December 31, 2022). Key outcome measures within 30 days post-surgery included emergency department visit rates (defined as any visit to the emergency department for any reason within 30 days after surgery) and readmission rates (recording unplanned rehospitalizations occurring within 30 days after surgery).
All adverse events were clinically evaluated by the attending physicians. Intraoperative body movement was defined as flexion of one or more limbs or head movement immediately following skin incision, excluding facial expressions (e.g., grimacing) or physiological reflex actions (e.g., coughing or swallowing). Hypoxemia was diagnosed based on arterial blood gas analysis, with a definitive threshold of arterial oxygen partial pressure (PaO2) < 60 mmHg. Postoperative agitation was assessed according to characteristic clinical manifestations, including crying, agitated behavior, irritability, and disorientation. Nausea was evaluated based on patient-reported subjective symptoms such as gastric discomfort, dry mouth, or increased salivation, with confirmation by the attending physician through clinical observation. Vomiting episodes were defined as the active expulsion of gastric contents through the mouth via retrograde esophageal movement and were monitored in real time by nursing staff. Pruritus was diagnosed based on patient-reported localized or generalized itching sensation and objective clinical signs, such as scratch marks or skin excoriations.
Postoperative complications were clinically evaluated by attending physicians and classified according to the stan
Grade I: Minor complications requiring only basic interventions;
Grade II: Moderate complications necessitating pharmacological treatment or blood transfusion;
Grade IIIa: Complications requiring non-invasive intervention under local anesthesia (e.g., drainage or endoscopic procedures);
Grade IIIb: Complications demanding surgical reintervention under general anesthesia;
Grade IVa: Single-organ dysfunction;
Grade IVb: Multi-organ dysfunction or failure;
Grade V: Death attributable to postoperative complications.
To further quantify the overall burden of complications, we applied the Comprehensive Complication Index (CCI)[22]. The CCI assigns weighted scores to individual complications based on their severity, with weights normalized to ensure comparability across different types of complications (e.g., grade I: Weight = 0.5, grade II: Weight = 3, grade IIIa: Weight = 8, grade IIIb: Weight = 14, grade IVa: Weight = 25, grade IVb: Weight = 50, and grade V: Weight = 100). These weights were then aggregated to generate a continuous composite score ranging from 0 (no complications) to 100 (maximum complication severity), providing a precise assessment of postoperative morbidity.
Postoperative pain was assessed using the Visual Analog Scale (VAS), evaluated by a dedicated anesthesiologist at standardized follow-up intervals of 2, 4, 6, 24, and 48 hours post-surgery. The VAS score ranges from 0 to 100, where 0 indicates no pain and 100 represents the most severe pain. The score was determined based on the patient's use of the PCA pump. According to international standards, pain levels were categorized as follows: 0 = no pain; 1-30 = mild pain; 31-60 = moderate pain; and 61-100 = severe pain. In this study, the internal consistency of the VAS was excellent, with a Cronbach's α coefficient of 0.9117, indicating high reliability of this assessment tool in the study population[23].
OS is defined as the time interval from the date of surgery completion to the time of death from any cause. Endpoint events include, but are not limited to, death related to disease progression, death due to treatment complications, death from comorbidities, or death from other accidental events. For cases where no endpoint event occurred during the observation period, survival time was censored to ensure the accuracy of the survival analysis.
Progression-free survival (PFS) is defined as the time interval from the date of surgery completion to the occurrence of objective radiological disease progression confirmed by the attending physician using to the modified RECIST 1.1[24] criteria, or to all-cause death, whichever occurs first. This metric comprehensively reflects the control of the disease and the OS status of the patients.
All statistical analyses were performed using SPSS 29.0 statistical software (SPSS Inc, Chicago, IL, United States). Categorical variables were expressed as frequencies and percentages, with group comparisons selected based on sample characteristics. Pearson χ2 test was used when the sample size was ≥ 40 and all expected frequencies (T) were ≥ 5. Continuity-corrected χ2 test was applied when the sample size was ≥ 40 but some expected frequencies were 1 ≤ T < 5. Fisher's exact test was used for samples with a size < 40 or any expected frequency T < 1. Continuous variables were tested for normality using the Shapiro-Wilk test. Normally distributed data were presented as mean ± SD, and group comparisons were conducted using independent samples t-tests with adjusted variances. Survival analysis was performed using the Kaplan-Meier method to construct survival curves, and intergroup differences were assessed by log-rank tests. All statistical tests were two-tailed, with P < 0.05 considered statistically significant.
A total of 204 patients were included in this study, with 103 in the sevoflurane group and 101 in the propofol group. Baseline characteristics of the two groups were similar (Table 1). We found no statistically significant differences in age, sex distribution, BMI, ECOG performance status, ASA classification, baseline systolic and diastolic blood pressures, heart rate, hemoglobin concentration, or the prevalence of hypertension and diabetes. These results indicated successful baseline matching between the sevoflurane and propofol groups.
| Parameters | Sevoflurane group (n = 103) | Propofol group (n = 101) | t/χ2 | P value |
| Age (years) | 59.72 ± 9.79 | 60.21 ± 10.13 | 0.349 | 0.728 |
| Sex | 0.245 | 0.621 | ||
| Male | 67 (65.05) | 69 (68.32) | ||
| Female | 36 (34.95) | 32 (31.68) | ||
| BMI (kg/m2) | 23.79 ± 2.25 | 24.10 ± 2.53 | 0.910 | 0.364 |
| ECOG | 0.003 | 0.954 | ||
| 0 | 88 (85.44) | 86 (85.15) | ||
| 1 | 15 (14.56) | 15 (14.85) | ||
| ASA classification | 0.420 | 0.811 | ||
| I | 44 (42.72) | 41 (40.59) | ||
| II | 58 (56.31) | 58 (57.43) | ||
| III | 1 (0.97) | 2 (1.98) | ||
| Systolic blood pressure (mmHg) | 115.01 ± 11.62 | 114.09 ± 11.28 | 0.572 | 0.568 |
| Diastolic blood pressure (mmHg) | 75.33 ± 10.12 | 72.70 ± 11.01 | 1.778 | 0.077 |
| Heart rate (bpm) | 129.78 ± 24.25 | 135.41 ± 25.30 | 1.622 | 0.106 |
| Hemoglobin (g/dL) | 12.98 ± 1.28 | 13.10 ± 1.33 | 0.659 | 0.510 |
| Underlying diseases | ||||
| Hypertension | 79 (76.70) | 71 (70.30) | 1.074 | 0.300 |
| Diabetes | 37 (35.92) | 46 (54.46) | 1.956 | 0.162 |
Intraoperative indicators were similar between the sevoflurane and propofol groups. We found no statistically significant differences in total anesthesia time, total operation time, intraoperative blood loss, or urine output between the two groups (Table 2).
| Parameters | Sevoflurane group (n = 103) | Propofol group (n = 101) | t | P value |
| Total anesthesia time (minute) | 181.54 ± 29.23 | 174.95 ± 26.33 | 1.691 | 0.092 |
| Total operation time (minute) | 161.85 ± 19.12 | 158.25 ± 17.13 | 1.416 | 0.158 |
| Bleeding (mL) | 106.95 ± 52.12 | 116.18 ± 52.33 | 1.263 | 0.208 |
| Urine (mL) | 757.98 ± 299.85 | 762.11 ± 312.31 | 0.096 | 0.923 |
At 30 days post-surgery, no statistically significant differences were observed in clinical outcomes between the sevoflurane and propofol groups (Table 3). In-hospital mortality was 0% in the sevoflurane group and 1.98% in the propofol group. The length of hospital stay was similar between groups. The incidence of emergency department visits and readmission rates were also similar between the two groups.
| Parameters | Sevoflurane group (n = 103) | Propofol group (n = 101) | t/χ2 | P value |
| In-hospital mortality | 0 (0) | 2 (1.98) | 0.525 | 0.469 |
| Length of hospital stay, day | 7.58 ± 1.12 | 7.39 ± 0.98 | 1.282 | 0.201 |
| Emergency department visit | 22 (21.36) | 28 (27.72) | 1.116 | 0.291 |
| Readmission rate | 4 (3.88) | 8 (7.92) | 1.501 | 0.220 |
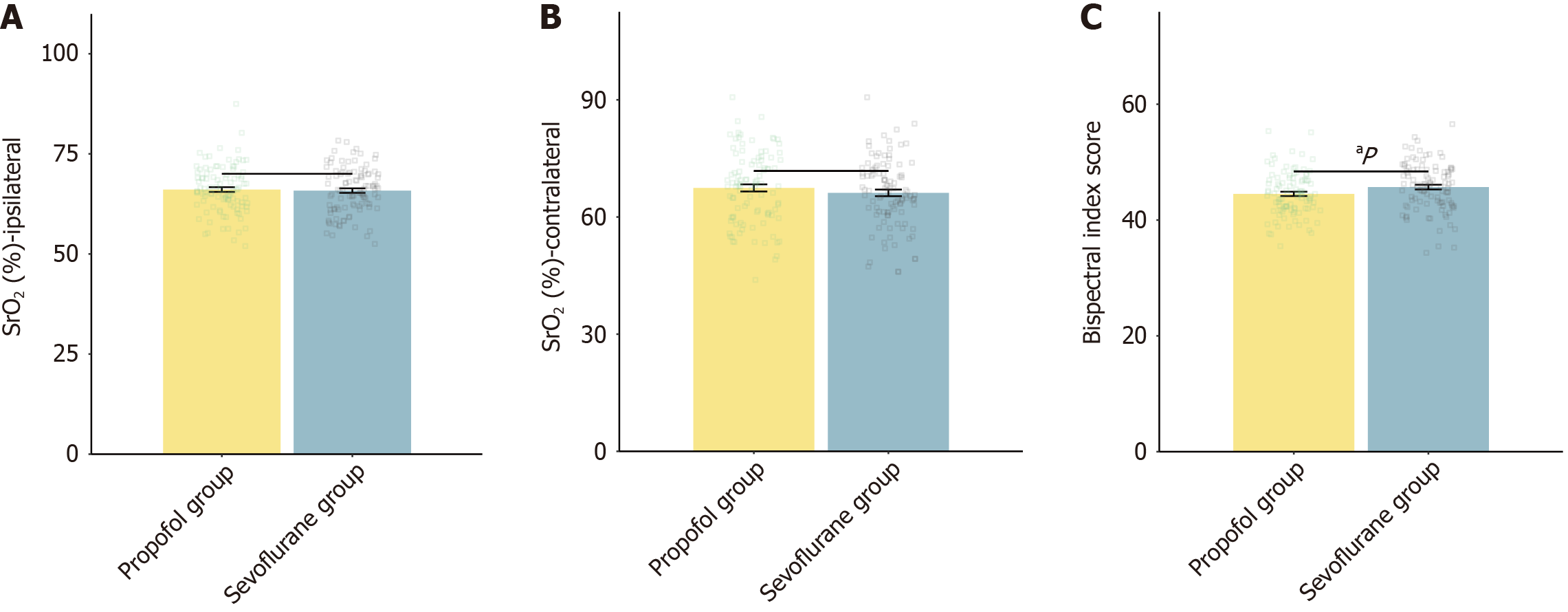
The assessment of anesthesia depth during the perioperative period demonstrated that the mean BIS score was significantly higher in the sevoflurane group than in the propofol group (Figure 1). We found no significant differences in ipsilateral SrO2 or contralateral SrO2 between the two groups.
During the intraoperative period, the propofol group exhibited significantly higher systolic blood pressure and diastolic blood pressure at the time of incision compared with the sevoflurane group (Table 4). The propofol group also had higher heart rates at the time of gastrectomy, at skin suturing, and at the end of surgery than the sevoflurane group (P = 0.043, 0.041, and 0.049, respectively). No significant differences were observed in systolic or diastolic blood pressure at the time of gastrectomy, skin suturing, or the end of surgery, nor in heart rate at the time of incision between the two groups.
| Parameters | Sevoflurane group (n = 103) | Propofol group (n = 101) | t | P value |
| At the time of incision | ||||
| Systolic blood pressure (mmHg) | 114.21 ± 12.09 | 118.02 ± 14.12 | 2.074 | 0.039 |
| Diastolic blood pressure (mmHg) | 70.68 ± 10.84 | 74.50 ± 11.90 | 2.401 | 0.017 |
| Heart rate (bpm) | 121.52 ± 17.24 | 123.81 ± 16.70 | 0.963 | 0.337 |
| At the time of gastrectomy | ||||
| Systolic blood pressure (mmHg) | 111.28 ± 11.71 | 112.35 ± 11.50 | 0.661 | 0.509 |
| Diastolic blood pressure (mmHg) | 69.54 ± 9.84 | 71.27 ± 9.35 | 1.282 | 0.201 |
| Heart rate (bpm) | 116.82 ± 12.33 | 120.89 ± 15.97 | 2.038 | 0.043 |
| At the time of skin suturing | ||||
| Systolic blood pressure (mmHg) | 108.90 ± 14.02 | 108.91 ± 13.17 | 0.003 | 0.998 |
| Diastolic blood pressure (mmHg) | 69.71 ± 11.65 | 70.61 ± 12.18 | 0.541 | 0.589 |
| Heart rate (bpm) | 119.03 ± 13.04 | 123.05 ± 14.83 | 2.055 | 0.041 |
| At the end of the surgery | ||||
| Systolic blood pressure (mmHg) | 102.82 ± 13.21 | 105.70 ± 13.74 | 1.522 | 0.129 |
| Diastolic blood pressure (mmHg) | 66.35 ± 10.53 | 67.08 ± 10.64 | 0.494 | 0.622 |
| Heart rate (bpm) | 119.89 ± 12.64 | 123.64 ± 14.35 | 1.984 | 0.049 |
Incidences of intraoperative and postoperative adverse events are summarized in Table 5. The propofol group experienced a significantly higher incidence of postoperative nausea compared with the sevoflurane group (P = 0.015). We found no statistically significant differences between groups for intraoperative body movement, intraoperative or postoperative hypoxia, agitation, vomiting, pruritus, or intraoperative hypotensive episodes.
| Parameters | Sevoflurane group (n = 103) | Propofol group (n = 101) | χ2 | P value |
| Intraoperative | ||||
| Body movement | 24 (23.3) | 34 (33.66) | 2.691 | 0.101 |
| Hypoxia | 3 (2.91) | 6 (5.94) | 0.507 | 0.476 |
| Postoperative | ||||
| Hypoxia | 3 (2.91) | 0 (0) | 1.314 | 0.252 |
| Agitation | 8 (7.77) | 8 (7.92) | 0.002 | 0.967 |
| Nausea | 29 (28.16) | 45 (44.55) | 5.933 | 0.015 |
| Vomiting | 12 (11.65) | 14 (13.86) | 0.224 | 0.636 |
| Pruritus | 14 (13.59) | 17 (13.59) | 0.415 | 0.519 |
| Hypotensive episode | 24 (23.3) | 14 (13.86) | 2.998 | 0.083 |
No statistically significant differences were observed between the sevoflurane and propofol groups in the incidence or severity of postoperative complications (Table 6). The proportion of patients without complications was similar. Distribution according to the Clavien-Dindo classification showed no significant differences in complication severity between groups. The mean CCI was also comparable.
| Parameters | Sevoflurane group (n = 103) | Propofol group (n = 101) | t/χ2 | P value |
| Patients with no complications | 35 (33.98) | 42 (41.58) | 1.255 | 0.263 |
| Clavien-Dindo classification of complication severity | 1.965 | 0.854 | ||
| I | 12 (11.65) | 8 (7.92) | ||
| II | 42 (40.78) | 36 (35.64) | ||
| IIIa | 28 (27.18) | 33 (32.67) | ||
| IVa | 8 (7.77) | 10 (9.9) | ||
| IVb | 5 (4.85) | 6 (5.94) | ||
| V | 8 (7.77) | 8 (7.92) | ||
| CCI of complication severity | 19.85 ± 2.50 | 20.40 ± 2.55 | 1.550 | 0.123 |
Postoperative pain, as assessed by VAS scores, was similar between the sevoflurane and propofol groups at most time points (Table 7). VAS scores at 2, 4, 6, and 48 hours postoperatively showed no statistically significant differences between groups. However, at 24 hours after surgery, the propofol group reported a significantly higher mean VAS score compared with the sevoflurane group.
| Parameters | Sevoflurane group (n = 103) | Propofol group (n = 101) | t | P value |
| Postoperative 2 hours | 24.35 ± 7.79 | 25.16 ± 7.52 | 0.761 | 0.447 |
| Postoperative 4 hours | 22.54 ± 6.58 | 23.61 ± 6.77 | 1.141 | 0.255 |
| Postoperative 6 hours | 21.51 ± 6.17 | 22.92 ± 6.05 | 1.650 | 0.101 |
| Postoperative 24 hours | 22.93 ± 6.13 | 24.61 ± 6.02 | 1.977 | 0.049 |
| Postoperative 48 hours | 24.52 ± 8.17 | 24.70 ± 8.12 | 0.152 | 0.880 |
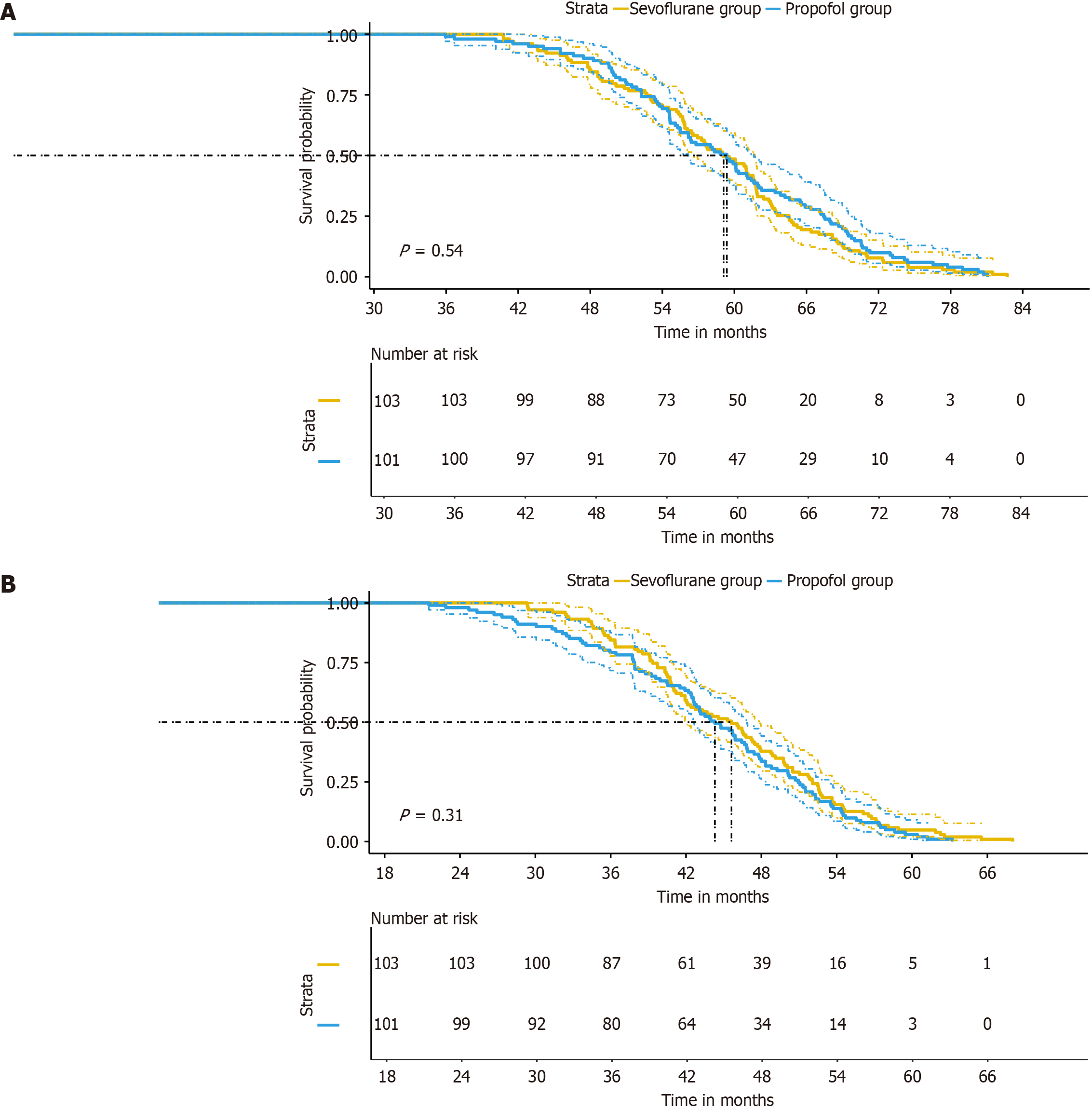
Long-term outcomes, as depicted by OS and PFS were similar between the sevoflurane and propofol groups (Figure 2). The mean OS was 58.74 months in the sevoflurane group and 59.52 months in the propofol group. The mean PFS was 45.54 months in the sevoflurane group and 43.85 months in the propofol group. No statistically significant differences in OS or PFS were observed between the groups.
In this single-center retrospective cohort study, we compared sevoflurane inhalation anesthesia and propofol TIVA in adult patients undergoing radical gastrectomy for gastric cancer. Our findings revealed a pattern of similar short- and long-term clinical outcomes between the two anesthetic modalities, with specific differences in immediate postoperative profiles and intraoperative physiological responses. These results warrant further analysis in the context of pharmacological processes, perioperative physiology, and existing clinical evidence.
Our study provides critical insight into the generally similar profile of major perioperative and long-term outcomes observed between the groups. This was consistent with previous literature[25] suggesting that sevoflurane and propofol are suitable anesthetic agents for major abdominal oncologic surgery when administered carefully and accompanied with strict intraoperative monitoring. The lack of a significant difference in OS and PFS between the groups challenges pre
From a molecular basis, sevoflurane and propofol possess unique immunomodulatory properties, but their clinical translation appears limited in the real-world setting of well-controlled perioperative care[26]. Sevoflurane, a volatile inhalational agent, has been reported to exert immunosuppressive and immunoprotective effects[27]. For example, sevoflurane can modulate the balance of pro- and anti-inflammatory cytokine release, reducing interleukin-6 and tumor necrosis factor-alpha levels[28]. These actions are expected to decrease the systemic inflammatory response and reduce oxidative stress during surgical trauma, potentially preserving immune competence and mitigating perioperative immunosuppression[28]. Conversely, some in vitro and in vivo studies suggest that sevoflurane may increase the metastatic potential of certain tumor cells via upregulation of the HIF-1α pathways and suppression of NK cell cytotoxicity[29]. However, these effects are often dose- and context-dependent, and their clinical implications in the perioperative period, especially with modern, balanced anesthesia techniques, may be less pronounced[30].
By contrast, propofol has received attention for its potential antitumor characteristics[30]. Propofol acts as an antioxidant and has been found in preclinical studies to inhibit cyclooxygenase activity and prostaglandin E2 synthesis, reducing tumor cell proliferation and invasion[30]. Additionally, propofol can enhance apoptotic pathways in tumor cells and may help preserve perioperative cellular immunity, especially by attenuating surgical stress-induced suppression of NK cell function[31]. However, large-scale clinical studies rarely show significant differences in oncologic outcomes solely due to anesthetic method, as demonstrated by the results of our study[32]. This may be attributed to the multidimensional nature of cancer recurrence, where tumor biology, stage, surgical technique, adjuvant therapies, and patient comorbidities often play a more dominant role than perioperative pharmacological influences alone[32].
The observed similarities in immediate postoperative outcomes, including emergency visits, readmission rates, and incidence/severity of postoperative complications, suggest that both anesthetic modalities provide safe and effective care when used in accordance with standardized perioperative protocols. An important factor contributing to these outcomes is the uniformity of perioperative management observed in our study—patients were subjected to strictly standardized anesthesia induction, intraoperative monitoring, and postoperative care, including use of multimodal analgesia and diligent follow-up. These factors may buffer any slight differences caused by the anesthetic agents themselves.
Interestingly, propofol was associated with an increased incidence of postoperative nausea; this finding contradicted the widely held belief that propofol possesses antiemetic effects[33]. This could be due to population-specific changes, variations in intraoperative opioid use, or undetected factors such as preoperative risk profiles or perioperative antiemetic strategies[34]. Alternatively, the effect size might have been influenced by statistical noise in the absence of significant between-group differences for other unfavorable outcomes[35]. Propofol and sevoflurane are generally well-tolerated, but volatile anesthetics like sevoflurane have historically been associated with high rates of PONV, likely due to their emetogenic potential[35]. However, the combined effects of antiemetic prophylaxis, opioid-sparing techniques, and patient-controlled analgesia regimes, which were implemented here[36], may outweigh the overall effect of anesthetic choice on PONV.
The differences observed in intraoperative hemodynamics and BIS values warrant further investigation into the mechanisms involved. Propofol TIVA is recognized for its potent vasodilatory and negative inotropic effects, which often lead to reductions in systemic vascular resistance and cardiac output[37]. However, our study found that propofol was associated with increased systolic and diastolic blood pressures at the time of incision. This might reflect a more robust stress response immediately after surgical stimulation in propofol-maintained patients, which could be mediated by decreased sedative depth (as suggested by the BIS)[38]. Alternatively, subtle changes in intraoperative opioid titration, timing of incision, or individual patient autonomic tone may account for these findings[38]. The increased BIS values in the sevoflurane group could suggest light anesthesia planes or altered EEG signatures induced by volatile agents[25]. Notably, sevoflurane is known to affect EEG differently from propofol, resulting in increased BIS readings despite similar clinical depth of anesthesia[39]. This highlights a limitation in relying solely on processed EEG indices to measure awareness levels between different anesthetic agents, a finding consistent with previous neurophysiological studies[38].
Another issue that requires consideration is the lack of a significant difference in perioperative complications, as measured by the Clavien-Dindo system and CCI. These results support the idea that, in the context of modern perioperative care, the overall morbidity of complex oncologic surgery may be more closely related to patient- and surgery-specific factors than to the precise choice of maintenance anesthetic. This is particularly relevant in the era of enhanced recovery protocols, multidisciplinary perioperative teams, and widespread use of intraoperative monitoring and standardized analgesic approaches, all of which were features of our institution’s management.
Our analysis of postoperative pain outcomes, as assessed by VAS, revealed subtle between-group differences at specific time points. Sevoflurane and propofol provide effective analgesia when combined with opioid-based PCA, but the differential influence of anesthetics on postoperative pain perception is a complex phenomenon. Sevoflurane may provide mild residual analgesic effects via its modulation of central nervous system transmission and anti-nociceptive pathways, whereas propofol’s influence is more transient due to rapid redistribution and clearance. The elevated VAS scores observed in the propofol group at 24 hours suggested an early waning of analgesic synergy, although this did not translate into differences in PCA usage or the need for rescue analgesia.
The study’s retrospective design introduced limitations related to residual confounding, selection bias, and the completeness of real-world data. Although baseline characteristics were well-matched, results may still be affected by unmeasured variables, such as subtle surgical factors, differences in stress response, or inflammatory milieu. The single-center nature of the study offers standardized protocols but may limit generalizability to broad patient populations or healthcare systems with differing perioperative practices. Additionally, the relatively short median follow-up period, dictated by the study’s temporal cutoff, restricts conclusions regarding long-term oncologic outcomes. Given the potential for later recurrence of gastric cancer, a long follow-up period, such as 5 years, would be required to fully assess the long-term oncologic outcomes. Furthermore, the small sample size of only 204 patients may have limited our ability to detect rare results or subtle differences between the two groups. Increasing the sample size in future research could help reveal these rare results and enhance the robustness of our findings. Future studies will also include an analysis of relevant biomarkers to explore evidence of immune regulation in the two groups of patients, which could provide thorough insights into potential immunomodulatory effects.
In summary, our study’s findings indicate that sevoflurane inhalation anesthesia and propofol TIVA are equally effective in short-term and long-term outcomes for patients undergoing gastrectomy for gastric cancer within the framework of standardized perioperative care. Therefore, the selection of these anesthetics should prioritize patient comorbidities, individual risk profiles, and institutional logistical factors, rather than projected effects on oncologic outcomes. Further prospective, multicenter studies with long follow-up and integration of biomarker analysis are necessary to fully elucidate the complex interactions among anesthesia, immune function, and cancer biology during the perioperative period.
| 1. | Gingrich A, Manguso N, Zuckerman R. Treatment of Gastric Cancer Carcinomatosis. Surg Clin North Am. 2025;105:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Hwang J, Carr J. Lymphadenectomy for Gastric Cancer. Surg Clin North Am. 2025;105:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Leja M. Where are we with gastric cancer screening in Europe in 2024? Gut. 2024;73:2074-2082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Liu-Burdowski J, Park J. Treatment of Early Gastric Cancer. Surg Clin North Am. 2025;105:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Nakahari H, Wilton NCT, Ikeda M, Kojima T. Low-dose sevoflurane co-administered with propofol-based general anaesthesia obliterates intra-operative neurophysiological monitoring in an infant. Anaesth Rep. 2023;11:e12244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Cen S, Yang G, Bao H, Yu Z, Liang L. Impact of propofol versus sevoflurane anesthesia on molecular subtypes and immune checkpoints of glioma during surgery. Health Sci Rep. 2023;6:e1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Duan GY, Duan ZX, Chen H, Chen F, Chen F, Du ZY, Chen LY, Lu KZ, Zuo ZY, Li H. Cognitive function and delirium following sevoflurane or propofol anesthesia for valve replacement surgery: A multicenter randomized controlled trial. Kaohsiung J Med Sci. 2023;39:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 8. | Chang JE, Kim H, Won D, Lee JM, Kim TK, Kang Y, Huh J, Hwang JY. Comparison of the effect of sevoflurane and propofol on the optic nerve sheath diameter in patients undergoing middle ear surgery. J Anesth. 2023;37:880-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Hu C, Wang B, Liu Z, Chen Q, Ishikawa M, Lin H, Lian Q, Li J, Li JV, Ma D; ESA-IC Onco-Anaesthesiology Research Group. Sevoflurane but not propofol enhances ovarian cancer cell biology through regulating cellular metabolic and signaling mechanisms. Cell Biol Toxicol. 2023;39:1395-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 10. | Cao SJ, Zhang Y, Zhang YX, Zhao W, Pan LH, Sun XD, Jia Z, Ouyang W, Ye QS, Zhang FX, Guo YQ, Ai YQ, Zhao BJ, Yu JB, Liu ZH, Yin N, Li XY, Ma JH, Li HJ, Wang MR, Sessler DI, Ma D, Wang DX; First Study of Perioperative Organ Protection (SPOP1) investigators. Long-term survival in older patients given propofol or sevoflurane anaesthesia for major cancer surgery: follow-up of a multicentre randomised trial. Br J Anaesth. 2023;131:266-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 11. | Zhu J, Chen C, Wu J, He M, Li S, Fang Y, Zhou Y, Xu H, Sadigh-Eteghad S, Manyande A, Zheng F, Chen T, Xu F, Ma D, Wang J, Zhang Z. Effects of propofol and sevoflurane on social and anxiety-related behaviours in sleep-deprived rats. Br J Anaesth. 2023;131:531-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 12. | Li C, Zhu Y. Impact of Sevoflurane and Propofol on Perioperative Respiratory Adverse Events in Pediatrics: A Systematic Review and Meta-analysis. J Perianesth Nurs. 2025;40:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Yan R, Song T, Wang W, Tian J, Ma X. Immunomodulatory roles of propofol and sevoflurane in murine models of breast cancer. Immunopharmacol Immunotoxicol. 2023;45:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Joys S, Panda NB, Ahuja CK, Luthra A, Tripathi M, Mahajan S, Kaloria N, Jain C, Singh N, Regmi S, Jangra K, Chauhan R, Soni SL, Bhagat H. Comparison of Effects of Propofol and Sevoflurane on the Cerebral Vasculature Assessed by Digital Subtraction Angiographic Parameters in Patients Treated for Ruptured Cerebral Aneurysm: A Preliminary Study. J Neurosurg Anesthesiol. 2023;35:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Hien VV, Tu NH, Thu ND. Propofol TCI or sevoflurane anesthesia without muscle relaxant for thoracoscopic thymectomy in myasthenia gravis patients: a prospective, observational study. BMC Anesthesiol. 2023;23:349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Reinoso-Barbero F, Sanabria-Carretero P, Alonso-Prieto M. Sevoflurane Versus Propofol for LMA® Removal in Awake Children: More Respiratory Adverse Effects or Faster Recovery of Airway Reflexes in More Awake Children? Anesth Analg. 2023;137:e4-e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Kanaya A, Mihara T, Tanaka S, Mikami M, Wagatsuma T, Yamauchi M. Association between the Depth of Sevoflurane or Propofol Anesthesia and the Incidence of Emergence Agitation in Children: A Single-Center Retrospective Study. Tohoku J Exp Med. 2023;260:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Kamal M, Chawriya SK, Kumar M, Kaloria N, Sharma A, Bhatia P, Singariya G, Paliwal B. Effect of sevoflurane, propofol and propofol with dexmedetomidine as maintenance agent on intracranial pressure in the Trendelenburg position during laparoscopic surgeries. J Anaesthesiol Clin Pharmacol. 2023;39:474-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Ji FH, Wang D, Zhang J, Liu HY, Peng K. Effects of propofol anesthesia versus sevoflurane anesthesia on postoperative pain after radical gastrectomy: a randomized controlled trial. J Pain Res. 2018;11:1247-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Langley MS, Heel RC. Propofol. A review of its pharmacodynamic and pharmacokinetic properties and use as an intravenous anaesthetic. Drugs. 1988;35:334-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 193] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26109] [Article Influence: 1186.8] [Reference Citation Analysis (2)] |
| 22. | Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 1431] [Article Influence: 110.1] [Reference Citation Analysis (0)] |
| 23. | Knop C, Oeser M, Bastian L, Lange U, Zdichavsky M, Blauth M. [Development and validation of the Visual Analogue Scale (VAS) Spine Score]. Unfallchirurg. 2001;104:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 22783] [Article Influence: 1340.2] [Reference Citation Analysis (1)] |
| 25. | Li AH, Bu S, Wang L, Liang AM, Luo HY. Impact of propofol and sevoflurane anesthesia on cognition and emotion in gastric cancer patients undergoing radical resection. World J Gastrointest Oncol. 2024;16:79-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (2)] |
| 26. | Choi EK, Kim S, Kim DY. Effects of propofol-remifentanil versus sevoflurane-remifentanil on acute postoperative pain after total shoulder arthroplasty: a randomized trial. J Yeungnam Med Sci. 2023;40:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Qiu GL, Peng LJ, Wang P, Yang ZL, Zhang JQ, Liu H, Zhu XN, Rao J, Liu XS. In vivo imaging reveals a synchronized correlation among neurotransmitter dynamics during propofol and sevoflurane anesthesia. Zool Res. 2024;45:679-690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Varsha AV, Unnikrishnan KP, Saravana Babu MS, Raman SP, Koshy T. Comparison of Propofol-Based Total Intravenous Anesthesia versus Volatile Anesthesia with Sevoflurane for Postoperative Delirium in Adult Coronary Artery Bypass Grafting Surgery: A Prospective Randomized Single-Blinded Study. J Cardiothorac Vasc Anesth. 2024;38:1932-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 29. | Taschner A, Fleischmann E, Kabon B, Sinner B, Eckhardt C, Horvath K, Adamowitsch N, Hantakova N, Hochreiter B, Zotti O, Fraunschiel M, Graf A, Reiterer C; RAPID II Trial investigators. Effect of desflurane, sevoflurane or propofol on the incidence of postoperative delirium in older adults undergoing moderate- to high-risk major non-cardiac surgery: study protocol for a prospective, randomised, observer-blinded, clinical trial (RAPID-II trial). BMJ Open. 2024;14:e092611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 30. | Hou Z, Luo D, Luo H, Hui Q, Xu Y, Lin X, Xu Z. Co-expression prognostic-related genes signature base on propofol and sevoflurane anesthesia predict prognosis and immunotherapy response in glioblastoma. Ann Med. 2023;55:778-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 31. | Bai X, Yin X, Hao N, Zhao Y, Ling Q, Yang B, Huang X, Long W, Li X, Zhao G, Tong Z. Effect of propofol and sevoflurane on postoperative fatigue after laparoscopic hysterectomy. J Psychosom Res. 2024;178:111605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Soukup J, Michel P, Christel A, Schittek GA, Wagner NM, Kellner P. Prolonged sedation with sevoflurane in comparison to intravenous sedation in critically ill patients - A randomized controlled trial. J Crit Care. 2023;74:154251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 33. | Monfort C, Oulehri W, Morisson L, Courgeon V, Harkouk H, Othenin-Girard A, Laferriere-Langlois P, Fortier A, Godin N, Idrissi M, Verdonck O, Richebe P. Using the nociception level index to compare the intraoperative antinociceptive effect of propofol and sevoflurane during clinical and experimental noxious stimulus in patients under general anesthesia. J Clin Anesth. 2024;96:111484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Oudejans E, Witkamp D, Schonewille P, Adelman MR, Hu-A-Ng GV, Hoogterp L, van Rooijen-van Leeuwen G, van der Laan R, Kerindongo RP, Witvliet JJ, Weber NC, Preckel B, Abbink TEM, van der Knaap MS. Effect of Propofol and Sevoflurane on Vanishing White Matter Models. Pediatr Neurol. 2025;167:66-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Xiong J, Wang M, Gao J, Zhou Y, Pang Y, Sun Y. Propofol suppresses hormones levels more obviously than sevoflurane in pediatric patients with craniopharyngioma: A prospective randomized controlled clinical trial. PLoS One. 2023;18:e0288863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 36. | Yao J, Gao Z, Qu W, Li J. Propofol total intravenous anesthesia vs. sevoflurane inhalation anesthesia: Effects on postoperative cognitive dysfunction and inflammation in geriatric patients undergoing laparoscopic surgery. Exp Ther Med. 2024;28:343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 37. | Martínez-Castro S, Monleón B, Puig J, Ferrer Gomez C, Quesada M, Pestaña D, Balvis A, Maseda E, de la Rica AS, Feijoo AM, Badenes R. Sedation with Sevoflurane versus Propofol in COVID-19 Patients with Acute Respiratory Distress Syndrome: Results from a Randomized Clinical Trial. J Pers Med. 2023;13:925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 38. | Lee B, Shin HJ, Kweon KH, Kim NY. Effect of sevoflurane-remifentanil and propofol-remifentanil anesthesia on glycocalyx shedding during deep inferior epigastric perforator flap breast reconstruction: a prospective randomized, controlled trial. Anesth Pain Med (Seoul). 2023;18:148-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 39. | Guo H, Hui H, He Y. Sevoflurane and propofol's effects on postoperative vomiting and nausea. J Gastrointest Oncol. 2023;14:1902-1903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/