Published online Oct 15, 2025. doi: 10.4251/wjgo.v17.i10.111509
Revised: August 20, 2025
Accepted: September 24, 2025
Published online: October 15, 2025
Processing time: 97 Days and 23.8 Hours
Distant metastasis causes most colorectal cancer (CRC) deaths. Gut microbiota (GM) dysbiosis and altered metabolites drive metastasis progression, serving as potential diagnostic biomarkers.
To investigate alterations in GM and metabolites between patients with non-metastatic and distant metastatic CRC.
According to the inclusion criteria, fresh fecal samples were collected from 14 non-metastatic CRC patients and 15 distant metastatic CRC patients. We perfor
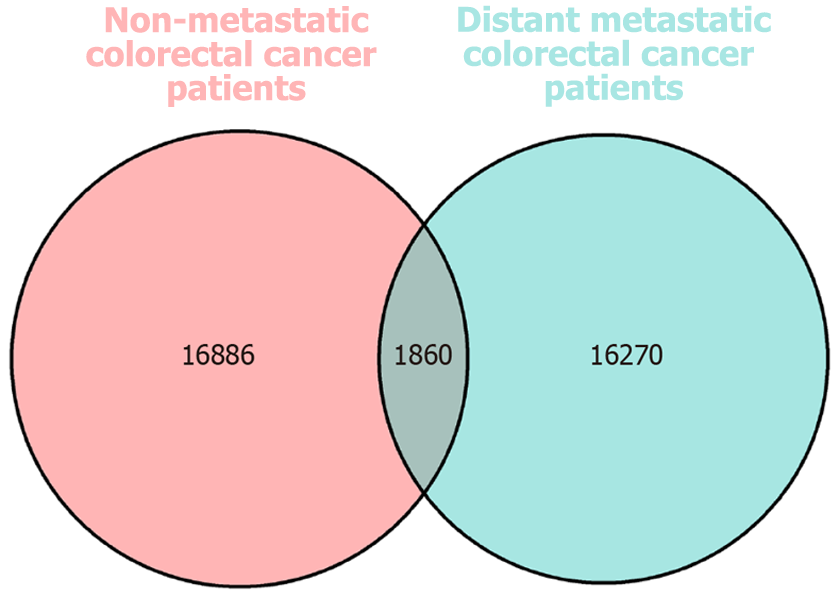
The cohort comprised 16 non-metastatic CRC patients (designated as the S group) and 16 distant metastatic CRC patients (designated as the DZ group). Sequence analysis (16S rRNA) identified a total of 35016 operational taxonomic units (OTUs) across both groups (16886 OTUs in the S group; 16270 in the DZ group; 1860 shared between groups). Inter-group microbial diversity analysis revealed notable differences in the β-diversity group (P < 0.05). Comparative analysis of GM revealed significant taxonomic composition differences between groups (P < 0.05), with higher relative abundances of Butyricicoccus, Ruminococcus_1, Coprococcus_2 in the S group, Pyramidobacter, Christensenellaceae_R-7_group, and Romboutsia in the DZ group (all P < 0.05). Functional analysis of differential microbiota revealed predominant enrichment in metabolic pathways. LC-MS-based untargeted metabolomics detected 91 differential metabolites in both positive and negative ionization modes. GM-derived metabolites showed significant alterations in the DZ group. Kyoto Encyclopedia of Genes and Genomes and Human Metabolome Database analyses revealed associated pathways involving nucleic acids, organic heterocyclic compounds, alkaloids, lipids/lipid-like molecules, and nucleotides. These metabolites may function synergistically, as evidenced by positive correlations between diazoxide, hy
GM and microbial metabolites differ significantly between CRC patients with distant metastasis and those without metastasis. Metabolites involved in nucleic acid, alkaloid, and lipid metabolism pathways potentially contribute to distant metastasis in CRC.
Core Tip: Colorectal cancer patients with different metastatic statuses exhibit distinct gut microbiota and metabolite profiles. These differences may contribute to cancer progression and metastasis, providing potential biomarkers for early diagnosis and therapeutic targets.
- Citation: Deng P, Lin H, Zeng H, Guo F. Gut microbiota and metabolite changes in metastatic colorectal cancer via 16S rRNA and metabolomics. World J Gastrointest Oncol 2025; 17(10): 111509
- URL: https://www.wjgnet.com/1948-5204/full/v17/i10/111509.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i10.111509
Colorectal cancer (CRC), which causes some of the most frequently seen malignant tumors of the digestive tract in clinical scenarios, severely impairs patients’ health and quality of life. According to global cancer epidemiology statistics released by the International Agency for Research on Cancer of the World Health Organization, CRC currently ranks third in global cancer incidence and second in mortality, accounting for 10.0% of total cancer cases and 9.4% of cancer-associated deaths worldwide[1]. Recent research has reported a trend of increasing incidence of CRC among younger populations, and the mortality rate is rising annually[2]. Furthermore, the majority of CRC patients are diagnosed at intermediate to advanced stages, posing significant challenges for treatment and prognosis[2].
CRC originates from colonic epithelial cells though its exact etiology remains incompletely understood multiple factors contribute to disease development, including diet, genetics, and living environment[3]. Epidemiological investigations indicate that genetic factors account for only a minor proportion of CRC cases, while dietary habits and environmental factors are responsible for the majority[4]. Research has shown that populations migrating to Western countries or adopting Western lifestyles exhibit increased CRC risk, demonstrating that CRC results from host-environment in
Gut microbiota (GM) refers to the microbial communities residing in the human intestinal tract. These microorganisms participate in nutrient absorption; they also significantly influence digestive capacity, immune function, and pharmacological responses. Current research demonstrates that GM dysbiosis can generate various harmful metabolites, triggering chronic intestinal inflammation, immune cell exhaustion, and immune evasion, thereby contributing to tumorigenesis[6]. The intestinal microbial ecosystem is intimately associated with multiple physiological and pathological processes, exerting profound impacts on disease initiation and progression[7].
Emerging research demonstrates that GM and their metabolites play a crucial role in maintaining host physiological homeostasis and driving disease pathogenesis (including cancer) through multiple mechanisms such as immune response modulation, genetic damage induction, and apoptosis regulation[8]. Notably, host dietary patterns profoundly influence both the composition of intestinal microbial communities and their metabolic profiles, thereby establishing gut microbes and metabolites as critical bridges connecting environmental exposures with host health status. Gut microbes and their metabolites become particularly significant in the development of CRC, which is strongly driven by environmental factors[9].
The human GM exhibits region-specific distribution patterns, with the colon and rectum harboring the highest microbial density. Current research has identified GM and their metabolites as a highly active area of investigation in CRC pathogenesis, with accumulating evidence demonstrating their profound influence on host immune regulation[10]. Alterations in dietary habits or environmental exposures may disrupt the ecological equilibrium between host and microbiota, leading to the production of deleterious secondary metabolites that promote CRC progression. Notably, while CRC development follows a protracted multistep process, metastatic progression occurs relatively rapidly, frequently resulting in advanced-stage diagnosis at initial clinical presentation. The mechanisms underlying CRC metastasis remain incompletely understood owing to their complexity; however, certain microbial metabolites such as N-nitroso com
A total of 32 patients diagnosed with CRC in the Department of General Surgery at the Xiangya Changde Hospital between December 2021 and November 2024 were enrolled in this study. The cohort comprised 16 non-metastatic CRC patients (designated as the S group) and 16 distant metastatic CRC patients (designated as the DZ group). All participants met the following criteria: (1) Absence of severe comorbidities and infectious diseases; (2) No antibiotic, probiotic, or immunosuppressive therapy within the preceding three months; and (3) Voluntary provision of written informed consent. The study protocol was approved by the Institutional Ethics Committee of our hospital.
Fecal specimens were collected from all participants using sterile fecal collection devices, with strict avoidance of urine contamination or contact with other potentially contaminating substances during the collection process. Immediately after collection, each specimen was thoroughly mixed with intestinal preservation solution to ensure homogeneity, followed by storage at -80 °C to maintain sample integrity.
Extraction of total microbial DNA: Total genomic DNA was extracted from all samples using the E.Z.N.A.® Soil DNA Kit (Omega Bio-tek, United States), with DNA concentration quantified by NanoDrop spectrophotometry and quality assessed through 1.2% agarose gel electrophoresis.
Target fragment polymerase chain reaction amplification: The bacterial 16S rRNA V4 region was amplified using primers 515F/806R in a reaction system containing 2.5 μL each primer (forward and reverse), 25 μL 2 × Master Mix, and 10 μL DNA template, with the volume adjusted to 50 μL using nuclease-free water. Amplification conditions included initial denaturation at 98 °C for 1 minute, followed by 30 cycles of denaturation at 98 °C for 10 seconds, annealing at 55 °C for 30 seconds, and extension at 72 °C for 60 seconds. The polymerase chain reaction products were then analyzed by 2.0% agarose gel electrophoresis, purified using magnetic beads, and quantified fluorometrically with the Quant-iT PicoGreen dsDNA Assay Kit on a Microplate reader (BioTek, FLx800).
Library preparation and sequencing: Sequencing libraries were constructed using the TruSeq Nano DNA LT Library Prep Kit, followed by paired-end sequencing (200-450 bp) on the MiSeq platform (Reagent Kit V3, 600 cycles). Raw sequences underwent initial quality filtering before being demultiplexed based on indexes and barcodes. Subsequent processing included denoising and operational taxonomic unit (OTU) clustering performed with QIIME2 (dada2) and Vsearch, followed by analysis and visualization using QIIME2 and R.
Metabolite extraction: Samples were ground in liquid nitrogen, and 50 mg aliquots were weighed and mixed with 200 μL ice-cold water plus 800 μL chilled methanol/acetonitrile (1:1, v/v). The mixture was vortexed thoroughly, followed by 1-hour ultrasonication in an ice bath and 2-hour incubation at -20 °C. Subsequent centrifugation (16000 × g, 20 minutes,
Chromatographic separation: Samples were maintained at 4 °C in the autosampler and analyzed using a SHIMADZU LC-30A ultra-high performance LC system equipped with a HILIC column. The separation was performed with a 3 μL injection volume at 25 °C column temperature and 0.3 mL/minute flow rate, using mobile phase A (water containing 25 mmol/L ammonium acetate) and mobile phase B (acetonitrile). The gradient elution program was executed as follows: 0-1 minute at 95% B (isocratic), 1-7 minutes linear gradient from 95% to 65% B, 7-9 minutes linear gradient from 65% to 35% B, 9-10.5 minutes at 35% B (isocratic), 10.5-11 minutes linear gradient from 35% to 95% B, and 11-15 minutes at 95% B (isocratic).
MS acquisition: Each sample was analyzed using electrospray ionization in both positive and negative ionization modes. Following ultra-performance LC separation, mass spectrometric analysis was performed on a QE Plus mass spectrometer (Thermo Scientific) equipped with a heated electrospray ionization source. The ionization parameters were set as follows: Spray voltage: 3.8 kv (+) and 3.2 kv (-); Capillary temperature: 320 (±); Sheath gas: 30 (±); Aux gas: 5 (±); Probe heater temp: 350 (±); S-lens RF level: 50.
Data preprocessing: Raw data were processed using MS data independent analysis for lipidomics (MSDIAL) software for peak alignment, retention time correction, and peak area extraction. Metabolite identification was performed by matching exact mass numbers (mass tolerance < 20 ppm) and secondary spectra (mass tolerance < 0.02 Da) against public databases, including the Human Metabolome Database (HMDB) and MassBank and an in-house metabolite standards library. For the extracted data, ion peaks with > 50% missing values within groups were excluded from subsequent statistical analysis. Both positive and negative ion data were normalized by total peak area, integrated, and then subjected to pattern recognition analysis using R software. Following unit variance scaling (UV) pretreatment, the processed data were used for downstream statistical analyses.
Raw data were processed using MSDIAL software to perform peak alignment, retention time correction, and peak area extraction. Metabolite identification was achieved by matching exact mass values (mass error < 20 ppm) and MS/MS spectra (mass error < 0.02 Da) against public databases (HMDB and MassBank) and an in-house standards library. Following the removal of ion peaks with > 50% missing values within groups, both positive and negative ionization mode data were normalized by total peak area. The integrated dataset was then subjected to pattern recognition analysis using R software, with UV pretreatment applied prior to subsequent statistical analyses.
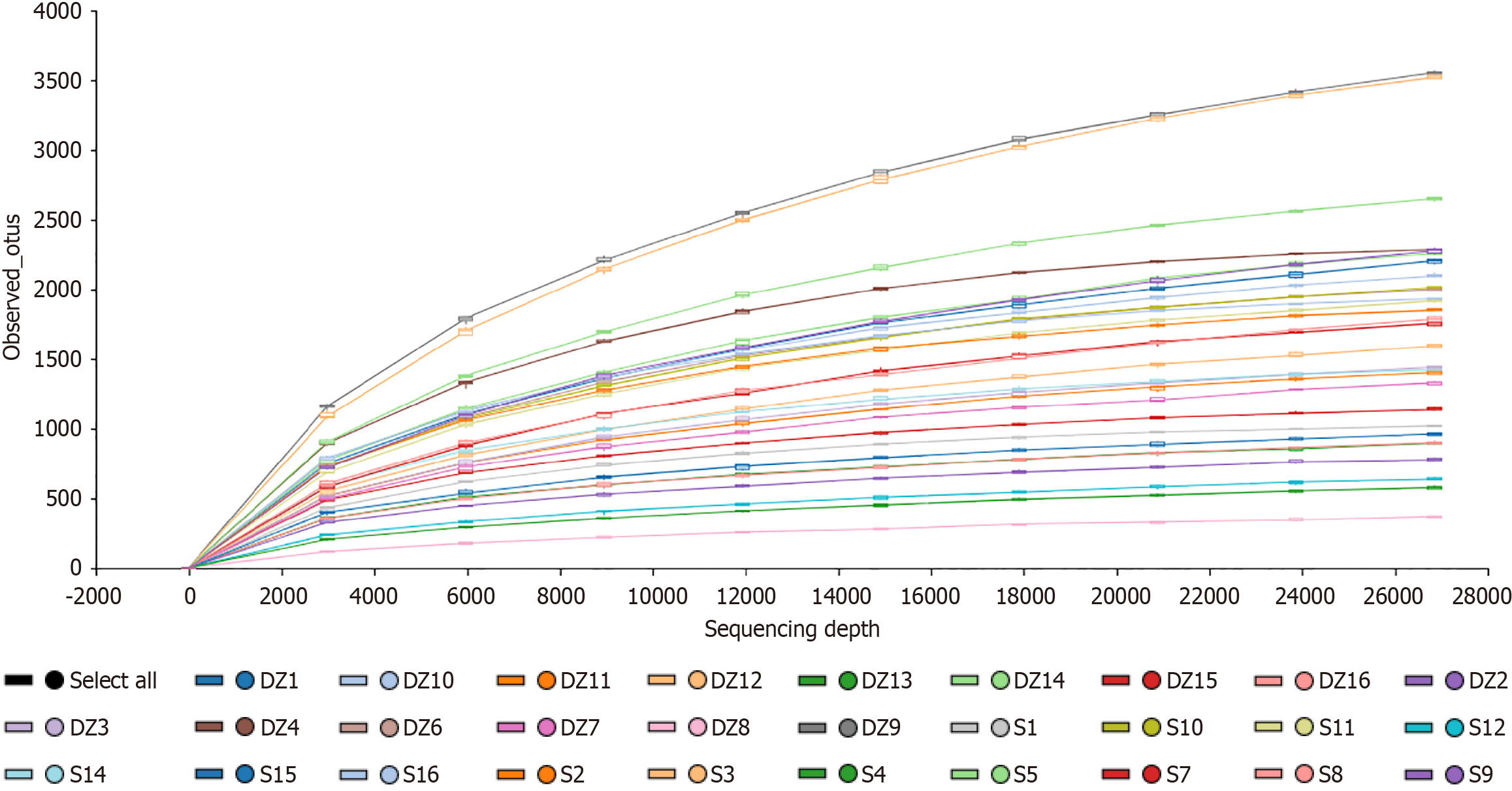
OTU analysis: Three enrolled patients were excluded due to non-compliant stool collection standards, resulting in 16S rRNA sequencing of 29 samples (14 in the S group and 15 in the DZ group). A total of 35016 OTUs were identified (Figure 1). Venn diagram analysis identified 16886 OTUs unique to the S group, 16270 unique to the DZ group, and 1860 shared between groups. Rarefaction curves generated by random sequence subsampling demonstrated plateau trends (Figure 2), indicating adequate sequencing depth to capture the majority of microbial diversity present in the samples.
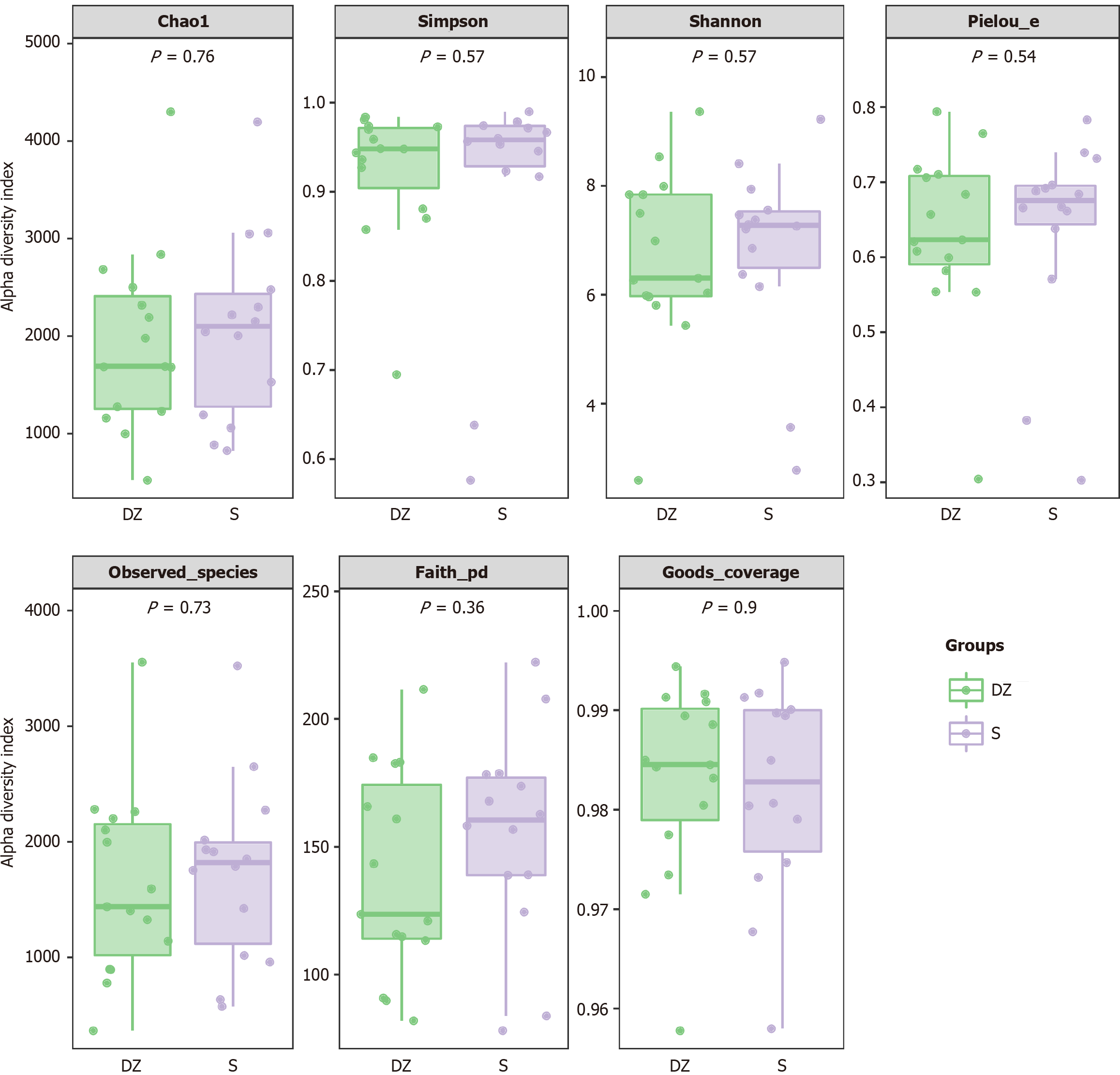
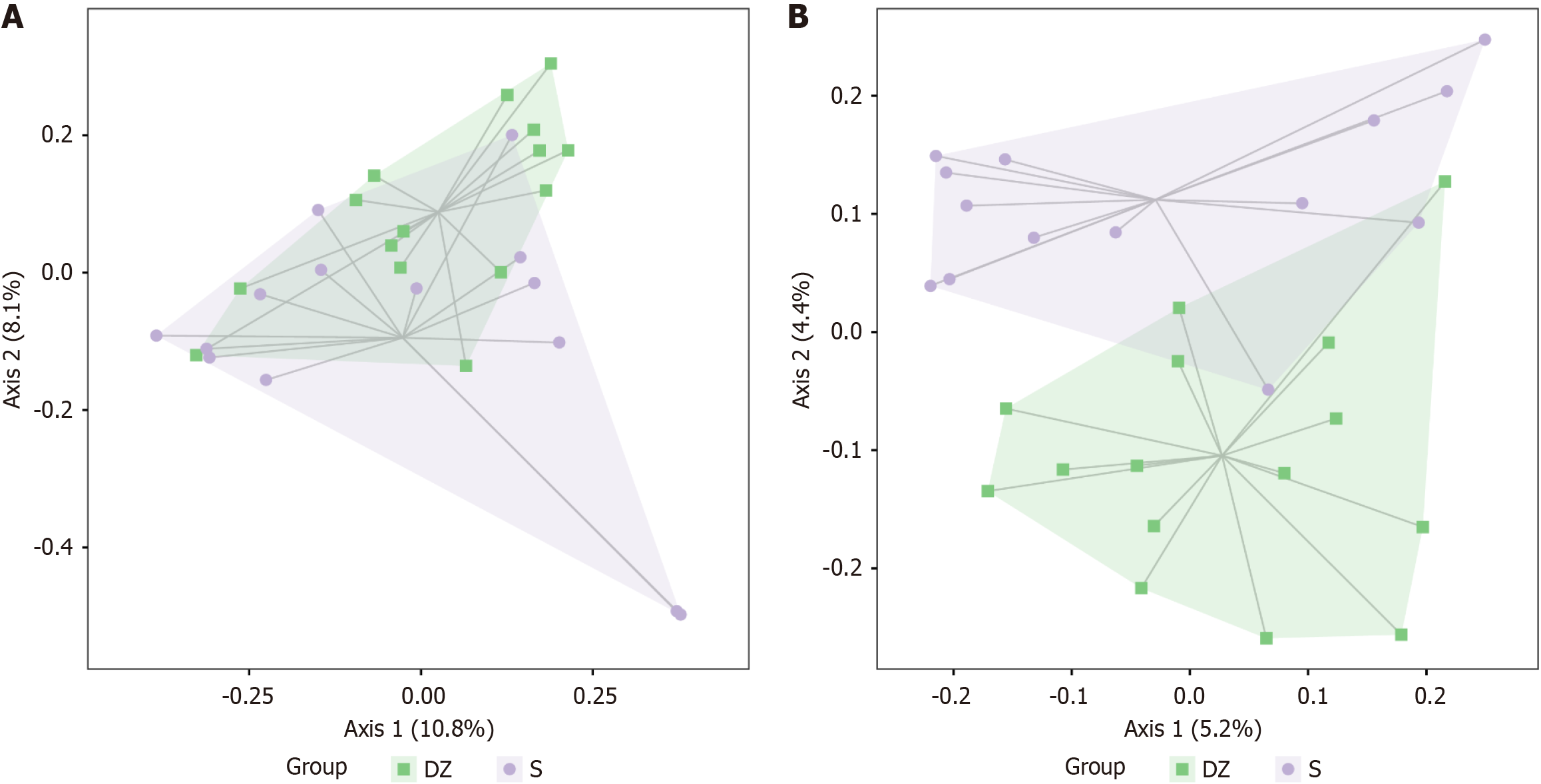
No notable differences were observed in α-diversity between the S and DZ groups (P > 0.05, Figure 3), but β-diversity analysis revealed significant intergroup differences (P < 0.05). Distance matrix and principal co-ordinates analysis analyses revealed significantly large Bray-Curtis distances between the two groups (Figure 4A), indicating substantial differences in microbial species composition; similarly, the Jaccard distance metrics demonstrated pronounced separation (Figure 4B), confirming distinct species assemblages within their ecological communities.
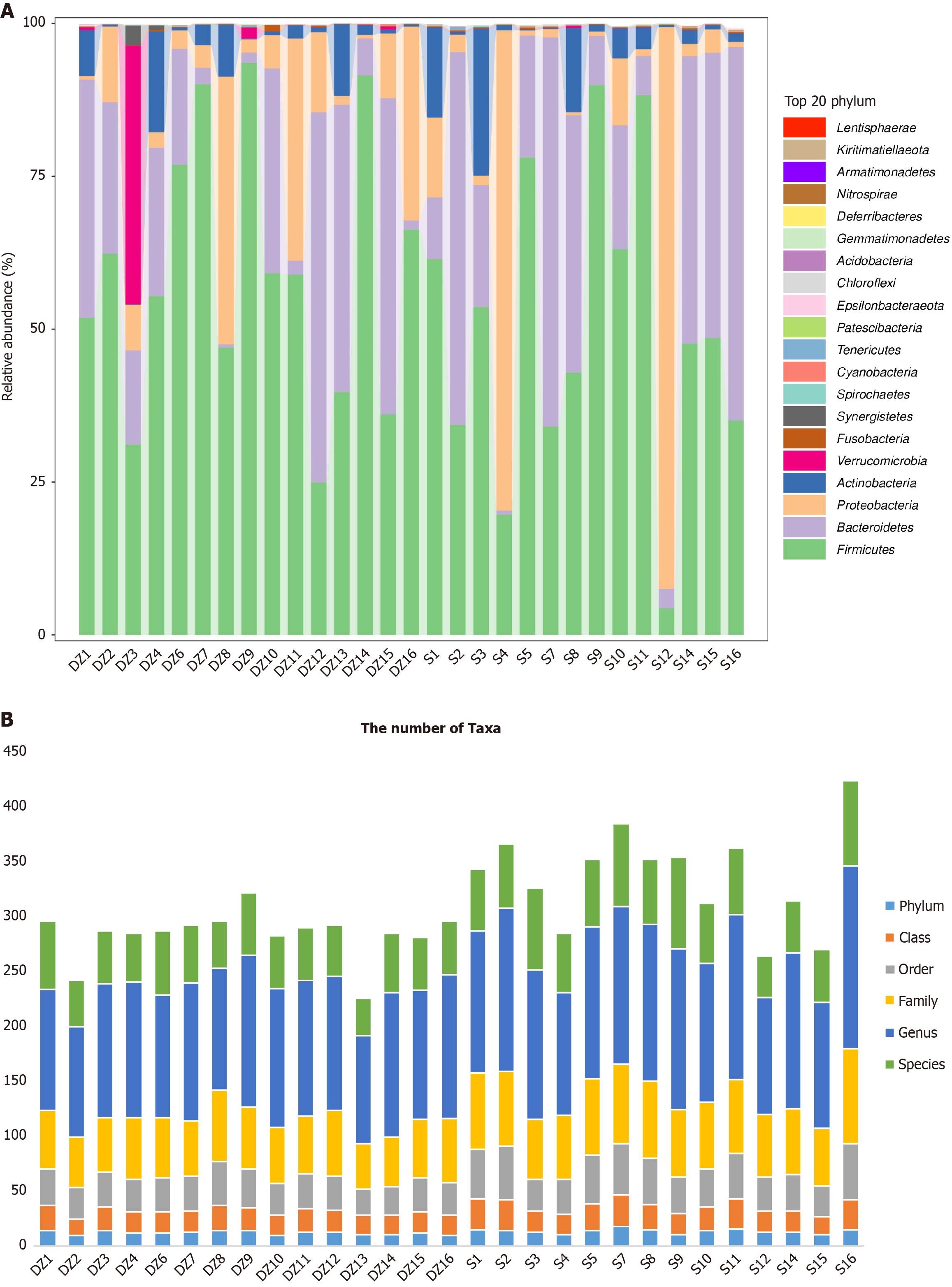
The dominant phyla in both groups were primarily Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria. In the DZ group, the Verrucomicrobia were notably dominant in the sample DZ3, though the difference was not significant (P < 0.05). Similarly, in samples S4 and S12, the Proteobacteria showed relatively higher dominance, but again without statistical significance (P < 0.05, Figure 5A). Statistical analysis of the GM taxonomic units in both groups revealed the highest abundance at the genus level (Figure 5B).
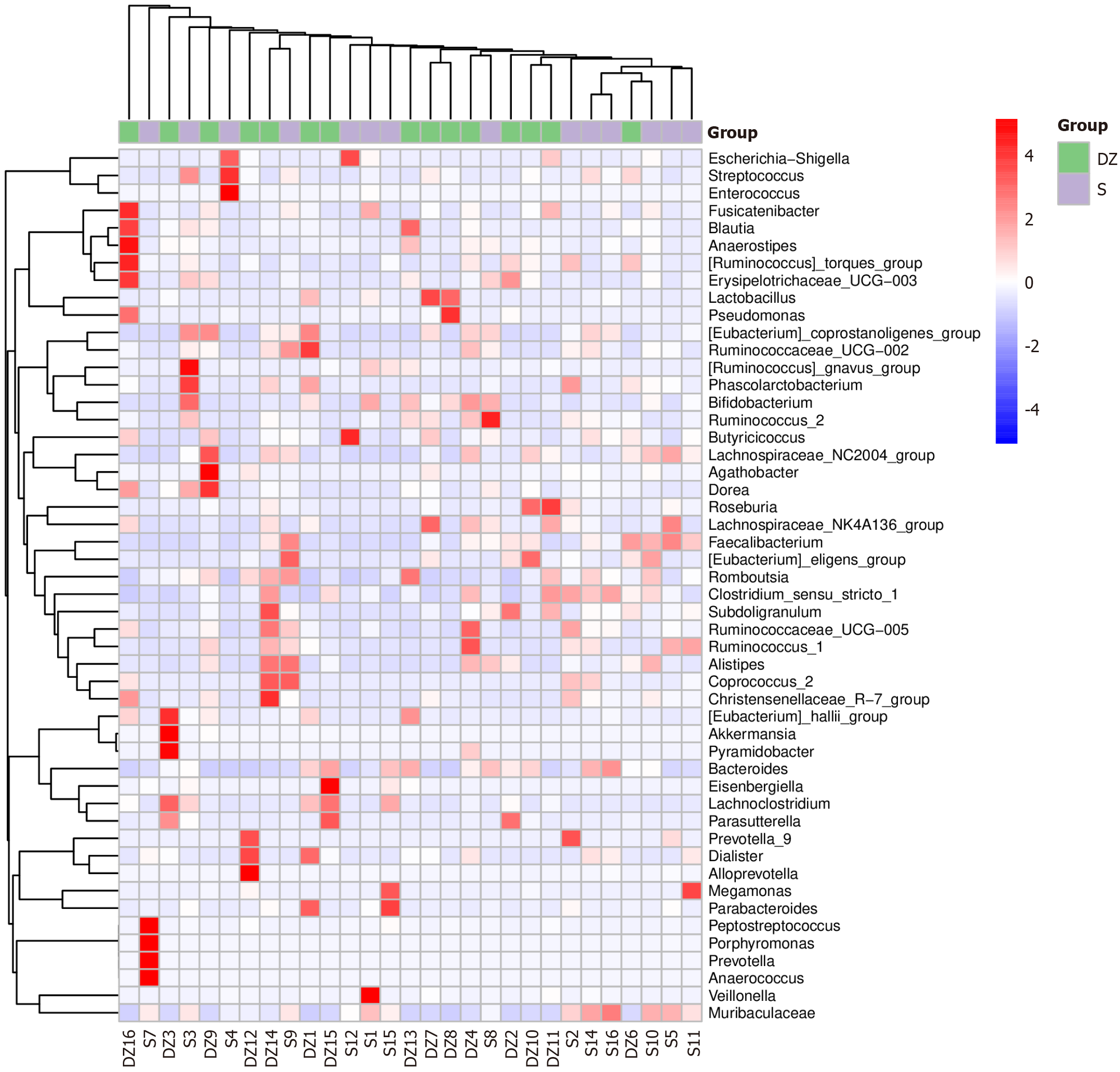
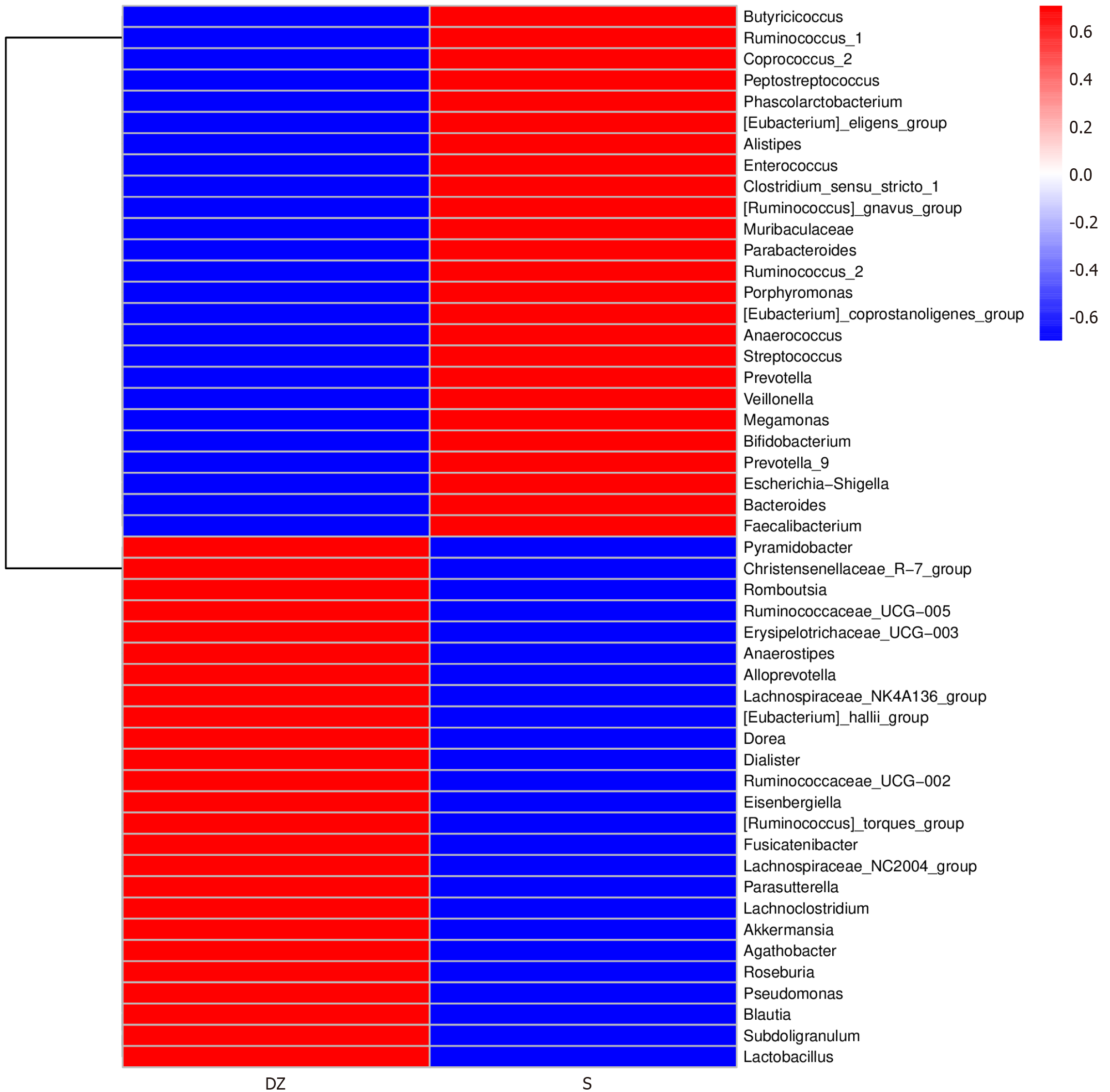
According to comparative analysis of differential GM between the two groups, the S group exhibited notably higher relative abundances of Butyricicoccus, Ruminococcus_1, and Coprococcus_2 (all P < 0.05). In contrast, the DZ group showed significant enrichment of Pyramidobacter, Christensenellaceae_R-7_group, and Romboutsia (all P < 0.05, Figures 6 and 7).
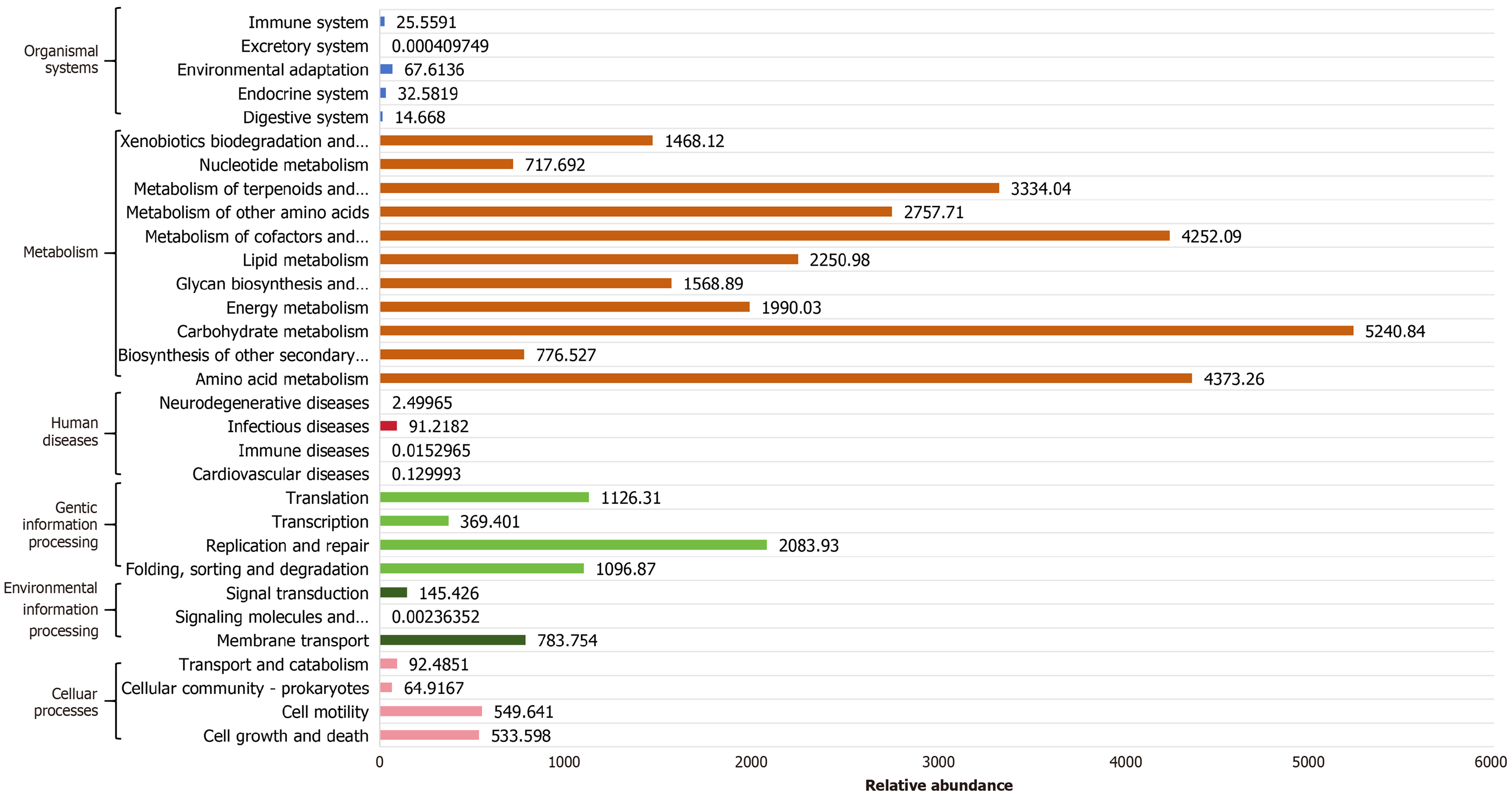
Functional annotation of prokaryotic genomes using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database revealed enriched functional pathways potentially associated with the differential microbiota (Figure 8). The analysis identified cell growth and death, cell motility, cellular community-prokaryotes, transport and catabolism, membrane transport, signal transduction, folding/sorting/degradation, replication/repair, transcription, translation, infectious diseases, amino acid metabolism, biosynthesis of other secondary metabolites, carbohydrate metabolism, energy metabolism, glycan biosynthesis and metabolism, lipid metabolism, the metabolism of cofactors and vitamins, the metabolism of other amino acids, the metabolism of terpenoids and polyketides, nucleotide metabolism, xenobiotics biodegradation and metabolism, digestive system, endocrine system, environmental adaptation, and immune system. The primary functional roles of these differential microbial communities are predominantly concentrated in metabolic pathways.
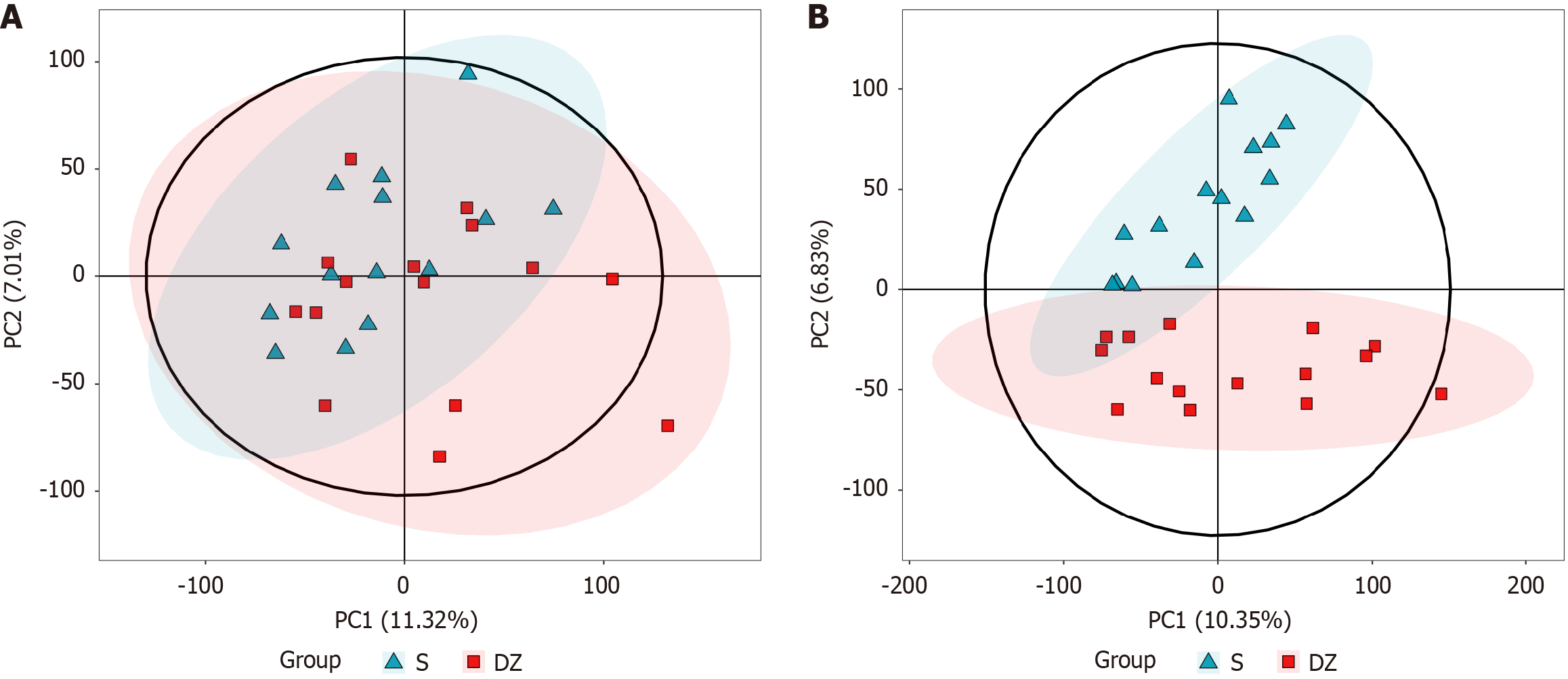
The LC-MS untargeted metabolomics analysis included 29 samples, consisting of 14 non-metastatic CRC cases (S group) and 15 cases with distant metastasis (DZ group). Multivariate statistical analysis using principal component analysis (PCA) was performed in both positive and negative ion modes, revealing distinct metabolic profiles between the two groups. In positive mode, PCA showed PC1 and PC2 accounted for 11.32% and 7.01% of variance, respectively (Figure 9A), while in negative mode, PC1 and PC2 explained 10.35% and 6.86% (Figure 9B). The PCA score plots (Figure 9) demonstrated clear separation between groups along both PC1 and PC2 axes, indicating significant differences in GM-derived metabolites.
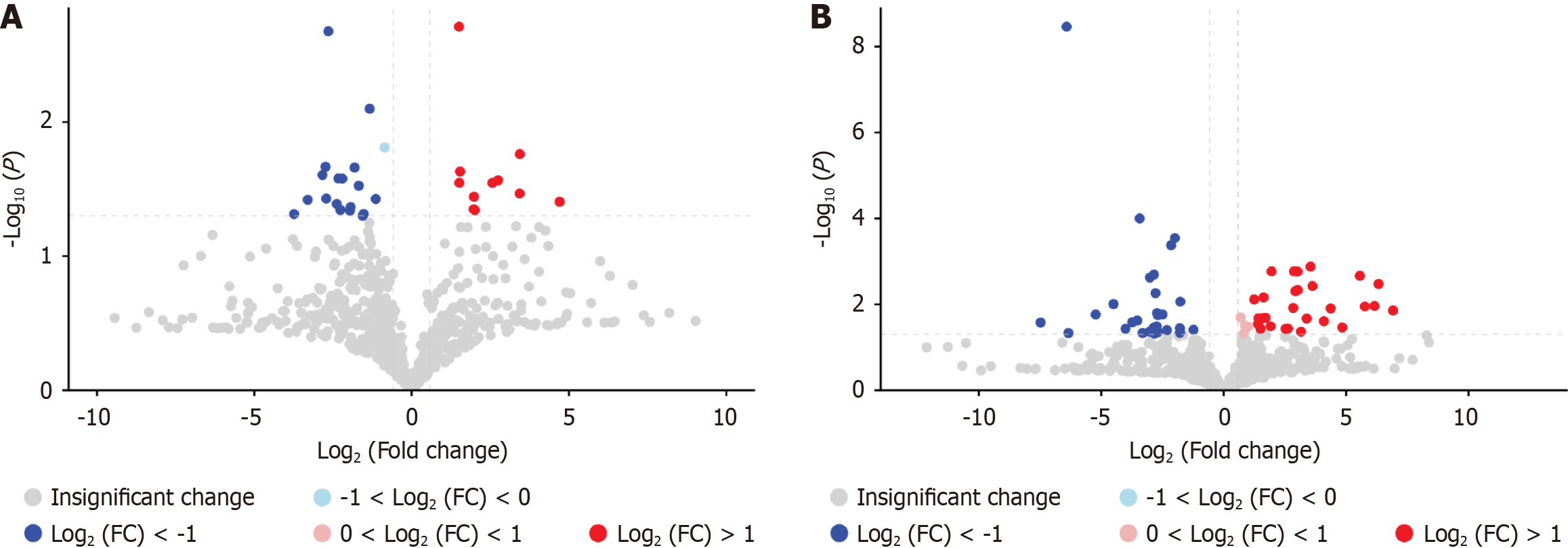
Univariate statistical analysis was further conducted on the differential metabolites in both positive and negative ion modes, including fold change (FC) analysis and Student’s t-tests, with results visualized in volcano plots. The results revealed significant intergroup differences in metabolite profiles, identifying a total of 91 differentially expressed metabolites. Specifically, 30 metabolites were altered in positive mode (10 upregulated and 20 downregulated), while 61 metabolites showed significant changes in negative mode (32 upregulated and 29 downregulated), as detailed in Figure 10.
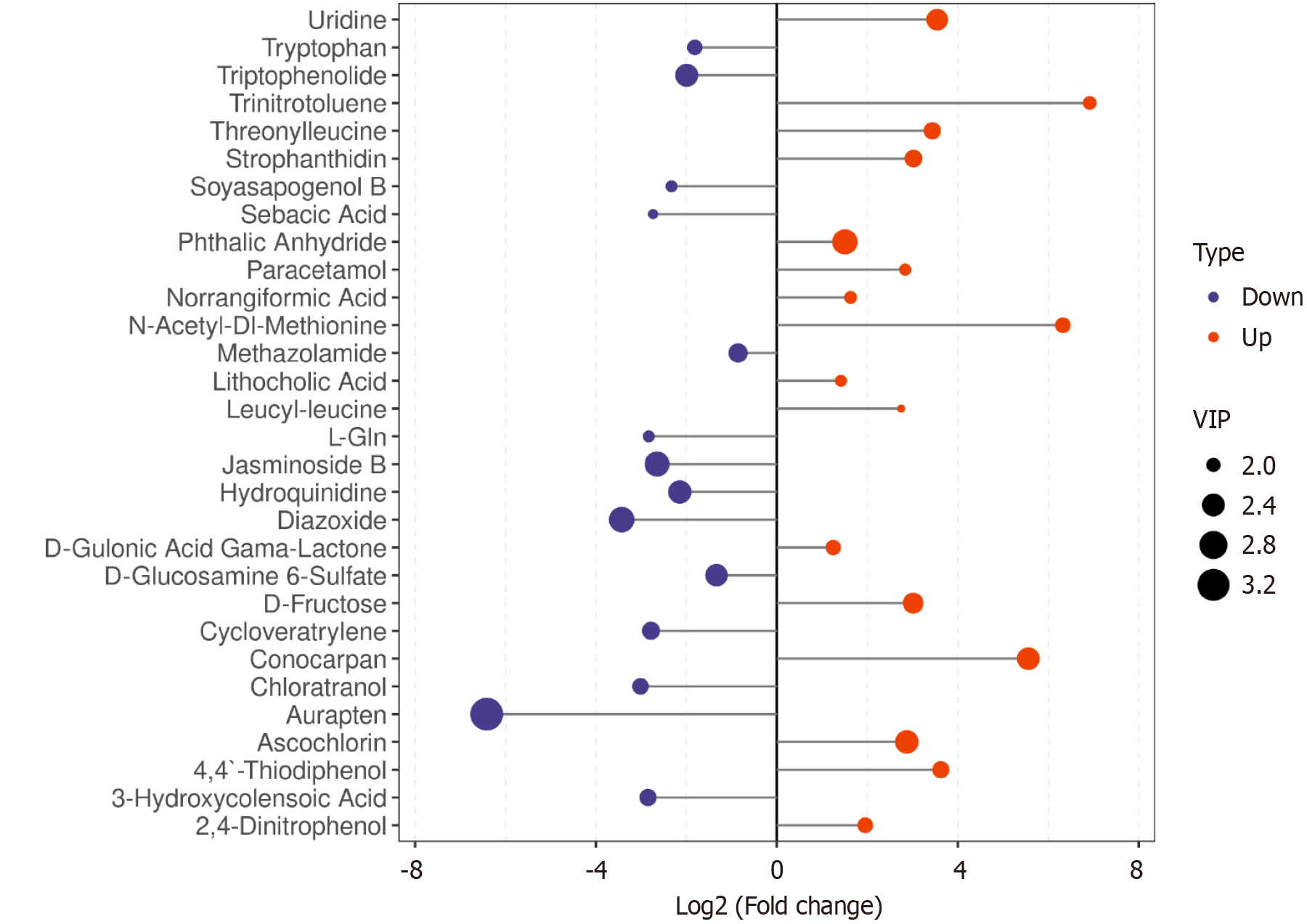
We further evaluated the identified differential metabolites for significance based on variable importance in projection (VIP) scores, using an FC threshold of > 2 or < 0.5 (Figure 11). The most prominent downregulated metabolites included aurapten, triptophenolide, jasminoside B, and diazoxide, while the top upregulated metabolites comprised phthalic anhydride, uridine, conocarpan, and ascochlorin.
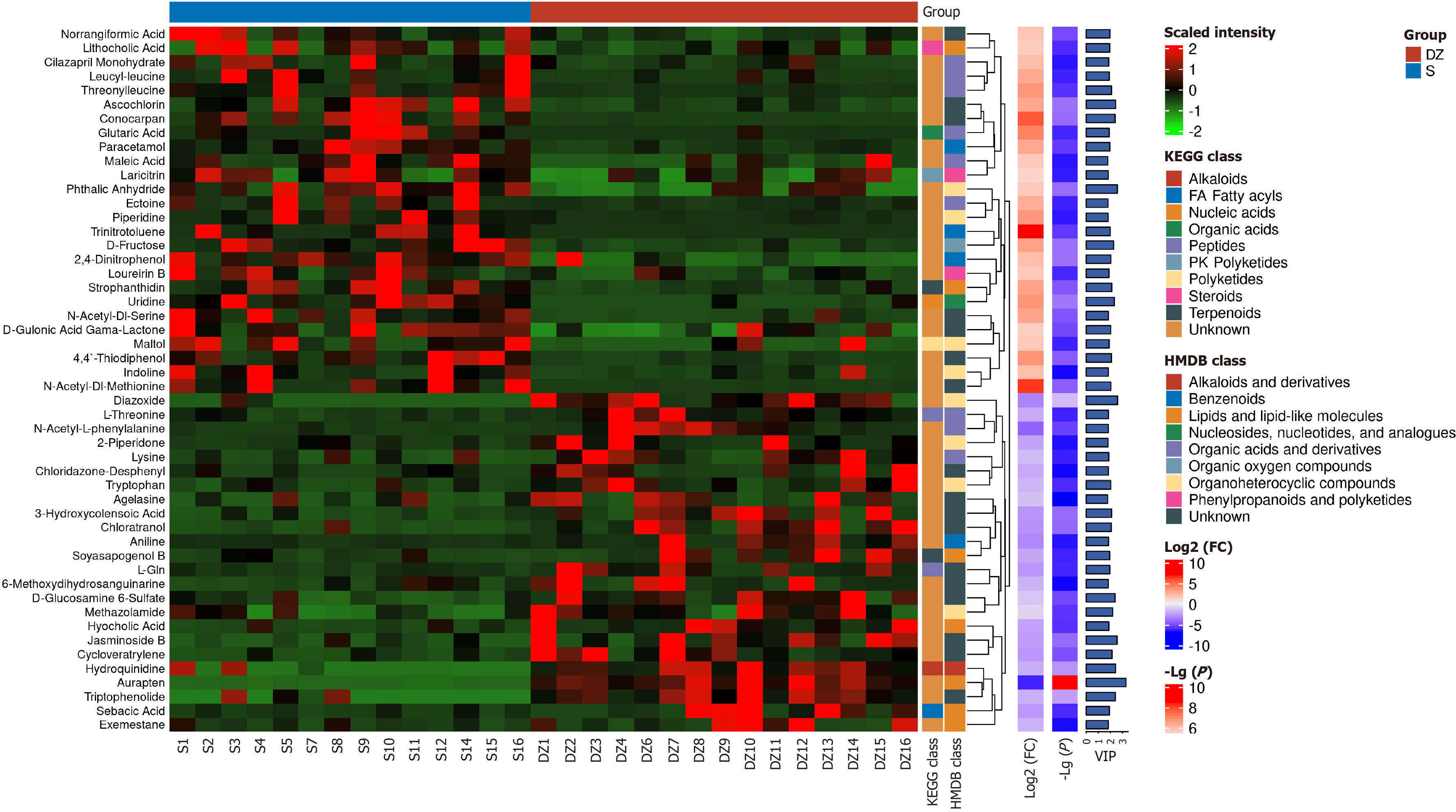
Using screening criteria of VIP > 1, P < 0.05, and FC > 2 or < 0.5, hierarchical clustering was performed on the significantly differential metabolites based on their expression. The top 50 metabolites were visualized in a heatmap integrating relative expression values, FC, P values, and classification information from KEGG and HMDB databases (Figure 12). The analysis revealed that the most significantly altered and biologically important metabolites in the DZ group included diazoxide, hydroquinidine, aurapten, and triptophenolide. Through integrated analysis with KEGG and HMDB databases, the potential metabolic pathways involved were identified as diazoxide associated with nucleic acids or organoheterocyclic compounds; hydroquinidine linked to alkaloids or alkaloids and derivatives; aurapten related to nucleic acids or lipids and lipid-like molecules; and triptophenolide connected to nucleic acids pathways (Figure 12).
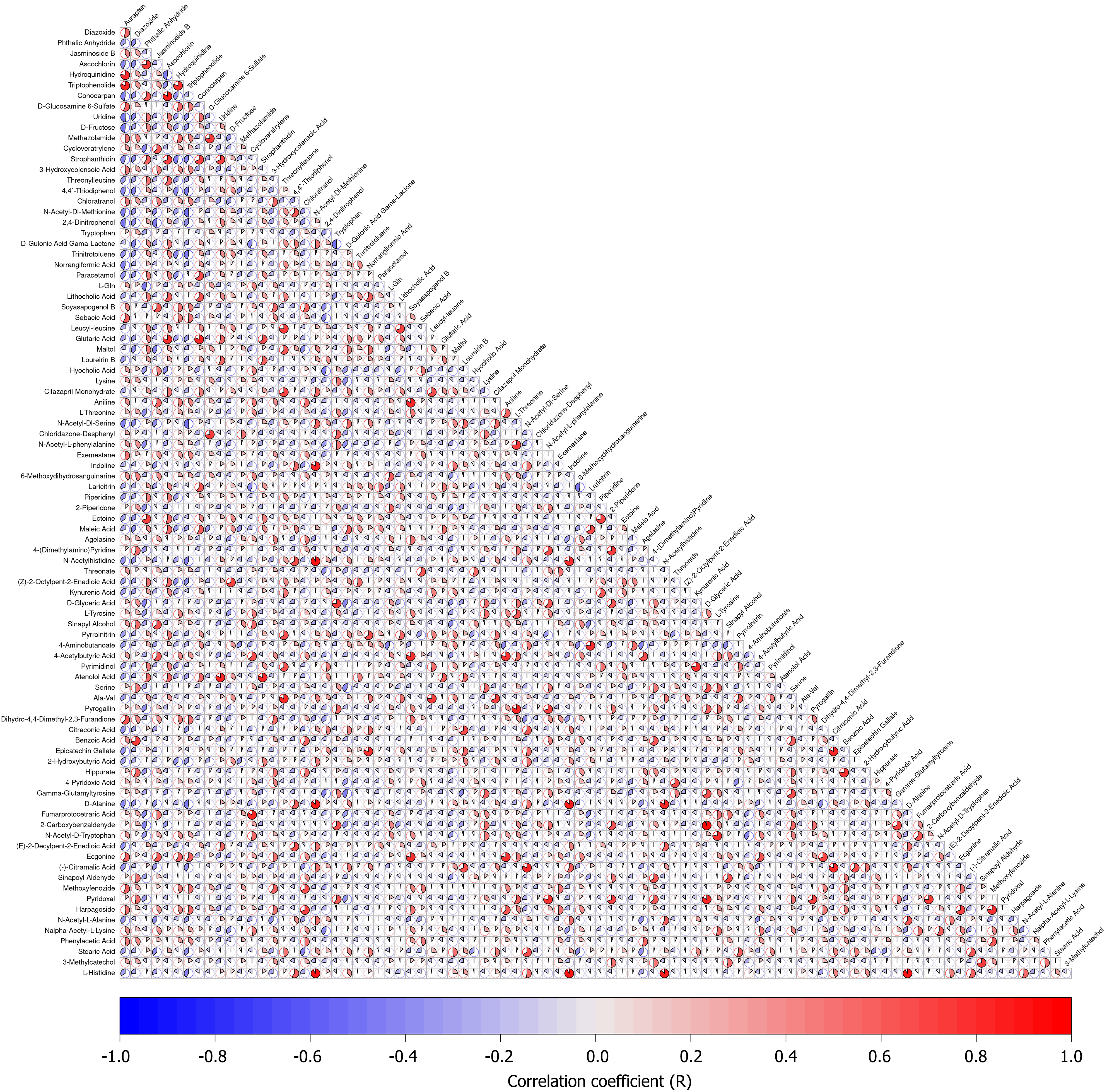
To elucidate the relationships between differential metabolites and understand the metabolic network and regulatory mechanisms among GM, correlation analysis of the differential metabolites was performed. The Pearson correlation coefficients between metabolites were visualized as a correlation matrix heatmap (Figure 13). Key findings revealed significant positive correlations between diazoxide and auraptene, hydroquinidine and auraptene, triptophenolide and auraptene, diazoxide and hydroquinidine, diazoxide and triptophenolide, and hydroquinidine and triptophenolide.
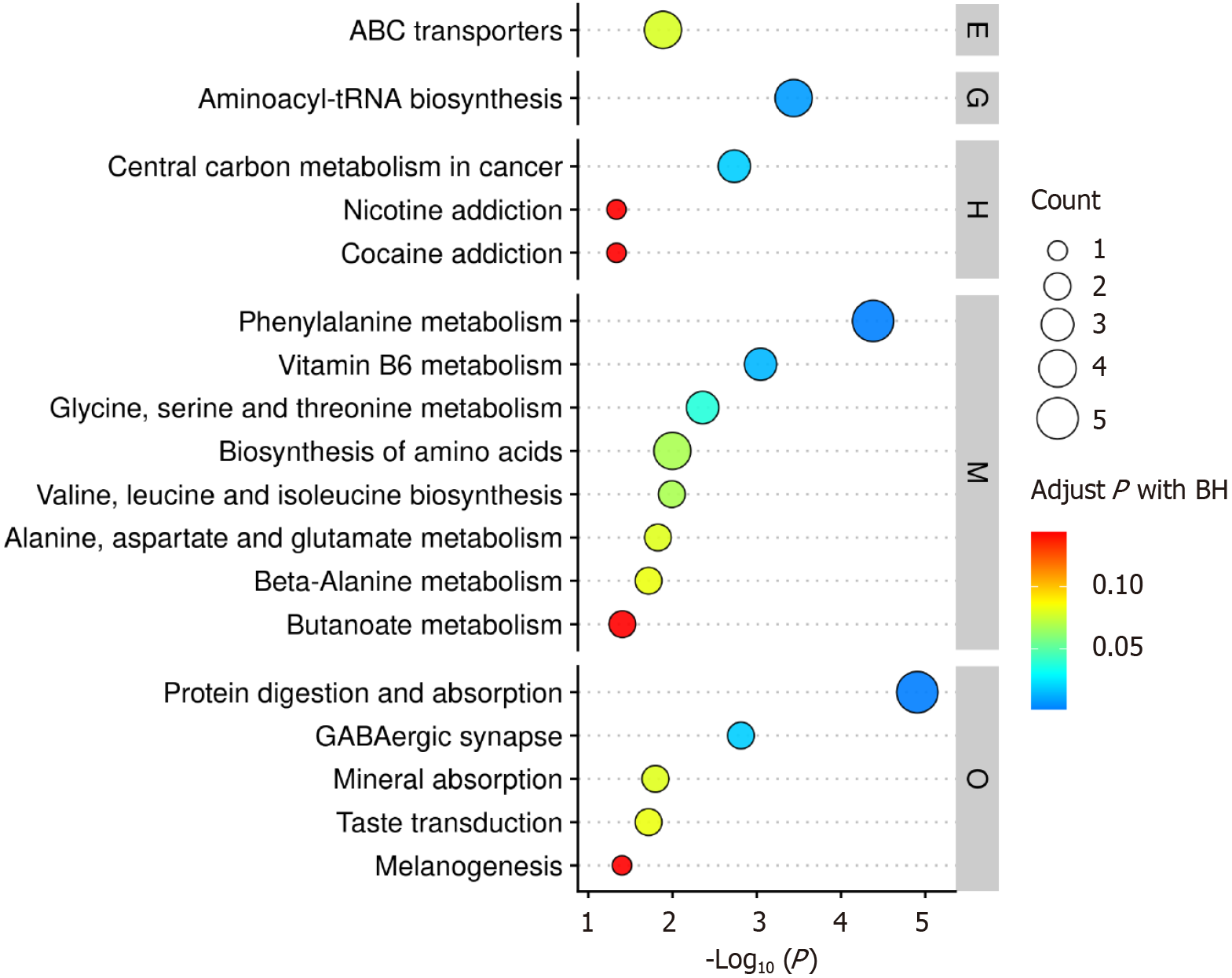
The differentially clustered metabolites were submitted to the MetaboAnalyst 5.0 online platform for pathway analysis to identify significantly altered metabolic pathways under experimental conditions. Results were visualized via bubble plot, revealing prominently dysregulated pathways including aminoacyl-tRNA biosynthesis, carbon metabolism in cancer, phenylalanine metabolism, vitamin B6 metabolism, and protein digestion and absorption (Figure 14).
In the human microbiome ecosystem, the GM represents the most critical and complex component. This system comprises intestinal microbial communities, gut mucosa, and the intestinal immune system, playing a vital role in human health and the occurrence and progression of CRC[12,13]. The pathogenesis of CRC may be strongly linked to alterations in GM structure and function induced by factors such as intestinal inflammation, dietary habits, and environmental variations[14,15]. Research has demonstrated that gut dysbiosis in patients is characterized by increased abundance of cancer-associated bacteria alongside decreased levels of beneficial bacteria[16]. The current investigation examined changes in GM and their metabolites in CRC patients, with subsequent predictive functional analysis of differential microbial species and metabolites.
In the present study, the analysis of GM diversity between the two groups revealed similar α-diversity but significantly different β-diversity, indicating comparable overall species richness yet distinct microbial compositions between groups. Notably, rarefaction curve-based analysis confirmed adequate sequencing depth, demonstrating that the observed α-diversity similarity was not attributable to uneven sequencing coverage.
Comparative analysis of the GM between the S and DZ groups revealed that S shared the same dominant phyla at the phylum level. Although some individual samples exhibited differences in GM composition, these variations were not statistically significant when considering the overall sample population. This observation may be attributed to factors such as host genetic background, immune status, dietary habits, and other related influences[17].
Statistical analysis of taxonomic units between the two groups revealed that the GM exhibited the highest microbial diversity at the genus level, prompting our selection of this genus level as the default research plane for conducting differential species analysis of the intestinal flora between the two groups. This approach identified distinct genus-level signatures: The S group exhibited significant enrichment of butyrate-producing genera, including Butyricicoccus, Ruminococcus_1, and Coprococcus_2, whereas the DZ group demonstrated predominance of Pyramidobacter, Christensenellaceae_R-7_group, and Romboutsia. Comparative analysis with previous studies revealed that these microbial taxa may contribute to CRC metastasis through multiple mechanisms, including promoting pro-inflammatory cytokine expression, modulating the tumor microenvironment, generating metabolites such as bile acids, immune regulation, and altering intercellular adhesion molecule activity[18-20].
The current study conducted KEGG pathway analysis on differentially abundant GM, revealing a comprehensive functional profile encompassing multiple categories, including cellular processes, environmental information processing, genetic information processing, human diseases, metabolism, and organismal systems. Notably, metabolic pathways demonstrated the highest relative abundance among all functional categories based on sample abundance analysis, with carbohydrate metabolism representing the most predominant subcategory, followed by amino acid metabolism and metabolism of cofactors/vitamins.
These findings warrant further investigation regarding their potential associations with CRC metastasis. A growing body of evidence in recent years has demonstrated that GM-mediated metabolic processes significantly influence the initiation, progression, and metastasis of CRC[21]. Carbohydrate metabolism, as the most active functional pathway of microbial communities, shows particular relevance to CRC metastasis risk. Through carbohydrate fermentation, gut microbes produce short-chain fatty acids, including butyrate, propionate, and acetate. While these metabolites are generally recognized for maintaining intestinal homeostasis, their metabolic dysregulation may facilitate procarcinogenic microenvironment formation.
Notably, butyrate exhibits anti-inflammatory and anti-tumor properties; however, its deficiency coupled with elevated harmful metabolites can compromise intestinal barrier function, exacerbate chronic inflammation, and ultimately create favorable conditions for cancer cell invasion and distant metastasis[22]. Amino acid metabolism plays an equally critical role in GM functionality. While amino acids serve as fundamental building blocks for protein synthesis, their metabolic dysregulation can disrupt multiple cellular processes. Certain gut bacteria metabolize amino acids to produce ammonia, amines, and phenolic compounds, with elevated levels of these metabolites being associated with colonic oxidative stress and DNA damage potential drivers of malignant transformation and metastasis. Furthermore, dysregulated amino acid metabolism can perturb immune homeostasis in the gut microenvironment, inducing a pro-inflammatory state that creates favorable conditions for cancer cell dissemination[23].
The metabolism of cofactors and vitamins relies on synergistic interactions with GM, and its dysregulation may facilitate CRC metastasis by impairing fundamental cellular processes. Specifically, vitamin D and B-complex vitamins participate in critical pathways including cellular proliferation, apoptosis, and immune regulation. Impaired microbial metabolism of these substances can lead to inhibition of key enzymatic reactions, disruption of redox homeostasis, or defects in DNA repair mechanisms, consequently weakening the host’s surveillance of cancer cells and accelerating metastatic progression[24].
Based on the metabolic pathway enrichment characteristics predicted from microbial functional analysis, this study identified 30 and 61 differential metabolites in positive and negative ionization modes, respectively, through full-spectrum metabolomic analysis, demonstrating the chemical diversity of GM-derived metabolites. Although the PCA showed relatively low contribution rates for PC1 and PC2 (indicating limited explanation of global variance), the distinct separation between groups in principal component space was consistent with the reported characteristics of “inter-group metabolic variance dispersion”[25]. In fact, intergroup differences in metabolic phenotypes are often driven by the synergistic effects of multiple weakly correlated pathways[26], which precisely aligns with the “global metabolic pathway enrichment” pattern observed in KEGG analysis. This phenomenon reflects how differential microbial communities collectively shape a procarcinogenic metabolic microenvironment through subtle perturbations across multiple pathways, including carbohydrate metabolism, amino acid metabolism, and cofactor metabolism.
The integration of univariate analysis with volcano plots further elucidated directional changes in metabolites: The observed downregulation of short-chain fatty acids (e.g., butyrate) in positive mode and upregulation of bile acids (e.g., deoxycholic acid) in negative mode revealed a characteristic metabolic axis imbalance. This pattern aligns with the previously reported procarcinogenic model of “beneficial metabolite depletion-toxic metabolite accumulation”[27]. Notably, the significant group separation along PC1 in this study suggests the existence of key metabolic modules undetected by conventional pathway analysis, providing critical leads for subsequent targeted validation.
In this study, employing stringent screening criteria (VIP > 1, P < 0.05, and FC > 2 or < 0.5) combined with KEGG and HMDB database analysis, we identified several highly expressed metabolites in the DZ group, including diazoxide, hydroquinidine, aurapten, and triptophenolide, which demonstrated critical potential in regulating CRC metastasis. Notably, diazoxide, as an organoheterocyclic compound, may be associated with its inhibitory effect on CRC cell proliferation through activation of the mitochondrial apoptosis pathway, including inhibition of respiratory chain complex I and induction of caspase-3 activation, which could be related to its enrichment in nucleic acid metabolism and organoheterocyclic compound pathways[28].
Hydroquinidine, as a quinoline alkaloid, may exert its antitumor effects through upregulation of cell cycle inhibitors p21/p27 to arrest tumor cells in G1 phase and inhibition of protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling pathway to reduce invasive capacity, consistent with Hongwiangchan et al[29] report on alkaloid-mediated cell cycle regulation. Aurapten, a coumarin-derived lipid-like molecule, demonstrates dual enrichment in nucleic acid and lipid metabolism pathways, suggesting potential mechanisms involving caspase-9-mediated apoptosis induction and fatty acid synthase inhibition to disrupt tumor energy metabolism[30]. Triptophenolide, as a diterpenoid alkaloid, exhibits potential effects on nucleic acid metabolism pathways that align with the RNA polymerase II inhibitory activity identified by Song et al[31] through proteomic analysis. This mechanism may induce G2/M phase arrest by interfering with transcription processes, subsequently reducing matrix metalloproteinase secretion. These metabolites collectively influence CRC metastasis through direct and indirect modulation of multiple pathways, including nucleic acid synthesis, alkaloid metabolism, and lipid metabolism, closely mirroring current research trends in the “microbiota-metabolite-tumor” interaction network.
Research on the relationship between differential GM and differential metabolites remains limited, with current studies primarily relying on inferences based on microbial metabolic features, chemical properties of compounds, and general patterns of the gut microenvironment. In our findings, the differential metabolites identified in the DZ group were predominantly exogenous or host-derived compounds rather than typical gut microbial metabolites. Moreover, their complex chemical structures require specific enzyme systems for synthesis or transformation, suggesting that their interaction with the microbiota is more likely to involve “indirect regulation”.
The study revealed that Pyramidobacter may influence the stability or activity of compounds through inflammatory microenvironments and amino acid metabolism, thereby participating in the transformation of hydroquinidine to alter its bioactivity. Furthermore, this bacterium could also induce intestinal inflammation by secreting lipopolysaccharide or activating the Toll-like receptor 4/nuclear factor kappa-B pathway, accelerating the degradation or hydroxylation mo
In this study, Pearson correlation analysis revealed extensive positive synergistic relationships among significantly upregulated metabolites in the DZ group. Key metabolite pairs demonstrated strong positive correlations, including diazoxide and aurapten (r = 0.72, P < 0.01), hydroquinidine and aurapten (r = 0.68, P < 0.01), and triptophenolide and aurapten (r = 0.75, P < 0.01). These findings suggest these metabolites may cooperatively promote CRC metastasis via functional interactions. Mechanistically, this synergistic effect may operate through multiple key pathways: (1) The metabolite network may cooperatively induce epithelial-mesenchymal transition (EMT). Research demonstrates that aurapten suppresses E-cadherin expression to initiate EMT, while diazoxide potentiates this process by activating snail transcription factors. Their combined action significantly enhances invasive capacity in CRC cells[36]; (2) Metabolite interactions may promote angiogenesis and immune evasion. Hydroquinidine upregulates vascular endothelial growth factor A to stimulate neovascularization, whereas aurapten creates an immunosuppressive niche by inhibiting cluster of differentiation (CD) 8 + T cell infiltration aligning with the “metabolite-immunity axis” carcinogenesis model[37]; (3) Metabolic reprogramming via cross-activation represents a key mechanism. Triptophenolide compels tumor cells to rely on glycolysis by suppressing mitochondrial oxidative phosphorylation, while diazoxide further enhances glycolytic flux through hexokinase 2 activation. This synergistic regulation promotes tumor cell survival in hypoxic microenvironments[38]; and (4) Metabolites may synergistically promote metastatic initiation by maintaining cancer stem cell (CSC) pro
Pathway analysis of differential metabolites in this study revealed significant enrichment of adenosine triphosphate-binding cassette (ABC) transporters, aminoacyl-tRNA biosynthesis, phenylalanine metabolism, biosynthesis of amino acids, and protein digestion and absorption pathways. Emerging evidence indicates these pathways drive CRC metastasis through multidimensional mechanisms: ABC transporters (e.g., P-glycoprotein, multidrug resistance protein 1) enhance tumor cell invasiveness by promoting EMT, with their overexpression being strongly associated with circulating tumor cell survival and CSC self-renewal[40].
The aminoacyl-tRNA biosynthesis pathway contributes to metastasis regulation through non-canonical functions of aminoacyl-tRNA synthetases[41]. The amino acid biosynthesis pathway provides critical metabolic substrates for tumor metastasis: Cancer cells can generate intracellular glycine and one-carbon units through the serine synthesis pathway, known as serine-glycine-one-carbon (SGOC) metabolism, which is essential for tumorigenesis and metastasis. The SGOC pathway constitutes a complex metabolic network that promotes cancer cell survival and proliferation in aggressive cancers[42]. Additionally, branched-chain amino acids like leucine activate the mTOR complex pathway to enhance protein synthesis, thereby driving the initiation of CRC metastasis[43]. The protein digestion and absorption pathway promotes metastatic signaling by supplying free amino acids to activate pro-metastatic pathways (e.g., AKT/mTOR), while simultaneously modulating GM-derived metabolites to suppress CD8 + T cell function, thereby fostering an immune-evasive microenvironment[44]. Therapeutic strategies targeting the amino acid transporter SLC7A5 or inhibiting IDO1-mediated tryptophan metabolism have demonstrated anti-metastatic potential in preclinical models[45].
The homeostasis of GM and their metabolite profiles is susceptible to disruption by various internal and external environmental factors, which may obscure disease-associated microbial/metabolite signatures. Diet stands out as one of the most influential determinants of gut microbial composition and metabolic activity, directly regulating bacterial proliferation and metabolic pathways through substrate availability, thereby altering metabolite profiles. For instance, high-fiber diets promote the enrichment of short-chain fatty acids-producing bacteria such as Christensenellaceae_R-7_group, leading to increased butyrate and propionate production. These metabolites lower intestinal pH and suppress pro-inflammatory bacterial growth. Conversely, high-fat/high-protein diets tend to enrich lipid-degrading bacteria like Romboutsia and proteolytic bacteria like Pyramidobacter, resulting in the accumulation of free fatty acids and branched-chain amino acid metabolites that compromise intestinal barrier integrity[46,47].
Pharmaceutical agents significantly influence GM and metabolites through antibacterial effects, interference with host metabolism, or inhibition of microbial enzymes. This interaction becomes particularly complex in CRC patients due to the combined effects of chemotherapeutic drugs and medications for underlying conditions. Broad-spectrum antibiotics can non-specifically suppress sensitive bacteria like Christensenellaceae_R-7_group, while potentially enriching antibiotic-resistant strains like certain Romboutsia species. This disruption leads to reduced short-chain fatty acids production and altered secondary bile acid metabolism, consequently modifying intestinal absorption and host metabolic pathways of compounds like diazoxide[48,49]. Furthermore, fluorouracil-based chemotherapeutics commonly used in CRC, not only directly target tumor cells but may also inhibit nucleic acid synthesis in commensal gut bacteria. This results in decreased abundance of gram-positive bacteria, such as Romboutsia, and abnormal accumulation of lipid metabolites, like aurapten[50].
From a sample collection standpoint, fecal specimens offer a non-invasive advantage over traditional invasive examinations like tissue biopsies, significantly reducing patient discomfort and psychological stress while improving compliance, which makes them particularly suitable for large-scale screening and long-term monitoring. When integrated with technologies such as 16S rRNA sequencing and LC-MS metabolomics, these samples enable precise detection and analysis of GM and metabolites, allowing identification of specific microbial and metabolic signatures that may serve as reliable diagnostic biomarkers.
However, their clinical application faces several challenges: Gut microbial communities and metabolites are highly susceptible to confounding factors like diet, lifestyle habits, and medication use. Significant inter-individual variations in dietary patterns may lead to fluctuations in microbial composition and metabolite levels, potentially compromising the accuracy of diagnostic results. Furthermore, the mechanistic links between GM/metabolites and CRC metastasis remain incompletely elucidated, and standardized diagnostic criteria are currently lacking. Discrepancies in identified differential microbial and metabolic signatures across studies pose significant challenges for clinical standardization and implementation. These limitations underscore the necessity for large-scale, multicenter studies to establish universally applicable diagnostic biomarkers.
Targeting GM and their metabolites as therapeutic interventions, particularly through probiotic administration, holds significant potential value. Probiotics can modulate microbial balance by increasing beneficial bacteria while suppressing pathogenic species, offering high safety profiles with minimal side effects that are suitable for long-term use. Compared to conventional therapies like chemotherapy or radiation, probiotic interventions cause substantially less physical damage, demonstrate better patient tolerance, and may improve quality of life.
However, several challenges persist in this therapeutic approach. The extreme complexity of gut microbial ecosystems exhibits remarkable interindividual variability (host-specific heterogeneity), leading to inconsistent treatment outcomes where a specific probiotic formulation may show efficacy in some patients but prove ineffective in others. This necessitates personalized probiotic regimens based on individual microbial profiles, inevitably increasing therapeutic complexity and costs. Furthermore, the precise colonization mechanisms and modes of action remain incompletely understood. Probiotics need to successfully colonize and proliferate in the intestines to exert their regulatory effects on microbiota and metabolism. However, the gut environment is complex, with various factors influencing probiotic colonization, such as pH levels and competition from other microorganisms. Crucially, the specific metabolic pathways and signaling cascades through which probiotics affect CRC metastasis require deeper mechanistic elucidation.
This study compared GM and metabolite profiles between non-metastatic and metastatic CRC patients, revealing significant associations between microbial structural alterations, metabolic signatures, and cancer progression. The results demonstrate that gut dysbiosis may directly and indirectly drive tumor cell invasion and distant metastasis through multiple mechanisms, including tumor microenvironment modulation, metabolic reprogramming, and immune evasion. These findings not only validate the critical role of the “microbiota-metabolite-metastasis” interaction network in CRC progression but also provide important insights for elucidating metastatic mechanisms and developing novel intervention strategies.
While our results align with certain existing reports, observed discrepancies may stem from cohort heterogeneity (e.g., geographic origin, dietary patterns, treatment history), diversity in metastatic sites and stages, and variations in detection sensitivity. Several study limitations should be noted: (1) The relatively small sample size may reduce statistical power and lead to missed detection of key associations; (2) Lack of systematic integration with clinicopathological data (e.g., tumor node metastasis staging, circulating tumor cell levels) limits comprehensive quantification of microbiota-me
These limitations reflect common challenges in current microbiome research and highlight the need for future mu
Building upon these findings, subsequent research will focus on three key directions: First, expanding cohort sizes with comprehensive clinical data to further validate the clinical utility of differential microbiota and metabolites as predictive biomarkers for CRC metastasis. Second, employing germ-free mouse transplantation models and organoid systems to elucidate the molecular mechanisms by which key microbial species (e.g., Fusobacterium nucleatum) and their metabolites (e.g., secondary bile acids) drive metastatic progression. Third, conducting functional cellular assays to delineate how differential metabolites precisely regulate EMT, angiogenesis, and immune evasion at the molecular target level. These investigations will establish a theoretical foundation for microbiota-targeted interventions against CRC metastasis, advancing the development of microbiome-based precision therapeutic strategies.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68587] [Article Influence: 13717.4] [Reference Citation Analysis (201)] |
| 2. | Xu K, Jiang B. Analysis of Mucosa-Associated Microbiota in Colorectal Cancer. Med Sci Monit. 2017;23:4422-4430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Li J, Ma X, Chakravarti D, Shalapour S, DePinho RA. Genetic and biological hallmarks of colorectal cancer. Genes Dev. 2021;35:787-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 383] [Article Influence: 76.6] [Reference Citation Analysis (8)] |
| 4. | Hampel H, Kalady MF, Pearlman R, Stanich PP. Hereditary Colorectal Cancer. Hematol Oncol Clin North Am. 2022;36:429-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 5. | Zhang W, An Y, Qin X, Wu X, Wang X, Hou H, Song X, Liu T, Wang B, Huang X, Cao H. Gut Microbiota-Derived Metabolites in Colorectal Cancer: The Bad and the Challenges. Front Oncol. 2021;11:739648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 6. | Yin Y, Wan J, Yu J, Wu K. Molecular Pathogenesis of Colitis-associated Colorectal Cancer: Immunity, Genetics, and Intestinal Microecology. Inflamm Bowel Dis. 2023;29:1648-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Messaritakis I, Koulouridi A, Boukla E, Sfakianaki M, Vogiatzoglou K, Karagianni M, Gouvas N, Tsiaoussis J, Xynos E, Athanasakis E, Mavroudis D, Tzardi M, Souglakos J. Investigation of Microbial Translocation, TLR and VDR Gene Polymorphisms, and Recurrence Risk in Stage III Colorectal Cancer Patients. Cancers (Basel). 2022;14:4407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Kovács T, Mikó E, Ujlaki G, Sári Z, Bai P. The Microbiome as a Component of the Tumor Microenvironment. Adv Exp Med Biol. 2020;1225:137-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Wang Y, Zhang X, Li J, Zhang Y, Guo Y, Chang Q, Chen L, Wang Y, Wang S, Song Y, Zhao Y, Wang Z. Sini Decoction Ameliorates Colorectal Cancer and Modulates the Composition of Gut Microbiota in Mice. Front Pharmacol. 2021;12:609992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Bertocchi A, Carloni S, Ravenda PS, Bertalot G, Spadoni I, Lo Cascio A, Gandini S, Lizier M, Braga D, Asnicar F, Segata N, Klaver C, Brescia P, Rossi E, Anselmo A, Guglietta S, Maroli A, Spaggiari P, Tarazona N, Cervantes A, Marsoni S, Lazzari L, Jodice MG, Luise C, Erreni M, Pece S, Di Fiore PP, Viale G, Spinelli A, Pozzi C, Penna G, Rescigno M. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell. 2021;39:708-724.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 375] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 11. | Abdulla MH, Agarwal D, Singh JK, Traiki TB, Pandey MK, Ahmad R, Srivastava SK. Association of the microbiome with colorectal cancer development (Review). Int J Oncol. 2021;58:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Cheng Y, Ling Z, Li L. The Intestinal Microbiota and Colorectal Cancer. Front Immunol. 2020;11:615056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 377] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 13. | Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019;16:690-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 906] [Article Influence: 129.4] [Reference Citation Analysis (0)] |
| 14. | Shan Y, Lee M, Chang EB. The Gut Microbiome and Inflammatory Bowel Diseases. Annu Rev Med. 2022;73:455-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 253] [Article Influence: 63.3] [Reference Citation Analysis (1)] |
| 15. | Illiano P, Brambilla R, Parolini C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020;287:833-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 241] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 16. | Janney A, Powrie F, Mann EH. Host-microbiota maladaptation in colorectal cancer. Nature. 2020;585:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 305] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 17. | Qin Y, Havulinna AS, Liu Y, Jousilahti P, Ritchie SC, Tokolyi A, Sanders JG, Valsta L, Brożyńska M, Zhu Q, Tripathi A, Vázquez-Baeza Y, Loomba R, Cheng S, Jain M, Niiranen T, Lahti L, Knight R, Salomaa V, Inouye M, Méric G. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat Genet. 2022;54:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (1)] |
| 18. | Chen S, Zhang L, Li M, Zhang Y, Sun M, Wang L, Lin J, Cui Y, Chen Q, Jin C, Li X, Wang B, Chen H, Zhou T, Wang L, Hsu CH, Zhuo W. Fusobacterium nucleatum reduces METTL3-mediated m(6)A modification and contributes to colorectal cancer metastasis. Nat Commun. 2022;13:1248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 178] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 19. | Kong C, Yan X, Zhu Y, Zhu H, Luo Y, Liu P, Ferrandon S, Kalady MF, Gao R, He J, Yin F, Qu X, Zheng J, Gao Y, Wei Q, Ma Y, Liu JY, Qin H. Fusobacterium Nucleatum Promotes the Development of Colorectal Cancer by Activating a Cytochrome P450/Epoxyoctadecenoic Acid Axis via TLR4/Keap1/NRF2 Signaling. Cancer Res. 2021;81:4485-4498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 20. | Li Q, Xiao Y, Han L, Luo W, Dai W, Fang H, Wang R, Xu Y, Cai S, Goel A, Bai F, Cai G. Microbiome dysbiosis, neutrophil recruitment and mesenchymal transition of mesothelial cells promotes peritoneal metastasis of colorectal cancer. Nat Cancer. 2025;6:493-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 21. | Hou H, Chen D, Zhang K, Zhang W, Liu T, Wang S, Dai X, Wang B, Zhong W, Cao H. Gut microbiota-derived short-chain fatty acids and colorectal cancer: Ready for clinical translation? Cancer Lett. 2022;526:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 164] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 22. | Thulasinathan B, Suvilesh KN, Maram S, Grossmann E, Ghouri Y, Teixeiro EP, Chan J, Kaif JT, Rachagani S. The impact of gut microbial short-chain fatty acids on colorectal cancer development and prevention. Gut Microbes. 2025;17:2483780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 23. | Zhu M, Hu Y, Gu Y, Lin X, Jiang X, Gong C, Fang Z. Role of amino acid metabolism in tumor immune microenvironment of colorectal cancer. Am J Cancer Res. 2025;15:233-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 24. | Frost Z, Bakhit S, Amaefuna CN, Powers RV, Ramana KV. Recent Advances on the Role of B Vitamins in Cancer Prevention and Progression. Int J Mol Sci. 2025;26:1967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 25. | Qian SZ, Jiang YM, Yan QL, Wu DH, Zhang WX, Chung JP. Visualization OPLS class models of GC-MS-based metabolomics data for identifying agarwood essential oil extracted by hydro-distillation. Sci Rep. 2025;15:5421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Lamaudière MTF, Arasaradnam R, Weedall GD, Morozov IY. The Colorectal Cancer Microbiota Alter Their Transcriptome To Adapt to the Acidity, Reactive Oxygen Species, and Metabolite Availability of Gut Microenvironments. mSphere. 2023;8:e0062722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Zhuang F, Bai X, Shi Y, Chang L, Ai W, Du J, Liu W, Liu H, Zhou X, Wang Z, Hong T. Metabolomic profiling identifies biomarkers and metabolic impacts of surgery for colorectal cancer. Front Surg. 2022;9:913967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 28. | Kumar A, Ali A, Kapardar RK, Dar GM, Nimisha, Apurva, Sharma AK, Verma R, Sattar RSA, Ahmad E, Mahajan B, Saluja SS. Implication of gut microbes and its metabolites in colorectal cancer. J Cancer Res Clin Oncol. 2023;149:441-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Hongwiangchan N, Sriratanasak N, Wichadakul D, Aksorn N, Chamni S, Chanvorachote P. Hydroquinone 5-O-Cinnamoyl Ester of Renieramycin M Suppresses Lung Cancer Stem Cells by Targeting Akt and Destabilizes c-Myc. Pharmaceuticals (Basel). 2021;14:1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Tayarani-Najaran Z, Tayarani-Najaran N, Eghbali S. A Review of Auraptene as an Anticancer Agent. Front Pharmacol. 2021;12:698352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Song X, He H, Zhang Y, Fan J, Wang L. Mechanisms of action of triptolide against colorectal cancer: insights from proteomic and phosphoproteomic analyses. Aging (Albany NY). 2022;14:3084-3104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Koppel N, Maini Rekdal V, Balskus EP. Chemical transformation of xenobiotics by the human gut microbiota. Science. 2017;356:eaag2770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 453] [Cited by in RCA: 724] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 33. | Al-Qadami GH, Secombe KR, Subramaniam CB, Wardill HR, Bowen JM. Gut Microbiota-Derived Short-Chain Fatty Acids: Impact on Cancer Treatment Response and Toxicities. Microorganisms. 2022;10:2048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 103] [Reference Citation Analysis (0)] |
| 34. | Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 2146] [Article Influence: 306.6] [Reference Citation Analysis (2)] |
| 35. | Kean IRL, Wagner J, Wijeyesekera A, De Goffau M, Thurston S, Clark JA, White DK, Ridout J, Agrawal S, Kayani R, O'Donnell R, Ramnarayan P, Peters MJ, Klein N, Holmes E, Parkhill J, Baker S, Pathan N. Profiling gut microbiota and bile acid metabolism in critically ill children. Sci Rep. 2022;12:10432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Zhang Q, Zhao Q, Li T, Lu L, Wang F, Zhang H, Liu Z, Ma H, Zhu Q, Wang J, Zhang X, Pei Y, Liu Q, Xu Y, Qie J, Luan X, Hu Z, Liu X. Lactobacillus plantarum-derived indole-3-lactic acid ameliorates colorectal tumorigenesis via epigenetic regulation of CD8(+) T cell immunity. Cell Metab. 2023;35:943-960.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 256] [Reference Citation Analysis (0)] |
| 37. | Kampoli K, Foukas PG, Ntavatzikos A, Arkadopoulos N, Koumarianou A. Interrogating the interplay of angiogenesis and immunity in metastatic colorectal cancer. World J Methodol. 2022;12:43-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (2)] |
| 38. | Hon KW, Zainal Abidin SA, Othman I, Naidu R. The Crosstalk Between Signaling Pathways and Cancer Metabolism in Colorectal Cancer. Front Pharmacol. 2021;12:768861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 39. | Melnik BC. Leucine signaling in the pathogenesis of type 2 diabetes and obesity. World J Diabetes. 2012;3:38-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 87] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 40. | Kim BH, Oh HK, Kim DW, Kang SB, Choi Y, Shin E. Clinical Implications of Cancer Stem Cell Markers and ABC Transporters as a Predictor of Prognosis in Colorectal Cancer Patients. Anticancer Res. 2020;40:4481-4489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Zhou Z, Sun B, Huang S, Yu D, Zhang X. Roles of aminoacyl-tRNA synthetase-interacting multi-functional proteins in physiology and cancer. Cell Death Dis. 2020;11:579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 42. | Huang D, Cai H, Huang H. Serine metabolism in tumor progression and immunotherapy. Discov Oncol. 2025;16:628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 43. | Jung MK, Okekunle AP, Lee JE, Sung MK, Lim YJ. Role of Branched-chain Amino Acid Metabolism in Tumor Development and Progression. J Cancer Prev. 2021;26:237-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 44. | Sun XZ, Zhao DY, Zhou YC, Wang QQ, Qin G, Yao SK. Alteration of fecal tryptophan metabolism correlates with shifted microbiota and may be involved in pathogenesis of colorectal cancer. World J Gastroenterol. 2020;26:7173-7190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 45. | Shi D, Wu X, Jian Y, Wang J, Huang C, Mo S, Li Y, Li F, Zhang C, Zhang D, Zhang H, Huang H, Chen X, Wang YA, Lin C, Liu G, Song L, Liao W. USP14 promotes tryptophan metabolism and immune suppression by stabilizing IDO1 in colorectal cancer. Nat Commun. 2022;13:5644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 46. | Waters JL, Ley RE. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019;17:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 427] [Cited by in RCA: 512] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 47. | Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1566] [Cited by in RCA: 2603] [Article Influence: 260.3] [Reference Citation Analysis (1)] |
| 48. | Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019;570:462-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 427] [Cited by in RCA: 826] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 49. | Nakashima M, Suga N, Ikeda Y, Yoshikawa S, Matsuda S. Relevant MicroRNAs of MMPs and TIMPs with Certain Gut Microbiota Could Be Involved in the Invasiveness and Metastasis of Malignant Tumors. Innov Discov. 2024;1:10. [DOI] [Full Text] |
| 50. | Yuan L, Zhang S, Li H, Yang F, Mushtaq N, Ullah S, Shi Y, An C, Xu J. The influence of gut microbiota dysbiosis to the efficacy of 5-Fluorouracil treatment on colorectal cancer. Biomed Pharmacother. 2018;108:184-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 167] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/