Published online Oct 15, 2025. doi: 10.4251/wjgo.v17.i10.110415
Revised: June 29, 2025
Accepted: August 26, 2025
Published online: October 15, 2025
Processing time: 130 Days and 9.5 Hours
Biliary tract cancer (BTC) is a rare disease with few available treatment options. Tumor malignancy and surgical invasiveness vary depending on the site of the lesion. Perioperative mortality remains high, particularly in patients with hilar cholangiocarcinoma and gallbladder cancer. Benchmark cases from high-volume centers have reported high surgical complications (87%) and 3-month mortality rates (13%). Japanese studies of hepatopancreatoduodenectomy have reported that although the complication rate is higher in high-volume centers than in other institutions, the mortality rate is low; operative safety depends on adequate liver volume after resection by portal vein embolization, cholangitis reduction, and comprehensive management of postoperative complications. Robot-assisted surgery is increasingly common in patients treated with pancreaticoduodenectomy even after distal pancreatectomy. However, many challenges exist due to device and visibility issues. Recently, adjuvant chemotherapies have been developed for the treatment of BTC. The introduction of immune checkpoint inhibitors and discovery of oncogenic driver genes have increased the number of promising treatment options. Innovations in targeted drug therapy, including fibroblast growth factor receptor inhibitors and immune checkpoint inhibitors, have shown efficacy and broadened the treatment options for unresectable BTC. Therefore, a multidisciplinary treatment strategy based on surgical intervention is desirable.
Core Tip: Radical resection is an important treatment for biliary tract cancer (BTC). However, radical resection for hilar cholangiocarcinoma is associated with a high incidence of complications and mortality. Preoperative assessment of the resectability and meticulous perioperative management are essential. Adjuvant chemotherapy has recently been developed for cases that are deemed unresectable. Additionally, immune checkpoint inhibitors and molecular-target agents are recent additions to the treatment options. Improvements in the overall treatment outcomes for BTC are expected. A multidisciplinary treatment strategy centered on surgical treatment of BTC will contribute to more successful outcomes.
- Citation: Nakagawa K, Tsujinaka S, Katayose Y, Yambe K, Sakurai H, Takami K, Kondo N, Yamamoto K, Shibata C. Current insights and future perspectives of treatment strategies for biliary tract cancer. World J Gastrointest Oncol 2025; 17(10): 110415
- URL: https://www.wjgnet.com/1948-5204/full/v17/i10/110415.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i10.110415
Biliary tract cancer (BTC) is a rare cancer[1,2] that is classified according to the primary site of the legion. BTCs comprise a diverse group of malignant diseases, with the current classifications encompassing intrahepatic bile duct, extrahepatic bile duct (hilar and distal), gallbladder, and papillary duodenum[1,2]. In some countries, however, cancers of the duodenal papillary region are not included in the BTC classification. Driver genes also vary depending on the site of the lesion[3,4].
Surgical resection is the primary curative approach for BTC. Still, surgical techniques and risks differ greatly depending on the site of the primary lesion. Recurrence after resection is common, and drug therapy is limited. Because BTC is a rare cancer, it has been considered collectively in both epidemiological statistics and treatment developments[1-3]. Therefore, a personalized treatment approach is needed for BTC. This minireview provides a comprehensive and multifaceted summary of recent advances and achievements in the treatment of BTC. Multiple innovations in treatment may lead to a fundamental change in the surgical indications for BTC treatment.
Regional differences exist in BTC incidence, particularly in some areas of Southeast Asia. However, the incidence of BTC is increasing in many countries due to an aging population and increases in obesity as well as liver diseases[3,4]. The BTC disease itself has a high mortality rate (relative to morbidity) and an overall poor prognosis. The risk factors for each site of the primary lesion are listed in Table 1[4-11]. Early detection and treatment of BTC requires screening patients with these risk factors. Other risk factors include occupational cholangiocarcinoma, which is caused by long-term occupational exposure to specific chemicals. In Japan’s printing industry, exposure to organic solvents such as 1,2-dichloropropane and dichloromethane are associated with an increased risk of cholangiocarcinoma[12].
| Cancer type | Candidate risk factors | Ref. |
| Intrahepatic cholangiocarcinoma | Liver fluke infection (Opisthorchis, Clonorchis); chronic liver disease (HBV, HCV, cirrhosis); PSC; hepatolithiasis; exposure to thorotrast or chemicals | [4,5] |
| Gallbladder cancer | Gallstones; large gallbladder polyps (> 10 mm); porcelain gallbladder; PBM; chronic Salmonella infection | [4,6-9,11] |
| Extrahepatic cholangiocarcinoma | PSC; PBM; liver fluke infection; chronic biliary inflammation or strictures | [4,5,10,11] |
| Ampullary cancer | FAP; Lynch syndrome; PBM | [4,11] |
Differentiation from benign diseases such as cholelithiasis is necessary. Computed tomography and magnetic resonance imaging are useful for diagnosing BTC[13]. The sensitivity of biopsy-based diagnosis of BTC is low. However, a recent report provided evidence that biopsy under the observation of a biliary speculum had a sensitivity of 95.8% and specificity of 94.7%[13]. It is difficult to improve sensitivity in the close examination of BTC in primary sclerosing cholangitis[14]. Many studies have been conducted to diagnose early-stage cancer and determine the efficacy of treatment by detecting circulating tumor DNA in blood and bile samples[15-18]. It is expected that circulating tumor DNA could be used clinically as a biomarker in the future. Today, BTC remains difficult to diagnose at an early stage, and the prognosis for advanced cases is poor. Gaining a thorough understanding of the epidemiological background and identifying new biomarkers are essential for improving the prognosis of BTC.
Surgical techniques for BTC differ greatly depending on the primary site of the lesion. The two main types of surgery are hepatectomy [for hilar bile duct cancer, gallbladder cancer, and intrahepatic cholangiocarcinoma (ICC)] and pancreatoduodenectomy (for distal bile duct cancer and papillary duodenal cancer). Hepatopancreatoduodenectomy (HPD) is also performed for extensive cholangiocarcinoma spanning several regions[19]. It is important to determine the extent of the tumor preoperatively, to achieve R0 during surgical resection[20,21]. Lymph node dissection is essential for radical surgery in the treatment of BTC.
Patients with ICC, hilar region cholangiocarcinoma, or gallbladder cancer are potential candidates for hepatic resection. Laparoscopic or robot-assisted surgery is a good option for treating peripheral ICC. A meta-analysis of 17 studies showed that laparoscopic liver resection for ICC provided short-term benefits (compared with open surgery), including decreased intraoperative blood loss, shorter postoperative hospital stay, and improved R0 resection rate. No significant differences were observed in long-term survival[22]. Robot-assisted surgery has been widely reported to be safe for hepatocellular carcinoma and is likely to become more widespread in the treatment of ICC due to the importance of lymph node dissection in ICC. In the future, both laparoscopic and robot-assisted surgery for ICC will require a thorough under
Benchmark cases of hilar cholangiocarcinoma were recently defined in several high-volume centers, and general surgical invasiveness and outcomes were evaluated[23]. In this international multicenter retrospective study on the surgical outcomes of hepatobiliary cancer in the hepatic hilum region, data were compiled from 24 institutions across three continents. In total, 1829 patients with locally resectable hilar cholangiocarcinoma without distant metastasis who underwent two or more liver resections between 2014 and 2018 were included in the analysis. Benchmark cases with a low-risk status were evaluated by excluding patients with an American Society of Anesthesiologists class III (severe systemic disease) or higher, obesity (body mass index > 35 kg/m2), chronic cardiovascular, pulmonary, or renal disease, diabetes mellitus, and anticoagulant use. The benchmark population comprised 708 patients (39% of the total population). The median planned residual liver dose was 45%, and 221 patients (31%) underwent portal vein embolization. Caudate lobectomy was performed in 538 patients (76%), and R0 resection was performed in 502 patients (71%).
The procedure-related complication rate was 87%, the hospitalization mortality rate was 8%, and the 3-month mortality rate was 13%, showing a high rate compared with other diseases. The procedure time was 8 hours with 4 Lymph node resections, blood loss of 1100 mL, intensive care unit stay of 2 days, and hospital stay of 19 days. The upper limit for all complications at 3 months was 87%. The outcome benchmark for post-hepatectomy liver failure (International Study Group of Liver Surgery B/C) was 22.5%, and the outcome benchmark for postoperative bile leak was 47%. Facilities that treated a higher proportion of difficult cases and a lower percentage of benchmark cases tended to have lower rates of postoperative liver failure and mortality. Asian centers also reported lower mortality rates for hilar cholangiocarcinoma. Even if hilar cholangiocarcinoma is limited to low-risk cases, the invasiveness of resection and complication and mortality rates are high. It is important to aim for R0 in surgical resection and to evaluate the patient’s ability to tolerate surgery.
The results of BTC resections were reported from an analysis of the National Clinical Database, which collects surgical cases in Japan[19]. Overall, 101475 Liver resections, 62720 pancreatectomies, and 422 HPDs registered in the National Clinical Database between 2011 and 2014 were reviewed by the hepatobiliary and pancreatic advanced-skills accredited facilities. The mortality rates for hepatobiliary resection, pancreatoduodenectomy, and HPD were the lowest at facility A and higher in facility B and non-accredited facilities. The mortality rates associated with HPD varied widely. However, the complication rate for liver resection and HPD was highest at facility A and lower in facility B and non-accredited facilities. Although non-accredited facilities had a higher mortality rate, there was no significant difference in the incidence of serious complications, such as liver failure and postoperative bleeding, which are directly related to surgical mortality. The increase in complications may be enhanced in facilities with greater amounts of cases and the inadequate control of local infectious complications may be the cause of death. The complication and mortality rates of HPD are worse with greater extent of hepatic resection. These reports indicated that the number of procedures performed at an institution and familiarity with difficult procedures are important in the treatment of BTC with hepatic resection. It has also been suggested that the mortality rate tends to decrease in cases where portal vein embolization is performed. It is important to ensure sufficient liver volume after resection via portal vein embolization, cholangitis reduction, and management of postoperative complications.
The results of robot-assisted surgery in the perihilar region have also been reported to support the viability of its use as a minimally invasive surgical approach[24-26]. In a comparison of laparoscopic and robot-assisted surgery, laparoscopic surgery required less time for hepatic resection, and robot-assisted surgery required less time for biliary reconstruction[27]. Future improvements in the field of view and devices are needed for robot-assisted hepatic resection because of the significant differences in anastomosis. Moreover, robot-assisted surgeries like open surgery will require a sufficient number of patients and proficiency to lead to consistently successful outcomes.
The most recent change in pancreaticoduodenectomy (PD) has been the widespread use of robot-assisted surgery. A multicenter trial (8 centers in 5 countries) comparing open PD, laparoscopic PD, and robot-assisted PD of distal bile duct cancer has been reported[28]. A total of 478 patients were enrolled, of whom 97 underwent minimally invasive PD (laparoscopic and robot-assisted PD). Minimally invasive PD was associated with less blood loss (300 mL vs 420 mL, P = 0.025), shorter operative time (453 minutes vs 340 minutes, P < 0.001), and lower rate of surgical site infection (7.8% vs 19.3%, P = 0.042) compared with open PD. Minimally invasive PD had a median overall survival (OS) (30 months vs 25 months) and disease-free survival (29 months vs 18 months) that were not significantly different between minimally invasive PD and open PD. When comparing robot-assisted PD and laparoscopic PD, robot-assisted PD had a higher lymph node dissection rate (18.0 vs 13.5, P = 0.008) and lower rate of serious complications (Clavien-Dindo 3b-5, 8.1% vs 32.1%, P = 0.005).
Robot-assisted surgery and open surgery have also been reported in five centers in China[29]. Between January 2014 and June 2019 distal cholangiocarcinoma cases were analyzed. In total, 217 robot-assisted PD cases and 228 open PD cases were enrolled, and 1:1 propensity score matching (PSM) was performed. After PSM, 180 patients were enrolled into each group. There were no significant differences in operative time, lymphadenectomy, intraoperative blood transfusion, vasectomy, R0 resection, major postoperative complications, reoperation, 90-day mortality, or long-term survival between the two groups before and after PSM. Compared with the open PD group, the robot-assisted PD group had significantly less estimated blood loss (150.0 mL vs 250.0 mL, P < 0.000). Pre-PSM and post-PSM comparisons were also made, but bias, cluster effects, etc could not be completely resolved. Therefore, one should be cautious in interpreting the results.
A study involving a relatively large number of cases determined that the safety and curative efficacy of robot-assisted surgery are comparable to those of open surgery. Although verification in prospective interventional studies is desirable in the future, the use of robot-assisted PD is expected to increase for the treatment of BTC. Minimally invasive surgery may allow perioperative drug therapy and radiotherapy to be administered more effectively within a shorter period. These findings may be useful in future multidisciplinary treatment strategies for BTC.
The combination of gemcitabine (GEM) + cisplatin (GC) is the primary choice for chemotherapy treatment of BTC[30]. There are few options for secondary chemotherapy treatment of BTC. Adjuvant perioperative therapy has also been developed. However, GEM[31], which is effective in pancreatic cancer, failed to show efficacy in BTC. However, many useful regimens that include GEM have been proposed for the treatment of BTC.
Recurrence is common after BTC resection, and adjuvant chemotherapy is administered in a small percentage of cases without evidence of recurrence[32-34]. In 2019, the BILCAP trial, conducted at 44 centers in the United Kingdom, proposed that capecitabine may be useful as adjuvant therapy after surgery. This trial failed to meet the primary endpoint of intention-to-treat (ITT). However, per protocol set was effective, suggesting that capecitabine could improve OS in patients with resected BTC as adjuvant chemotherapy[35]. Long-term results have also been reported[36]. Although the quality of evidence must be considered, this is the first time that an effective adjuvant therapy has been proposed for BTC.
In Asia, where BTC is more common, a phase III trial on adjuvant therapy was conducted in Japan. The JCOG1202 trial was a phase III study evaluating the effect of S-1 adjuvant therapy on OS compared with resection alone. The trial enrolled 440 patients aged 20-80 years with PS 0-1, R 0/1, and D1 or higher lymph node dissection[37]. ITT analysis demonstrated the superiority of S-1 therapy over post-resection with a hazard ratio (HR) of 0.694 (one-sided P = 0.0080), proving the efficacy of adjuvant therapy. The 3-year survival rates were 67.6% and 77.1% in the resection alone group and the S-1 group, respectively. Although the results in the resection alone group were excellent, the S-1 group had an additional benefit. Some of the clinical trials of postoperative therapy for BTC are tabulated in Table 2[32,35,37-39].
| Trial | Regimen | Country/no. of sites | Sample size | Primary endpoint | Enrollment period | Results | Ref. |
| BILCAP | Capecitabine | United Kingdom/44 | 447 | OS | 2006-2014 | No OS difference in ITT (HR = 0.81, P = 0.097); sensitivity and PPS analysis suggested that capecitabine can improve OS (HR = 0.71, P = 0.01) | [35] |
| BCAT | GEM | Japan/33 | 225 | OS | 2007-2011 | No OS or RFS difference (HR = 1.01, P = 0.964) | [32] |
| PRODIGE 12-ACCORD 18 | GEMOX | France/33 | 196 | RFS | 2009-2014 | No RFS benefit (HR = 0.88, P = 0.48) | [38] |
| JCOG1202 (ASCOT) | S-1 | Japan/38 | 440 | OS | 2013-2018 | OS benefit (HR = 0.69, P = 0.008); RFS not significant | [37] |
| ACTICCA-1 | GEM + CDDP vs capecitabine | Germany, United Kingdom, Netherlands, Austria, Denmark/90+ | 594 | DFS | 2014-2022 | Follow-up ongoing1 | [39] |
Regimens for non-resected BTC have also seen new developments in Asia, and the JCOG1113 trial randomized patients with unresectable or recurrent BTC to GC or GEM/S-1(GS) in a 1:1 ratio[40]. A total of 354 individuals were evaluated after a follow-up period of 1 year and 4 months. At the cutoff point, 287 deaths (81%) were recorded. The median OS was 13.4 months in the GC group and 15.1 months in the GS group (HR = 0.945; 95% confidence interval (CI): 0.777-1.149, one-sided P = 0.046). The 90%CI upper limit for HR was below the noninferiority margin of 1.155, indicating that the GS group was noninferior to the GC group. However, as the upper 90%CI of the HR was below the noninferiority margin of 1.155, the superiority of GS was not verified. Grade 3-4 adverse events in the group included neutropenia (59.9%), leukopenia (24.9%), biliary tract infection (20.9%), and thrombocytopenia (7.3%). Adverse events were within the acceptable range, indicating that GS may be a new treatment option for advanced or recurrent BTC without the need for hydration at the time of administration.
The results of the KHBO1401 study, which verified the superiority of triplet GC + S1 (GCS) over GC, were reported[41]. The median OS was 12.6 months in the GC group and 13.5 months in the GCS group (HR = 0.79, one-sided P < 0.046), indicating the superiority of GCS. In countries where S-1 is available, GCS therapy may be a new option for the primary treatment of advanced BTC. A regimen of standard GC plus nab-paclitaxel (GAP), which is effective in pancreatic cancer (SWOG1815 study)[42], was also validated in a 2:1 randomized trial of GAP to GC treatment. The median OS for GAP was 14.0 months and 13.6 months for GC, with no significant difference in OS (HR = 0.91, 95%CI: 0.72-1.14, P = 0.41).
The addition of immune checkpoint inhibitors (ICIs) to GC therapy is effective for BTC. Durvalumab is a human IgG1 monoclonal antibody that primarily targets programmed death ligand 1 and has shown efficacy as maintenance therapy after chemoradiation in stage III non-small cell lung cancer (PACIFIC study)[43]. GC therapy, the standard of care for BTC, was validated in a phase III trial (the TOPAZ-1 trial)[44]. At the time of the interim analysis, the GC + durvalumab group showed significantly prolonged survival with a median OS of 12.8 months compared with 11.5 months in the GC + placebo group (HR = 0.80, P = 0.021). The OS rate at 24 months was 24.9% in the GC + durvalumab group and 10.4% in the GC + placebo group. The long tail (tail plateau), which is a characteristic of ICI treatment, was observed at a certain point where the survival curve stopped moving down[45].
The KEYNOTE-966 study, which examined the effects of adding the programmed death 1 antibody, pembrolizumab, to GC, reported similar results[46]. This study differed from the TOPAZ-1 study because GEM treatment continued after completion of GC treatment. A total of 1069 patients with unresectable locally advanced or metastatic BTC were randomized in a 1:1 ratio. With a median follow-up of 25.6 months, the median OS was 10.9 months in the GC group vs 12.7 months in the GC + pembrolizumab group (HR = 0.83, P = 0.0034). Both trials showed that some patients could benefit from additional ICI for BTC treatment. The incidence of immune-mediated adverse events was 2.7% for all grades in the TOPAZ-1 trial compared with 22.0% in the KEYNOTE-966 trial. However, this ICI-specific side effect requires careful attention. The phase III trials for advanced BTC are summarized in Table 3[30,40,41,44,46].
| Trial | Sample size | Primary endpoint | Regimen and median OS | Enrollment period | Results | Ref. |
| ABC-02 | 410 | OS | GC: 11.7 months; GEM: 8.1 months | 2005-2008 | Significant OS benefit with GC (HR = 0.64, P < 0.001) | [30] |
| JCOG1113 | 354 | OS | GS: 15.1 months; GC: 13.4 months | 2013-2017 | GS noninferior to GC (HR = 0.945, P = 0.046) | [40] |
| KHBO1401 | 246 | OS | GCS: 13.5 months; GC: 12.6 months | 2013-2017 | OS benefit with GCS (HR = 0.79, P = 0.046) | [41] |
| TOPAZ-1 | 685 | OS | D + GC: 12.8 months; GC: 11.5 months | 2019-2021 | OS benefit with D + GC (HR = 0.80, P = 0.021) | [44] |
| KEYNOTE-966 | 1069 | OS | P + GC: 12.7 months; GC: 10.9 months | 2019-2022 | OS benefit with P + GC (HR = 0.83, P = 0.0034) | [46] |
The ABC-06 trial reported the efficacy of FOLFOX (FFX) as a second-line treatment in 2021[47]. This study compared the efficacy of FFX plus active symptom control (ASC) over ASC alone in patients with BTC after GC failure. Median OS was 5.3 months in the ASC group vs 6.2 months in the FFX group. The HR was 0.69 (P = 0.0031), significantly better than in the FFX group[47]. Chemotherapy for BTC has seen an increase in promising innovative options in recent years. However, its role in clinical practice remains exploratory and based solely on first-line results. Going forward, clinical experience and follow-up clinical studies are needed to establish priority treatments and second-line therapies.
In 2015, a BTC genome sequencing study revealed that 40% of BTCs had genetic abnormalities that could be therapeutic targets[48]. Therefore, drug discovery targeting genetic mutations has been pursued. Several molecular-target drugs have been developed for this purpose.
The fibroblast growth factor receptor 2 (FGFR2) fusion genes are found in 10%-15% of patients with ICC. When fibroblast growth factor binds to FGFR, it forms a dimer that activates the intracellular tyrosine kinase domain, which is thought to be involved in cell growth by activating downstream mitogen-activated protein kinases and phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathways[49]. The FGFR2 fusion gene is a ligand-independent receptor that contributes to tumor cell proliferation and survival[50]. These mechanisms have led to the development of several FGFR inhibitors. The major FGFR inhibitors used in the development are summarized in Table 4. They are broadly classified as either reversible or irreversible.
| Drug name | Target FGFR | Clinical status (for CCA) | Type |
| Pemigatinib | FGFR1-3 | Approved (United States/European Union/Japan) | Reversible |
| Infigratinib | FGFR1-3 | Withdrawn (formerly approved in United States) | Reversible |
| Zoligratinib (Debio 1347) | FGFR1-3 | Phase 2 ongoing | Reversible |
| Fexagratinib (AZD4547) | FGFR1-3 | Phase 1-2 completed | Reversible |
| Erdafitinib | FGFR1-4 | Approved (bladder CA); exploratory in CCA | Reversible |
| Derazantinib | FGFR1-3 | Phase 2 (FIDES-01) | Reversible |
| Tasurgratinib (E7090) | FGFR1-3 | Approved (Japan); phase 2 completed | Reversible |
| Futibatinib | FGFR1-4 | Approved (United States/Japan) | Irreversible |
| Lirafugratinib (RLY-4008) | FGFR2 selective | Phase 1/2 with high ORR | Irreversible |
| PRN1371 | FGFR1-4 | Early phase trials | Irreversible |
| H3B-6527 | FGFR4 selective | FGFR4 focus (HCC) | Irreversible |
| Ponatinib | Multitarget (BCR-ABL, FGFR) | Not specific for CCA | Multitarget |
| Lucitanib | Multitarget (VEGFR, FGFR) | Exploratory | Multitarget |
| Nintedanib | Multitarget (VEGFR, FGFR, PDGFR) | Exploratory | Multitarget |
Currently, several FGFR inhibitors are used clinically. Pemigatinib inhibits tumor growth by blocking the phosphorylation of FGFR fusion proteins and other downstream signaling molecules. The phase II FIGHT-202 trial demonstrated a response rate of 35.5% and a median survival of 21.06 months[51]. Fucibatinib exerts antitumor effects by irreversibly inhibiting FGFR1-4 and suppressing intracellular signaling. The phase I/II trial showed an objective response rate of 41.7% and a median OS of 21.7 months in the phase II portion of the trial[52].
Taslugratinib is an ATP-competitive, reversible inhibitor of FGFR1-3. A multicenter, open-label, single-arm phase II study conducted in Japan and China showed an objective response rate of 30% and a clinical efficacy rate of 51%. RLY-4008 tested lirafugratinib and showed high selectivity and response rates in FGFR2 fusion-positive ICC with an objective response rate of 88% in patients with FGFR2 fusion in the phase I/II trial. FGFR inhibitors have several possible mechanisms of resistance acquisition depending on the binding sites and pathways[53]. FGFR inhibitors have side effects, such as hyperphosphatemia and retinal detachment. The sequential administration of FGFR inhibitors may be possible in the future.
Isocitrate dehydrogenase (IDH) mutations are targeted in diseases such as acute myeloid leukemia, and IDH inhibitors are used as therapeutic agents. IDH is an energy-metabolizing enzyme involved in the citric acid cycle. 2-hydroxyglutaric acid, produced by IDH mutants, is a carcinogenic metabolite that causes abnormal cellular metabolism and is involved in tumor formation[54]. IDH1 mutations are found in 13% of patients with ICC.
The ClarIDHy trial was a randomized, controlled, phase III trial in which patients with advanced cholangiocarcinoma with IDH1 mutations who had progressed during prior therapy were randomized 2:1 to receive ivosidenib or placebo[55]. The primary endpoint was progression-free survival, which was 2.7 months in the ivosidenib group and 1.4 months in the placebo group, representing a statistically significant prolongation. In that trial, approximately 70% of the placebo group crossed over to ivosidenib after progression. Therefore, both analyses based on the initial randomized treatment assignment and crossover-adjusted analysis were performed in the OS investigation. ITT analysis showed no OS advantage in the ivosidenib group compared with the placebo group (HR = 0.69, 95%CI: 0.44-1.10, P = 0.060). The adjusted analysis, which eliminated the effect of crossover to ivosidenib in the placebo group, revealed an OS advantage of ivosidenib over placebo (HR = 0.46, 95%CI: 0.28-0.75, P = 0.0008). Ivosidenib has been clinically tested for BTC treatment in the United States.
Abnormalities in the MDM2, HER2 (ERBB2), and BRCA1/2 genes have attracted attention as potential therapeutic targets for BTC. High microsatellite instability/high tumor mutational burden and BRAFV600E mutations are also promising therapeutic options for BTC. It is still necessary to perform appropriate formalin fixation of specimens and search for gene mutations using next-generation sequencing or fluorescence in situ hybridization in a large number of patients.
In advanced BTC, several multidisciplinary treatments, such as transplantation after chemoradiation, have been explored. However, the efficacy of S1 has been demonstrated only as adjuvant therapy after resection[37], and there is no evidence for neoadjuvant therapy. The GAIN trial (AIO/CALGP/ACO-GAIN), a phase III randomized trial targeting intrahepatic bile duct cancer/bile duct cancer/gallbladder cancer, has initiated patient enrollment and plans to enroll 330 patients. The trial aims to compare preoperative 3-course GC plus surgery plus postoperative chemotherapy (arm A) with immediate surgery plus postoperative chemotherapy (arm B) to evaluate OS. However, as of February 2024 only 68 cases had been enrolled, and the trial was terminated early due to insufficient case accumulation. At the time of discontinuation, the median follow-up period was 11.8 months. OS for arm A vs arm B was 27.8 months vs 14.6 months, (HR = 0.46, P = 0.04), respectively, and the R0 resection rate was 62.5% vs 33.3%, respectively[56].
The JCOG1920 (NABICAT) clinical trial, a phase III trial using GCS, is currently the most promising regimen in Japan. It has been ongoing since March 2021[57]. The trial aims to enroll 330 patients with resectable or borderline resectable BTC (intrahepatic, hilar, distal bile duct cancer, and gallbladder cancer). The intervention group will receive GEM 1000 mg/m² + GC 25 mg/m² (day 1) + S-1 (80-120 mg/day on days 1-7) every 2 weeks for three courses and followed by surgery. The control group will undergo surgery alone. The OS of the two groups will be compared. Additionally, a phase II/III trial using GAP therapy is underway in Italy[58]. The objective is to evaluate neoadjuvant GAP therapy for high-risk resectable BTC (cholangiocarcinoma and gallbladder cancer). Case accumulation of 108 patients in the phase II portion began in November 2023. Depending on the results of these clinical trials, preoperative therapy may become standard treatment for BTC.
Meanwhile, various treatments have been developed to convert unresectable BTC to resectable with an increasing number of patients undergoing conversion resection[59-62]. If R0 resection is achievable, improved survival is anticipated. There are reports of conversion surgery after the use of ICI[61]. These are retrospective reports, and it is currently difficult to determine the appropriate patient populations or treatment regimens. This remains an exploratory treatment strategy that should be investigated further at high-volume centers.
The development of chemotherapy and molecular-target drugs has led to the emergence of new treatments in clinical practice. However, even when reviewing clinical trial results, it cannot be expected that all patients with unresectable BTC will experience improved prognosis. Considering the possibility of achieving long-term disease control with effective treatment, it is crucial to select appropriate surgical indications. For conversion surgery in unresectable BTC cases, meticulous surgical planning and surgical techniques are required. In cases where resection is deemed feasible, it is important to monitor recurrence over an adequate period post-resection. Identifying recurrence and intervening with appropriate treatment should improve the prognosis of cases that undergo resection.
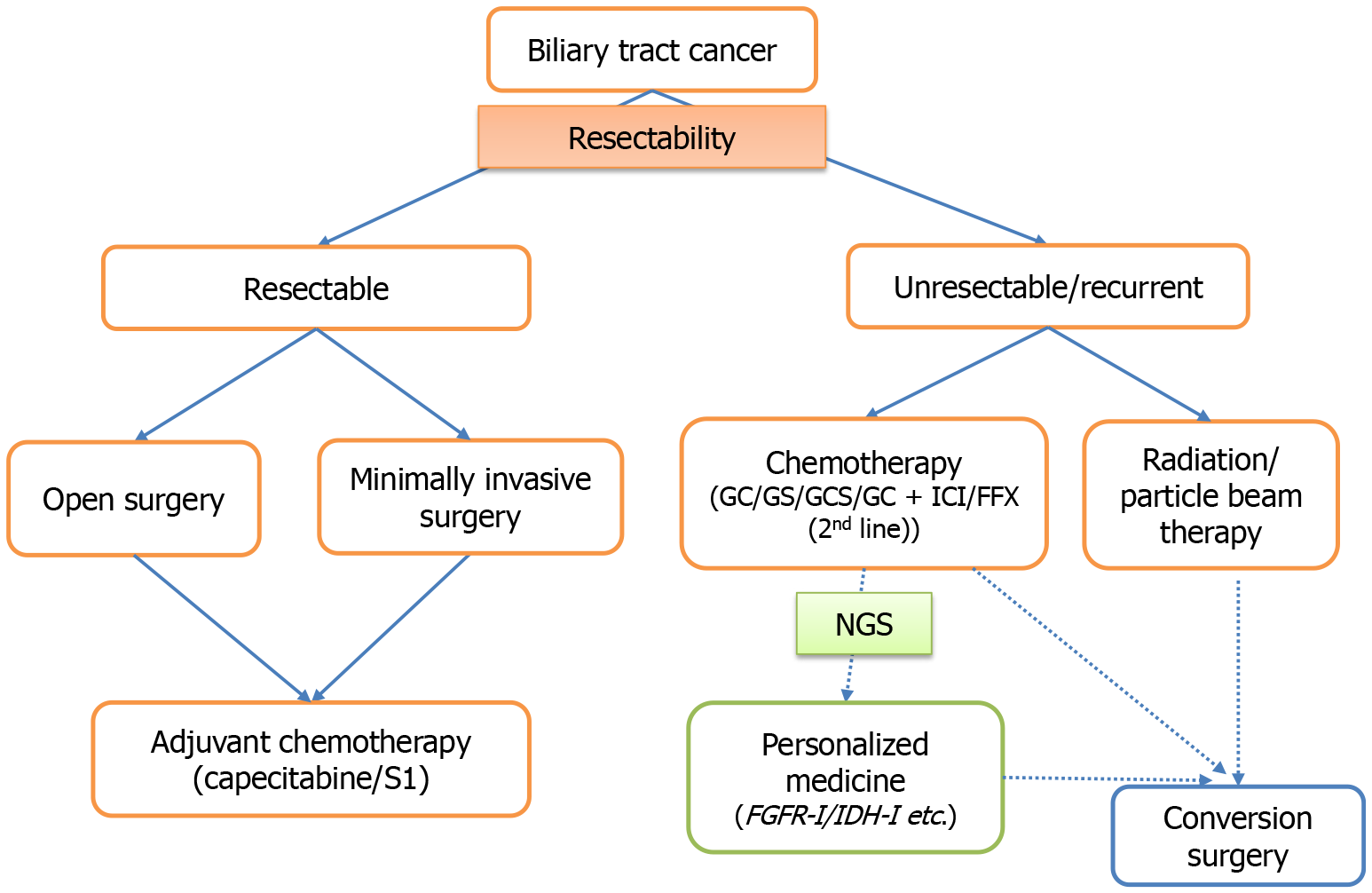
Surgical and pharmacological treatments for BTC have been developed for BTCs. It is ideal to select treatment options tailored to the patient. This article discussed observations from daily clinical practice in BTC management to offer a point of reference for clinicians treating BTC. Figure 1 presents a schematic overview of the current treatment approach for BTC. In addition to the treatments described here, diagnostic and therapeutic techniques for gastrointestinal endoscopists, such as endoscopic ultrasound-guided hepaticogastrostomy, are rapidly progressing for BTC management. Appropriate drainage and drug therapy may improve the prognosis in patients with advanced BTC. Particle beam therapy is expected to be effective in some patients with BTC. Simultaneously, the prognosis of resected cases is expected to improve with the enhancement of postoperative adjuvant therapy and treatment after recurrence. Future studies are required to determine the surgical indications and treatment strategies for BTC. Ultimately, the collective findings to date highlight the importance of refining multidisciplinary treatment strategies that incorporate surgery as the central component.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12562] [Article Influence: 6281.0] [Reference Citation Analysis (6)] |
| 2. | Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 364] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 3. | Ouyang G, Liu Q, Wu Y, Liu Z, Lu W, Li S, Pan G, Chen X. The global, regional, and national burden of gallbladder and biliary tract cancer and its attributable risk factors in 195 countries and territories, 1990 to 2017: A systematic analysis for the Global Burden of Disease Study 2017. Cancer. 2021;127:2238-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 4. | Miyazaki M, Takada T, Miyakawa S, Tsukada K, Nagino M, Kondo S, Furuse J, Saito H, Tsuyuguchi T, Chijiiwa K, Kimura F, Yoshitomi H, Nozawa S, Yoshida M, Wada K, Amano H, Miura F; Japanese Association of Biliary Surgery; Japanese Society of Hepato-Pancreatic Surgery; Japan Society of Clinical Oncology. Risk factors for biliary tract and ampullary carcinomas and prophylactic surgery for these factors. J Hepatobiliary Pancreat Surg. 2008;15:15-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Clements O, Eliahoo J, Kim JU, Taylor-Robinson SD, Khan SA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J Hepatol. 2020;72:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 392] [Article Influence: 65.3] [Reference Citation Analysis (1)] |
| 6. | Pérez-Moreno P, Riquelme I, García P, Brebi P, Roa JC. Environmental and Lifestyle Risk Factors in the Carcinogenesis of Gallbladder Cancer. J Pers Med. 2022;12:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Piovani D, Nikolopoulos GK, Aghemo A, Lleo A, Alqahtani SA, Hassan C, Repici A, Bonovas S. Environmental Risk Factors for Gallbladder Cancer: Field-Wide Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2025;23:1500-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | Afzal A, Liu YY, Noureen A, Rehman A, Iftikhar M, Afzal H, Azam F, Saddozai UAK, Jan T, Asif Z, Zhang L, Ji XY, Khawar MB. Epidemiology of gall bladder cancer and its prevalence worldwide: a meta-analysis. Orphanet J Rare Dis. 2025;20:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Ahn DH, Bekaii-Saab T. Ampullary cancer: an overview. Am Soc Clin Oncol Educ Book. 2014;112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 10. | Xiong J, Wang Y, Huang H, Bian J, Wang A, Long J, Zheng Y, Sang X, Xu Y, Lu X, Zhao H. Systematic review and meta-analysis: cholecystectomy and the risk of cholangiocarcinoma. Oncotarget. 2017;8:59648-59657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Kamisawa T, Kuruma S, Tabata T, Chiba K, Iwasaki S, Koizumi S, Kurata M, Honda G, Itoi T. Pancreaticobiliary maljunction and biliary cancer. J Gastroenterol. 2015;50:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Kubo S, Takemura S, Tanaka S, Shinkawa H, Kinoshita M, Hamano G, Ito T, Koda M, Aota T. Occupational cholangiocarcinoma caused by exposure to 1,2-dichloropropane and/or dichloromethane. Ann Gastroenterol Surg. 2018;2:99-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Van Beers BE. Diagnosis of cholangiocarcinoma. HPB (Oxford). 2008;10:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Xiong Z, Wang K, Zhang H, Fang Y, Li F, Huang J. Improved fluoroscopy-guided biopsies in the diagnosis of indeterminate biliary strictures: a multi-center retrospective study. Sci Rep. 2023;13:13152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Kaura K, Sawas T, Bazerbachi F, Storm AC, Martin JA, Gores GJ, Abu Dayyeh BK, Topazian MD, Levy MJ, Petersen BT, Chandrasekhara V. Cholangioscopy Biopsies Improve Detection of Cholangiocarcinoma When Combined with Cytology and FISH, but Not in Patients with PSC. Dig Dis Sci. 2020;65:1471-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Liao W, Wang Q, Jiang Q, Wu X, Yang Y, Yang A. Enhancing diagnostic strategies for biliary strictures: an evolving landscape. Hepatobiliary Surg Nutr. 2024;13:885-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Ito S, Ando M, Aoki S, Soma S, Zhang J, Hirano N, Kashiwagi R, Murakami K, Yoshimachi S, Sato H, Kusaka A, Iseki M, Inoue K, Mizuma M, Kume K, Nakagawa K, Masamune A, Asano N, Yasuda J, Unno M. Usefulness of multigene liquid biopsy of bile for identifying driver genes of biliary duct cancers. Cancer Sci. 2024;115:4054-4063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Kokuryo T, Sunagawa M, Yamaguchi J, Baba T, Kawakatsu S, Watanabe N, Onoe S, Mizuno T, Ebata T. Whole-genome Sequencing Analysis of Bile Tract Cancer Reveals Mutation Characteristics and Potential Biomarkers. Cancer Genomics Proteomics. 2025;22:34-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Endo I, Hirahara N, Miyata H, Yamamoto H, Matsuyama R, Kumamoto T, Homma Y, Mori M, Seto Y, Wakabayashi G, Kitagawa Y, Miura F, Kokudo N, Kosuge T, Nagino M, Horiguchi A, Hirano S, Yamaue H, Yamamoto M, Miyazaki M. Mortality, morbidity, and failure to rescue in hepatopancreatoduodenectomy: An analysis of patients registered in the National Clinical Database in Japan. J Hepatobiliary Pancreat Sci. 2021;28:305-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Akamatsu N, Sugawara Y, Hashimoto D. Surgical strategy for bile duct cancer: Advances and current limitations. World J Clin Oncol. 2011;2:94-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Fairweather M, Balachandran VP, D'Angelica MI. Surgical management of biliary tract cancers. Chin Clin Oncol. 2016;5:63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Zhao X, Gao FW, Jiang KY, Yang J, Xie QY, Gong J, Yang MY, Mao TY, Lei ZH. Laparoscopic or open liver resection for intrahepatic cholangiocarcinoma: A meta-analysis and systematic review. Front Oncol. 2023;13:1096714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 23. | Mueller M, Breuer E, Mizuno T, Bartsch F, Ratti F, Benzing C, Ammar-Khodja N, Sugiura T, Takayashiki T, Hessheimer A, Kim HS, Ruzzenente A, Ahn KS, Wong T, Bednarsch J, D'Silva M, Koerkamp BG, Jeddou H, López-López V, de Ponthaud C, Yonkus JA, Ismail W, Nooijen LE, Hidalgo-Salinas C, Kontis E, Wagner KC, Gunasekaran G, Higuchi R, Gleisner A, Shwaartz C, Sapisochin G, Schulick RD, Yamamoto M, Noji T, Hirano S, Schwartz M, Oldhafer KJ, Prachalias A, Fusai GK, Erdmann JI, Line PD, Smoot RL, Soubrane O, Robles-Campos R, Boudjema K, Polak WG, Han HS, Neumann UP, Lo CM, Kang KJ, Guglielmi A, Park JS, Fondevila C, Ohtsuka M, Uesaka K, Adam R, Pratschke J, Aldrighetti L, De Oliveira ML, Gores GJ, Lang H, Nagino M, Clavien PA. Perihilar Cholangiocarcinoma - Novel Benchmark Values for Surgical and Oncological Outcomes From 24 Expert Centers. Ann Surg. 2021;274:780-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 24. | Li J, Tan X, Zhang X, Zhao G, Hu M, Zhao Z, Liu R. Robotic radical surgery for hilar cholangiocarcinoma: A single-centre case series. Int J Med Robot. 2020;16:e2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Jacoby H, Rayman S, Ross S, Crespo K, Syblis C, Rosemurgy A, Sucandy I. Robotic resection of hilar cholangiocarcinoma: a single institution experience. Mini-invasive Surg. 2022;6:58. [DOI] [Full Text] |
| 26. | Liu J, Dou C, Chen J, Lu Y, Liang L, Wei F, Zhang C. Evaluation of the outcomes of biliary-enteric reconstruction in robotic radical resection of hilar cholangiocarcinoma: a single-center propensity score matching analysis. Sci Rep. 2024;14:14836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 27. | Uijterwijk BA, Lemmers DHL, Bolm L, Luyer M, Koh YX, Mazzola M, Webber L, Kazemier G, Bannone E, Ramaekers M, Ielpo B, Wellner U, Koek S, Giani A, Besselink MG, Abu Hilal M; ISGACA consortium, the International study group on non-pancreatic periampullary cancer. Long-term Outcomes After Laparoscopic, Robotic, and Open Pancreatoduodenectomy for Distal Cholangiocarcinoma: An International Propensity Score-matched Cohort Study. Ann Surg. 2023;278:e570-e579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Xu S, Zhang XP, Zhao GD, Zou WB, Zhao ZM, Hu MG, Gao YX, Tan XL, Liu Q, Liu R. Robotic versus open pancreaticoduodenectomy for distal cholangiocarcinoma: a multicenter propensity score-matched study. Surg Endosc. 2022;36:8237-8248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 29. | Kone LB, Bystrom PV, Maker AV. Robotic Surgery for Biliary Tract Cancer. Cancers (Basel). 2022;14:1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3327] [Article Influence: 207.9] [Reference Citation Analysis (15)] |
| 31. | Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1421] [Article Influence: 109.3] [Reference Citation Analysis (0)] |
| 32. | Ebata T, Hirano S, Konishi M, Uesaka K, Tsuchiya Y, Ohtsuka M, Kaneoka Y, Yamamoto M, Ambo Y, Shimizu Y, Ozawa F, Fukutomi A, Ando M, Nimura Y, Nagino M; Bile Duct Cancer Adjuvant Trial (BCAT) Study Group. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg. 2018;105:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 311] [Article Influence: 38.9] [Reference Citation Analysis (1)] |
| 33. | Nakeeb A, Pitt HA. Radiation therapy, chemotherapy and chemoradiation in hilar cholangiocarcinoma. HPB (Oxford). 2005;7:278-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Boutin M, Krishnan T, Safro M, Yang J, Jafari H, Davies JM, Gill S. Real-world experience supporting the role of oncologic resection and adjuvant chemotherapy in biliary tract cancers. Ther Adv Med Oncol. 2024;16:17588359241247008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 35. | Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans JTR, Stocken D, Praseedom R, Ma YT, Davidson B, Neoptolemos JP, Iveson T, Raftery J, Zhu S, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater J; BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 901] [Article Influence: 128.7] [Reference Citation Analysis (0)] |
| 36. | Bridgewater J, Fletcher P, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans TR, Stocken D, Stubbs C, Praseedom R, Ma YT, Davidson B, Neoptolemos J, Iveson T, Cunningham D, Garden OJ, Valle JW, Primrose J; BILCAP study group. Long-Term Outcomes and Exploratory Analyses of the Randomized Phase III BILCAP Study. J Clin Oncol. 2022;40:2048-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 37. | Nakachi K, Ikeda M, Konishi M, Nomura S, Katayama H, Kataoka T, Todaka A, Yanagimoto H, Morinaga S, Kobayashi S, Shimada K, Takahashi Y, Nakagohri T, Gotoh K, Kamata K, Shimizu Y, Ueno M, Ishii H, Okusaka T, Furuse J; Hepatobiliary and Pancreatic Oncology Group of the Japan Clinical Oncology Group (JCOG-HBPOG). Adjuvant S-1 compared with observation in resected biliary tract cancer (JCOG1202, ASCOT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet. 2023;401:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 172] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 38. | Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly JP, Boudjema K, Fartoux L, Bouhier-Leporrier K, Jouve JL, Faroux R, Guerin-Meyer V, Kurtz JE, Assénat E, Seitz JF, Baumgaertner I, Tougeron D, de la Fouchardière C, Lombard-Bohas C, Boucher E, Stanbury T, Louvet C, Malka D, Phelip JM. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J Clin Oncol. 2019;37:658-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 396] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 39. | Stein A, Arnold D, Bridgewater J, Goldstein D, Jensen LH, Klümpen HJ, Lohse AW, Nashan B, Primrose J, Schrum S, Shannon J, Vettorazzi E, Wege H. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC Cancer. 2015;15:564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 40. | Morizane C, Okusaka T, Mizusawa J, Katayama H, Ueno M, Ikeda M, Ozaka M, Okano N, Sugimori K, Fukutomi A, Hara H, Mizuno N, Yanagimoto H, Wada K, Tobimatsu K, Yane K, Nakamori S, Yamaguchi H, Asagi A, Yukisawa S, Kojima Y, Kawabe K, Kawamoto Y, Sugimoto R, Iwai T, Nakamura K, Miyakawa H, Yamashita T, Hosokawa A, Ioka T, Kato N, Shioji K, Shimizu K, Nakagohri T, Kamata K, Ishii H, Furuse J; members of the Hepatobiliary and Pancreatic Oncology Group of the Japan Clinical Oncology Group (JCOG-HBPOG). Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol. 2019;30:1950-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 236] [Article Influence: 33.7] [Reference Citation Analysis (1)] |
| 41. | Ioka T, Kanai M, Kobayashi S, Sakai D, Eguchi H, Baba H, Seo S, Taketomi A, Takayama T, Yamaue H, Takahashi M, Sho M, Kamei K, Fujimoto J, Toyoda M, Shimizu J, Goto T, Shindo Y, Yoshimura K, Hatano E, Nagano H; Kansai Hepatobiliary Oncology Group (KHBO). Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401- MITSUBA). J Hepatobiliary Pancreat Sci. 2023;30:102-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 160] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 42. | Shroff RT, King G, Colby S, Scott AJ, Borad MJ, Goff L, Matin K, Mahipal A, Kalyan A, Javle MM, El Dika I, Tan B, Cheema P, Patel A, Iyer R, Kelley RK, Thumar J, El-Khoueiry A, Guthrie KA, Chiorean EG, Hochster H, Philip PA. SWOG S1815: A Phase III Randomized Trial of Gemcitabine, Cisplatin, and Nab-Paclitaxel Versus Gemcitabine and Cisplatin in Newly Diagnosed, Advanced Biliary Tract Cancers. J Clin Oncol. 2025;43:536-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 43. | Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M; PACIFIC Investigators. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2750] [Cited by in RCA: 3419] [Article Influence: 379.9] [Reference Citation Analysis (9)] |
| 44. | Oh DY, Ruth He A, Qin S, Chen LT, Okusaka T, Vogel A, Kim JW, Suksombooncharoen T, Ah Lee M, Kitano M, Burris H, Bouattour M, Tanasanvimon S, McNamara MG, Zaucha R, Avallone A, Tan B, Cundom J, Lee CK, Takahashi H, Ikeda M, Chen JS, Wang J, Makowsky M, Rokutanda N, He P, Kurland JF, Cohen G, Valle JW. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022;1:EVIDoa2200015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 688] [Article Influence: 172.0] [Reference Citation Analysis (1)] |
| 45. | Michielin O, Lalani AK, Robert C, Sharma P, Peters S. Defining unique clinical hallmarks for immune checkpoint inhibitor-based therapies. J Immunother Cancer. 2022;10:e003024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 46. | Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, Yau T, Klümpen HJ, Chan SL, Ozaka M, Verslype C, Bouattour M, Park JO, Barajas O, Pelzer U, Valle JW, Yu L, Malhotra U, Siegel AB, Edeline J, Vogel A; KEYNOTE-966 Investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401:1853-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 660] [Article Influence: 220.0] [Reference Citation Analysis (0)] |
| 47. | Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, Falk S, Gillmore R, Wadsley J, Patel K, Anthoney A, Maraveyas A, Iveson T, Waters JS, Hobbs C, Barber S, Ryder WD, Ramage J, Davies LM, Bridgewater JA, Valle JW; Advanced Biliary Cancer Working Group. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22:690-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 544] [Article Influence: 108.8] [Reference Citation Analysis (0)] |
| 48. | Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, Hama N, Hosoda F, Urushidate T, Ohashi S, Hiraoka N, Ojima H, Shimada K, Okusaka T, Kosuge T, Miyagawa S, Shibata T. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 1002] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 49. | Zugman M, Botrus G, Pestana RC, Uson Junior PLS. Precision Medicine Targeting FGFR2 Genomic Alterations in Advanced Cholangiocarcinoma: Current State and Future Perspectives. Front Oncol. 2022;12:860453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Vogel A, Segatto O, Stenzinger A, Saborowski A. FGFR2 Inhibition in Cholangiocarcinoma. Annu Rev Med. 2023;74:293-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 51. | Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, Paulson AS, Borad MJ, Gallinson D, Murphy AG, Oh DY, Dotan E, Catenacci DV, Van Cutsem E, Ji T, Lihou CF, Zhen H, Féliz L, Vogel A. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 1178] [Article Influence: 196.3] [Reference Citation Analysis (0)] |
| 52. | Goyal L, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic TB, Abrams TA, Furuse J, Kelley RK, Cassier PA, Klümpen HJ, Chang HM, Chen LT, Tabernero J, Oh DY, Mahipal A, Moehler M, Mitchell EP, Komatsu Y, Masuda K, Ahn D, Epstein RS, Halim AB, Fu Y, Salimi T, Wacheck V, He Y, Liu M, Benhadji KA, Bridgewater JA; FOENIX-CCA2 Study Investigators. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N Engl J Med. 2023;388:228-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 367] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 53. | Zhou Y, Wu C, Lu G, Hu Z, Chen Q, Du X. FGF/FGFR signaling pathway involved resistance in various cancer types. J Cancer. 2020;11:2000-2007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 54. | Golub D, Iyengar N, Dogra S, Wong T, Bready D, Tang K, Modrek AS, Placantonakis DG. Mutant Isocitrate Dehydrogenase Inhibitors as Targeted Cancer Therapeutics. Front Oncol. 2019;9:417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 187] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 55. | Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DV, Borad MJ, Bridgewater J, Harris WP, Murphy AG, Oh DY, Whisenant J, Lowery MA, Goyal L, Shroff RT, El-Khoueiry AB, Fan B, Wu B, Chamberlain CX, Jiang L, Gliser C, Pandya SS, Valle JW, Zhu AX. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:796-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 796] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 56. | Goetze TO, Vogel A, Pratschke J, Behrend M, Reim D, Schnitzbauer AA, Bleckmann A, Becker S, Rahbari N, Brunner SM, Manekeller S, Luley KB, Lang SA, Gutsche K, Habibzada T, Klagges J, Schaaf M, Pauligk C, Bankstahl US, Al-batran S. Neoadjuvant chemotherapy with gemcitabine plus cisplatin followed by radical liver resection versus immediate radical liver resection alone followed adjuvant therapy in biliary tract cancer: Final results from the phase III AIO/CALGP/ACO-GAIN-Trial. J Clin Oncol. 2025;43:4008-4008. [DOI] [Full Text] |
| 57. | Nara S, Ioka T, Ogawa G, Kataoka T, Sano Y, Esaki M, Nagano H, Kudo M, Ikeda M, Kanai M, Yasuda I, Yamazaki K, Shirakawa H, Kobayashi S, Ozaka M, Gotohda N, Hatano E, Furuse J, Okusaka T, Ueno M. Randomized multicenter phase III trial of neoadjuvant gemcitabine + cisplatin + S-1 (GCS) versus surgery first for resectable biliary tract cancer (JCOG1920: NABICAT). J Clin Oncol. 2023;41:TPS621-TPS621. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 58. | Niger M, Nichetti F, Fornaro L, Pircher C, Morano F, Palermo F, Rimassa L, Pressiani T, Berardi R, Gardini AC, Sperti E, Salvatore L, Melisi D, Bergamo F, Siena S, Mosconi S, Longarini R, Arcangeli G, Corallo S, Delliponti L, Tamberi S, Fea E, Brandi G, Rapposelli IG, Salati M, Baili P, Miceli R, Ljevar S, Cavallo I, Sottotetti E, Martinetti A, Busset MDD, Sposito C, Di Bartolomeo M, Pietrantonio F, de Braud F, Mazzaferro V. Correction to: A phase II/III randomized clinical trial of CisPlatin plUs Gemcitabine and Nabpaclitaxel (GAP) as pReoperative chemotherapy versus immediate resection in patIents with resecTable BiliarY tract cancers (BTC) at high risk for recurrence: PURITY study. BMC Cancer. 2024;24:563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 59. | Murakami T, Matsuyama R, Yabushita Y, Homma Y, Sawada Y, Miyake K, Kumamoto T, Takeda K, Maeda S, Yamanaka S, Endo I. Efficacy of Conversion Surgery for Initially Unresectable Biliary Tract Cancer That Has Responded to Down-Staging Chemotherapy. Cancers (Basel). 2025;17:873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Kato A, Shimizu H, Ohtsuka M, Yoshidome H, Yoshitomi H, Furukawa K, Takeuchi D, Takayashiki T, Kimura F, Miyazaki M. Surgical resection after downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer: a retrospective single-center study. Ann Surg Oncol. 2013;20:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 61. | Wang S, Wang Y, Zhu C, Liu K, Chao J, Zhang N, Piao M, Yang X, Zhang L, Long J, Xun Z, Zhang T, Sang X, Yang X, Zhao H. Conversion surgery intervention versus continued systemic therapy in patients with a response after PD-1/PD-L1 inhibitor-based combination therapy for initially unresectable biliary tract cancer: a retrospective cohort study. Int J Surg. 2024;110:4608-4616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 62. | Zhou Y, Wang Q, Lin M, Wang S. Survival benefit of conversion surgery for initially unresectable biliary tract cancer: a systematic review and meta-analysis. Langenbecks Arch Surg. 2025;410:63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/