INTRODUCTION
Hepatocellular carcinoma (HCC) remains a major global health concern, particularly in the context of the increasing incidence of metabolic liver diseases and shifting immune landscapes[1-3]. Although traditional research has focused on viral etiologies and oncogenic mutations, recent insights have emphasized the pivotal role of metabolic enzymes in orchestrating tumor development and immune responses[1,4]. One such enzyme, uridine diphosphate (UDP)-glucose 6-dehydrogenase (UGDH), catalyzes the conversion of UDP-glucose to UDP-glucuronic acid, a key precursor in the glucuronidation pathway, which plays essential roles in the detoxification of bile acids, xenobiotics, and microbial metabolites[5].
In a recent study, published in the World Journal of Gastrointestinal Oncology, Cao et al[6] identified UGDH as a prognostically unfavorable factor in HCC. Using an integrative approach that combined pan-cancer bioinformatics, immunological analyses, and experimental validation, these authors demonstrated that the overexpression of UGDH is linked to increases in tumor proliferation and immunosuppressive signatures. These findings accordingly highlight the need to broaden our understanding of UGDH, recognizing it not only as a key regulator of hepatic metabolism but also as an important player in gut–liver immune communication.
UGDH IN CANCER AND LIVER IMMUNOMETABOLISM: BEYOND DETOXIFICATION
Traditionally, UGDH has been recognized for its role in hepatic glucuronidation, a phase II metabolic process that is essential for the conversion of lipophilic compounds to excretable forms[7]. Cao et al[6] revealed that UGDH is upregulated in multiple gastrointestinal malignancies, particularly in HCC, in which its elevated expression was found to be correlated with poor overall survival and an immune microenvironment enriched in Th2 cells and depleted in cluster of differentiation 8 + cytotoxic T lymphocytes.
Functional experiments confirmed that silencing UGDH in HCC cells (e.g., Huh7) suppresses tumor proliferation and attenuates xenograft tumor growth[8], thereby providing evidence to indicate that UGDH contributes to both tumor metabolic flexibility and immune evasion, which is consistent with a broader model in which metabolic enzymes orchestrate multiple hallmarks of cancer progression. Similar conclusions have been reported in other studies investigating UGDH in the context of cancer cell survival and immune modulation[7,8].
The upregulation of UGDH in HCC tissues and hepatoma cells, as reported by Cao et al[6], may reflect a compensatory metabolic adaptation to heightened demand for glucuronidation under conditions of bile acid accumulation and microbial stress, and in turn, this metabolic reprogramming may exacerbate immune tolerance by facilitating the clearance of immunogenic ligands, thereby highlighting the role of UGDH as a functional mediator of gut–liver immune crosstalk.
THE GUT-LIVER AXIS AND GLUCURONIDATION: A TWO-WAY STREET
The liver and gut maintain a tightly regulated bidirectional relationship mediated via portal circulation[9,10]. The liver receives a constant input of gut-derived microbial metabolites, including short-chain fatty acids, secondary bile acids, and pathogen-associated molecular patterns, such as lipopolysaccharides (LPS), which necessitate effective detoxification to maintain immune tolerance and physiological homeostasis[11-13].
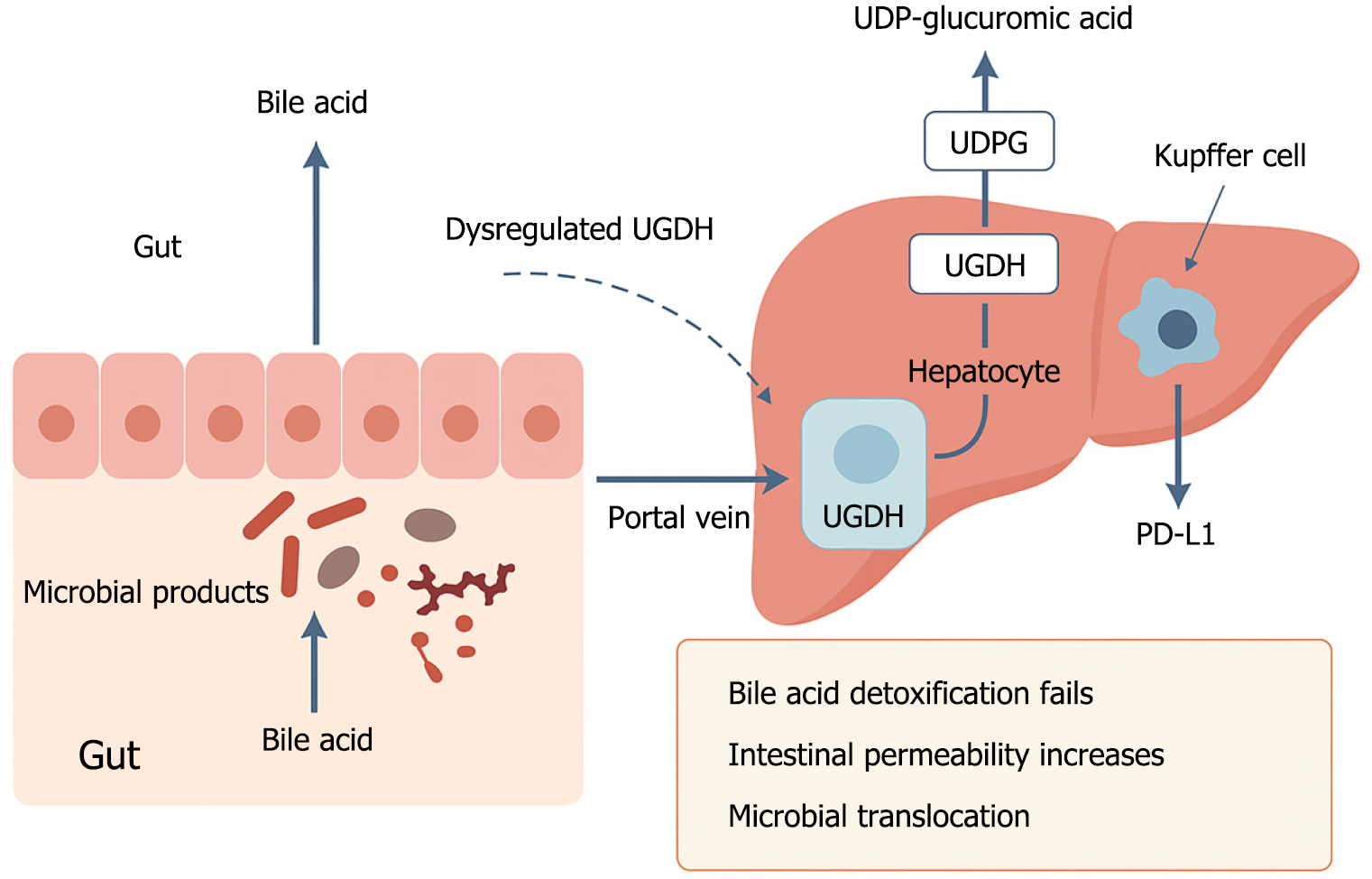
UGDH produces UDP-glucuronic acid, an essential substrate for conjugating bile acids and microbial products, thereby facilitating their excretion via bile[14]. Under conditions in which UGDH activity is impaired or insufficient, glucuronidation is disrupted. The ensuing accumulation of unconjugated bile acids leads to epithelial injury and an increase in intestinal permeability (Figure 1), thereby enabling microbial products, such as LPS, to undergo translocation to the liver, wherein they stimulate innate immune cells and can thus induce chronic inflammation[15-18].
Figure 1 Schematic illustration of how dysregulated uridine diphosphate-glucose 6-dehydrogenase disrupts gut-liver immune homeostasis.
Under normal conditions, hepatic uridine diphosphate (UDP)-glucose 6-dehydrogenase (UGDH) converts UDP-glucose to UDP-glucuronic acid, which supports the glucuronidation and detoxification of bile acids and microbial metabolites. This process protects the intestinal barrier and prevents harmful microbial products from reaching the liver. However, when UGDH is downregulated or impaired, detoxification fails. Bile acids accumulate, intestinal permeability increases, and microbial products such as lipopolysaccharide translocate to the liver via the portal vein. These signals activate Kupffer cells, leading to programmed cell death ligand 1 upregulation and immune suppression, thereby promoting hepatocarcinogenesis. UDP: Uridine diphosphate; UGDH: Uridine diphosphate-glucose 6-dehydrogenase; PD-L1: Programmed cell death ligand 1.
Upon sensing elevated levels of LPS, Kupffer cells initiate an upregulated production of immunosuppressive cytokines, such as interleukin-10, and co-inhibitory ligands, such as programmed cell death ligand 1 (PD-L1), thus generating an immune-tolerant microenvironment that is conducive to tumor growth[18,19]. UGDH thus functions as a pivotal regulatory node linking metabolic detoxification with immune surveillance at the gut-liver interface.
MICROBIAL REGULATION OF HEPATIC UGDH EXPRESSION: A FEEDBACK LOOP
Emerging data is providing evidence to indicate that the hepatic expression of UGDH is responsive to gut microbial composition and metabolic activity. For example, short-chain fatty acids such as butyrate can influence histone acetylation and modulate hepatic gene transcription[20]. Similarly, secondary bile acids transmit signals via nuclear receptors (e.g., farnesoid X receptor and Takeda G-protein-coupled receptor 5) to regulate liver metabolism[21].
Alterations in the composition of the gut microbiota, driven by dietary changes, liver disease, or antibiotic exposure, can promote a corresponding shift in the composition of microbial metabolites reaching the liver[22], which in turn can lead to a downregulation of UGDH expression or enzymatic activity, thereby compromising the capacity of the liver to glucuronidate and excrete toxic compounds. The resulting accumulation of bile acids and pathogen-associated molecular patterns contributes to exacerbating epithelial injury and inflammation, and thus perpetuates a state of dysbiosis[23].
In response to these conditions, a reciprocal feedback loop is established in which the microbiota modulate UGDH, whereas UGDH activity shapes microbial selection via the detoxification of bile acids. A disruption of this balance may be one of the key factors contributing to the development of metabolic dysfunction-associated steatotic liver disease (MASLD)-associated HCC and other gut-liver axis-related cancers.
METABOLIC IMMUNE CHECKPOINTS: UGDH AS A NOVEL REGULATOR
By targeting immune checkpoint molecules, such as programmed cell death 1 and cytotoxic T-lymphocyte-associated protein 4, immunotherapy has revolutionized cancer care[24-26], and, recently, metabolic checkpoints, defined as those enzymes that influence antigen presentation, cytokine secretion, and immune cell function, have garnered growing attention. It is conceivable that UGDH may represent one such checkpoint.
UGDH not only contributes to regulating the glucuronidation of bile acids and microbial ligands[6] but can also intervene in the immune microenvironment and activate immune effector cells[27,28], plausibly indirectly influencing the immune activation threshold of the liver. Impaired detoxification enhances the immunosuppressive properties of these molecules, reduces antigen presentation, and promotes regulatory T cell phenotypes[29], thereby tending to mirror the mechanisms of MASLD, in which mitochondrial DNA leakage activates the cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon genes (cGAS-STING) pathway and enhances the expression of PD-L1[30].
We hypothesize that a reduction in UGDH activity would exacerbate innate immune signaling pathways, such as cGAS-STING, and act in synergy with PD-L1 induction to enhance immune tolerance and accelerate tumor progression, thereby highlighting the role of UGDH as a key regulator of immune escape mechanisms, which play pivotal roles in metabolically driven liver cancers. Although to date there has been no evidence to indicate a direct mechanistic link between UGDH and the cGAS-STING pathway, we envisage a potential convergence in their roles based on common features in immune suppression. It is thus plausible that a suppression of UGDH-mediated detoxification may promote mitochondrial stress and microbial translocation, which are established activators of cGAS-STING signaling. Consequently, although UGDH may not directly regulate this pathway, its metabolic consequences could function synergistically in conjunction with innate immune signaling cascades that converge on PD-L1 expression and immune tolerance.
THERAPEUTIC AND DIAGNOSTIC IMPLICATIONS
The findings reported by Cao et al[6] have laid a strong foundation for further characterization of UGDH as a potential therapeutic target, and have several important clinical implications, including the following: (1) Direct pharmacological inhibition of UGDH may restrict tumor growth and potentially restore immune activation; (2) Microbiota modulation strategies (e.g., probiotics, prebiotics, and dietary interventions) could indirectly enhance hepatic UGDH activity and the efficiency of glucuronidation; (3) Therapies combining the inhibition of UGDH with immune checkpoint blockade may contribute to improving the outcomes of patients with non-viral HCC or MASLD-related tumors; and (4) Diagnostic assays quantifying UGDH activity or downstream metabolites may serve as biomarkers for tumor immunogenicity and therapeutic responses.
These potential strategies are consistent with precision medicine approaches and reinforce the importance of integrating metabolic and immune paradigms in gastrointestinal oncology.
CONCLUSION
Cao et al[6] not only identified UGDH as a driver of HCC but also opened up new avenues for our understanding of its role in immune modulation. By bridging hepatic metabolism and intestinal homeostasis, UGDH has emerged as a metabolic immune checkpoint that plays an essential role in regulating the gut-liver axis. We propose that dysregulated UGDH activity contributes to bile acid accumulation, intestinal barrier dysfunction, microbial translocation, and hepatic immunosuppression. This multifaceted role places UGDH at the crossroads between metabolism and immunity, thereby offering opportunities for novel therapeutic interventions. Further research is warranted to validate these mechanisms and assess the translational feasibility of targeting UGDH in gastrointestinal cancers. Nevertheless, although the inhibition of UGDH may offer therapeutic benefits in the context of cancer, its physiological role in maintaining hepatic detoxification must be carefully considered. Accordingly, future strategies involving any modification of UGDH activity may require selective or tumor-specific targeting to avoid adverse metabolic consequences.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade A, Grade B, Grade B, Grade C
Novelty: Grade A, Grade A, Grade B, Grade C
Creativity or Innovation: Grade A, Grade B, Grade B, Grade C
Scientific Significance: Grade A, Grade B, Grade B, Grade C
P-Reviewer: Jain BP, PhD, Assistant Professor, India; Xie YF, PhD, Professor, China; Zao XB, MD, Professor, China S-Editor: Fan M L-Editor: A P-Editor: Zhao S