Published online Oct 15, 2025. doi: 10.4251/wjgo.v17.i10.110203
Revised: July 17, 2025
Accepted: September 8, 2025
Published online: October 15, 2025
Processing time: 136 Days and 6.2 Hours
Well-differentiated rectal neuroendocrine tumors (rNETs) represent approximately 28% of gastrointestinal neuroendocrine tumors, with a rising incidence over recent decades. However, data from Perú remains limited.
To assess overall survival (OS) in patients with rNETs and describe the clinical and pathological characteristics of the study population.
This retrospective study included patients diagnosed with rNETs at the Instituto Nacional de Enfermedades Neoplásicas between 2009 and 2024. Qualitative variables were evaluated using the χ2 test through contingency tables. OS was estimated using the Kaplan-Meier method, and differences between groups were assessed with the log-rank test. Cox proportional hazards models were used to evaluate variables associated with OS. All statistical analyses were conducted using R software.
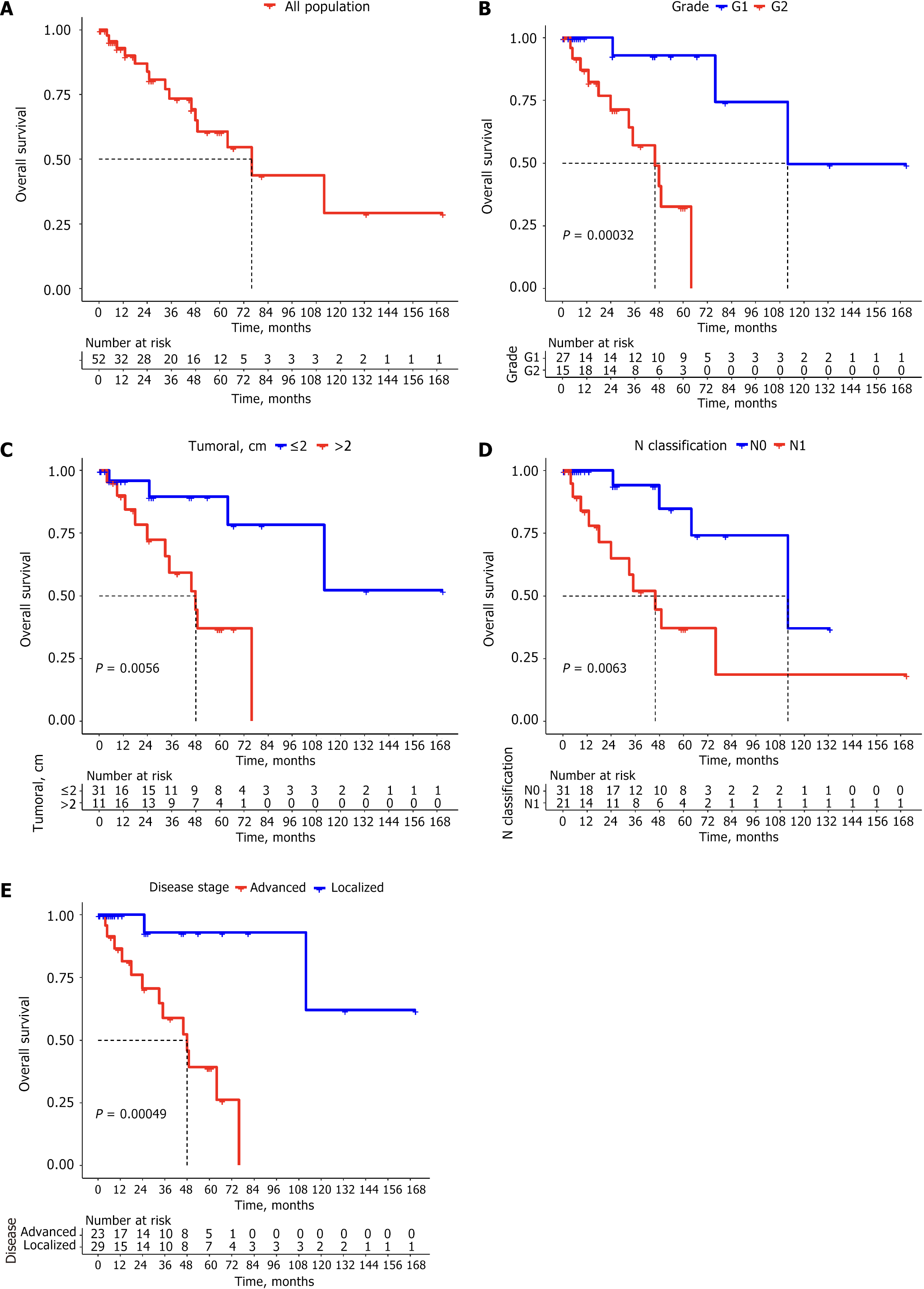
A total of 52 patients were included, with a mean age of 51.9 years (range: 27-74 years) and composed of 65.4% females. The most common stage at diagnosis was stage I (48.1%), followed by stage IV (36.5%). The median OS within the study population was 76 months. The 5-year OS for grade 1 tumors was 92.9% compared to 32.6% for grade 2 tumors (P = 0.00032). The median OS was 48 months for tumors exceeding 20 mm in size, whereas it was not reached for tumors measuring 20 mm or less (P = 0.0056). Similarly, the median OS for patients classified as lymph node involvement 1 was 46 months, while it was estimated at 112 months for those classified as lymph node involvement 0 (P = 0.0063).
rNETs exceeding 2 cm in size, classified as grade 2, or presenting with lymph node involvement 1 status were correlated with advanced disease stages and diminished survival outcomes.
Core Tip: This retrospective study encompassed the largest known Peruvian patients with well-differentiated rectal neuroendocrine tumors. With a median overall survival of 76 months and a 5-year survival rate of 60.6%, outcomes were significantly affected by tumor size, grade, and nodal status. Grade 2 tumors, lesions > 20 mm, and nodal involvement were linked to poorer survival. These findings provide important regional data and support the integration of these factors into risk-stratification strategies for rectal neuroendocrine tumors in Perú.
- Citation: Cruz-Diaz WE, Leonardo A, Saavedra A, Paitan V, Haro-Varas J, Mantilla R, Macetas J, Veramendi E, Pacheco C, Calderón M, Vidaurre T, Castro-Oliden V. Rectal neuroendocrine tumors in a Latin American population: Insights from a Peruvian national cancer institute. World J Gastrointest Oncol 2025; 17(10): 110203
- URL: https://www.wjgnet.com/1948-5204/full/v17/i10/110203.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i10.110203
Well-differentiated rectal neuroendocrine tumors (rNETs) are neoplasms arising from the diffuse neuroendocrine system of the gastrointestinal tract, comprising approximately 28% of all gastroenteropancreatic (GEP) neuroendocrine tumors (NETs). According to Surveillance, Epidemiology, and End Results program data, the incidence of rNETs was approximately 2.65 per 100000 individuals in 2015, with a significant upward trend over recent decades, reflected by an annual percentage change of 6.43 (P < 0.001) between 1975 and 2015[1]. This rise is largely attributed to the expanded use of endoscopic procedures, colorectal cancer screening campaigns, routine histological assessments using neuroendocrine markers, and improved hospital registry data collection[2]. The prevalence of rNETs detected through England’s bowel cancer screening program was 29 per 100000 colonoscopies[3], while a Polish bowel cancer screening cohort of 50148 participants reported a prevalence of 50-70 per 100000 colonoscopies[4].
Data on rNETs in Latin America remain limited. A Chilean NET registry reported that 115 out of 166 cases (64%) were GEP-NETs, though the specific proportion of rNETs was not provided[5]. An observational study from Argentina documented 532 NET cases, including 461 GEP-NETs and 71 bronchial NETs; of the GEP-NETs, 12.4% were located in the colon, rectum, or anus, but rectal cases were not separately reported[6]. A hospital-based study in Ecuador found an age-adjusted annual incidence of rNETs in 2020 of 0.49 per 100000 individuals, with rNETs comprising 6.4% of all NETs. Notably, patients with rNET had the most favorable prognosis, with a median overall survival (OS) of 43.2 months[7]. In Panama, a study reported two NET cohorts, with colorectal tumors as the most common primary site, accounting for 27 out of 157 cases (17.2%)[8]. At the Instituto Nacional de Enfermedades Neoplásicas (INEN) in Perú, 650 neuroendocrine neoplasms (NENs) were recorded between 2009 and 2018, with the rectum identified as the most frequent primary site, accounting for 15% of all cases[9].
Accurate diagnosis of rNETs is critical for guiding treatment and assessing prognosis. The 2022 World Health Organization classification of NENs bases tumor grading on proliferative activity, measured by mitotic count and Ki-67 index. This system categorizes NETs into grade 1, grade 2, and grade 3, corresponding to low-, intermediate-, and high-grade tumor, respectively. Grade 1 tumors, with a Ki-67 index of < 3% and/or < 2 mitoses per 2 mm², are typically indolent and associated with favorable outcomes. Grade 2 tumors, defined by a Ki-67 index of 3%-20% and/or 2-20 mitoses per 2 mm², show more variable clinical behavior. Grade 3 tumors are aggressive, with a Ki-67 index > 20% and/or > 20 mitoses per 2 mm²[10]. This updated classification enhances risk stratification and supports personalized treatment strategies for patients with rNET.
The therapeutic approach to rNETs depends on disease stage: Localized vs advanced. Tumors smaller than 10 mm are typically managed with endoscopic resection, given their low risk of local invasion, nodal involvement, and distant metastasis[11]. For tumors larger than 20 mm or those with lymph node involvement 1 (N1), and in the absence of distant metastases, radical surgical resection is recommended. A multidisciplinary team (MDT) discussion is advised for intermediate-sized tumors measuring 10-20 mm[12]. Advanced-stage rNETs are usually treated with systemic therapies, including somatostatin analogs (SSAs), targeted agents, peptide receptor radionuclide therapy (PRRT), and chemotherapy. Treatment decisions are guided by somatostatin receptor expression status, tumor burden, tumor grade, and proliferation index. However, in many Latin American countries, access to these therapies is limited due to high costs, restricting availability to a small subset of patients. This study aims to evaluate OS in a Peruvian rNET cohort, identify factors associated with prognosis, and characterize the clinical and pathological features of this increasingly recognized disease.
A retrospective study was conducted to identify patients diagnosed with rNETs at the INEN between 2009 and 2024. Registry data were obtained from the Department of Epidemiology and Statistics. A total of 59 patients were identified, of whom 7 were excluded due to incomplete pathological assessment, as their medical records did not include a Ki-67 index. Ultimately, the final analysis included 52 patients.
Inclusion criteria: Patients diagnosed with well-differentiated rNETs of grade 1 or grade 2, with a Ki-67 index ≤ 20%, at any clinical stage, and with complete clinical records and pathological assessments reviewed at INEN.
Exclusion criteria: Patients were excluded if they had incomplete clinical data, no pathological assessment reviewed at INEN, grade 3 or poorly differentiated tumors, or a Ki-67 index > 20%.
The primary objective was to assess OS in the entire study. The secondary objectives included assessing OS across subgroups defined by key clinical and pathological variables, describing the demographic, clinical, and pathological characteristics of the study population, and detailing the treatment modalities employed.
Primary tumor resection was performed via endoscopic or surgical approaches, depending on tumor characteristics. Patients with advanced disease were treated with systemic therapy, primarily chemotherapy. Treatment response was evaluated using computed tomography imaging according to the Response Evaluation Criteria in Solid Tumours 1.1 criteria.
Descriptive statistics were used to summarize qualitative variables as frequencies and percentages, and quantitative variables using means with ranges or medians with interquartile ranges, depending on data distribution assessed by normality tests. Associations between categorical variables were evaluated with χ2 tests; when more than 20% of expected cell counts were < 5, categories were combined. For 2 × 2 contingency tables, Yates’ correction was applied. OS was calculated from diagnosis to death or last follow-up, with censoring for patients without the event. Survival curves were estimated using the Kaplan-Meier method, and differences between groups were assessed by the log-rank test. Variables significantly associated with OS in univariate analysis were included in multivariate Cox proportional hazards models, with the proportional hazards assumption tested. Statistical significance was set at P < 0.05. Analyses were performed using R software (R Core Team, 2023).
Among the 52 patients included, the mean age was 51.9 years (range: 27-74 years) and 65.4% were female. The most common birthplaces were Lima (26.9%), Ancash (11.5%) and Junin (9.6%). The majority of patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 (48.1%), followed by ECOG 1 (44.2%). The most frequent presenting symptom was rectal bleeding (36.5%), followed by abdominal pain (25.0%) and anorectal pain (19.2%; Table 1). Regarding clinical staging at diagnosis according to the tumor-nodes-metastasis classification, the most frequent tumor stage (T stage) was T1 (55.7%), followed by T3 (21.2%). In the regional lymph node staging (N stage) and distant metastasis staging (M stage), most patients had N0 (59.6%) and M0 (63.46%). Stage I was the most common overall (48.1%), followed by stage IV (36.5%). At the time of analysis, 29 patients (55.8%) had localized disease, while 23 (44.2%) had advanced disease. Among those with advanced disease (n = 23), 19 (82.6%) had metastatic disease, 3 (13.0%) had recurrent disease, and 1 (4.3%) had unresectable disease (Table 2).
| Variables | n = 52 |
| Age in years | |
| Mean (minimum-maximum) | 51.9 (27-74) |
| Sex | |
| Female | 34 (65.4) |
| Male | 18 (34.6) |
| Region of birth | |
| Lima | 14 (26.9) |
| Ancash | 6 (11.5) |
| Junín | 5 (9.6) |
| Cajamarca | 4 (7.7) |
| Piura | 4 (7.7) |
| La Libertad | 3 (5.8) |
| Arequipa | 2 (3.8) |
| Cusco | 2 (3.8) |
| Huánuco | 2 (3.8) |
| Loreto | 2 (3.8) |
| San Martin | 2 (3.8) |
| Apurímac | 1 (1.9) |
| Ayacucho | 1 (1.9) |
| Ica | 1 (1.9) |
| Lambayeque | 1 (1.9) |
| Pasco | 1 (1.9) |
| Puno | 1 (1.9) |
| ECOG scale | |
| 0 | 25 (48.1) |
| 1 | 23 (44.2) |
| 2 | 3 (5.8) |
| 3 | 1 (1.9) |
| Symptoms | |
| Rectal bleeding | 19 (36.5) |
| Abdominal pain | 13 (25.0) |
| Anorectal pain | 10 (19.2) |
| Constipation | 10 (19.2) |
| Weight loss | 8 (15.4) |
| Diarrhea | 3 (5.8) |
| Anorectal tumor | 1 (1.9) |
| Variables | n = 52 |
| Classification T | |
| T1 | 29 (55.7) |
| T2 | 8 (15.4) |
| T3 | 11 (21.2) |
| T4 | 4 (7.7) |
| Classification N | |
| N0 | 31 (59.6) |
| N1 | 21 (40.4) |
| Classification M | |
| M0 | 33 (63.5) |
| M1 | 19 (36.5) |
| Stage | |
| I | 25 (48.1) |
| II | 3 (5.8) |
| III | 5 (9.6) |
| IV | 19 (36.5) |
| Disease stage | |
| Localized | 29 (55.8) |
| Advanced | 23 (44.2) |
| Status of advanced disease, n = 23 | |
| Metastatic | 19 (82.6) |
| Recurrent | 3 (13.0) |
| Unresectable | 1 (4.3) |
A significant association was observed between tumor grade and disease stage, tumor size, and nodal status. A higher proportion of patients with advanced disease was observed in the grade 2 group compared to the grade 1 group (84.0% vs 7.4%, respectively). Similarly, patients with grade 2 tumors were more likely to have tumor sizes > 20 mm than those with grade 1 tumors (76% vs 7.4%, respectively). Additionally, the proportion of patients with N1 was markedly higher in the grade 2 group compared to the grade 1 group (72.0% vs 11.1%, respectively; Table 3). Regarding tumor size, 24 patients (46.2%) had tumors measuring ≤ 1 cm, 21 patients (40.4%) had tumors > 2 cm, and 7 patients (13.5%) had tumors between > 1 cm and ≤ 2 cm. The median tumor size was 1.3 cm [interquartile range (IQR): 0.75-3.5 cm]. Among metastatic sites, the liver was most common (42.3%), followed by bone (17.3%), and non-regional lymph nodes (7.7%). Of the 22 patients with liver metastases, the vast majority (95.5%) had multiple lesions, and 59.1% presented with bulky tumors, defined as tumor volume involvement > 25% (Table 4).
| Variables | Grade 1, 27 (51.9) | Grade 2, 25 (48.1) | P value |
| Disease stage | |||
| Localized | 25 (92.6) | 4 (16.0) | |
| Advanced | 2 (7.4) | 21 (84.0) | < 0.001 |
| Tumor size in cm | |||
| ≤ 2 | 25 (92.6) | 6 (24.0) | |
| > 2 | 2 (7.4) | 19 (76.0) | < 0.001 |
| Classification N | |||
| N1 | 3 (11.1) | 18 (72.0) | |
| N0 | 24 (88.9) | 7 (28.0) | < 0.001 |
| Variables | n = 52 |
| Organ | |
| Liver | 22 (42.3) |
| Bone | 9 (17.3) |
| Non-regional lymph node | 4 (7.7) |
| Pancreas | 3 (5.8) |
| Lung | 3 (5.8) |
| Prostate | 2 (3.8) |
| Soft tissue | 1 (1.9) |
| Ovary | 1 (1.9) |
| Cervix | 1 (1.9) |
| Bladder | 1 (1.9) |
| Vagina | 1 (1.9) |
| Adrenal | 1 (1.9) |
| Number of metastatic organs | |
| 0 | 29 (55.8) |
| 1 | 10 (19.2) |
| 2 | 6 (11.5) |
| 3 | 3 (5.8) |
| 4 | 2 (3.8) |
| 5 | 2 (3.8) |
| Liver lesions, n = 22 | |
| Single | 1 (4.5) |
| Multiples | 21 (95.5) |
| Bulky liver tumor, as volume involvement > 25%, n = 22 | |
| Yes | 13 (59.1) |
| No | 9 (40.9) |
A total of 35 patients (67.3%) underwent primary tumor resection. Of these, 25 patients (48.1%) underwent endoscopic resection, while 10 patients (19.2%) underwent surgical resection. Among the surgical cases (n = 10), the most common procedure was low anterior resection (LAR) of the rectum, representing 50% of surgeries. Additionally, three procedures (5.8%) were performed in an advanced disease setting.
A total of 20 patients with advanced rNETs (38.5%) received first-line chemotherapy. The most commonly used regimen was capecitabine plus temozolomide (TEMCAP), administered in 80.0% of cases. The median number of treatment cycles was 9.5 (IQR: 5.5-20), with a median treatment duration of 11 months (IQR: 6-18). Treatment responses included 1 patient (5%) achieving complete response, 6 patients (30%) with stable disease, and 10 patients (50%) experiencing progressive disease. Of those receiving first-line chemotherapy, 9 patients (45.0%) proceeded to second-line treatment. The most frequently used second-line regimens (n = 9) were capecitabine plus oxaliplatin, dacarbazine, and TEMCAP, each administered in 22.2% of cases. Response rates in the second-line setting included 1 patient (11.1%) with complete response, 1 (11.1%) with partial response, 1 (11.1%) with stable disease, 4 (44.4%) with progressive disease, and 2 (22.2%) who were not evaluable. Details of systemic treatment are summarized in Table 5.
| Variables | n = 52 |
| First-line chemotherapy | |
| Yes | 20 (38.5) |
| No | 32 (61.5) |
| First chemotherapy scheme, n = 20 | |
| TEMCAP | 16 (80.0) |
| Cisplatin/etoposide | 3 (15.0) |
| Octreotide | 1 (5.0) |
| Cycles of the 1st chemotherapy, n = 20 | |
| Median (IQR) | 9.5 (5.5-20) |
| Duration of the 1st chemotherapy, n = 20 | |
| Median (IQR) | 11 (6-18) |
| Unregistered | 1 |
| Response to the 1st chemotherapy, n = 20 | |
| CR | 1 (5.0) |
| SD | 6 (30.0) |
| PD | 10 (50.0) |
| Not evaluated | 3 (15.0) |
| Second-line chemotherapy, n = 20 | |
| Yes | 9 (45.0) |
| No | 11 (55.0) |
| 2nd Chemotherapy scheme, n = 9 | |
| CAPOX | 2 (22.2) |
| Dacarbazine | 2 (22.2) |
| TEMCAP | 2 (22.2) |
| Carboplatin/etoposide | 1 (11.1) |
| FOLFOX | 1 (11.1) |
| Interferon alpha | 1 (11.1) |
| 2nd chemotherapy cycles, n = 9 | |
| Median (IQR) | 3 (2-5) |
| Duration of the 2nd chemotherapy, n = 9 | |
| Median (IQR) | 1 (0-5) |
| Response to 2nd chemotherapy, n = 9 | |
| CR | 1 (11.1) |
| PR | 1 (11.1) |
| SD | 1 (11.1) |
| PD | 4 (44.4) |
| Not evaluated | 2 (22.2) |
At the time of analysis, 15 patients (28.8%) had died. The median follow-up for the 52 patients was 25 months (range: 1-171 months). Median OS was estimated at 76 months (Figure 1A), with estimated OS rates of 92.9%, 73.4%, and 60.6% at 12 months, 36 months, and 60 months, respectively. Survival differed significantly by tumor grade. Although median OS was not formally reached in either subgroup, it was estimated at 112 months for grade 1 tumors and 46 months for grade 2 tumors (Figure 1B). The 5-year OS rates were 92.9% for grade 1 and 32.6% for grade 2 tumors (P = 0.00032). For tumors > 20 mm, the estimated median OS was 48 months, whereas median OS could not be calculated for tumors ≤ 20 mm due to an insufficient number of events (P = 0.0056) (Figure 1C). Patients with N1 had a median OS of 46 months, compared to an estimated 112 months for N0 patients, where median OS was not formally reached (P = 0.0063) (Figure 1D). Among patients with advanced disease, the estimated median OS was 48 months, while median OS was not reached in localized disease due to the limited number of deaths at the time of analysis (P = 0.00049; Figure 1E; Table 6).
| Variables | n (events) | 1-year | 3-year | 5-year | P value |
| All patients | 52 (15) | 92.9% | 73.4% | 60.6% | N/A |
| Age in years | 0.86 | ||||
| < 65 | 44 (13) | 93.9% | 71.1% | 61.6% | |
| ≥ 65 | 8 (2) | 87.5% | 87.5% | 43.8% | |
| Sex | 0.34 | ||||
| Female | 34 (9) | 100% | 71.2% | 64.7% | |
| Male | 18 (6) | 78.0% | 78.0% | 52.0% | |
| Grade | 0.00032 | ||||
| G1 | 27 (3) | 100% | 92.9% | 92.9% | |
| G2 | 25 (12) | 87.1% | 57.0% | 32.6% | |
| Ki-67 (%) | 0.00032 | ||||
| < 3 | 27 (3) | 100% | 92.9% | 92.9% | |
| 3-20 | 25 (12) | 87.1% | 57.0% | 32.6% | |
| Tumor size (cm) | 0.048 | ||||
| ≤ 1 | 24 (3) | 100% | 91.7% | 91.7% | |
| > 1 | 28 (12) | 87.6% | 62.8% | 43.9% | |
| Tumor size (cm) | 0.0056 | ||||
| ≤ 2 | 31 (4) | 95.8% | 89.4% | 89.4% | |
| > 2 | 21 (11) | 89.9% | 59.1% | 37.0% | |
| Classification N | 0.0063 | ||||
| N0 | 31 (4) | 100% | 94.1% | 84.7% | |
| N1 | 21 (11) | 83.9% | 51.9% | 37.1% | |
| Disease stage | 0.00049 | ||||
| Advanced | 23 (13) | 86.5% | 58.8% | 39.2% | |
| Localized | 29 (2) | 100% | 92.9% | 92.9% | |
| First-line chemotherapy | 0.086 | ||||
| No | 32 (5) | 92.0% | 76.7% | 76.7% | |
| Yes | 20 (10) | 94.4% | 69.6% | 46.4% |
The Cox proportional hazards model included tumor grade, tumor size (≤ 2 cm vs > 2 cm), type of primary tumor resection, N classification, and M classification as covariates. The analysis revealed that grade 2 was significantly associated with an increased risk of death. Patients with grade 2 tumors had an approximately 22-fold higher risk of mortality compared to those with grade 1 tumors [hazard ratio (HR) = 22.0; 95% confidence interval (CI): 1.01-472.41; P = 0.049]. Although statistically significant, the wide CI indicates substantial uncertainty, and this result should be interpreted with caution (Table 7).
| Variables | Univariate | Multivariate | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Grade | ||||||
| G1 | 1.01 | 1.01 | ||||
| G2 | 19.42 | 2.34-161.2 | 0.006 | 21.89 | 1.01-472.41 | 0.049 |
| Ki-67 as% | ||||||
| < 3 | 1.01 | |||||
| 3-20 | 19.42 | 2.34-161.2 | 0.006 | |||
| Tumor size in cm | ||||||
| ≤ 1 | 1.01 | |||||
| > 1 | 3.39 | 0.94-12.16 | 0.062 | |||
| Tumor size in cm | ||||||
| ≤ 2 | 1.01 | 1.01 | ||||
| > 2 | 5.34 | 1.44-19.73 | 0.012 | 0.53 | 0.05-5.51 | 0.598 |
| Classification N | ||||||
| N0 | 1.01 | 1.01 | ||||
| N1 | 4.39 | 1.38-13.95 | 0.012 | 1.34 | 0.18-9.92 | 0.774 |
| Classification M | ||||||
| M0 | 1.01 | 1.01 | ||||
| M1 | 5.08 | 1.59-16.23 | 0.006 | 0.65 | 0.09-4.65 | 0.67 |
| Disease stage | ||||||
| Localized | 1.01 | |||||
| Advanced | 16.22 | 2.07-127 | 0.008 | |||
| Number of metastatic sites | ||||||
| None | 1.01 | |||||
| ≥ 1 | 16.22 | 2.07-127 | 0.008 | |||
The most common treatment-related toxicity was constipation, reported by 4 patients (20%), with 3 having experienced grade 1 and 1 having experienced grade 2 toxicity. The most severe adverse events included grade 3 thrombocytopenia in 2 patients and grade 3 neutropenia in another 2 patients. Additionally, 1 patient developed grade 4 anemia, and another experienced grade 4 thrombocytopenia.
The median OS in our study was 76 months, with a 5-year OS rate of 60.6%, aligning with results reported in other Latin American cohorts[5,6]. By comparison, a study from Ecuador reported a shorter median OS of 43.2 months for rNET patients[7]. In the United States, data on localized rNETs indicate that median OS was not reached, with a 5-year OS rate of 99.3%, while metastatic disease was associated with a median OS of 11 months and a 5-year OS rate of 17.2%[8]. These stage-specific survival differences are consistent with our findings, where the 5-year OS was 92.9% for localized disease vs 39.2% for advanced stages (P = 0.00049). Additionally, Asian registries have reported 5-year OS rates of 69.8% and 86% in China and Taiwan, respectively[13,14].
Twenty-five patients (48%) with localized disease in our study underwent endoscopic resection. Among these, 21 patients (84%) had tumors measuring ≤ 10 mm, with no evidence of distant metastasis or recurrence during follow-up. Only 1 patient (4%) in this group had N1. This aligns with standard treatment guidelines recommending endoscopic resection for lesions ≤ 10 mm due to their low recurrence risk[12]. Reported rates of N1 in rNETs ≤ 10 mm vary from 0% for tumors ≤ 6 mm to 10.3% for tumors measuring 7-10 mm[15]. Similarly, in lesions with submucosal invasion, metastasis rates have been reported as 9.7% for tumors 6-10 mm, and only 1.3% for tumors < 5 mm[16]. Twenty-one patients (40.4%) had tumors measuring > 20 mm. Of these, 4 patients (19%) were diagnosed at a localized stage, while 17 patients (81%) presented with advanced disease. Lymph N1 was observed in 17 patients (81%) within this subgroup. These findings align with the multivariate analysis by Concors et al[17], which identified tumor size as a significant predictor of lymph node metastasis, with odds ratio of 5.1 for tumors measuring 10-19.9 mm (P < 0.005) and 4.9 for tumors ≥ 20 mm (P < 0.005). Tumor size was also strongly associated with distant metastasis, with odds ratios of 16.6 for tumors 11-19.9 mm and 77.7 for tumors > 20 mm (P < 0.005). Among the patients with tumors > 20 mm, 4 (19%) underwent surgery, including 2 with localized and 2 with advanced disease. Current clinical guidelines recommend LAR or abdominoperineal resection, typically with total mesorectal excision, for rNETs > 20 mm or those with nodal involvement[12]. Seven patients (13.5%) had tumors measuring > 10 mm and ≤ 20 mm. Among these, 3 patients (42.9%) underwent endoscopic resection, and 3 (42.9%) underwent surgical resection. Only 1 patient was diagnosed with M1 disease; the remainder were classified as having localized disease at diagnosis. At the time of analysis, none of these patients had experienced disease recurrence during follow-up. For rNETs in this size range, cross-sectional imaging should inform the MDT discussions regarding endoscopic vs surgical management[12]. In our study, grade 2 tumors were significantly associated with poorer survival and a higher frequency of N1 and advanced-stage disease compared to grade 1 tumors. These findings are consistent with previous studies. For example, Li et al[18] compared 515 grade 1 and 86 grade 2 rNETs, reporting substantially higher rates of lymph node and distant metastases in grade 2 tumors (44.2% and 31.4%, respectively) vs grade 1 tumors (5.2% and 2.1%).
In advanced disease, systemic therapy selection is individualized and typically guided by MDT discussions. SSAs have demonstrated efficacy in hindgut GEP-NETs, as shown in the CLARINET trial, which included a small proportion of rNET patients (11% in the lanreotide group and 3% in the placebo group)[19]. At 24 months, estimated progression-free survival (PFS) was 65.1% with lanreotide vs 33.0% with placebo (HR = 0.47, P = 0.0003)[19]. The RADIANT-4 trial evaluated everolimus against placebo and included a subgroup of 40 rNET patients, including 25 (12%) in the everolimus group and 15 (16%) in the placebo group. Everolimus reduced the risk of progression by 52% (HR = 0.48; P < 0.00001)[20]. PRRT targeting somatostatin receptors has become a cornerstone in advanced NET treatment. The NETTER-1 trials assessed 177Lu-DOTATATE in patients with metastatic midgut NETs who progressed on octreotide LAR. Although rNET patients were not included, the study demonstrated significant improvement in PFS (HR = 0.21, P < 0.0001), supporting its relevance in GEP-NET[21]. The NETTER-2 trials compared 177Lu-DOTATATE to high-dose octreotide LAR in patients with advanced grade 2 and grade 3 somatostatin receptor-positive GEP-NET, including 7 (5%) rNET patients in the experimental arm and 4 (5%) in the control arm. Median PFS at 22.8 vs 8.5 months favored PRRT (HR = 0.276, P < 0.0001)[22].
Access to first-line therapies such as SSAs, everolimus, or PRRT remains limited in many developing countries. As a result, chemotherapy often becomes the only available treatment option for patients with advanced disease, regardless of somatostatin receptor status, tumor grade, disease burden, or growth rate. In our study, 20 patients with advanced-stage disease (38.5%) received first-line chemotherapy, with TEMCAP being the most frequently used regimen (80%). A systematic review and meta-analysis by Lamarca et al[23] had evaluated the efficacy of chemotherapy in well-differentiated non-pancreatic NETs, including rNETs. The pooled analysis yielded an overall response rate of 11.5% (95%CI: 5.8-17.2), a median PFS of 16.9 months (95%CI: 3.8-30.04), and a median OS of 32.2 months (95%CI: 10.4-54.2).
This study has several limitations. Its retrospective and single-center design may limit the generalizability of the findings to broader populations. Additionally, the relatively small sample size reduces the statistical power to detect subtle differences between subgroups. To address these limitations, larger prospective, multicenter studies are needed to validate our findings and to better define prognostic factors and treatment outcomes for patients with rNETs in this region.
To our knowledge, this is the largest reported study of patients with rNETs from Perú. Our findings align with international data and add valuable regional evidence to the limited literature on this tumor type. Tumor size > 2 cm, grade 2, and N1 were significantly associated with advanced-stage disease and poorer survival outcomes.
| 1. | Xu Z, Wang L, Dai S, Chen M, Li F, Sun J, Luo F. Epidemiologic Trends of and Factors Associated With Overall Survival for Patients With Gastroenteropancreatic Neuroendocrine Tumors in the United States. JAMA Netw Open. 2021;4:e2124750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 2. | Andrés-Asenjo B, Solórzano-Aurusa FJO, Borrego-Pintado H, Blanco-Antona F, Romero-de Diego A, Heredia-Rentería JB. [Tumor neuroendocrino ano-rectal: desde un pólipo con buen pronóstico hasta un carcinoma letal]. Cir Cir. 2018;86:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Basuroy R, O'Donnell CM, Srirajaskanthan R, Ramage JK. Ileocolonic neuroendocrine tumours identified in the English bowel cancer screening programme. Colorectal Dis. 2018;20:O85-O91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Kaminski M, Polkowski M, Regula J. Prevalence and endoscopic features of rectal neuroendocrine tumors (carcinoids) among 50148 participants of the Polish colorectal-cancer screening programme. Gut. 2007;56:A310. |
| 5. | Pinto MP, Muñoz Medel M, Carrillo D, Retamal IN, Bravo ML, Valenzuela Y, Nervi B, Sánchez C, Galindo H, Ibañez C, Peña J, Balmaceda C, Madrid J, Briones J, Torres J, Nilo F, Guarda FJ, Quintana JC, Orellana P, Mondaca S, Acevedo F, Vicentini D, Cordova-Delgado M, Owen GI, Garrido M. Chilean Registry for Neuroendocrine Tumors: A Latin American Perspective. Horm Cancer. 2019;10:3-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | O'Connor JM, Marmissolle F, Bestani C, Pesce V, Belli S, Dominichini E, Mendez G, Price P, Giacomi N, Pairola A, Loria FS, Huertas E, Martin C, Patane K, Poleri C, Rosenberg M, Cabanne A, Kujaruk M, Caino A, Zamora V, Mariani J, Dioca M, Parma P, Podesta G, Andriani O, Gondolesi G, Roca E. Observational study of patients with gastroenteropancreatic and bronchial neuroendocrine tumors in Argentina: Results from the large database of a multidisciplinary group clinical multicenter study. Mol Clin Oncol. 2014;2:673-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Escobar KM, Vicente-Villardon JL, Villacís Gonzalez RE, Castillo Cordova PH, Sánchez Rodríguez JM, De la Cruz-Velez M, Siteneski A. Neuroendocrine Tumors: An Analysis of Prevalence, Incidence, and Survival in a Hospital-Based Study in Ecuador. Healthcare (Basel). 2022;10:1569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Cukier M, Vergara R, Mendez-Rios JD, Castillo O, Barrera I, Tello E, El Achtar O, Loo Y, Tapia H, Perez G, Peña M. Neuroendocrine tumors in Panama: A nationwide database analysis. Mol Clin Oncol. 2021;15:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Instituto Nacional de Enfermedades Neoplásicas. Resolución Jefatural N.° 026-2021-J-INEN. 25 Jan 2021. Available from: https://www.gob.pe/institucion/inen/normas-legales/1736246-026-2021-j-inen. |
| 10. | Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M, Asa SL. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol. 2022;33:115-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 619] [Article Influence: 154.8] [Reference Citation Analysis (2)] |
| 11. | Gallo C, Rossi RE, Cavalcoli F, Barbaro F, Boškoski I, Invernizzi P, Massironi S. Rectal neuroendocrine tumors: Current advances in management, treatment, and surveillance. World J Gastroenterol. 2022;28:1123-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (8)] |
| 12. | Rinke A, Ambrosini V, Dromain C, Garcia-Carbonero R, Haji A, Koumarianou A, van Dijkum EN, O'Toole D, Rindi G, Scoazec JY, Ramage J. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for colorectal neuroendocrine tumours. J Neuroendocrinol. 2023;35:e13309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (1)] |
| 13. | Zheng R, Zhao H, An L, Zhang S, Chen R, Wang S, Sun K, Zeng H, Wei W, He J. Incidence and survival of neuroendocrine neoplasms in China with comparison to the United States. Chin Med J (Engl). 2023;136:1216-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 14. | Chang JS, Chen LT, Shan YS, Chu PY, Tsai CR, Tsai HJ. An updated analysis of the epidemiologic trends of neuroendocrine tumors in Taiwan. Sci Rep. 2021;11:7881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Inada Y, Yoshida N, Fukumoto K, Hirose R, Inoue K, Dohi O, Murakami T, Ogiso K, Tomie A, Kugai M, Yoriki H, Inagaki Y, Hasegawa D, Okuda K, Okuda T, Morinaga Y, Kishimoto M, Itoh Y. Risk of lymph node metastasis after endoscopic treatment for rectal NETs 10 mm or less. Int J Colorectal Dis. 2021;36:559-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Soga J. Carcinoids of the rectum: an evaluation of 1271 reported cases. Surg Today. 1997;27:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 115] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Concors SJ, Sinnamon AJ, Folkert IW, Mahmoud NN, Fraker DL, Paulson EC, Roses RE. Predictors of Metastases in Rectal Neuroendocrine Tumors: Results of a National Cohort Study. Dis Colon Rectum. 2018;61:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Li YW, He YP, Liu FQ, Peng JJ, Cai SJ, Xu Y, Wang MH. Grade G2 Rectal Neuroendocrine Tumor Is Much More Invasive Compared With G1 Tumor. Front Oncol. 2021;11:646536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1352] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 20. | Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB, Rouyrre N, Sachs C, Valle JW, Fave GD, Van Cutsem E, Tesselaar M, Shimada Y, Oh DY, Strosberg J, Kulke MH, Pavel ME; RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 943] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 21. | Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1702] [Cited by in RCA: 2437] [Article Influence: 270.8] [Reference Citation Analysis (0)] |
| 22. | Singh S, Halperin D, Myrehaug S, Herrmann K, Pavel M, Kunz PL, Chasen B, Tafuto S, Lastoria S, Capdevila J, García-Burillo A, Oh DY, Yoo C, Halfdanarson TR, Falk S, Folitar I, Zhang Y, Aimone P, de Herder WW, Ferone D; all the NETTER-2 Trial Investigators. [(177)Lu]Lu-DOTA-TATE plus long-acting octreotide versus high-dose long-acting octreotide for the treatment of newly diagnosed, advanced grade 2-3, well-differentiated, gastroenteropancreatic neuroendocrine tumours (NETTER-2): an open-label, randomised, phase 3 study. Lancet. 2024;403:2807-2817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 174] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 23. | Lamarca A, Elliott E, Barriuso J, Backen A, McNamara MG, Hubner R, Valle JW. Chemotherapy for advanced non-pancreatic well-differentiated neuroendocrine tumours of the gastrointestinal tract, a systematic review and meta-analysis: A lost cause? Cancer Treat Rev. 2016;44:26-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/