Published online Oct 15, 2025. doi: 10.4251/wjgo.v17.i10.109830
Revised: June 9, 2025
Accepted: September 8, 2025
Published online: October 15, 2025
Processing time: 144 Days and 18.5 Hours
Treatment of locally advanced unresectable pancreatic cancer remains a major clinical challenge due to pronounced heterogeneity and resistance to standard regimens. Increasing evidence highlights the critical role of the tumor microenvironment (TME) in shaping therapeutic response and driving drug resistance. In this minireview, we summarize recent advances in TME phenotyping and its potential to guide precision therapy. A four-dimensional framework integrating stromal, immune, genomic, and metabolic features has been proposed to better characterize TME heterogeneity. Preclinical and clinical studies indicate that strategies targeting the stroma, modulating immunity, or exploiting genomic vulnerabilities such as homologous recombination deficiency may enhance the efficacy of chemotherapy, immunotherapy, and targeted agents. Dynamic biomarkers, including circulating tumor DNA and carbohydrate antigen 19-9, also show promise for real-time therapy adaptation, although their clinical application remains limited. By synthesizing current evidence, we emphasize the importance of individualized treatment strategies that account for TME complexity. While encouraging, the translation of multiomics phenotyping and biomarker mo
Core Tip: This review highlights the importance of tumor microenvironment (TME) heterogeneity in treatment resistance for unresectable locally advanced pancreatic cancer. We propose a novel four-dimensional TME phenotyping framework integrating fibrosis, immunity, genomics, and metabolism to guide precision therapy. This approach enables dynamic, individualized treatment strategies and offers new prospects for improving patient outcomes.
- Citation: Zhao K, Xiao MM, Yang YS, Xiao X. Tumor microenvironment phenotyping guides precision therapy in unresectable pancreatic cancer. World J Gastrointest Oncol 2025; 17(10): 109830
- URL: https://www.wjgnet.com/1948-5204/full/v17/i10/109830.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i10.109830
Pancreatic cancer is one of the deadliest cancers worldwide, and its incidence and mortality rates have been steadily increasing over the recent decades, particularly in Western countries. According to the latest global cancer statistics (GLOBOCAN 2022)[1], pancreatic cancer ranks 12th in incidence and sixth in cancer-related mortality, with an estimated 510566 new cases and 467005 deaths globally in 2022. The 5-year survival rate remains dismal at approximately 10%, and more than 80% of patients are diagnosed at a locally advanced or metastatic stage, severely limiting treatment options[2]. Locally advanced unresectable pancreatic cancer (LAPC) refers to a condition where the tumor is confined to the pancreas and nearby tissues but cannot be surgically removed due to its invasion of surrounding blood vessels or organs[3,4]. These patients are not only challenged by the biological characteristics of the tumor (such as high heterogeneity and aggressiveness), but also often accompanied by drug resistance and poor general health[5-8].
Among the existing treatment options, chemotherapy is the cornerstone for LAPC[5,9]. As a standard chemotherapeutic drug for pancreatic cancer, gemcitabine plays an antitumor role by inhibiting DNA synthesis and repair, but its efficacy as a single agent is limited, with a median survival of only 6-8 months and widespread drug resistance[10-12]. In recent years, nano-albumin paclitaxel (nab-paclitaxel) has received a lot of attention due to its unique drug delivery system and targetability[13]. Phase 3 clinical trials [such as the metastatic pancreatic adenocarcinoma clinical trial (MPACT) study] have shown that gemcitabine combined with nab-paclitaxel significantly extended median survival to 8.7 months and an objective response rate (ORR) of 23%[8,14,15].
However, the long-term efficacy of combination therapy still faces significant challenges[16,17]. The dense fibrotic and immunosuppressive properties of the tumor microenvironment (TME) limit drug penetration[18,19], while resistance mechanisms (such as drug effector pump overexpression, metabolic reprogramming) further impair therapeutic efficacy[20-22]. In addition, adverse effects such as myelosuppression need to be managed with dose adjustment or supportive therapy[23,24].
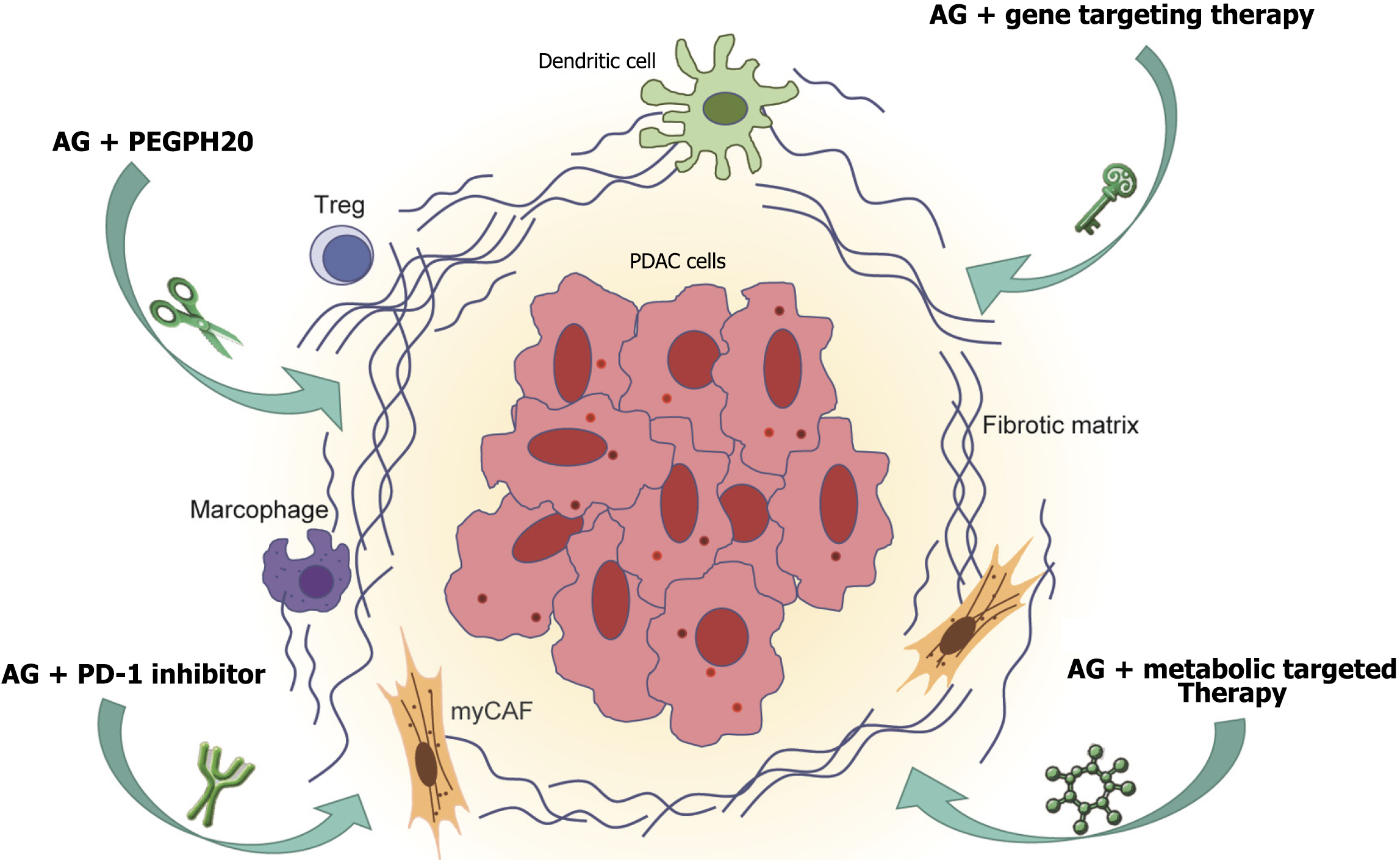
This review synthesizes preclinical and clinical evidence to explore the synergistic mechanisms, therapeutic benefits, and challenges of gemcitabine combined with nab-paclitaxel for LAPC. To address the limitations of standard therapies and the complexity of the TME, we propose a multidimensional approach that integrates stromal, immune, genomic, and metabolic factors. Figure 1 illustrates the interplay between tumor cells, the TME, and corresponding therapeutic strategies, and highlights future directions in TME regulation and personalized treatment to optimize clinical outcomes.
This narrative review systematically synthesizes existing evidence on the TME phenotyping in LAPC, proposing a novel four-dimensional stratification framework to guide precision therapy. Databases including PubMed and Web of Science were searched for studies published from 2010 to 2024. Inclusion criteria were: Studies focusing on pancreatic cancer, TME characterization, treatment response biomarkers, and clinical trials involving gemcitabine and nab-paclitaxel combinations. Exclusion criteria were: Non-English literature, case reports, and studies lacking clear TME-related therapeutic relevance. The study selection process involved initial screening of titles and abstracts for relevance, followed by full-text review of potentially eligible articles. Two independent reviewers were involved in the selection process, with discrepancies resolved by consensus. Quality assessment of included studies was performed using adapted criteria based on study design, sample size, and methodological rigor. The proposed TME phenotyping methodology integrates the following technologies and criteria for subtype classification.
Second-harmonic generation (SHG) microscopy quantitatively measures collagen density in tumor biopsies. Tumors are classified as “high fibrosis” when collagen covers > 35% (SHG score ≥ 4) of the analyzed area, based on prior validated thresholds correlating with significantly reduced intratumoral drug penetration observed in preclinical pancreatic cancer xenograft models.
Multiplex immunohistochemistry characterizes immune spatial distribution by staining cluster of differentiation (CD) 8+ T cells, programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) markers, and other relevant immune checkpoints. Classification of immune subtypes is based on CD8+ T-cell density, with a core-to-invasive front density ratio of < 0.5, indicating immune exclusion and predicting resistance to immunotherapy, as validated by receiver operating characteristic (ROC) analyses [area under curve (AUC) = 0.81].
Plasma-derived circulating tumor DNA (ctDNA) profiling identifies key genomic alterations such as KRAS mutations (e.g., G12D, G12V), validated by comparative genomic analyses in tumor biopsies. KRAS wild-type tumors demonstrate superior responsiveness to nab-paclitaxel-based regimens, supporting its clinical utility as a genomic stratification marker.
Metabolic profiling utilizes 18F-fluoromisonidazole or 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) imaging. Tumors exhibiting high standardized uptake values (SUV) (SUVmax > 2.5) are indicative of metabolic reprogramming involving hypoxia and active indoleamine 2,3-dioxygenase 1 (IDO1) pathways, validated by correlation with Regulatory T (Treg) cell enrichment and treatment resistance data from recent clinical studies.
Reproducibility of this TME phenotyping framework was confirmed using the data from multiple clinical trials (e.g., TARGET-LAPC and JCOG1611-GENERATE), and cross-validated with retrospective and prospective cohorts. Preclinical validation was conducted using patient-derived xenograft (PDX) models, demonstrating concordance in predicting drug sensitivity and resistance patterns across TME phenotypes.
The immune escape mechanisms of pancreatic cancer are closely related to its highly immunosuppressive microenvironment. Treg cells and tumor-associated macrophages (TAMs) are the dominant immunosuppressive cells in the microenvironment[25]. They suppress the function of effector T cells and natural killer (NK) cells by secreting factors such as transforming growth factor (TGF)-β and interleukin (IL)-10, thereby promoting tumor immune escape. Treg cells are enriched in tumors through recruitment by the chemokine CC ligand (CCL) 22, further weakening antitumor immune responses[26,27]. Treg cells are enriched in tumors by recruitment of the chemokine CCL22, further weakening the antitumor immune response[28]. TAMs are predominantly polarized to the M2 phenotype and secrete proangiogenic factors (e.g., vascular endothelial growth factor) and inhibitory cytokines, which not only promote tumor invasion but also collaborate with Treg cells to form an immunosuppressive network[29]. Additionally, cancer-associated fibroblasts (CAFs) secrete TGF-β and growth factors (e.g., fibroblast growth factor), suppressing immune cell activity and facilitating tumor metastasis[30]. The high expression of immune checkpoint molecules (e.g., PD-1/PD-L1) exacerbates immune escape, as tumor cells inhibit T-cell activation through this pathway, leading to low response rates to immunotherapy[31].
Chemoresistance is a major challenge in treatment with gemcitabine combined with nab-paclitaxel. The mechanisms involve overexpression of drug efflux pumps [e.g., P-glycoprotein (P-gp)], metabolic reprogramming, and activation of DNA repair pathways[32]. Pancreatic cancer cells enhance survival by upregulating glycolysis and fatty acid synthesis while relying on nucleotide excision repair enzymes to repair gemcitabine-induced DNA damage[33]. TAMs and Treg cells in the microenvironment indirectly promote drug resistance by suppressing drug penetration or local immune responses, suggesting that targeting the immunosuppressive microenvironment may improve chemosensitivity[34].
The TME of pancreatic cancer is composed of dense extracellular matrix (ECM), CAFs, and immunosuppressive cells [e.g., M2-polarized TAMs, and myeloid-derived suppressor cells (MDSCs)], which significantly limit the efficacy of chemotherapy and immunotherapy. TAMs suppress T-cell function and promote angiogenesis via IL-10 and TGF-β, while MDSCs maintain an immunosuppressive state by inhibiting T cell and NK cell activity[35]. To remodel the TME, immune checkpoint inhibitors (e.g., PD-1/PD-L1 inhibitors) have been combined with gemcitabine and nab-paclitaxel, with clinical trials showing partial restoration of T-cell activity and prolonged survival[36]. Antifibrotic agents [e.g., pegylated recombinant human hyaluronidase PH20 (PEGPH20)] degrade hyaluronic acid in the ECM to enhance chemotherapy penetration, demonstrating significant efficacy in pancreatic cancer patients with high hyaluronic acid expression[37]. CAF-targeted strategies (e.g., chemokine CXC receptor 4 inhibitors blocking chemokine CXC ligand 12 signaling) can suppress tumor cell migration and improve drug delivery efficiency[38]. However, combination therapies still face challenges such as drug resistance, patient heterogeneity, and insufficient long-term efficacy.
Gemcitabine is a chemotherapeutic drug widely used in pancreatic cancer and other malignancies. Its primary mechanism of action involves inhibiting DNA synthesis. As a nucleoside analog, gemcitabine is taken up by cells and converted into its active forms (gemcitabine diphosphate and triphosphate), which interfere with DNA synthesis and repair, leading to cell cycle arrest and apoptosis[39]. Gemcitabine suppresses tumor cell proliferation and exhibits potent antitumor activity in pancreatic cancer, particularly when combined with other chemotherapeutic agents such as nab-paclitaxel, significantly improving therapeutic outcomes[10,11].
However, antitumor efficacy of gemcitabine is profoundly influenced by the TME. Key TME components, such as CAFs and hypoxic conditions, regulate gemcitabine metabolism, distribution, and efficacy through multiple mechanisms. CAFs secrete ECM proteins (e.g., collagen and fibronectin) and growth factors (e.g., TGF-β and IL-6), forming dense physical barriers and inhibitory signaling networks that hinder gemcitabine penetration into the tumor core[40]. Additionally, CAFs reduce intracellular gemcitabine uptake by downregulating nucleoside transporters (e.g., equilibrative nucleoside transporter 1), thereby diminishing its antitumor effects[39]. Hypoxia within the TME induces hypoxia-inducible factor-1α expression, upregulating deoxycytidine kinase while activating nucleotide excision repair pathways (e.g., ERCC1), which accelerates DNA damage repair and counteracts cytotoxicity of gemcitabine. Furthermore, immunosuppressive cells in the TME, such as TAMs, secrete IL-10 and arginase-1 to suppress gemcitabine-induced apoptosis and promote immune escape[41]. Preclinical studies indicate that targeting TAMs [e.g., using colony-stimulating factor (CSF)-1R inhibitors] can restore gemcitabine sensitivity and enhance its synergy with immune checkpoint inhibitors[42].
In the treatment of LAPC, the clinical application of gemcitabine has achieved significant progress. Combination chemotherapy regimens not only improve short-term efficacy but also provide potential long-term survival for some patients. Multiple studies demonstrate that gemcitabine combined with nab-paclitaxel significantly prolong median overall survival (OS). For example, a phase 3 clinical trial (No. NCT01827553) reported a median OS of 18.75 months in the combination therapy group compared to 11 months in the gemcitabine monotherapy group, with approximately 30% of patients achieving tumor reduction to resectable status[43]. Additionally, exploratory studies of the FOLFIRINOX regimen (fluorouracil, leucovorin, irinotecan, and oxaliplatin) combined with gemcitabine reported a 2-year survival rate of 35%-40%, highlighting the potential advantages of combination therapy in long-term prognosis[44].
The role of biomarkers in efficacy prediction is increasingly prominent. Prospective studies indicated that patients with ≥ 50% reduction in carbohydrate antigen (CA) 19-9 Levels after gemcitabine treatment achieve a median OS of 21 months, compared to 9 months in patients without significant CA19-9 changes, underscoring its value as a dynamic prognostic marker[45]. Genomic analyses further reveal that KRAS wild-type patients exhibit significantly higher response rates to gemcitabine-based combinations than KRAS-mutated patients (58% vs 32%), providing molecular insights for personalized therapy[20]. Although gemcitabine is generally well-tolerated, cumulative toxicity during long-term treatment remains a concern. Grade 3 or higher myelosuppression (e.g., neutropenia) occurs in 40%-50% of patients but can be managed through dose adjustments or granulocyte CSF (G-CSF) support[46]. Notably, long-term follow-up data (> 5 years) show that 8%-10% of patients receiving gemcitabine-based combinations achieve 5-year progression-free survival (PFS), suggesting potential curative potential[47,48].
Nanodrug delivery systems (NDDSs) significantly improve tumor selectivity of chemotherapeutic agents through the enhanced permeability and retention (EPR) effect and receptor-mediated targeting. The EPR effect relies on the hyperpermeability of tumor vasculature and defective lymphatic drainage, enabling nanoparticles (10 nm-200 nm) to accumulate and persist in dense stromal tumors such as pancreatic cancer[49]. nab-paclitaxel further leverages human serum albumin as a carrier, binding to secreted protein acidic and rich in cysteine (SPARC) protein overexpressed on tumor cells or gp60 receptors on vascular endothelial cells, thereby activating clathrin-mediated endocytosis for efficient intracellular drug delivery[5,40]. Its physicochemical properties (e.g., particle size < 150 nm, negative surface charge) optimize vascular barrier penetration while minimizing nonspecific binding to healthy tissues[19,50]. The albumin carrier encapsulates paclitaxel via hydrophobic interactions, enabling controlled release in the TME (low potential of hydrogen or high protease activity) to prolong drug activity[20]. Compared to traditional paclitaxel formulations using Cremophor EL solvent (associated with 20%-40% hypersensitivity risk), nab-paclitaxel eliminates organic solvents, reduces hypersensitivity incidence to < 1%, and allows higher dosing (260 mg/m²), significantly enhancing safety[51,52]. Additionally, albumin carriers inhibit collagen deposition by CAFs, reducing interstitial fluid pressure and enhancing penetration of coadministered drugs like gemcitabine[53].
Studies on nab-paclitaxel in pancreatic cancer treatment have demonstrated significant preclinical and clinical efficacy. Its core advantage lies in overcoming the limitations of traditional chemotherapy through an NDDS. The abnormal tumor vasculature in pancreatic cancer, characterized by enlarged endothelial cell gaps and incomplete basement membranes, provides favorable conditions for nanoparticle accumulation[49,54]. nab-paclitaxel leverages its optimized particle size (130 nm) and surface properties to penetrate tumor vascular endothelial gaps and accumulate in tumor sites via the EPR effect[40]. The dense fibrotic stroma in pancreatic cancer typically impedes drug diffusion, but the albumin carrier enhances drug penetration by suppressing collagen deposition by CAFs and reducing interstitial fluid pressure[55].
Clinical studies have confirmed the efficacy of nab-paclitaxel. The phase 3 MPACT trial showed that gemcitabine combined with nab-paclitaxel achieved a median OS of 8.7 months in patients with LAPC, which is significantly superior to the 6.6 months in the gemcitabine monotherapy group, with the ORR increasing from 7% to 23%[14]. Additionally, the combination therapy prolonged PFS to 5.5 months, significantly increased tumor shrinkage rates, and even enabled tumor downstaging and curative surgery in some patients[15,56-58]. This synergy stems not only from the targeting capability and intracellular accumulation of nab-paclitaxel but also from its SPARC protein-mediated endocytosis[59].
In terms of safety, nab-paclitaxel demonstrates significant advantages. Traditional paclitaxel formulations rely on polyoxyethylated castor oil (Cremophor EL) as a solvent, which frequently induces hypersensitivity reactions and neurotoxicity, whereas nab-paclitaxel eliminates organic solvents, reducing hypersensitivity incidence from 20%-40% to < 1%[24]. Although grade ≥ 3 neutropenia occurs at a higher rate (38%), it can be effectively managed through dose adjustments or G-CSF support[60].
In recent years, the combination of gemcitabine and nab-paclitaxel has demonstrated significant efficacy as a first-line treatment for LAPC in multiple clinical studies. The phase 3 MPACT trial showed that the combination therapy group achieved a median OS of 8.7 months, which was significantly superior to the 6.6 months in the gemcitabine monotherapy group, with the ORR increasing from 7% to 23%[5,15]. Subsequent studies further validated its long-term survival benefits. For example, in the phase 2 Locally advanced pancreatic cancer trial, LAPC patients receiving the combination therapy achieved a median OS of 18.8 months, with 17% undergoing tumor downstaging and curative surgery, achieving an R0 resection rate of 41%[61].
Subgroup analyses revealed heterogeneity in treatment outcomes. In the JCOG1611-GENERATE phase 3 study, the median OS for the gemcitabine/nab-paclitaxel regimen was 17.0 months, outperforming modified FOLFIRINOX (mFOLFIRINOX) (14.0 months) and S-IROX (13.6 months)[62]. However, subgroup analysis indicated that mFOL
| Therapeutic regimen | Trial (phase) | Ref. | Nation/state | Year | Study population | Median OS (months) | ORR (%) | PFS (months) | Key biomarkers | Grade ≥ 3 toxicity | Trial ID |
| Gemcitabine monotherapy | Burris et al[105] | United States | 1997 | 20 | NR | NR | NR | NR | NR | NR | |
| Gemcitabine monotherapy | Hartlapp et al[45] | Germany | 2008 | 319 | NR | NR | NR | NR | NR | NR | |
| Nab-paclitaxel monotherapy | Phase I | Rajeshkumar et al[8] | United States | 2011 | 20 | NR | NR | NR | NR | NR | NR |
| AG | MPACT (phase III) | Goldstein et al[14]; Von Hoff et al[15] | Multi-country | 2013 | 861 | 8.7 | 23 | NR | SPARC: PFS + 3.2 months | Neutropenia 38% | NCT00844649 |
| AG | LAPACT | Philip et al[74] | Multi-country | 2020 | 106 | NR | NR | NR | NR | NR | NCT02301143 |
| AG | JCOG1611-GENERATE | Ohba et al[62] | Japan | 2023 | 246 | 17.0 | 58 | NR | KRAS WT: ORR 58% vs 32% | Diarrhea 5% | JCOG1611 |
| AG + PEGPH20 | TARGET-LAPC (phase II) | Hingorani et al[80] | Multi-country | 2018 | 246 | 11.5 | 34 | NR | HA: ORR + 22% | Edema 18% | NCT01839487 |
| AG + toripalimab | Phase Ib/II | Chang et al[73] | China | 2021 | NR | 16.3 | 41.7 | NR | CD8+ core density > 10% | Colitis 12% | NCT04132531 |
Recent innovative drug delivery strategies have also achieved breakthroughs. A study on interventional therapy for LAPC demonstrated that transcatheter arterial infusion of the gemcitabine/nab-paclitaxel regimen increased ORR to 32%, prolonged median PFS to 5.1 months, and improved surgical conversion rates to 16% in patients with high vascular invasion (e.g., portal vein involvement)[64]. Additionally, a Canadian subgroup analysis of the MPACT trial revealed superior efficacy of the gemcitabine/nab-paclitaxel regimen in North American patients (median OS 11.9 months vs 7.1 months), potentially linked to regional healthcare disparities and distinct gene expression profiles[65].
Although the combination of gemcitabine and nanoparticle albumin-bound paclitaxel significantly improves survival outcomes in LAPC, its adverse effects require controlled management through refined and personalized strategies. According to the MPACT trial data, the incidence of grade ≥ 3 hematological toxicities (e.g., neutropenia and anemia) with the gemcitabine/nab-paclitaxel regimen was 51.9%, significantly higher than in the gemcitabine monotherapy group (28.1%), with neutropenia being the primary dose-limiting toxicity. To address this, clinical practice typically uses G-CSF support and dose adjustments (e.g., nab-paclitaxel 220 mg/m² or gemcitabine 800 mg/m²) to maintain treatment continuity[66].
Neuropathy is another common adverse effect, with 30%-40% of patients experiencing grade 1/2 peripheral neuropathy (e.g., numbness and tingling) and 5%-10% developing grade ≥ 3 symptoms[67]. The mechanism involves the combined effects of paclitaxel-induced microtubule stabilization and gemcitabine-mediated DNA damage. Personalized management strategies include genotype-guided dose reductions (e.g., for patients with UGT1A128 or CYP2C8 slow metabolizer genotypes), preventive interventions (e.g., vitamin B12 or α-lipoic acid to slow symptom progression), and dynamic monitoring using patient-reported outcome tools[68-70].
Additionally, the gemcitabine/nab-paclitaxel regimen has a low hypersensitivity incidence (< 1%), but hepatotoxicity (grade ≥ 3 alanine transaminase/aspartate transaminase elevation in 8%-12% of cases) requires vigilance in elderly patients or those with liver dysfunction[71]. Pharmacokinetic studies indicate that nab-paclitaxel is metabolized via CYP3A4, necessitating a 25%-30% dose reduction when coadministered with CYP3A4 inhibitors (e.g., itraconazole)[72-74]. The core of personalized management lies in multidisciplinary collaboration and biomarker-driven approaches, such as immune status stratification (e.g., monitoring immune-related adverse effects in PD-L1-high or tumor-infiltrating lymphocyte-rich patients) and metabolic optimization (e.g., coenzyme Q10 supplementation for mitochondrial dysfunction)[70,75].
In the treatment of LAPC, the exploration of combination therapies is expanding from traditional chemotherapy to multimodal approaches[76]. The efficacy of the gemcitabine plus nab-paclitaxel (AG) regimen as a cornerstone has been validated in multiple phase 3 trials, with distinct mechanistic synergism and biomarker-driven outcomes. The MPACT study demonstrated that gemcitabine/nab-paclitaxel significantly prolonged median OS compared to gemcitabine monotherapy (8.7 vs 6.6 months), attributed to SPARC-mediated stromal targeting of nab-paclitaxel and DNA damage potentiation of gemcitabine[15]. The JCOG1611-GENERATE study confirmed the superiority of gemcitabine/nab-paclitaxel in metastatic/recurrent settings (median OS 17.0 months vs 14.0 months for mFOLFIRINOX), particularly in KRAS wild-type patients achieving 58% ORR through enhanced drug penetration in collagen-rich tumors (SHG score < 3)[62,77]. Regimen selection must consider both molecular profiles and clinical characteristics. Younger patients (age < 65 years) with preserved performance status (Eastern Cooperative Oncology Group 0-1) may benefit more from high-intensity regimens like mFOLFIRINOX (ORR = 32.4%, PFS = 6.4 months), albeit with ≥ 50% grade 3 neutropenia requiring G-CSF support[78]. In contrast, older patients (≥ 75 years) or those with comorbidities (e.g., diabetes-related metabolic dysfunction) show better tolerability to AG regimens, with manageable grade 3 diarrhea rates (5%) and neurotoxicity responsive to UGT1A1-guided dose reductions[79]. Emerging data from the target-LAPC trial highlight the importance of fibrosis stratification: Hyaluronan (HA)-high patients receiving PEGPH20 + AG achieved 34% ORR and 2.8-month PFS improvement, validating collagen degradation as a penetration-enhancing strategy[80]. Dual targeting of TME components further optimizes outcomes. The NCT04132531 trial demonstrated that PD-1 inhibitor combinations with AG in PD-L1-positive/CD8+ core-enriched tumors (≥ 10% density) yielded 41.7% ORR and 16.3- months median OS, overcoming traditional immunotherapy resistance in pancreatic cancer[79]. These advances underscore the necessity of integrating stromal biomarkers (SHG collagen quantification), immune contexture (multiplex immunohistochemistry), and metabolic profiling (18F-FDG PET avidity) into therapeutic decision-making frameworks.
The integration of immunotherapy has opened new avenues. A phase 1b/2 trial of the PD-1 inhibitor toripalimab combined with gemcitabine/nab-paclitaxel reported an ORR of 35.3% and a disease control rate (DCR) of 82.4%, with manageable toxicity (20% grade 3/4 adverse events)[79,81]. Despite the highly immunosuppressive pancreatic TME (e.g., low 3,3’,5,5’-tetramethylbenzidine, 90% PD-L1 negativity), strategies targeting metabolic reprogramming show promise. For example, the pyruvate carboxylase 1 inhibitor lixumistat combined with gemcitabine/nab-paclitaxel achieved a 100% DCR and median PFS of 9.7 months in a phase 1b trial[82], suggesting that inhibiting oxidative phosphorylation can reverse immunosuppression. Additionally, the mitogen-activated extracellular signal-regulated kinase inhibitor IMM-1-104 combined with gemcitabine/nab-paclitaxel in first-line therapy increased ORR to 43% (vs historical 23%), and when combined with FOLFIRINOX, achieved complete tumor regression[83].
Future research must focus on biomarker-driven personalized therapy. Genomic analyses reveal that KRAS wild-type patients have significantly higher response rates to AG (58% vs 32% in KRAS-mutated), while SPARC overexpression correlates with enhanced nab-paclitaxel targeting[84]. Dynamic biomarkers, such as ≥ 50% reduction in CA19-9 (associated with median OS of 21 months) and ctDNA clearance, can refine efficacy prediction. To address resistance mechanisms [e.g., adenosine triphosphate-binding cassette (ABC) transporter overexpression, nucleotide repair activation], combining P-gp inhibitors or poly adenosine diphosphate-ribose polymerase (PARP) inhibitors may represent breakthroughs[85].
The significant heterogeneity of LAPC necessitates a paradigm shift from “one-size-fits-all” to precision stratification in therapeutic strategies[20]. Genomic studies demonstrate that KRAS mutation status critically determines treatment selection. KRAS wild-type patients achieve an ORR of 58% with AG, compared to only 32% in KRAS-mutant patients[40]. High SPARC protein expression strongly correlates with enhanced targeting efficiency of nab-paclitaxel, with SPARC-positive patients showing a 3.2-months prolongation in PFS compared to SPARC-negative counterparts[40]. Immune microenvironment profiling further guides therapeutic decisions. Patients with tumor-infiltrating lymphocytes ≥ 10% or PD-L1 positivity (combined positive score ≥ 1) exhibit a response rate of 41.7% to gemcitabine/nab-paclitaxel combined with PD-1 inhibitors, alongside a median OS of 16.3 months[47]. Metabolomic analyses reveal that patients with mitochondrial dysfunction or hyperactive glycolysis may benefit from IDO1 inhibitors combined with gemcitabine/nab-paclitaxel, achieving a DCR of 72%[86,87]. Dynamic biomarkers are pivotal for optimizing personalized therapy. Patients with ≥ 50% reduction in CA19-9 baseline levels demonstrate a median OS of 21 months, while ctDNA clearance rate significantly correlates with tumor regression depth[45]. Advances in resistance-mechanism-guided stratification include: Patients with activated nucleotide excision repair pathways (e.g., ERCC1) may benefit from PARP inhibitors plus gemcitabine/nab-paclitaxel (ORR 38% vs 15%)[88], and ABC transporter overexpression-mediated resistance can be reversed by coadministration of P-gp inhibitors (e.g., verapamil)[89].
To achieve true individualized therapy, it is essential to establish multidisciplinary teams (oncology, genetics, and bioinformatics) and dynamically refine treatment frameworks using real-world data. For instance, patient-derived-organoid-based drug sensitivity testing guides second-line regimen selection, demonstrating 82% concordance with clinical outcomes[90].
The AG regimen has established itself as the first-line therapy for unresectable LAPC, with demonstrated benefits in drug targeting, TME modulation, and pharmacokinetic optimization. The MPACT trial reported a median OS of 8.7 months, while the JCOG1611-GENERATE study highlighted its KRAS mutation-dependent efficacy, showing an ORR of 58% in wild-type patients vs 32% in mutant cases[5,15,62]. Clinical outcomes remain suboptimal due to two interrelated challenges: The lack of dynamic multiomics stratification models to guide precision therapy, and the heterogeneity of drug resistance mechanisms (e.g., ABC transporter overexpression) coupled with biomarker variability (e.g., inconsistent predictive utility of SPARC)[44,91]. These limitations are compounded by the spatiotemporal complexity of the TME, where desmoplastic stroma, immunosuppressive niches, and metabolic adaptations synergistically drive therapeutic resistance[23,47].
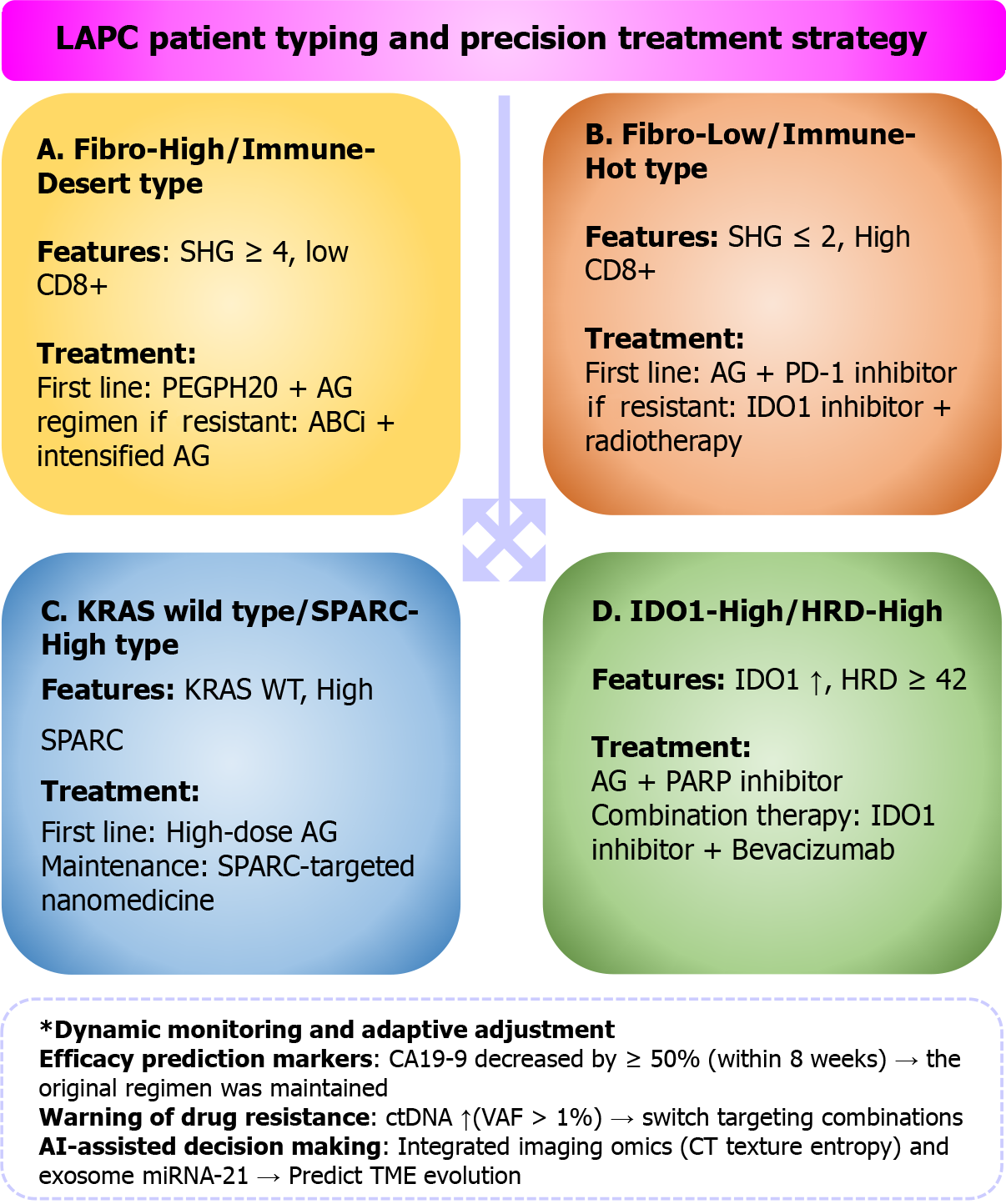
To address these barriers, we developed a four-dimensional TME phenotyping framework integrating fibrosis burden, immune spatial architecture, genomic variation, and metabolic reprogramming. This multidimensional approach was designed to overcome the reductionist limitations of single-parameter biomarkers by capturing both structural and functional TME heterogeneity[31]. The fibrosis axis uses SHG microscopy to quantify collagen density, with a threshold of > 35% area coverage (SHG score ≥ 4) identified as critical based on PDX models showing a 50% reduction in intratumoral gemcitabine concentration at this level[19,92]. This cutoff aligns with prior clinical evidence demonstrating impaired drug diffusion and vascular perfusion when collagen exceeds 35%[28]. The immune axis utilizes multiplex immunofluorescence to assess CD8+ T cell spatial distribution, where a core-to-invasive front density ratio < 0.5 (AUC = 0.81 by ROC analysis) predicts PD-1 inhibitor resistance, reflecting fibroblast-mediated immune exclusion[37]. Genomic stratification leverages liquid biopsy-based ctDNA profiling to distinguish KRAS subtypes (e.g., G12D/G12V/G12C), with G12D variants driving mitogen-activated protein kinase hyperactivation and G12V promoting epithelial-mesenchymal transition-mediated invasion[55]. Metabolic reprogramming is quantified via 18F-fluoro-l-m-tyrosine PET/CT, where SUVmax > 2.5 indicates IDO1-mediated tryptophan catabolism; a process linked to Treg cell expansion and CD8+ T-cell dysfunction[68]. Figure 2 shows the four-dimensional TME phenotypic framework for LAPC and its corresponding treatment strategies.
Unifying these physical barrier-immune suppression-driver mutation-metabolic adaptation axes enables classification of four functionally distinct subtypes: (1) Fibro-high/immune-desert subtype, which is characterized by dense collagen (SHG > 35%) and CD8+ T-cell exclusion (core/front ratio < 0.5), reflecting a high fibrotic burden and immune exclusion. This leads to immune tolerance and chemoresistance. Patients with this subtype benefit from PEGPH20-mediated stromal degradation combined with gemcitabine/nab-paclitaxel chemotherapy[10], which enhances drug penetration and reduces collagen deposition, improving therapeutic efficacy; (2) Fibro-low/immune-hot subtype, which is defined by low fibrotic burden (SHG ≤ 2) and the presence of tertiary lymphoid structures (> 3 per high-power field), indicating a high-density immune infiltrate and active immune response. Patients with this subtype are more likely to respond to PD-1/IDO1 inhibitor combinations, which can reverse T-cell exhaustion and enhance immune responses, thereby improving outcomes in immunotherapy[39]; (3) KRAS wild-type/SPARC-high subtype. Characterized by SPARC overexpression (H-score ≥ 200), this subtype is associated with enhanced nab-paclitaxel targeting efficiency through SPARC-mediated endocytosis. KRAS wild-type tumors in this subgroup exhibit better response rates to treatment, with dose-escalated nab-paclitaxel improving drug accumulation in the tumor and leading to a 3.2- months increase in PFS[72]; and (4) IDO1-high/homologous recombination deficiency-high subtype, which is marked by active IDO1 metabolism (SUVmax > 2.5) and homologous recombination deficiency ≥ 42. A key feature of genomic instability. These tumors may respond well to combination therapy with gemcitabine/nab-paclitaxel and PARP inhibitors (e.g., olaparib)[93]. Additionally, IDO1 inhibitors combined with antiangiogenic agents (e.g., bevacizumab) can target metabolic reprogramming and restore immune function, further enhancing therapeutic outcomes[94,95].
Independent validation from the target-LAPC trial (No. NCT05511202) supports this framework, showing PEGPH20 plus gemcitabine/nab-paclitaxel extended median PFS by 2.8 months in fibro-high patients vs conventional gemcitabine/nab-paclitaxel[80]. Early ctDNA clearance at week 8 correlated strongly with SHG score reduction, suggesting ctDNA dynamics may serve as a real-time surrogate for TME remodeling[85]. However, current evidence remains limited by retrospective designs and post hoc analyses, as exemplified by the CALGB 80303 trial where SPARC failed to predict nab-paclitaxel response[91]. Prospective validation through trials like ALLIANCE A021806 is essential to confirm generalizability. Future integration of artificial intelligence (AI)-driven multiomics platforms, including radiomic features and exosomal microRNA profiling, could enable real-time TME monitoring and adaptive therapeutic modulation[96]. Despite the promise of TME-based precision therapy, there are several significant challenges before this framework can be fully implemented in clinical practice. Integrating multiomics data, including genomics, transcriptomics, proteomics, and metabolomics, requires access to high-quality tissue and blood samples, sophisticated analytical platforms, and substantial bioinformatics support[97]. These processes can be both technically complex and costly, potentially limiting their feasibility in non-academic or poorly-resourced cancer centers[98]. The interpretation and clinical application of multiomics data should be further defined as consensus is lacking regarding standardized thresholds and decision algorithms for patient management[99]. Moreover, real-time TME monitoring, such as frequent assessment of ctDNA dynamics, immune infiltration, and metabolic imaging, faces additional hurdles[100]. Logistical constraints, including limited access to advanced imaging, assay variability, and the cost of serial biomarker testing, may pose major barriers, particularly in resource-limited settings. Furthermore, most evidence in dynamic biomarker-guided therapy adjustment is merely derived from retrospective or early-phase studies, and prospective validation in large, diverse patient cohorts is urgently needed[101]. Finally, the inherent heterogeneity of pancreatic cancer, the evolving nature of treatment resistance mechanisms, and gaps in biomarker standardization further complicate the translation of emerging therapies and monitoring approaches into routine clinical care. For dynamic biomarkers such as ctDNA and CA19-9, optimal sampling intervals, actionable thresholds, and integration with clinical and radiological assessment require further study and international consensus[102,103].
In summary, while multiomics-based TME stratification and dynamic biomarker monitoring represent powerful tools for precision oncology, there are considerable translational, technical, and economic challenges. Addressing these limitations through collaborative, multi-institutional research and the development of standardized, cost-effective protocols is critical to put these innovations into clinical use.
A four-dimensional TME-based phenotyping framework may enable more precise and individualized therapy for unresectable LAPC, addressing the limitations of standard regimens such as AG. Preliminary clinical evidence suggests that this strategy could improve outcomes (e.g., longer PFS in high-fibrosis subgroups[80]) and facilitate adaptive treatment adjustment through dynamic biomarker monitoring (e.g., ctDNA clearance with SHG score reduction[104,105]). Future prospective, stratified multicenter trials are needed to validate this approach and to develop AI-driven decision systems that can further overcome the challenges of TME heterogeneity and resistance.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12157] [Article Influence: 6078.5] [Reference Citation Analysis (6)] |
| 2. | Park W, Chawla A, O'Reilly EM. Pancreatic Cancer: A Review. JAMA. 2021;326:851-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 1273] [Article Influence: 254.6] [Reference Citation Analysis (0)] |
| 3. | Huguet F, Mukherjee S, Javle M. Locally advanced pancreatic cancer: the role of definitive chemoradiotherapy. Clin Oncol (R Coll Radiol). 2014;26:560-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Massucco P, Capussotti L, Magnino A, Sperti E, Gatti M, Muratore A, Sgotto E, Gabriele P, Aglietta M. Pancreatic resections after chemoradiotherapy for locally advanced ductal adenocarcinoma: analysis of perioperative outcome and survival. Ann Surg Oncol. 2006;13:1201-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Wolfe AR, Robb R, Hegazi A, Abushahin L, Yang L, Shyu DL, Trevino JG, Cruz-Monserrate Z, Jacob JR, Palanichamy K, Chakravarti A, Williams TM. Altered Gemcitabine and Nab-paclitaxel Scheduling Improves Therapeutic Efficacy Compared with Standard Concurrent Treatment in Preclinical Models of Pancreatic Cancer. Clin Cancer Res. 2021;27:554-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Borsoi C, Leonard F, Lee Y, Zaid M, Elganainy D, Alexander JF, Kai M, Liu YT, Kang Y, Liu X, Koay EJ, Ferrari M, Godin B, Yokoi K. Gemcitabine enhances the transport of nanovector-albumin-bound paclitaxel in gemcitabine-resistant pancreatic ductal adenocarcinoma. Cancer Lett. 2017;403:296-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Liang C, Shi S, Meng Q, Liang D, Ji S, Zhang B, Qin Y, Xu J, Ni Q, Yu X. Do anti-stroma therapies improve extrinsic resistance to increase the efficacy of gemcitabine in pancreatic cancer? Cell Mol Life Sci. 2018;75:1001-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Rajeshkumar NV, Yabuuchi S, Pai SG, Tong Z, Hou S, Bateman S, Pierce DW, Heise C, Von Hoff DD, Maitra A, Hidalgo M. Superior therapeutic efficacy of nab-paclitaxel over cremophor-based paclitaxel in locally advanced and metastatic models of human pancreatic cancer. Br J Cancer. 2016;115:442-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Azmi AS, Khan HY, Muqbil I, Aboukameel A, Neggers JE, Daelemans D, Mahipal A, Dyson G, Kamgar M, Al-Hallak MN, Tesfaye A, Kim S, Shidham V, M Mohammad R, Philip PA. Preclinical Assessment with Clinical Validation of Selinexor with Gemcitabine and Nab-Paclitaxel for the Treatment of Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. 2020;26:1338-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Versteijne E, van Dam JL, Suker M, Janssen QP, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, Buijsen J, Busch OR, Creemers GM, van Dam RM, Eskens FALM, Festen S, de Groot JWB, Groot Koerkamp B, de Hingh IH, Homs MYV, van Hooft JE, Kerver ED, Luelmo SAC, Neelis KJ, Nuyttens J, Paardekooper GMRM, Patijn GA, van der Sangen MJC, de Vos-Geelen J, Wilmink JW, Zwinderman AH, Punt CJ, van Tienhoven G, van Eijck CHJ; Dutch Pancreatic Cancer Group. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J Clin Oncol. 2022;40:1220-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 491] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 11. | Beutel AK, Halbrook CJ. Barriers and opportunities for gemcitabine in pancreatic cancer therapy. Am J Physiol Cell Physiol. 2023;324:C540-C552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 12. | Halbrook CJ, Pontious C, Kovalenko I, Lapienyte L, Dreyer S, Lee HJ, Thurston G, Zhang Y, Lazarus J, Sajjakulnukit P, Hong HS, Kremer DM, Nelson BS, Kemp S, Zhang L, Chang D, Biankin A, Shi J, Frankel TL, Crawford HC, Morton JP, Pasca di Magliano M, Lyssiotis CA. Macrophage-Released Pyrimidines Inhibit Gemcitabine Therapy in Pancreatic Cancer. Cell Metab. 2019;29:1390-1399.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 322] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 13. | Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Control Release. 2013;170:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 369] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 14. | Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S, Tabernero J, Teixeira L, Tortora G, Van Laethem JL, Young R, Penenberg DN, Lu B, Romano A, Von Hoff DD. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107:dju413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 466] [Article Influence: 42.4] [Reference Citation Analysis (3)] |
| 15. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 5121] [Article Influence: 393.9] [Reference Citation Analysis (12)] |
| 16. | Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 1517] [Article Influence: 189.6] [Reference Citation Analysis (0)] |
| 17. | Missiaen R, Mazzone M, Bergers G. The reciprocal function and regulation of tumor vessels and immune cells offers new therapeutic opportunities in cancer. Semin Cancer Biol. 2018;52:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Liu Y, Liu Z, Yang Y, Cui J, Sun J, Liu Y. The prognostic and biology of tumour-infiltrating lymphocytes in the immunotherapy of cancer. Br J Cancer. 2023;129:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Guo M, Sheng W, Yuan X, Wang X. Neutrophils as promising therapeutic targets in pancreatic cancer liver metastasis. Int Immunopharmacol. 2024;140:112888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Pergolizzi RG, Brower ST. Molecular Targets for the Diagnosis and Treatment of Pancreatic Cancer. Int J Mol Sci. 2024;25:10843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 21. | Lee S, Rauch J, Kolch W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int J Mol Sci. 2020;21:1102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 561] [Article Influence: 93.5] [Reference Citation Analysis (0)] |
| 22. | Yin X, Xu R, Song J, Ruze R, Chen Y, Wang C, Xu Q. Lipid metabolism in pancreatic cancer: emerging roles and potential targets. Cancer Commun (Lond). 2022;42:1234-1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 82] [Reference Citation Analysis (1)] |
| 23. | Zhang QL, Wu TT, Han Y, Zheng ZM, Zhang Y. Chemotherapy-Induced Myelosuppression in Esophageal Cancer Patients: Risks and Suggestions for Its Management. Curr Med Sci. 2022;42:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 24. | Saito K, Michihata N, Hamada T, Jo T, Matsui H, Fushimi K, Nakai Y, Yasunaga H, Fujishiro M. Gemcitabine plus nab-paclitaxel for pancreatic cancer and interstitial lung disease: A nationwide longitudinal study. Cancer Sci. 2023;114:3996-4005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Kaufman DL. GABA molecules made by B cells can dampen antitumour responses. Nature. 2021;599:374-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Nicolini A, Ferrari P. Involvement of tumor immune microenvironment metabolic reprogramming in colorectal cancer progression, immune escape, and response to immunotherapy. Front Immunol. 2024;15:1353787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 81] [Reference Citation Analysis (0)] |
| 27. | Martinez-Bosch N, Vinaixa J, Navarro P. Immune Evasion in Pancreatic Cancer: From Mechanisms to Therapy. Cancers (Basel). 2018;10:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 28. | Bischoff L, Alvarez S, Dai DL, Soukhatcheva G, Orban PC, Verchere CB. Cellular mechanisms of CCL22-mediated attenuation of autoimmune diabetes. J Immunol. 2015;194:3054-3064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Jarosz-Biej M, Smolarczyk R, Cichoń T, Kułach N. Tumor Microenvironment as A "Game Changer" in Cancer Radiotherapy. Int J Mol Sci. 2019;20:3212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 397] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 30. | Liu J, Huang Z, Chen HN, Qin S, Chen Y, Jiang J, Zhang Z, Luo M, Ye Q, Xie N, Zhou ZG, Wei Y, Xie K, Huang C. ZNF37A promotes tumor metastasis through transcriptional control of THSD4/TGF-β axis in colorectal cancer. Oncogene. 2021;40:3394-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, El Dika IH, Segal N, Shcherba M, Sugarman R, Stadler Z, Yaeger R, Smith JJ, Rousseau B, Argiles G, Patel M, Desai A, Saltz LB, Widmar M, Iyer K, Zhang J, Gianino N, Crane C, Romesser PB, Pappou EP, Paty P, Garcia-Aguilar J, Gonen M, Gollub M, Weiser MR, Schalper KA, Diaz LA Jr. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med. 2022;386:2363-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1058] [Article Influence: 264.5] [Reference Citation Analysis (0)] |
| 32. | Comandatore A, Immordino B, Balsano R, Capula M, Garajovà I, Ciccolini J, Giovannetti E, Morelli L. Potential Role of Exosomes in the Chemoresistance to Gemcitabine and Nab-Paclitaxel in Pancreatic Cancer. Diagnostics (Basel). 2022;12:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Ma J, Hui P, Meng W, Wang N, Xiang S. Ku70 inhibits gemcitabine-induced DNA damage and pancreatic cancer cell apoptosis. Biochem Biophys Res Commun. 2017;484:746-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Monteleone G, Franzè E, Maresca C, Colella M, Pacifico T, Stolfi C. Targeted Therapy of Interleukin-34 as a Promising Approach to Overcome Cancer Therapy Resistance. Cancers (Basel). 2023;15:971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 35. | Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17:527-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 854] [Cited by in RCA: 905] [Article Influence: 150.8] [Reference Citation Analysis (0)] |
| 36. | Miyanaga A, Asahina H, Watanabe S, Shukuya T, Tsubata Y, Hosomi Y, Sugawara S, Maemondo M, Okano T, Morita S, Matsuyama K, Kobayashi K, Seike M. A Phase I/II Study of Necitumumab Plus Pembrolizumab, Nab-Paclitaxel, and Carboplatin for Previously Untreated Advanced Squamous Non-Small Cell Lung Cancer Study: (NEJ048A/NEXUS). Clin Lung Cancer. 2023;24:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 37. | Ko AH, Kim KP, Siveke JT, Lopez CD, Lacy J, O'Reilly EM, Macarulla T, Manji GA, Lee J, Ajani J, Alsina Maqueda M, Rha SY, Lau J, Al-Sakaff N, Allen S, Lu D, Shemesh CS, Gan X, Cha E, Oh DY. Atezolizumab Plus PEGPH20 Versus Chemotherapy in Advanced Pancreatic Ductal Adenocarcinoma and Gastric Cancer: MORPHEUS Phase Ib/II Umbrella Randomized Study Platform. Oncologist. 2023;28:553-e472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 38. | de Nigris F, Schiano C, Infante T, Napoli C. CXCR4 inhibitors: tumor vasculature and therapeutic challenges. Recent Pat Anticancer Drug Discov. 2012;7:251-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Lee JC, Shin DW, Park H, Kim J, Youn Y, Kim JH, Kim J, Hwang JH. Tolerability and safety of EUS-injected adenovirus-mediated double-suicide gene therapy with chemotherapy in locally advanced pancreatic cancer: a phase 1 trial. Gastrointest Endosc. 2020;92:1044-1052.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 40. | Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 1715] [Article Influence: 122.5] [Reference Citation Analysis (0)] |
| 41. | Carvalho TMA, Di Molfetta D, Greco MR, Koltai T, Alfarouk KO, Reshkin SJ, Cardone RA. Tumor Microenvironment Features and Chemoresistance in Pancreatic Ductal Adenocarcinoma: Insights into Targeting Physicochemical Barriers and Metabolism as Therapeutic Approaches. Cancers (Basel). 2021;13:6135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 42. | Anfray C, Ummarino A, Andón FT, Allavena P. Current Strategies to Target Tumor-Associated-Macrophages to Improve Anti-Tumor Immune Responses. Cells. 2019;9:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 234] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 43. | Wittel UA, Lubgan D, Ghadimi M, Belyaev O, Uhl W, Bechstein WO, Grützmann R, Hohenberger WM, Schmid A, Jacobasch L, Croner RS, Reinacher-Schick A, Hopt UT, Pirkl A, Oettle H, Fietkau R, Golcher H. Consensus in determining the resectability of locally progressed pancreatic ductal adenocarcinoma - results of the Conko-007 multicenter trial. BMC Cancer. 2019;19:979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Sütcüoğlu O, Doğan A, Yilmaz F, Şahin AB, Şahin TK, Esen SA, Erol C, Üner A, Özet A, Turan N, Eraslan E, Deligönül A, Odabaş H, Günel N, Uçar G, Dede DŞ, Dizdar Ö, Çubukçu E, Öksüzoğlu ÖB, Emre Yildirim M, Yazici O, Özdemir N. Retrospective Evaluation of the Efficacy of Gemcitabine-Based Therapies After FOLFIRINOX Failure in Advanced Pancreatic Cancer, Multi-Center Real-Life Data. Pancreas. 2023;52:e235-e240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Hartlapp I, Valta-Seufzer D, Siveke JT, Algül H, Goekkurt E, Siegler G, Martens UM, Waldschmidt D, Pelzer U, Fuchs M, Kullmann F, Boeck S, Ettrich TJ, Held S, Keller R, Anger F, Germer CT, Stang A, Kimmel B, Heinemann V, Kunzmann V; German Pancreatic Cancer Group (AIO-PAK) and NEOLAP investigators. Prognostic and predictive value of CA 19-9 in locally advanced pancreatic cancer treated with multiagent induction chemotherapy: results from a prospective, multicenter phase II trial (NEOLAP-AIO-PAK-0113). ESMO Open. 2022;7:100552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Rogers SJ, Datta NR, Puric E, Timm O, Marder D, Khan S, Mamot C, Knuchel J, Siebenhüner A, Pestalozzi B, Guckenberger M, Bodis S, Riesterer O. The addition of deep hyperthermia to gemcitabine-based chemoradiation may achieve enhanced survival in unresectable locally advanced adenocarcinoma of the pancreas. Clin Transl Radiat Oncol. 2021;27:109-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Shibata Y, Uemura K, Kondo N, Sumiyoshi T, Okada K, Seo S, Otsuka H, Murakami Y, Arihiro K, Takahashi S. Long-term survival after distal pancreatectomy with celiac axis resection and hepatic artery reconstruction in the setting of locally advanced unresectable pancreatic cancer. Clin J Gastroenterol. 2022;15:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Kunzmann V, Siveke JT, Algül H, Goekkurt E, Siegler G, Martens U, Waldschmidt D, Pelzer U, Fuchs M, Kullmann F, Boeck S, Ettrich TJ, Held S, Keller R, Klein I, Germer CT, Stein H, Friess H, Bahra M, Jakobs R, Hartlapp I, Heinemann V; German Pancreatic Cancer Working Group (AIO-PAK) and NEOLAP investigators. Nab-paclitaxel plus gemcitabine versus nab-paclitaxel plus gemcitabine followed by FOLFIRINOX induction chemotherapy in locally advanced pancreatic cancer (NEOLAP-AIO-PAK-0113): a multicentre, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 49. | Tseng SW, Chen WM, Shia BC, Chen MC, Wu SY. Correlation of maintenance chemotherapy and improved survival in patients with locally advanced unresectable pancreatic head adenocarcinoma receiving neoadjuvant chemotherapy and concurrent chemoradiotherapy. Am J Cancer Res. 2024;14:2313-2325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 50. | Cao J, Huang D, Peppas NA. Advanced engineered nanoparticulate platforms to address key biological barriers for delivering chemotherapeutic agents to target sites. Adv Drug Deliv Rev. 2020;167:170-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 51. | Cho H, Jeon SI, Ahn CH, Shim MK, Kim K. Emerging Albumin-Binding Anticancer Drugs for Tumor-Targeted Drug Delivery: Current Understandings and Clinical Translation. Pharmaceutics. 2022;14:728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 52. | Han X, Gong F, Chi L, Feng C, Sun J, Chen Y, Liu J, Shen Y. Cancer-targeted and glutathione-responsive micellar carriers for controlled delivery of cabazitaxel. Nanotechnology. 2019;30:055601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Sarma K, Akther MH, Ahmad I, Afzal O, Altamimi ASA, Alossaimi MA, Jaremko M, Emwas AH, Gautam P. Adjuvant Novel Nanocarrier-Based Targeted Therapy for Lung Cancer. Molecules. 2024;29:1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 54. | Kang M, Yoo S, Jung Y, Lim H, Lee MH, Ryu JK, Lee JI. Factors affecting peripheral neuropathy induced by nanoparticle albumin-bound paclitaxel in patients with pancreatic cancer. Br J Clin Pharmacol. 2024;90:3232-3241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 55. | Wang M, Xue W, Yuan H, Wang Z, Yu L. Nano-Drug Delivery Systems Targeting CAFs: A Promising Treatment for Pancreatic Cancer. Int J Nanomedicine. 2024;19:2823-2849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 56. | Mita J, Iguchi T, Iseda N, Takada K, Hirose K, Miura N, Honboh T, Emi Y, Akashi T, Kato S, Sadanaga N, Matsuura H. A case of successful conversion surgery for locally advanced pancreatic cancer with synchronous triple cancer of the lung and esophagus: a case report. Surg Case Rep. 2022;8:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 57. | Nichetti F, Rota S, Ambrosini P, Pircher C, Gusmaroli E, Droz Dit Busset M, Pusceddu S, Sposito C, Coppa J, Morano F, Pietrantonio F, Di Bartolomeo M, Mariani L, Mazzaferro V, de Braud F, Niger M. NALIRIFOX, FOLFIRINOX, and Gemcitabine With Nab-Paclitaxel as First-Line Chemotherapy for Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis. JAMA Netw Open. 2024;7:e2350756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 82] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 58. | Bodeker KL, Smith BJ, Berg DJ, Chandrasekharan C, Sharif S, Fei N, Vollstedt S, Brown H, Chandler M, Lorack A, McMichael S, Wulfekuhle J, Wagner BA, Buettner GR, Allen BG, Caster JM, Dion B, Kamgar M, Buatti JM, Cullen JJ. A randomized trial of pharmacological ascorbate, gemcitabine, and nab-paclitaxel for metastatic pancreatic cancer. Redox Biol. 2024;77:103375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 59. | Zhou X, Zhang L, Qierang C, Huang M, Yang X, Li L, Jiang J. Investigating the relationship between secreted protein acidic and rich in cysteine expression level and therapeutic efficacy of nab-paclitaxel: a meta-analysis. Transl Cancer Res. 2021;10:876-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 60. | Lapidari P, Vaz-Luis I, Di Meglio A. Side effects of using granulocyte-colony stimulating factors as prophylaxis of febrile neutropenia in cancer patients: A systematic review. Crit Rev Oncol Hematol. 2021;157:103193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang W, Yeap BY, Drapek LC, Ly L, Baglini CV, Blaszkowsky LS, Ferrone CR, Parikh AR, Weekes CD, Nipp RD, Kwak EL, Allen JN, Corcoran RB, Ting DT, Faris JE, Zhu AX, Goyal L, Berger DL, Qadan M, Lillemoe KD, Talele N, Jain RK, DeLaney TF, Duda DG, Boucher Y, Fernández-Del Castillo C, Hong TS. Total Neoadjuvant Therapy With FOLFIRINOX in Combination With Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5:1020-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 423] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 62. | Ohba A, Ozaka M, Ogawa G, Okusaka T, Kobayashi S, Yamashita T, Ikeda M, Yasuda I, Sugimori K, Sasahira N, Ikezawa K, Miki I, Okano N, Mizuno N, Furukawa M, Shirakawa H, Sano Y, Katayama H, Furuse J, Ueno M. 1616O Nab-paclitaxel plus gemcitabine versus modified FOLFIRINOX or S-IROX in metastatic or recurrent pancreatic cancer (JCOG1611, GENERATE): A multicentred, randomized, open-label, three-arm, phase II/III trial. Ann Oncol. 2023;34:S894. [DOI] [Full Text] |
| 63. | Sanomachi T, Ohba A, Ogura N, Hara H, Yagi S, Okada M, Maruki Y, Nagashio Y, Kondo S, Susumu H, Morizane C, Ueno H, Okusaka T. 341P Clinical experience with S-1 or FOLFOX as third-line treatment in patients with metastatic pancreatic cancer who received first-line gemcitabine + nab-paclitaxel and second-line nanoliposomal-irinotecan + 5-FU/leucovorin. Ann Oncol. 2024;35:S142. [DOI] [Full Text] |
| 64. | Huang C, Cheng CS, Shen Y, Chen H, Lin J, Hua Y, Feng L, Wu C, Wang P, Chen Z, Meng Z. Digital subtraction angiography-guided pancreatic arterial infusion of GEMOX chemotherapy in advanced pancreatic adenocarcinoma: a phase II, open-label, randomized controlled trial comparing with intravenous chemotherapy. BMC Cancer. 2024;24:941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 65. | Kang J, Hwang I, Yoo C, Kim KP, Jeong JH, Chang HM, Lee SS, Park DH, Song TJ, Seo DW, Lee SK, Kim MH, Hong SM, Shin SH, Hwang DW, Song KB, Lee JH, Kim SC, Ryoo BY. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: retrospective analysis. Invest New Drugs. 2018;36:732-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 66. | Carvalho de Brito AB, Riechelmann RP, Fonseca de Jesus VH. Impact of Granulocyte Colony-Stimulating Factor (G-CSF) on the Outcomes of Patients With Metastatic Pancreatic Adenocarcinoma (MPA) During First-Line Treatment With FOLFIRINOX: A Single-Center Retrospective Analysis. Cancer Control. 2023;30:10732748221149543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 67. | Postma TJ, Heimans JJ, Muller MJ, Ossenkoppele GJ, Vermorken JB, Aaronson NK. Pitfalls in grading severity of chemotherapy-induced peripheral neuropathy. Ann Oncol. 1998;9:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 162] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 68. | Konecny G, Untch M, Slamon D, Beryt M, Kahlert S, Felber M, Langer E, Lude S, Hepp H, Pegram M. Drug interactions and cytotoxic effects of paclitaxel in combination with carboplatin, epirubicin, gemcitabine or vinorelbine in breast cancer cell lines and tumor samples. Breast Cancer Res Treat. 2001;67:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Theodossiou C, Cook JA, Fisher J, Teague D, Liebmann JE, Russo A, Mitchell JB. Interaction of gemcitabine with paclitaxel and cisplatin in human tumor cell lines. Int J Oncol. 1998;12:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Passacantilli I, Panzeri V, Terracciano F, Delle Fave G, Sette C, Capurso G. Co-treatment with gemcitabine and nab-paclitaxel exerts additive effects on pancreatic cancer cell death. Oncol Rep. 2018;39:1984-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 71. | Lee KW, Chan SL. Hepatotoxicity of targeted therapy for cancer. Expert Opin Drug Metab Toxicol. 2016;12:789-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Hohmann N, Sprick MR, Pohl M, Ahmed A, Burhenne J, Kirchner M, Le Cornet L, Kratzmann M, Hajda J, Stenzinger A, Steindorf K, Delorme S, Schlemmer HP, Riethdorf S, van Schaik R, Pantel K, Siveke J, Seufferlein T, Jäger D, Haefeli WE, Trumpp A, Springfeld C. Protocol of the IntenSify-Trial: An open-label phase I trial of the CYP3A inhibitor cobicistat and the cytostatics gemcitabine and nab-paclitaxel in patients with advanced stage or metastatic pancreatic ductal adenocarcinoma to evaluate the combination's pharmacokinetics, safety, and efficacy. Clin Transl Sci. 2023;16:2483-2493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 73. | Chang C, Li X, Cao D. Combination of gemcitabine, nab-paclitaxel, and S-1(GAS) as the first-line treatment for patients with locally advanced or advanced pancreatic ductal adenocarcinoma: study protocol for an open-label, single-arm phase I study. BMC Cancer. 2021;21:545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Philip PA, Lacy J, Portales F, Sobrero A, Pazo-Cid R, Manzano Mozo JL, Kim EJ, Dowden S, Zakari A, Borg C, Terrebonne E, Rivera F, Sastre J, Bathini V, López-Trabada D, Asselah J, Saif MW, Shiansong Li J, Ong TJ, Nydam T, Hammel P. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study. Lancet Gastroenterol Hepatol. 2020;5:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 75. | Mucciolo G, Araos Henríquez J, Jihad M, Pinto Teles S, Manansala JS, Li W, Ashworth S, Lloyd EG, Cheng PSW, Luo W, Anand A, Sawle A, Piskorz A, Biffi G. EGFR-activated myofibroblasts promote metastasis of pancreatic cancer. Cancer Cell. 2024;42:101-118.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 76. | Del Chiaro M, Sugawara T, Karam SD, Messersmith WA. Advances in the management of pancreatic cancer. BMJ. 2023;383:e073995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 77. | Marschner N, Haug N, Hegewisch-Becker S, Reiser M, Dörfel S, Lerchenmüller C, Linde H, Wolf T, Hof A, Kaiser-Osterhues A, Potthoff K, Jänicke M; TPK‐Group (Tumour Registry Pancreatic Cancer). Head-to-head comparison of treatment sequences in advanced pancreatic cancer-Real-world data from the prospective German TPK clinical cohort study. Int J Cancer. 2024;155:1629-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | Lee YP, Oh SY, Kim KM, Go SI, Kim JH, Huh SJ, Kang JH, Ji JH. Modified FOLFIRINOX as a Second-Line Treatment for Patients with Gemcitabine-Failed Advanced Biliary Tract Cancer: A Prospective Multicenter Phase II Study. Cancers (Basel). 2022;14:1950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Galvano A, Castiglia M, Rizzo S, Silvestris N, Brunetti O, Vaccaro G, Gristina V, Barraco N, Bono M, Guercio G, Graceffa G, Fulfaro F, Gori S, Bazan V, Russo A. Moving the Target on the Optimal Adjuvant Strategy for Resected Pancreatic Cancers: A Systematic Review with Meta-Analysis. Cancers (Basel). 2020;12:534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 80. | Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS, Braiteh F, Ritch PS, Zalupski MM, Bahary N, Oberstein PE, Wang-Gillam A, Wu W, Chondros D, Jiang P, Khelifa S, Pu J, Aldrich C, Hendifar AE. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J Clin Oncol. 2018;36:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 362] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 81. | Mukherji R, Debnath D, Hartley ML, Noel MS. The Role of Immunotherapy in Pancreatic Cancer. Curr Oncol. 2022;29:6864-6892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 71] [Reference Citation Analysis (0)] |
| 82. | Pant S, Smaglo BG, Huey RW, Haldar SD, Morelli MP, Willis J, Xiao L, Yuan Y, Attanasio S, Shah R, Citron F, Zhao D, Kopetz S, Maitra A, Welsch D, Sheth R, Bhosale P, Wolff RA, Viale A. A phase 1b dose escalation trial of gemcitabine and nab-paclitaxel in combination with lixumistat in patients with advanced pancreatic cancer. J Clin Oncol. 2025;43:743-743. [DOI] [Full Text] |
| 83. | Deiana C, Agostini M, Brandi G, Giovannetti E. The trend toward more target therapy in pancreatic ductal adenocarcinoma. Expert Rev Anticancer Ther. 2024;24:525-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 84. | Bansod S, Dodhiawala PB, Lim KH. Oncogenic KRAS-Induced Feedback Inflammatory Signaling in Pancreatic Cancer: An Overview and New Therapeutic Opportunities. Cancers (Basel). 2021;13:5481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 85. | Chen XY, Wu ZX, Wang JQ, Teng QX, Tang H, Liu Q, Chen ZS, Chen W. Multidrug resistance transporters P-gp and BCRP limit the efficacy of ATR inhibitor ceralasertib in cancer cells. Front Pharmacol. 2024;15:1400699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 86. | Jin L, Kim HS, Shi J. Neutrophil in the Pancreatic Tumor Microenvironment. Biomolecules. 2021;11:1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 87. | Wang WQ, Liu L, Xu HX, Wu CT, Xiang JF, Xu J, Liu C, Long J, Ni QX, Yu XJ. Infiltrating immune cells and gene mutations in pancreatic ductal adenocarcinoma. Br J Surg. 2016;103:1189-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 88. | Chabanon RM, Muirhead G, Krastev DB, Adam J, Morel D, Garrido M, Lamb A, Hénon C, Dorvault N, Rouanne M, Marlow R, Bajrami I, Cardeñosa ML, Konde A, Besse B, Ashworth A, Pettitt SJ, Haider S, Marabelle A, Tutt AN, Soria JC, Lord CJ, Postel-Vinay S. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J Clin Invest. 2019;129:1211-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 263] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 89. | Tian Y, Lei Y, Wang Y, Lai J, Wang J, Xia F. Mechanism of multidrug resistance to chemotherapy mediated by P-glycoprotein (Review). Int J Oncol. 2023;63:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 91] [Reference Citation Analysis (0)] |
| 90. | Yao Y, Xu X, Yang L, Zhu J, Wan J, Shen L, Xia F, Fu G, Deng Y, Pan M, Guo Q, Gao X, Li Y, Rao X, Zhou Y, Liang L, Wang Y, Zhang J, Zhang H, Li G, Zhang L, Peng J, Cai S, Hu C, Gao J, Clevers H, Zhang Z, Hua G. Patient-Derived Organoids Predict Chemoradiation Responses of Locally Advanced Rectal Cancer. Cell Stem Cell. 2020;26:17-26.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 498] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 91. | Huang Y, Liang W, Yang Y, Zhao L, Zhao H, Wu X, Zhao Y, Zhang Y, Zhang L. Phase I/II dose-finding study of nanoparticle albumin-bound paclitaxel (nab®-Paclitaxel) plus Cisplatin as Treatment for Metastatic Nasopharyngeal Carcinoma. BMC Cancer. 2016;16:464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Pereira BA, Ritchie S, Chambers CR, Gordon KA, Magenau A, Murphy KJ, Nobis M, Tyma VM, Liew YF, Lucas MC, Naeini MM, Barkauskas DS, Chacon-Fajardo D, Howell AE, Parker AL, Warren SC, Reed DA, Lee V, Metcalf XL, Lee YK, O'Regan LP, Zhu J, Trpceski M, Fontaine ARM, Stoehr J, Rouet R, Lin X, Chitty JL, Porazinski S, Wu SZ, Filipe EC, Cadell AL, Holliday H, Yang J, Papanicolaou M, Lyons RJ, Zaratzian A, Tayao M, Da Silva A, Vennin C, Yin J, Dew AB, McMillan PJ, Goldstein LD, Deveson IW, Croucher DR, Samuel MS, Sim HW, Batten M, Chantrill L, Grimmond SM, Gill AJ, Samra J, Jeffry Evans TR, Sasaki T, Phan TG, Swarbrick A, Sansom OJ, Morton JP; Australian Pancreatic Cancer Matrix Atlas (APMA); Australian Pancreatic Cancer Genome Initiative (APGI), Pajic M, Parker BL, Herrmann D, Cox TR, Timpson P. Temporally resolved proteomics identifies nidogen-2 as a cotarget in pancreatic cancer that modulates fibrosis and therapy response. Sci Adv. 2024;10:eadl1197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 93. | Peng X, Zhao Z, Liu L, Bai L, Tong R, Yang H, Zhong L. Targeting Indoleamine Dioxygenase and Tryptophan Dioxygenase in Cancer Immunotherapy: Clinical Progress and Challenges. Drug Des Devel Ther. 2022;16:2639-2657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 94. | Fujiwara Y, Kato S, Nesline MK, Conroy JM, DePietro P, Pabla S, Kurzrock R. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat Rev. 2022;110:102461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 200] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 95. | Le Naour J, Galluzzi L, Zitvogel L, Kroemer G, Vacchelli E. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2020;9:1777625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 96. | Yang Z, Guan F, Bronk L, Zhao L. Multi-omics approaches for biomarker discovery in predicting the response of esophageal cancer to neoadjuvant therapy: A multidimensional perspective. Pharmacol Ther. 2024;254:108591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 97. | Huang S, Chaudhary K, Garmire LX. More Is Better: Recent Progress in Multi-Omics Data Integration Methods. Front Genet. 2017;8:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 385] [Cited by in RCA: 438] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 98. | Correa-Aguila R, Alonso-Pupo N, Hernández-Rodríguez EW. Multi-omics data integration approaches for precision oncology. Mol Omics. 2022;18:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 99. | Tebani A, Afonso C, Marret S, Bekri S. Omics-Based Strategies in Precision Medicine: Toward a Paradigm Shift in Inborn Errors of Metabolism Investigations. Int J Mol Sci. 2016;17:1555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 100. | Reina C, Šabanović B, Lazzari C, Gregorc V, Heeschen C. Unlocking the future of cancer diagnosis - promises and challenges of ctDNA-based liquid biopsies in non-small cell lung cancer. Transl Res. 2024;272:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 101. | Khalighi S, Reddy K, Midya A, Pandav KB, Madabhushi A, Abedalthagafi M. Artificial intelligence in neuro-oncology: advances and challenges in brain tumor diagnosis, prognosis, and precision treatment. NPJ Precis Oncol. 2024;8:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 85] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 102. | Diab HMH, Smith HG, Jensen KK, Jørgensen LN. The current role of blood-based biomarkers in surgical decision-making in patients with localised pancreatic cancer: A systematic review. Eur J Cancer. 2021;154:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 103. | Cox M 3rd, Vitello D, Chawla A. Translating the multifaceted use of liquid biopsy to management of early disease in pancreatic adenocarcinoma. Front Oncol. 2025;15:1520717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 104. | Malla M, Loree JM, Kasi PM, Parikh AR. Using Circulating Tumor DNA in Colorectal Cancer: Current and Evolving Practices. J Clin Oncol. 2022;40:2846-2857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 179] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 105. | Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4351] [Cited by in RCA: 4217] [Article Influence: 145.4] [Reference Citation Analysis (0)] |