Published online Oct 15, 2025. doi: 10.4251/wjgo.v17.i10.109506
Revised: July 10, 2025
Accepted: August 21, 2025
Published online: October 15, 2025
Processing time: 125 Days and 23.7 Hours
Contrast-enhanced ultrasound (CEUS) offers valuable reference data for the early diagnosis of hepatocellular carcinoma (HCC) through dynamic enhancement patterns and quantitative analysis.
To evaluate the clinical value, diagnostic accuracy, and imaging characteristics of CEUS in the early diagnosis of HCC and its correlation with HCC pathological findings.
This single-center retrospective study included 125 patients suspected of having primary liver cancer who underwent CEUS at the Department of Hepatobiliary Surgery and Imaging of our hospital from January 2022 to March 2024. All patients were diagnosed with HCC via postoperative pathology or puncture histology. All patients underwent conventional ultrasound examination and CEUS, while some underwent computed tomography or magnetic resonance imaging examination. Clinical data, liver function, serological indicators, and imaging results were collected. Key CEUS indicators, including arterial phase enhancement time (APT) and peak enhancement intensity (PEI), were analyzed.
Of the 125 patients, 66.40% were male, with a mean age of 56.74 ± 11.25 years. Conventional type HCC accounted for 71.20%, with histological grades I (14.40%), II (51.20%), and III-IV (34.40%). CEUS enhancement patterns included “fast-in and fast-out” (36%), “fast-in and slow-out” (40%), and “continuous enhancement” (24%). APT < 15 seconds was observed in 40% of patients, and PEI ≥ 1.5 in 56%. Correlation analysis revealed significant negative correlations between tumor differentiation grade and APT, washout completion time, and longest diameter (P < 0.01). Logistic regression identified PEI [odds ratio (OR) = 3.374], WIT (OR = 0.541), lesion boundary characteristics, and APT (OR = 0.471) as significant predictors. Receiver operating characteristic analysis demonstrated high diagnostic performance: PEI (area under the curve = 0.893), WIT (0.851), lesion boundary characteristics (0.876), and APT (0.864), all with Youden’s index > 0.4. Subgroup analysis showed comparable overall diagnostic performance between CEUS and computed tomography/magnetic resonance imaging, but computed tomography/magnetic resonance imaging had higher sensitivity and specificity for Liver Imaging Reporting and Data System 5 lesions (P = 0.032).
CEUS holds significant clinical value in the early diagnosis of HCC, as it effectively identifies the typical imaging characteristics of early-stage HCC through dynamic contrast enhancement and quantitative analysis, particularly during the arterial and portal phases. As a non-invasive, cost-effective, and efficient imaging modality, CEUS has a broad clinical application potential.
Core Tip: Contrast-enhanced ultrasound (CEUS) plays an important role in the early diagnosis of hepatocellular carcinoma. By observing characteristic enhancement patterns - such as arterial phase hyperenhancement and portal/delayed phase washout - CEUS provides critical insights into tumor vascularity and behavior. Quantitative indicators such as arterial phase time and peak enhancement intensity further improve diagnostic accuracy. Compared with computed tomography or magnetic resonance imaging, CEUS offers advantages including real-time imaging, lower cost, no ionizing radiation, and better sensitivity for small or early-stage lesions. These strengths support its use in early detection, pathological correlation, and timely clinical intervention for hepatocellular carcinoma.
- Citation: Dong Y, Chen LP, He JG, Yang QQ, Hu ZW. Clinical value of contrast-enhanced ultrasound in early diagnosis of hepatocellular carcinoma. World J Gastrointest Oncol 2025; 17(10): 109506
- URL: https://www.wjgnet.com/1948-5204/full/v17/i10/109506.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i10.109506
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor worldwide, and the third leading cause of cancer-related death, primarily because of the low rate of early detection[1]. In China, the incidence of HCC is particularly high, largely attributable to hepatitis B virus (HBV) infection and a high prevalence of liver cirrhosis. Consequently, both the incidence and mortality rates of liver cancer in China have remained among the highest globally. According to the China Tumor Registration Annual Report, there are over 400000 new cases of liver cancer annually in China, with a 5-year survival rate of less than 15%. However, when diagnosed at an early stage and treated with curative options such as radical surgery or radiofrequency ablation, the 5-year survival rate can rise to over 70%, underscoring the critical importance of early detection for patient survival. Traditionally, early screening for liver cancer has relied on a combination of serum alpha-fetoprotein (AFP) testing and routine abdominal B-mode ultrasound[2]. However, this approach has limited sensitivity, particularly in cases involving AFP-negative tumors or small liver lesion measuring less than 2 cm in diameter, which may not provide sufficient imaging information for accurate diagnosis[3]. Moreover, conventional ultrasound struggles to clearly distinguish between hypoechoic nodules and atypically enhanced lesions in the background of cirrhotic liver, leading to a higher risk of misdiagnosis or missed diagnosis[4]. In this context, contrast-enhanced ultrasound (CEUS) has emerged as a promising non-invasive diagnostic tool for early-stage liver cancer. After the intravenous injection of a microbubble contrast agent (e.g., SonoVue), CEUS enables the real-time observation of liver tissue blood perfusion, allowing for the dynamic assessment of the enhancement and washout characteristics of lesions during the arterial, portal venous, and delayed phases[5]. Compared with traditional imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI), CEUS offers bedside, real-time imaging without radiation exposure, as well as enhanced sensitivity to small lesions and superior visualization of blood perfusion details, making it especially suitable for repeated follow-ups and dynamic monitoring[6]. The characteristic “fast-in, fast-out” enhancement pattern of HCC on CEUS enhances lesion recognition, particularly for small tumors measuring 1-2 cm, and is especially valuable for patient with cirrhotic liver[7]. In recent years, major international guidelines and consensus documents [e.g., the European Association for the Study of the Liver, World Federation for Ultrasound in Medicine and Biology, and Liver Imaging Reporting and Data System (LI-RADS)] have incorporated CEUS as a recommended tool for early liver cancer screening. However, CEUS still faces several challenges in clinical practice. Some tumors display atypical enhancement patterns, such as lack of arterial phase enhancement or absent washout during the portal venous phase, making differentiation from well-differentiated HCC, hepatic adenoma, focal nodular hyperplasia (FNH), and other benign lesions more challenging[8]. Moreover, CEUS interpretation is subject to inter-operator variability, equipment differences, and interference from underlying liver diseases such as steatosis or liver cirrhosis[9]. Therefore, this study aims to systematically evaluate the clinical value of CEUS in the early diagnosis of liver cancer through a retrospective analysis of liver cancer cases diagnosed at our hospital in recent years, paying special attention to cases with AFP-negative status, lesion diameter < 2 cm, and underlying cirrhosis. CEUS findings will be compared with those from conventional imaging modalities such as CT and MRI. The study seeks to clarify the diagnostic accuracy of CEUS for early HCC in different clinical contexts, optimize the imaging screening process for high-risk groups, and improve early detection rates, ultimately providing evidence to support early diagnosis and treatment strategies for liver cancer.
This single-center retrospective study included 125 patients who underwent CEUS examination for suspected primary liver cancer between January 2022 and March 2024 at the Departments of Hepatobiliary Surgery and Imaging of our hospital. All patients were diagnosed with HCC by postoperative pathology or puncture histology. The cohort consisted of 83 males and 42 females, with an age range of 35-78 years. All patients underwent conventional ultrasound and CEUS prior to diagnosis, and some cases underwent CT or MRI examinations.
The inclusion criteria including: (1) Age ≥ 18 years with full civil capacity; (2) Presence of hepatic space-occupying lesion identified on CEUS; (3) History of high-risk factors for liver cancer (e.g., HBV positivity, liver cirrhosis); (4) HCC confirmed by surgical resection or puncture pathology; and (5) Availability of complete CEUS imaging data for retrospective analysis. The exclusion criteria including: (1) Non-HCC pathological types (e.g., intrahepatic cholangiocarcinoma, metastatic carcinoma): Fourteen cases; (2) Extrahepatic metastatic carcinoma: Six cases; (3) Inability to tolerate CEUS or absence of contrast-enhanced imaging data: Five cases; and (4) Severe cardiopulmonary insufficiency, active hemorrhage, or contrast agent allergy: Three cases. After applying these criteria, 125 eligible patients with HCC were included in the final analysis to systematically evaluate clinical data and imaging features.
In this study, the baseline data of included cases were systematically collected and organized through a retrospective review of the Hospital Information System, Picture Archiving and Communication System, and Electronic Medical Record System. All data were independently extracted by two trained researchers and verified by a third researcher to ensure accuracy and completeness. The baseline data collected included the following: (1) General demographic data: Sex, age, weight, height, body mass index, history of liver disease (e.g., HBV, hepatitis C, and alcoholic liver disease), and family history of liver cancer; (2) Liver function and serological indicators: Alanine aminotransferase, aspartate aminotransferase, albumin, total bilirubin, direct bilirubin, international normalized ratio, and Child-Pugh classification; (3) Tumor-related markers: AFP (ng/mL) level, carcinoembryonic antigen, carbohydrate antigen 19-9, and other tumor biomarkers; (4) Liver imaging data: Number, size, boundary clarity, and echogenicity of lesions detected by conventional ultrasound; (5) Medical history: Presence of diabetes, hypertension, and cardiovascular or cerebrovascular diseases; and (6) Antiviral treatment history: Whether the patients received antiviral therapy and the duration of treatment - used to evaluate the effect of hepatitis management on imaging findings. All data were analyzed in accordance with patient privacy protocols. Cases with more than 15% of missing data on key variables were excluded from the final analysis.
The CEUS examinations in this study were performed and interpreted by ultrasound professionals with intermediate or higher professional titles and extensive experience in liver ultrasound imaging. The equipment used included the Philips EPIQ7 and Mindray Resona 8 color Doppler ultrasound diagnostic systems, both equipped with real-time contrast imaging capabilities and a low mechanical index (< 0.1). A convex array probe with a frequency range of 1-5 MHz was selected to balance image resolution and tissue penetration. During the examination, patients were placed in the supine position. A conventional two-dimensional ultrasound was first conducted to assess the liver and identify the location, size, shape, boundary, and internal echogenicity of any suspicious lesions. These images were archived for reference. Next, an intravenous line was established via the elbow vein, and 2.4 mL of the second-generation ultrasound contrast agent SonoVue (BRACCO) was injected rapidly, followed by a 5 mL saline flush. The enhancement process was continuously observed for more than 5 minutes, covering the following key phases: (1) Arterial phase (10-30 seconds): Assessment of enhancement degree, speed, edge clarity, and the presence of a “fast-in” enhancement pattern compared with background liver parenchyma; (2) Portal venous phase (31-120 seconds): Evaluation of whether the lesion showed hypoenhancement compared with surrounding liver tissue, and whether the “washout” pattern began to appear; and (3) Delayed phase (121-300 seconds): Observation of lesion characteristics such as further washout, hypoechogenicity, and indistinct margins, used to determine the presence of a “fast-in and fast-out” pattern.
Key contrast parameters recorded included: Time to initial contrast enhancement (seconds), time to peak enhancement and peak intensity (via software analysis), relative enhancement intensity of the lesion compared with surrounding liver tissue, washout time (start and end), and washout pattern (homogeneous/heterogeneous, annular/patchy). Each patient underwent at least one preoperative CEUS examination. In cases where the initial contrast appearance was atypical or image quality was suboptimal, a repeat CEUS was scheduled. The rescan rate was 18.5%. Suspicious lesions identified by CEUS were confirmed by either CT/MRI or liver puncture biopsy. All imaging results were reviewed independently by two attending physicians. In cases of disagreement, a senior physician acted as an arbitrator. Arterial phase enhancement time (APT) was defined as the time (in seconds) from contrast agent injection to the onset of lesion enhancement. The starting point of enhancement was determined independently by two experienced ultrasound physicians using Philips QLAB quantitative analysis software. Enhancement was defined as a lesion intensity at least 15% greater than that of the background liver parenchyma. If the discrepancy between manual and software results exceeded 3 seconds, arbitration was performed by a third senior physician. Interobserver agreement for APT interpretation was assessed using the Kappa statistic, with a κ value of 0.82, indicating strong consistency.
Histological typing: In this study, HCC was classified into three main types based on the common histological morphology: (1) Conventional type HCC: Includes three basic structural patterns: Trabecular, pseudoglandular and solid; (2) Clear cell HCC: Characterized by tumor cells with cytoplasmic vacuoles and abundant glycogen or lipid content. A diagnosis of clear cell type is made when ≥ 50% of the tumor area exhibits clear cell characteristics; and (3) Fibrolamellar HCC: A rare variant, typically seen in younger patients, characterized by large lamellar fibrous septa and eosinophilic cytoplasm.
Determination of differentiation degree: The degree of HCC differentiation was assessed according to the Edmondson-Steiner classification criteria: (1) Grade I (highly differentiated): Tumor cells resemble normal hepatocytes with regular architecture, abundant cytoplasm, and rare mitotic figures; (2) Grade II (moderately differentiated): Tumor cells exhibit moderate atypia, disorganized arrangement, and visible mitotic figures; and (3) Grades III-IV (poorly differentiated): Tumor cells show prominent atypia, enlarged hyperchromatic nuclei, increased mitotic figures, disorganized architecture, necrosis, and common vascular invasion. The following pathological features were recorded in all reports: Cell morphology, differentiation grade, necrosis percentage, vascular invasion (including portal microvascular invasion), and hepatic capsule invasion.
Immunohistochemical markers for auxiliary diagnosis: In cases where histological classification was inconclusive or further diagnostic confirmation was required, liver-specific immunohistochemical markers were employed, including hepatocyte paraffin 1, glypican-3, AFP, cytokeratin 19 (CK19), and antigen Kiel 67 (Ki-67). A Ki-67 proliferation index > 20% indicated poor tumor differentiation, while CK19 positivity suggested a stem cell-like phenotype was associated with high invasiveness.
Imaging mode: Based on the typical imaging dynamic characteristics of liver cancer, lesion enhancement patterns were classified into three types: Fast-in and fast-out, fast-in and slow-out, and continuous enhancement. The term “fast-in and fast-out” refers to “obvious enhancement in the arterial phase followed by rapid washout in the portal venous and delayed phases”, and is considered a typical manifestation of early-stage liver cancer.
APT: Refers to the time (in seconds) between contrast agent injection and the onset of lesion enhancement. An APT of < 15 seconds suggests a rich blood supply and is commonly associated with well- or moderately-differentiated HCC.
Peak enhancement intensity: Peak enhancement intensity (PEI) represents the relative intensity of lesion enhancement, automatically calculated by software. It compares the echogenicity of the lesion to that of the surrounding liver tissue. A PEI ≥ 1.5 suggests abundant vascularity and may indicate malignancy.
Washout initiation time and washout completion time: Washout initiation time (WIT) < 60 seconds and washout completion time (WCT) < 180 seconds are typical angiographic features of HCC. WIT > 90 seconds generally suggests a benign lesion or highly differentiated HCC.
Longest diameter and enhanced area: Both were measured from CEUS dynamic plots. A lesion with a longest diameter (LD) > 3 cm but not exceeding 5 cm, without vascular invasion or distant metastasis, may correspond to Barcelona Clinic Liver Cancer stage A (early stage). Lesions > 5 cm or multiple lesions with a combined diameter > 3 cm may indicate Barcelona Clinic Liver Cancer stage B (intermediate stage). Enhanced area (EA) reflects the blood supply of the tumor and may indirectly reflect the tumor microvascular density.
Lesion boundary characteristics: These include “clear”, “fuzzy”, and “annular edge enhancement”. Fuzzy margins or annular enhancement are often indicative of infiltrative growth and are highly suggestive of malignancy.
Statistical analysis were performed using SPSS 25.0 statistical software. Measurement data were expressed as mean ± SD, while categorical (enumeration) data were expressed as frequencies and percentages. Intra-group comparisons were performed using the t-test or χ2 test. For comparisons between groups, the independent sample t-test or analysis of variance was used. For data not normally distributed, results were expressed as medians and interquartile ranges, and inter-group comparisons were conducted using the Mann-Whitney U test or Kruskal-Wallis H test. To explore the correlation between key CEUS indicators and pathological findings, Spearman correlation analysis was applied to assess the relationship between continuous variables (e.g., APT and washout time) and tumor differentiation grade. A correlation was considered statistically significant if the absolute value of the correlation coefficient (|r|) was > 0.4 and P < 0.05. Furthermore, binary logistic regression analysis was performed to identify independent predictors of early liver cancer diagnosis. Lesion type (0 = benign, 1 = malignant) was set as the dependent variable, and CEUS-related indicators (e.g., PEI, WIT, lesion boundary characteristics) were used as independent variables. Model outputs included odds ratios (ORs) with 95% confidence intervals and P-values. The level of statistical significance was set at P < 0.05. The diagnostic performance of each significant predictor was evaluated using receiver operating characteristic (ROC) curve analysis. The area under the ROC curve (AUC) was calculated, with an AUC greater than 0.85 considered indicative of high diagnostic value. Sensitivity, specificity, and Youden’s index were also calculated to determine the optimal cutoff values. A total of 125 patients who underwent both CEUS and CT/MRI examinations were included in the analysis. The diagnostic performance of the two modalities for detecting early-stage HCC (≤ 3 cm) was compared using pathological findings as the gold standard. All statistical tests were two-sided, and P < 0.05 indicated statistical significance.
A total of 125 patients were included in this study, with 66.40% being male. The mean age of participants was 56.74 ± 11.25 years, with a mean body mass index of 23.58 ± 3.45 kg/m2. Baseline characteristics including hepatitis B history (50.40%), lesion characteristics (single lesion 72.80%, maximum diameter 3.74 ± 1.85 cm, clear boundary 70.40%) are shown in Table 1.
| Characteristic | Value or case |
| Male | 83 (66.40) |
| Age (years) | 56.74 ± 11.25 |
| BMI (kg/m2) | 23.58 ± 3.45 |
| History of hepatitis B | 63 (50.40) |
| Alcoholic liver disease | 19 (15.20) |
| Family history of liver cancer | 28 (22.40) |
| ALT (U/L) | 48.73 ± 21.16 |
| AST (U/L) | 44.82 ± 19.87 |
| ALB (g/L) | 38.42 ± 4.37 |
| TBIL (μmol/L) | 22.48 ± 6.89 |
| AFP (ng/mL) | 392.35 ± 106.33 |
| Single lesion | 91 (72.80) |
| Maximum diameter of lesion (cm) | 3.74 ± 1.85 |
| Clear boundary | 88 (70.40) |
| Hypoechoic lesions | 72 (57.60) |
Among the 125 HCC patients, 71.20% had conventional type HCC, 19.20% clear cell type, and 9.60% fibrolamellar type. Differentiation grades were: Grade I (14.40%), grade II (51.20%), grades III-IV (34.40%). Immunohistochemical markers showed: Ki-67 PI > 20% (37.60%), CK19+ (23.20%), AFP+ (78.40%), glypican-3+ (90.40%). Microvascular invasion and capsule invasion rates were 33.60% and 24.80% respectively (Table 2).
| Project | n (%) |
| Conventional type HCC | 89 (71.20) |
| Clear cell HCC | 24 (19.20) |
| Fibrolamellar HCC | 12 (9.60) |
| Grade I (highly differentiated) | 18 (14.40) |
| Grade II (moderate differentiation) | 64 (51.20) |
| Grade III-IV (low differentiation) | 43 (34.40) |
| Ki-67 > 20% | 47 (37.60) |
| CK19 positive | 29 (23.20) |
| AFP positive | 98 (78.40) |
| Glypican-3 positive | 113 (90.40) |
| MVI positive | 42 (33.60) |
| Capsule invasion | 31 (24.80) |
Enhancement patterns distribution: Fast-in and fast-out (36%, 45 cases), fast-in and slow-out (40%, 50 cases), continuous enhancement (24%, 30 cases) (Table 3).
| Project | n (%) |
| Fast-in and fast-out | 45 (36) |
| Fast-in and slow-out | 50 (40) |
| Continuous enhancement | 30 (24) |
APT distribution: < 15 seconds (40%, 50 patients), ≥ 15 seconds (60%, 75 patients). Interobserver agreement was high (κ = 0.82) (Table 4).
| Project | n (%) |
| APT < 15 seconds | 50 (40) |
| APT ≥ 15 seconds | 75 (60) |
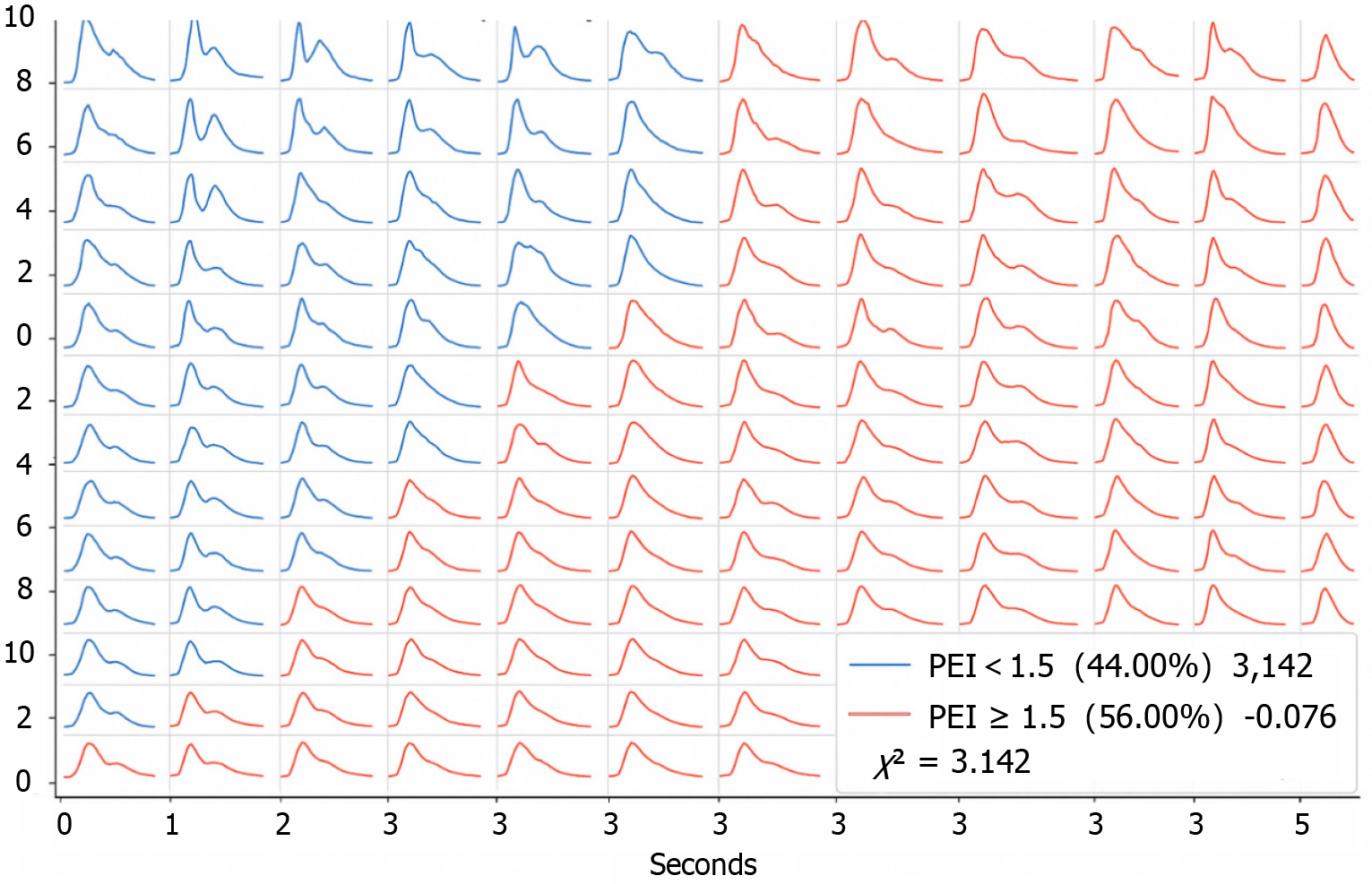
PEI distribution: < 1.5 (44%), ≥ 1.5 (56%) (Figure 1).
WIT distribution: < 60 seconds (44%), ≥ 60 seconds (56%). WCT distribution: < 180 seconds (48%), ≥ 180 seconds (52%) (Table 5).
| Project | n (%) |
| WIT < 60 seconds | 55 (44) |
| WIT ≥ 60 seconds | 70 (56) |
| WCT < 180 seconds | 60 (48) |
| WCT ≥ 180 seconds | 65 (52) |
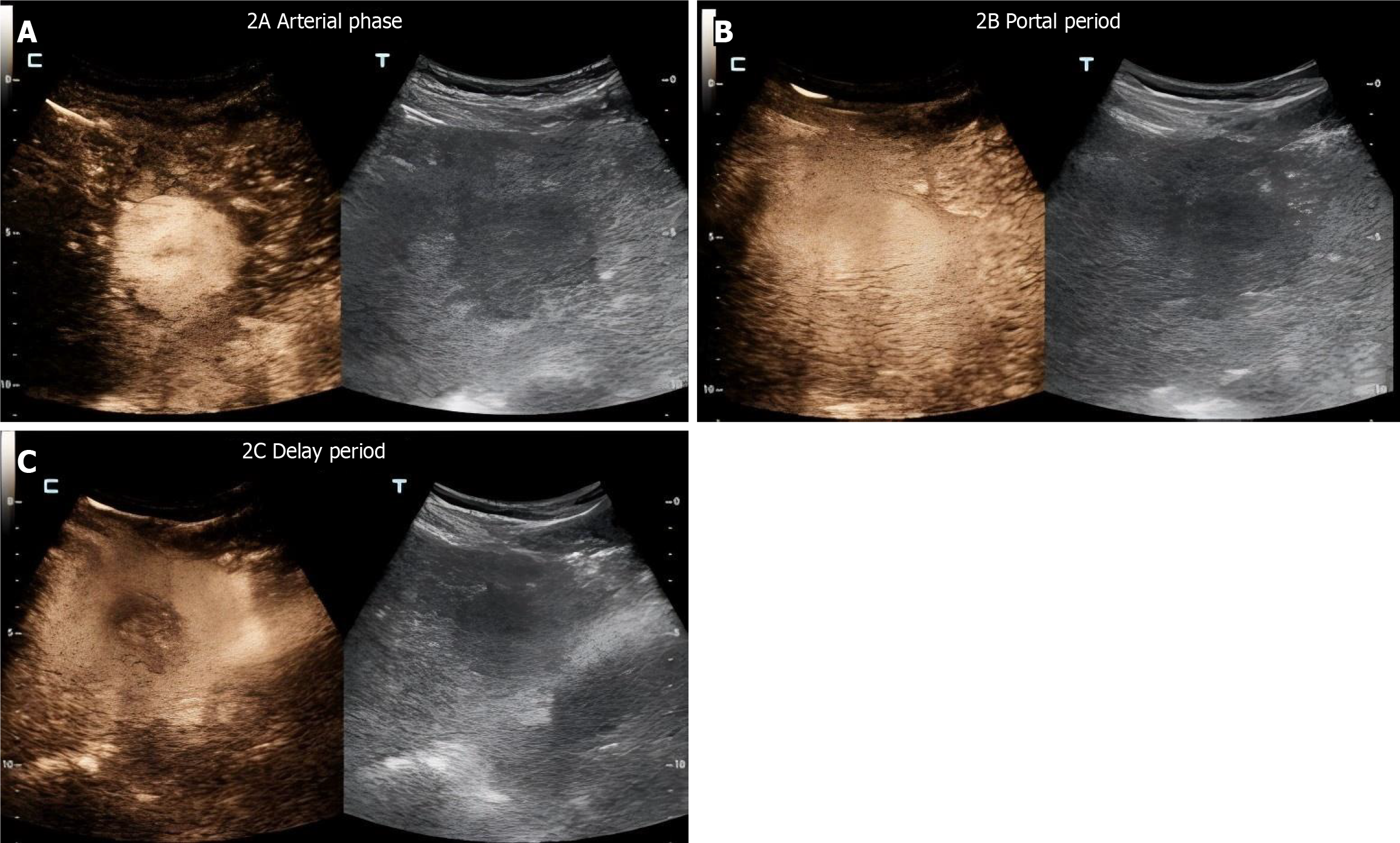
LD distribution: ≤ 3 cm (40%), > 3 cm (60%). EA distribution: ≤ 10 cm2 (41.60%), > 10 cm2 (58.40%) (Table 6, Figure 2).
| Project | n (%) |
| LD ≤ 3 cm | 50 (40) |
| LD > 3 cm | 75 (60) |
| EA ≤ 10 cm2 | 52 (41.60) |
| EA > 10 cm2 | 73 (58.40) |
Boundary clarity distribution: Clear (39.20%), fuzzy (33.60%), annular edge enhancement (27.20%) (Table 7).
| Boundary characteristics category | n (%) |
| Clear boundary | 49 (39.20) |
| Fuzzy boundary | 42 (33.60) |
| Annular enhancement at the edge | 34 (27.20) |
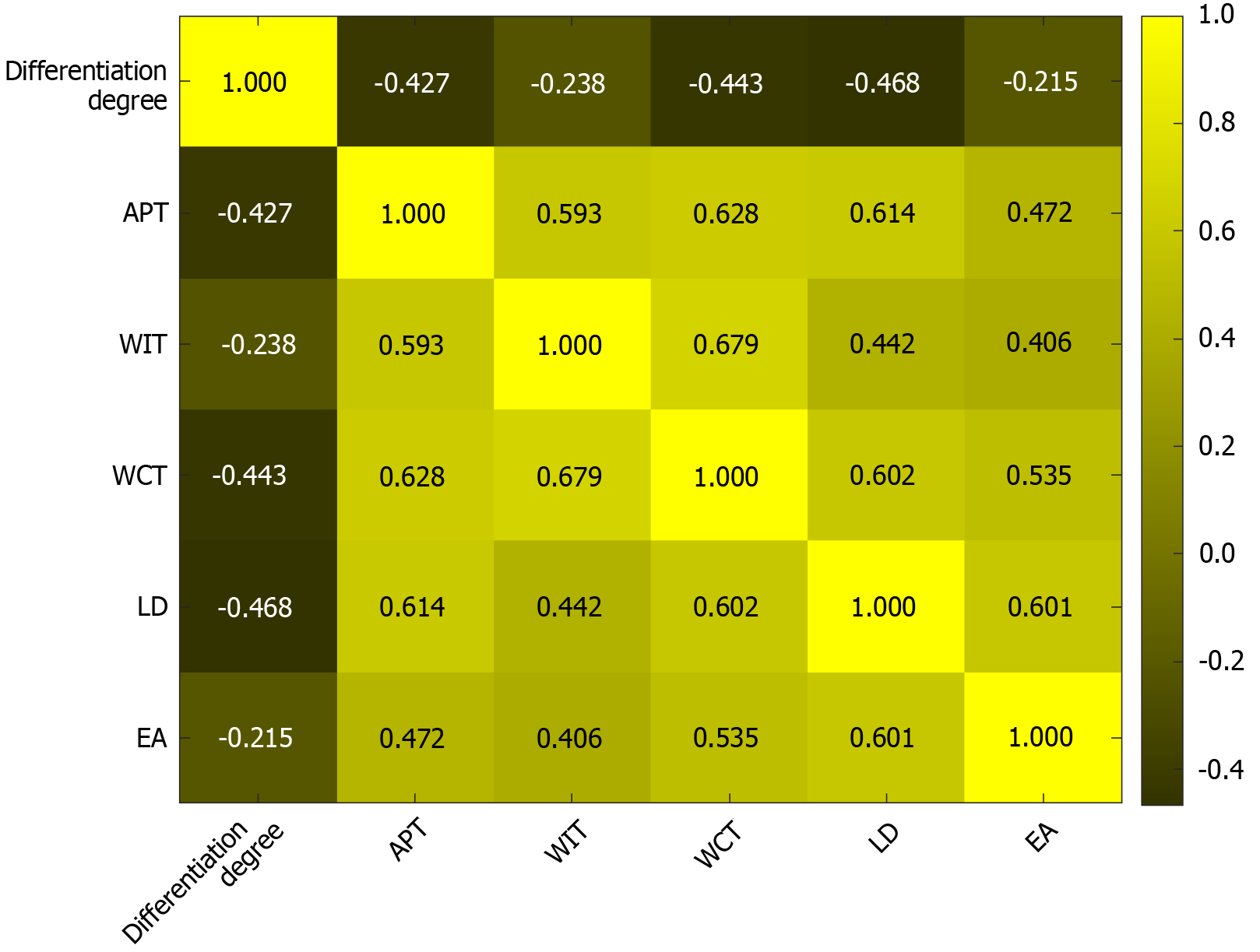
Correlation analysis showed that tumor differentiation degree was significantly negatively correlated with APT (r =
The results of logistic regression analysis showed that PEI had a significant impact on the diagnosis of liver cancer (OR = 3.374, P = 0.020), indicating that a higher PEI is associated with an increased risk of malignancy. WIT was negatively correlated with malignancy (OR = 0.541, P = 0.045), suggesting that a longer WIT corresponds to a lower likelihood of malignancy. Lesion boundary characteristics and APT also demonstrated predictive value, with P-values of 0.051 and 0.018, respectively (Table 9). Given that the boundary features approached statistical significance (P = 0.051), a sensitivity analysis was performed by excluding lesions with unclear boundaries (n = 42) and reconstructing the model. The results indicated that boundary features retained predictive significance (OR = 2.821, P = 0.038), supporting their stability as auxiliary diagnostic indicators. Although the original P-value for boundary features was close to the threshold of significance (P = 0.051), the sensitivity analysis confirms its robustness. This finding may be related to the invasive growth pattern of early HCC and should be interpreted in conjunction with other imaging characteristics.
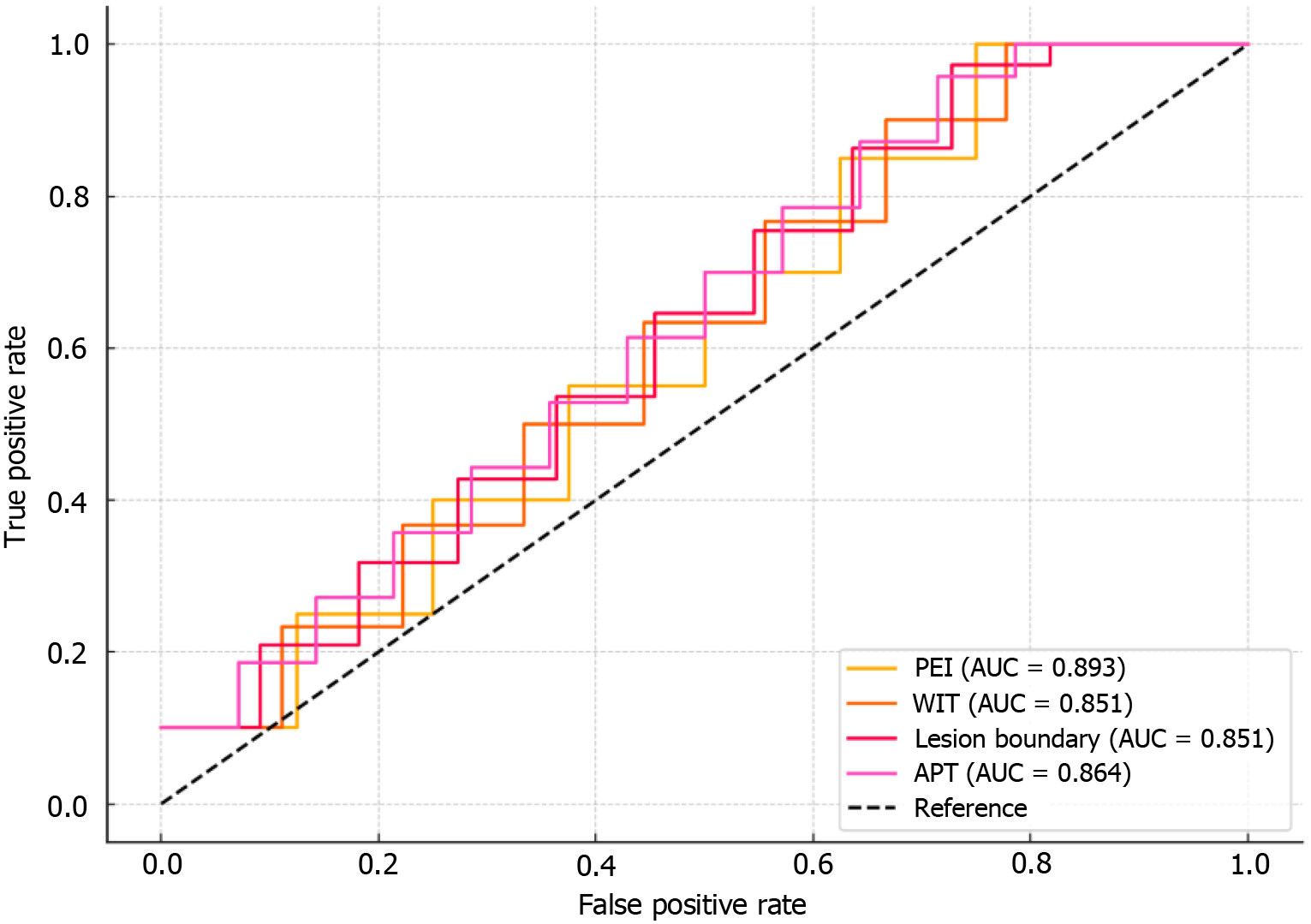
ROC curve analysis demonstrated that PEI had the best performance in the early diagnosis of HCC, with an AUC of 0.893, sensitivity of 85.2%, and specificity of 72.5%. Among the other parameters, WIT had an AUC of 0.851, lesion boundary characteristics had an AUC of 0.876, and APT had an AUC of 0.864, each showing good diagnostic efficacy. The Youden’s index for all indicators was higher than 0.4, indicating high clinical utility in the early diagnosis of HCC (Table 10, Figure 4).
| Indicator | Cutoff value | Sensitivity (%) | Specificity (%) | AUC | 95%CI | Youden’s index |
| PEI | 1.45 | 85.2 | 72.5 | 0.893 | 0.840-0.946 | 0.577 |
| WIT | 12.3 | 78.9 | 67.3 | 0.851 | 0.796-0.906 | 0.463 |
| Boundary characteristics of lesions | 0.98 | 80.5 | 69.1 | 0.876 | 0.822-0.930 | 0.495 |
| APT | 10.5 | 82.4 | 74.1 | 0.864 | 0.810-0.918 | 0.564 |
The results showed that overall, CEUS and CT/MRI had comparable sensitivity and specificity (P > 0.05). However, for LI-RADS 5 Lesions, CT/MRI demonstrated significantly higher sensitivity and specificity (P = 0.032) (Table 11).
The results of this study demonstrate that CEUS can effectively aid in the early diagnosis of HCC, with significant diagnostic advantages, particularly in liver lesions less than 3 cm in size. CEUS has been shown to exhibit high sensitivity and specificity in detecting early HCC, revealing significant differences in enhancement patterns during both the arterial and portal phases[10]. This finding is consistent with previous studies. For example, studies have reported that HCC typically exhibits rapid enhancement in the arterial phase, followed by rapid washout in the portal venous and delayed phases, a pattern described as “fast-in and fast-out”, which was also observed in this study[11]. Compared to conventional ultrasound, CEUS is superior in visualizing the blood supply and hemodynamic characteristics of lesions, especially in patients with small liver cancer and cirrhosis, which is crucial for early clinical diagnosis[12]. In the present study, the potential value of CEUS in early-stage liver cancer was further confirmed by analyzing key lesion parameters, including enhancement pattern, APT, WIT, and PEI. In particular, a PEI ≥ 1.5 was found to be closely associated with malignant lesions, supporting previous findings and reinforcing PEI as a reliable quantitative CEUS indicator for effectively screening early-stage HCC[13]. Unlike traditional imaging methods, PEI more accurately reflects the vascular distribution within lesions, providing strong support for the precise early diagnosis of liver cancer[14]. Another important finding in this study concerns lesion boundary characteristics, such as “clear”, “fuzzy”, or “annular enhancement at the edge”, which have been shown to have high accuracy in distinguishing malignant liver tumors[15]. This observation is consistent with previous studies that reported liver cancer often presents with irregular or fuzzy margins, whereas benign lesions tend to have well-defined borders[16]. Therefore, CEUS can provide a stronger diagnostic basis for clinical applications by evaluating lesion boundary characteristics, especially in early-stage liver cancer, thereby effectively distinguishing between malignant and benign lesions[17]. In this study, binary logistic regression analysis identified several key predictors, such as PEI, WIT, and lesion boundary characteristics, that were significantly correlated with pathological results. ROC curve analysis revealed that these factors exhibited high diagnostic accuracy, with an AUC value exceeding 0.85, indicating strong diagnostic performance. This result is consistent with other studies. For example, researchers have found that dynamic CEUS features can effectively predict the degree of malignancy in liver cancer. Notably, in lesions smaller than 3 cm, the diagnostic value of CEUS is significantly enhanced[18]. Moreover, CEUS has advantages in the early diagnosis of liver cancer, especially when used in conjunction with CT and MRI, in which CEUS can serve as a valuable complementary diagnostic tool[19]. Although CT and MRI offer high sensitivity, they are often associated with higher costs and greater equipment demands. In contrast, CEUS is non-invasive, radiation-free, and easy to operate, making it especially suitable for primary healthcare settings and pre-clinical diagnosis screening[20]. CEUS employs microbubble contrast agents to enhance blood flow signals, allowing real-time visualization of vascular changes during dynamic enhancement[21]. Typically, malignant tumors demonstrate rapid enhancement during the arterial phase and rapid washout during the portal venous and delayed phases, features attributed to abnormal angiogenesis and the rapid blood flow characteristics of liver cancer[22]. By analyzing these hemodynamic characteristics, CEUS can provide valuable insights into the malignancy of tumors. However, some early-stage HCC lesions may show an atypical enhancement pattern characterized by “continuous enhancement”, which can be easily mistaken for benign conditions such as hepatic adenoma or FNH, thereby complicating the differential diagnosis. Hepatic adenomas often demonstrate arterial phase enhancement that persists into the portal venous phase without significant washout. In contrast, FNH often presents with a characteristic “spoke-like” arterial perfusion pattern and delayed enhancement of a central scar. In contrast, HCC, even when showing continuous enhancement, generally exhibits structural irregularities and indistinct borders. To avoid misdiagnosis, it is essential to evaluate lesion morphology and boundary characteristics in combination with quantitative indicators such as PEI and WIT. Specifically, PEI serves as a quantitative index of tumor perfusion intensity and is effective in distinguishing between malignant and benign lesions[23]. By comparing parameters such as APT and WIT across different lesion types, this study highlights the diagnostic advantages of CEUS in identifying small HCC. Integrating these parameters with lesion boundary characteristics significantly improves diagnostic accuracy. CEUS offers a non-invasive, real-time, dynamic evaluation method with important clinical value, particularly in the early detection of HCC. Although CEUS has a slightly lower LI-RADS 5 conformity rate for early HCC (≤ 2 cm) than CT/MRI (P < 0.05), its real-time dynamic imaging ability provides a distinct advantage in detecting small nodules, especially in cirrhotic livers, such as the ability to capture rapid arterial phase enhancement. This finding suggests that CEUS can serve as a complementary tool to CT and MRI. Furthermore, CEUS shows promising value in laparoscopic liver resection. In the pilot phase of this study, five cases utilized intraoperative CEUS in combination with intraoperative ultrasound, which delineated tumor margins and identified satellite lesions, thereby aiding in determining resection boundaries (with pathological margins > 1 cm). For instance, in one case (patient A, a 58-year-old male with 2.1 cm HCC), CEUS detected a 2 mm satellite nodule that had been missed by CT, demonstrating higher detection efficiency than conventional MRI. This finding supports the potential of CEUS in intraoperative precision navigation and surgical planning[17,24].
The results of this study have significant clinical value, particularly in improving the efficiency and accuracy of diagnosis. By analyzing patient-related biomarkers, this study proposes a more accurate diagnostic method that enables physicians to identify and assess disease severity more rapidly. This approach not only reduces the subjectivity associated with traditional diagnostic methods but also enhances the early detection of diseases, thereby enabling timely intervention and leading to substantially better patient outcomes. Compared to conventional diagnostic methods, the technique proposed in this study offers notable economic advantages. Traditional diagnostic approaches often involve lengthy and high-cost procedures, such as complex imaging studies and repeated sampling[25]. In contrast, the biomarker-based detection method presented in this study is simple to perform, yields faster results, and delivers accurate diagnostic information within a short timeframe, thereby reducing patient waiting and treatment times, as well as hospital operating costs, with high cost-effectiveness[24].
However, this study has some limitations. First, the sample size is relatively small, necessitating further research with a larger cohort to improve the generalizability and reliability of the findings. Second, the detection of certain biomarkers may be influenced by external factors such as environmental conditions or individual patient differences. Therefore, these variables should be carefully considered in clinical applications. Moreover, the diagnostic method proposed in this study requires further validation regarding its applicability to various diseases and clinical settings. Future research should aim to expand the sample size and confirm the method’s utility with more extensive clinical data. Additionally, integrating this diagnostic technique with advanced technologies such as artificial intelligence and big data analytics may further enhance diagnostic precision and efficiency. Efforts should also focus on promoting the widespread adoption of this method across different regions and healthcare institutions to facilitate broader clinical implementation and practice.
In summary, this study demonstrated that CEUS has significant clinical value in the early diagnosis of HCC. Its ability to evaluate blood supply, microvessel density, and enhancement patterns of liver lesions offers strong diagnostic support in clinical practice, particularly for the early detection of small HCC, where it plays an irreplaceable role. With continued advancements and broader clinical adoption, CEUS is expected to play an increasingly important role in the early diagnosis of HCC, ultimately providing more opportunities for timely and effective treatment.
| 1. | Como G, Montaldo L, Baccarani U, Lorenzin D, Zuiani C, Girometti R. Contrast-enhanced ultrasound applications in liver transplant imaging. Abdom Radiol (NY). 2021;46:84-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Qiu Y, Wu Z, Chen Y, Liao J, Zhang Q, Wang Q, Duan Y, Gong K, Chen S, Wang L, Fan P, Duan Y, Wang W, Dong Y. Nano Ultrasound Contrast Agent for Synergistic Chemo-photothermal Therapy and Enhanced Immunotherapy Against Liver Cancer and Metastasis. Adv Sci (Weinh). 2023;10:e2300878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 3. | Francica G, Meloni MF, Riccardi L, Giangregorio F, Caturelli E, Terracciano F, de Sio I. Role of Contrast-Enhanced Ultrasound in the Detection of Complications After Ultrasound-Guided Liver Interventional Procedures. J Ultrasound Med. 2021;40:1665-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Zhou H, Zhang C, Du L, Jiang J, Zhao Q, Sun J, Li Q, Wan M, Wang X, Hou X, Wen Q, Liu Y, Zhou X, Huang P. Contrast-Enhanced Ultrasound Liver Imaging Reporting and Data System in Diagnosing Hepatocellular Carcinoma: Diagnostic Performance and Interobserver Agreement. Ultraschall Med. 2022;43:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Dong Y, Wang WP, Lee WJ, Meloni MF, Clevert DA, Chammas MC, Tannapfel A, Forgione A, Piscaglia F, Dietrich CF. Contrast-Enhanced Ultrasound Features of Histopathologically Proven Hepatocellular Carcinoma in the Non-cirrhotic Liver: A Multicenter Study. Ultrasound Med Biol. 2022;48:1797-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Francica G, Meloni MF, Riccardi L, de Sio I, Caturelli E, Terracciano F, Giangregorio F, Chiang J, Danzi R, Marra A, Niosi M, Ranalli TV, Pompili M. Contrast-Enhanced Ultrasound Findings in Patients with Rare Solitary Necrotic Nodule of the Liver - a Multicenter Report. Ultraschall Med. 2023;44:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Schellhaas B, Jesper D, Strobel D; DEGUM CEUS HCC Study Group. Contrast-enhanced ultrasound pattern of hepatocellular carcinoma in noncirrhotic liver - results from the prospective multicentre DEGUM CEUS HCC study. Eur J Gastroenterol Hepatol. 2023;35:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Huang JX, Shi CG, Xu YF, Fu J, Zhong Y, Liu LZ, Pei XQ. The benefit of contrast-enhanced ultrasound in biopsies for focal liver lesions: a retrospective study of 820 cases. Eur Radiol. 2022;32:6830-6839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 9. | Mostafa AG, Abramson Z, Ghbrial M, Biswas S, Chan S, Darji H, Gartrell J, Karol SE, Li Y, Mulrooney DA, Patni T, Zaghloul TM, McCarville MB. Contrast enhanced ultrasound of liver lesions in patients treated for childhood malignancies. Cancer Imaging. 2024;24:115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Rafailidis V, Fang C, Leenknegt B, Ballal K, Deganello A, Sellars ME, Yusuf GT, Huang DY, Sidhu PS. Contrast-Enhanced Ultrasound Quantification Assessment of Focal Fatty Variations in Liver Parenchyma: Challenging the Traditional Qualitative Paradigm of Uniform Enhancement With Adjacent Parenchyma. J Ultrasound Med. 2021;40:1137-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Chen M, Qiu M, Liu Y, Zhou W, Xie X, Zhou L. Utility of the pediatric liver contrast-enhanced ultrasound criteria in differentiating malignant and benign multifocal lesions. Pediatr Radiol. 2023;53:2004-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Luo Y, Yue W, Li Z, Wang P. Contrast-enhanced ultrasound and its differential diagnosis in 21 patients with intrahepatic space-occupying lesions under the background of fatty liver. Ann Palliat Med. 2021;10:3097-3104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Qin S, Cui R, Wang Y, Chen Y, Huang Y, Liu GJ. Contrast-Enhanced Ultrasound Imaging Features of Focal Chemotherapy-Induced Sinusoidal Injury in Patients With Colorectal Cancer: Initial Experience. J Ultrasound Med. 2021;40:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Dong Y, Chen S, Möller K, Qiu YJ, Lu XY, Zhang Q, Dietrich CF, Wang WP. Applications of Dynamic Contrast-Enhanced Ultrasound in Differential Diagnosis of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma in Non-cirrhotic Liver. Ultrasound Med Biol. 2023;49:1780-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Zhou G, Zhang Y, You Y, Wang B, Wang S, Yang C, Zhang Y, Liu J. Contrast-Enhanced Ultrasound and Magnetic Resonance Enhancement Based on Machine Learning in Cancer Diagnosis in the Context of the Internet of Things Medical System. Comput Intell Neurosci. 2022;2022:4378173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Zhou H, Ding J, Zhou Y, Wang Y, Zhao L, Shih CC, Xu J, Wang J, Tong L, Chen Z, Lin Q, Jing X. Malignancy diagnosis of liver lesion in contrast enhanced ultrasound using an end-to-end method based on deep learning. BMC Med Imaging. 2024;24:68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 17. | Li H, Shi M, Long X, Huang P, Peng C, He W, Li Y, Li B, Yuan Y, Qiu J, Zou R. Contrast-enhanced intraoperative ultrasound improved hepatic recurrence-free survival in initially unresectable colorectal cancer liver metastases. Dig Liver Dis. 2025;57:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Wang Z, Yao J, Jing X, Li K, Lu S, Yang H, Ding H, Li K, Cheng W, He G, Jiang T, Liu F, Yu J, Han Z, Cheng Z, Tan S, Wang Z, Qi E, Wang S, Zhang Y, Li L, Dong X, Liang P, Yu X. A combined model based on radiomics features of Sonazoid contrast-enhanced ultrasound in the Kupffer phase for the diagnosis of well-differentiated hepatocellular carcinoma and atypical focal liver lesions: a prospective, multicenter study. Abdom Radiol (NY). 2024;49:3427-3437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Tiyarattanachai T, Turco S, Eisenbrey JR, Wessner CE, Medellin-Kowalewski A, Wilson S, Lyshchik A, Kamaya A, Kaffas AE. A Comprehensive Motion Compensation Method for In-Plane and Out-of-Plane Motion in Dynamic Contrast-Enhanced Ultrasound of Focal Liver Lesions. Ultrasound Med Biol. 2022;48:2217-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Xian MF, Li W, Lan WT, Zeng D, Xie WX, Lu MD, Huang Y, Wang W. Strategy for Accurate Diagnosis by Contrast-Enhanced Ultrasound of Focal Liver Lesions in Patients Not at High Risk for Hepatocellular Carcinoma: A Preliminary Study. J Ultrasound Med. 2023;42:1333-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Huang Z, Zhou P, Li S, Li K. Evaluation of contrast-enhanced ultrasound LI-RADS version 2017: application on 271 liver nodules in individuals with non-alcoholic steatohepatitis. Eur Radiol. 2022;32:7146-7154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Eisenbrey JR, Gabriel H, Savsani E, Lyshchik A. Contrast-enhanced ultrasound (CEUS) in HCC diagnosis and assessment of tumor response to locoregional therapies. Abdom Radiol (NY). 2021;46:3579-3595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 23. | Ishikawa T, Ohno E, Mizutani Y, Iida T, Koya T, Sasaki Y, Ogawa H, Kinoshita F, Hirooka Y, Kawashima H. Comparison of contrast-enhanced transabdominal ultrasonography following endoscopic ultrasonography with GD-EOB-DTPA-enhanced MRI for the sequential diagnosis of liver metastasis in patients with pancreatic cancer. J Hepatobiliary Pancreat Sci. 2022;29:682-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Sun L, Yin S, Xing B, Li Z, Fan Z, Yan K. Contrast-Enhanced Ultrasound With SonoVue and Sonazoid for the Diagnosis of Colorectal Liver Metastasis After Chemotherapy. J Ultrasound Med. 2023;42:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Lou C, Li YX, Tan BB, Tao CJ, Xu CC, Liao YY. Clinical value of contrast-enhanced ultrasound versus conventional ultrasound in biopsy of focal liver lesions. Acta Radiol. 2024;65:700-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/