Published online Oct 15, 2025. doi: 10.4251/wjgo.v17.i10.108514
Revised: May 28, 2025
Accepted: August 15, 2025
Published online: October 15, 2025
Processing time: 181 Days and 16.5 Hours
Ulcerative colitis (UC) is associated with an increased risk of developing colitis-associated colorectal cancer (caCRC), a major complication of long-standing disease. In this review, we examined the pathogenic association between UC and caCRC, highlighting the risk factors, molecular mechanisms, and current strategies for prevention and management. Compared to sporadic colorectal cancer, caCRC tends to occur at a younger age and is more frequently characterized by mucinous or signet-ring cell histology, proximal colonic involvement, and a higher incidence of synchronous lesions. The risk of caCRC increases 8-10 years after UC diagnosis and is influenced by disease duration, extent of colonic involvement, inflammatory burden, family history of colorectal cancer, and coexisting primary sclerosing cholangitis. The inflammation-to-cancer progression follows a multistep pathway of genetic alterations, advancing from low-grade to high-grade dysplasia, and ultimately to carcinoma. While chemopreventive agents such as 5-aminosalicylates may offer some benefit, surveillance colo
Core Tip: Ulcerative colitis is a chronic inflammatory bowel disease with increased risk of colorectal cancer (CRC), particularly colitis-associated CRC (caCRC). The development of caCRC is influenced by disease duration, extent, severity, and factors such as primary sclerosing cholangitis and family history. Unlike sporadic CRC, caCRC tends to occur at a younger age and follows a distinct inflammation-dysplasia-carcinoma sequence. This review highlights the pathogenic link between ulcerative colitis and CRC and summarizes current strategies for prevention, surveillance, and management to improve patient outcomes.
- Citation: Catalano M, Mini E, Nobili S, Vascotto IA, Ravizza D, Amorosi A, Tonelli F, Roviello F, Roviello G, Nesi G. Ulcerative colitis and colorectal cancer: Pathogenic insights and precision strategies for prevention and treatment. World J Gastrointest Oncol 2025; 17(10): 108514
- URL: https://www.wjgnet.com/1948-5204/full/v17/i10/108514.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i10.108514
Ulcerative colitis (UC) is an idiopathic inflammatory bowel disease (IBD) that typically begins in the rectum and extends proximally throughout the colon[1,2]. The global incidence and prevalence of IBD exhibit considerable geographic variation and temporal trends. Once considered a disease confined to Western industrialized nations, IBD is now rising rapidly in newly industrialized countries[3-5]. Recent data indicate that North America and Europe continue to have the highest prevalence, with rates exceeding 0.3% of the population, although the incidence in some regions is stabilizing or declining. In contrast, countries in Asia, the Middle East, and Latin America are experiencing a sharp increase in new UC cases, with incidence rates in parts of East Asia reaching 1.5-3 per 100000 person-years - an increase largely attributed to urbanization, Westernized dietary patterns, and improved diagnostic capabilities.
UC incidence is comparable between males and females, with most diagnoses occurring between ages 30 and 40[5]. An urban lifestyle, characterized by factors such as pollution, dietary changes, improved hygiene, reduced infections, and antibiotic use, is often implicated in colonic microbiome alterations and disruption of the intestinal mucosa, potentially influencing the UC disease course[6,7]. Abdominal pain, diarrhea, and hematochezia are the primary clinical features, although extraintestinal manifestations, including arthropathies and mucocutaneous or ophthalmic disorders, affect approximately one-third of patients[8,9]. Diagnosis relies on clinical evaluations, endoscopic findings, and histological confirmation[10]. First-line treatment includes 5-aminosalicylic acid (5-ASA), with or without corticosteroids and infliximab, to induce and maintain remission[11]. Intravenous corticosteroids and surgical intervention are reserved for patients with severe or nonresponsive UC[11].
Colorectal cancer (CRC) is among the most serious complications in patients with UC. Chronic inflammation (CI) of the colorectal mucosa is a well-established risk factor for CRC development. In UC, colitis-associated CRC (caCRC) arises due to multiple factors, including disease duration, extent of colonic involvement, persistent colitis from childhood, severity of the initial flare, coexisting primary sclerosing cholangitis (PSC), and a family history of CRC[1,2,4,5]. Compared to sporadic CRC (sCRC), caCRC typically presents at a younger age[12,13]. Its development follows a multistep pathway of accumulating genetic mutations, leading to aggressive mucosal changes from low-grade dysplasia (LGD) to high-grade dysplasia (HGD), and ultimately cancer. Although patients with UC have an elevated risk of CRC, the precise magnitude of this risk remains debated, owing to variations in study populations, methodologies, and the periods of published data[5,8-11,14,15]. Additional complexity arises from colonoscopic surveillance practices, medical therapies, and surgical interventions[16].
This review synthesizes current knowledge of the molecular, immunological, microbial, and histopathological mechanisms underlying caCRC. It also highlights recent advances in chemoprevention, risk stratification, surveillance, and surgical management. Framed in the context of precision medicine, this review provides a comprehensive and forward-looking overview that builds on and extends prior research in the field.
CRC arising in IBD exemplifies inflammation-induced carcinogenesis. CI promotes the release of reactive oxygen and nitrogen species, leading to oxidative stress-mediated deoxyribonucleic acid (DNA) damage and the accumulation of mutations in carcinogenesis-related genes[17]. Beyond mutagenesis, CI triggers epigenetic changes, alters epithelial turnover, disrupts the intestinal barrier, and modifies gut microbiota (GM) composition. The host immune response further perpetuates this cycle, driving the inflammation-dysplasia-carcinoma sequence characteristic of caCRC[18,19].
CI contributes to carcinogenesis through two mechanisms: A direct pathway involving oxidative stress and DNA damage and an indirect pathway mediated by cytokines produced by inflammatory and intestinal epithelial cells[19-22]. In IBD, dysregulated immune responses promote the release of proinflammatory cytokines[21], notably interleukin (IL)-6, which activates the Janus kinase/signal transducer and activator of transcription 3 (STAT3) pathway[23-25]. This signaling pathway enhances epithelial cell proliferation and impairs apoptosis, thereby fostering a tumor-promoting environment[24,25].
Additional mediators, such as nuclear factor kappa B and suppressor of cytokine signaling 3, interact with STAT3 to sustain inflammation and drive carcinogenesis[25-32]. While anti-inflammatory cytokines such as transforming growth factor-beta exert protective effects in early disease, they might become dysregulated or inactive in later stages due to mutations[33-35]. Other signaling pathways, including the apoptosis signal-regulating kinase 1/mitogen-activated protein kinase cascade, further contribute to tumor progression by modulating cell survival and innate immune functions[36].
Immune cells, such as tumor-infiltrating leukocytes and tumor-associated macrophages, and immune proteins such as S100a9 might play a significant role in CI and carcinogenesis, serving as prognostic biomarkers and therapeutic targets for anticancer therapy[37-39]. However, clinical evidence supporting these roles remains lacking, and further investigation is warranted. In addition to the canonical IL-6/STAT3 and nuclear factor kappa B pathways, several other molecular alterations have been identified in caCRC. Epigenetic modifications, such as promoter hypermethylation, histone modifications, and dysregulated micro-ribonucleic acid (microRNA) expression, contribute to tumor initiation and progression by silencing tumor suppressor genes and impairing immune surveillance[40]. Dysregulation of Wnt/β-catenin signaling and cellular-MYC amplification has also been implicated, particularly in mucinous and signet-ring cell subtypes of caCRC[41]. These cumulative genetic and epigenetic alterations highlight the molecular heterogeneity of caCRC and represent promising targets for individualized therapeutic strategies. Table 1 summarizes the key molecular and cellular pathways implicated in caCRC.
| Pathway/cell type | Key mediators | Biological role | Impact on tumorigenesis |
| IL-6/JAK/STAT3 pathway | IL-6, JAK, STAT3 | Promotes epithelial proliferation and inhibits apoptosis | Facilitates CI and epithelial transformation |
| NF-κB signaling | NF-κB, TNF-α | Controls immune and inflammatory gene expression | Sustains pro-tumorigenic inflammation |
| TGF-β pathway | TGF-β, TGFBR2 | Regulates epithelial growth; normally anti-proliferative | Loss or mutation promotes tumor progression |
| SOCS3 regulation | SOCS3 | Inhibits STAT3-mediated signaling | Dysregulation leads to enhanced inflammation and carcinogenesis |
| MAPK/ASK1 signaling | ASK1 | Mediates stress response and immune modulation | ASK1 deficiency exacerbates inflammation and susceptibility to cancer |
| CD4+ T helper cells | IL-17, IL-22, IL-9 | Stimulate epithelial regeneration and immune activation | Promote dysplasia and tumor development |
| Macrophages (M1/M2) | IL-13, CCL17 (M2), TNF-α (M1) | M1: Antitumor; M2: Tumor-promoting via immunosuppression and cytokine release | High M2 infiltration is linked to poor prognosis and metastasis |
| Regulatory T cells | FOXP3, IL-10 | Maintain immune tolerance and suppress inflammation | Dual role: Contextually antitumor or tumor-permissive |
| Tumor-infiltrating leukocytes | Various cytokines and chemokines | Orchestrate local immune response | Serve as prognostic markers; role depends on cellular composition |
| S100 family proteins | S100A9, S100A8 | Mediate inflammation and immune cell recruitment | Correlated with tumor progression; potential therapeutic targeting |
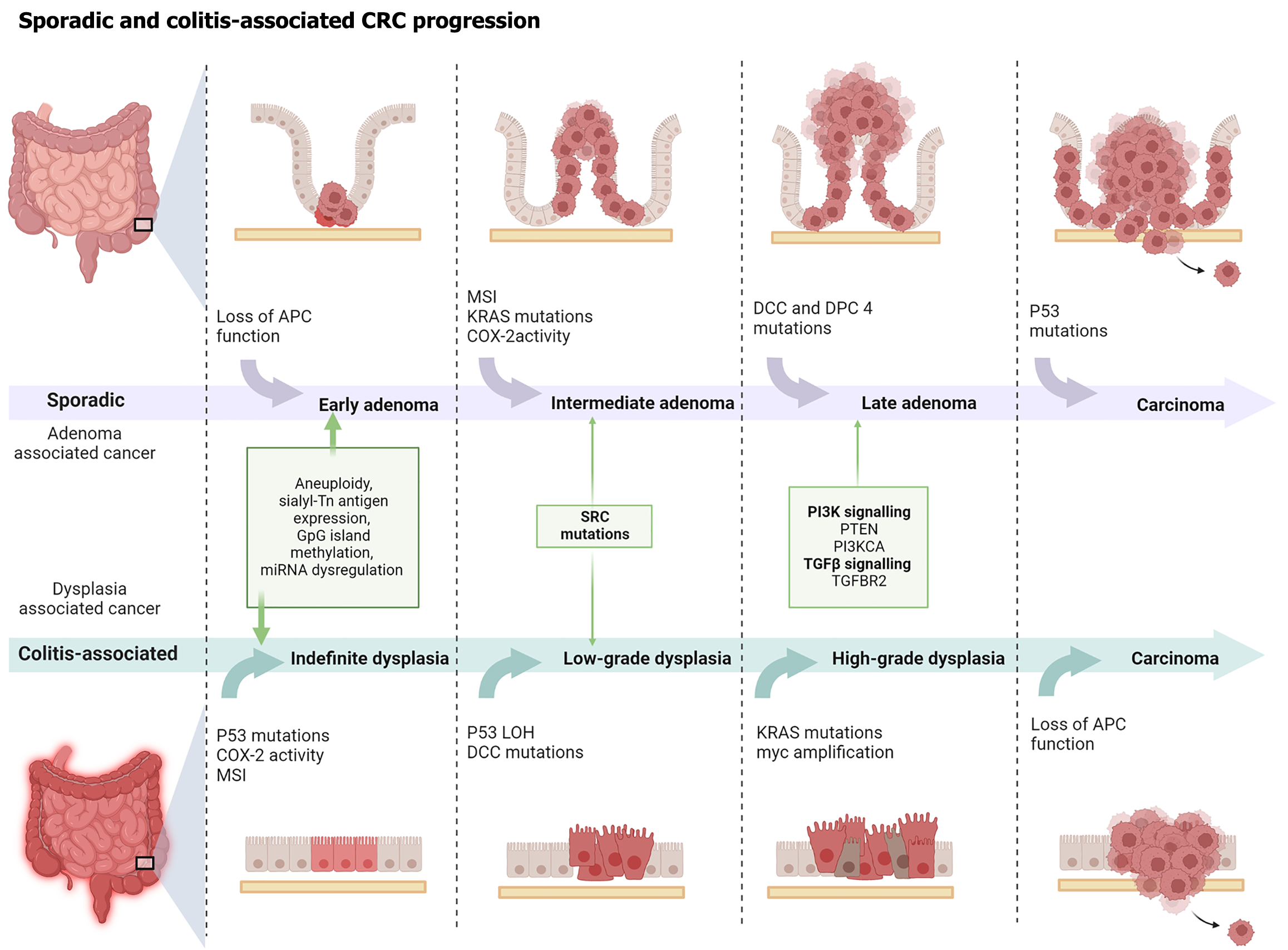
Unlike sCRC, caCRC develops from dysplastic lesions affecting extensive areas of chronically inflamed mucosa[42]. The diagnosis of dysplasia (intraepithelial neoplasia) relies on cytohistological criteria and is currently classified into LGD and HGD using the Riddell or Vienna system[43]. Given the limited interobserver agreement, any biopsy indicating dysplasia should be confirmed by an experienced gastrointestinal pathologist. Dysplasia in UC is often multifocal, and early neoplastic changes might be inconspicuous on colonoscopy because they could be flat and resemble inflamed mucosa[44,45]. However, dysplastic lesions may appear as elevated masses, dysplasia-associated lesions or masses, resembling pedunculated polyps or velvety plaques, similar to sporadic adenomatous or villous polyps, respectively[46].
IBD-associated dysplasia functions not only as a precursor but also as a potential marker of concurrent cancer[47]. The progression from early dysplasia to CRC involves a series of molecular alterations shared with sCRC and caCRC, though with distinct timing and frequency (Figure 1). More recently, non-conventional lesions resembling those found in hyperplastic/serrated polyposis syndrome have been identified in patients with IBD with long-standing disease. These findings suggest the presence of a serrated pathway of carcinogenesis in IBD, involving O(6)-methylguanine DNA methyltransferase silencing, likely due to promoter hypermethylation and Kirsten rat sarcoma viral oncogene homolog mutations[48].
Immune cell subsets, particularly cluster of differentiation 4+ T helper (Th) cells, play a pivotal role in caCRC pathogenesis. Proinflammatory Th17-associated cytokines, including IL-9, IL-17, and IL-22, reportedly promote dysplasia and neoplasia in murine models and human tissues[46-49].
Macrophages exhibit dual roles, with M1 macrophages exerting anti-tumoral effects and M2 macrophages facilitating tumor progression and metastasis through IL-13 production and chemokines such as chemokine ligand 17[50-55]. Recently, Kvorjak et al[56] identified a novel regulatory axis between macrophages and colonic cells in UC and caCRC. Their study demonstrated that chemokine ligand 17 and IL-13 secreted by M2 macrophages co-cultured with colon cells activated oncogenic signaling pathways, including protein kinase B and STAT6. These cytokines also induced aberrant ST6GALNAC1 glycosyltransferase overexpression, resulting in elevated expression of the tumor glycoform mucin1, cell surface associated with sialylated Thomsen-nouvelle antigen, which has been implicated in tumor progression, metastasis, and immune modulation[57].
Innate lymphoid cells and regulatory T cells (Tregs) contribute to local immune homeostasis; however, their roles in tumor regulation are context-dependent and subject to ongoing debate. Innate lymphoid cells maintain intestinal homeostasis by promoting protective immune responses, yet they are also implicated in IBD pathogenesis and cancer progression in caCRC[58]. Similarly, Tregs, which are key regulators of tissue inflammation and autoimmunity, exhibit a complex role in sCRC and caCRC[59]. While some studies have reported a positive correlation between high Treg infiltration and improved control, others have failed to replicate these findings[60]. Overall, current evidence favors an antitumorigenic function of Tregs in sCRC and caCRC[61,62].
Growing evidence indicates that GM plays a crucial role in sCRC and caCRC development[63]. However, whether carcinogenesis in the context of CI is driven by specific microbial species or by broader shifts in microbiota composition remains unclear. Comparative analyses have not identified any significant differences in microbiota composition between healthy individuals and patients with sCRC and caCRC. Nevertheless, caCRC is generally associated with a reduced abundance of Bacteroidetes and Firmicutes, and an increased prevalence of Proteobacteria, whereas sCRC is characterized by reduced Bacteroidetes and a marked expansion of Fusobacteria[64].
The tumorigenic potential of specific microorganisms is well-established in the case of Fusobacterium nucleatum in sCRC, where high bacterial loads correlate with enhanced tumor growth, distant metastasis, and poor prognosis[64]. Recent studies have also implicated Fusobacterium nucleatum in exacerbating tumorigenesis in a caCRC mouse model by activating the epidermal growth factor receptor signaling pathway and induction of epithelial-to-mesenchymal transition[65].
Enterobacteriaceae/Escherichia coli (E. coli) strains harboring the pks gene cluster have been detected more frequently in patients with IBD and CRC than in healthy individuals[66]. Certain strains, such as pks+ E. coli, are associated with oncogenic phenotypes and promote tumor formation in murine models of caCRC[67]. However, the role of pks+ E. coli in human caCRC pathogenesis remains to be fully elucidated.
Enterotoxigenic Bacteroides fragilis (B. fragilis) secretes B. fragilis toxin (BFT), which has been linked to diarrheal disease, IBD, and CRC. BFT directly interacts with epithelial cell receptors, inducing cell proliferation, proinflammatory mediator release, and DNA damage[68]. Inflammatory conditions characterized by a Th17 response might amplify this effect through BFT-induced STAT3 activation in epithelial and mucosal immune cells, thereby promoting tumorigenesis[69,70]. In contrast, animal studies suggest that non-toxigenic B. fragilis, also present in healthy GM, might exert protective effects against colitis and polyp formation[71].
Additionally, GM influences CI and tumor development through its metabolic byproducts. Among these, short-chain fatty acids, such as propionate, butyrate, and acetate, are abundant in starch-rich diets and have demonstrated anti-inflammatory effects in UC. Short-chain fatty acids regulate epithelial cell proliferation and induce apoptosis; their exogenous administration lowers IL-6, tumor necrosis factor-α, and IL-17A expression and reduces tumor cell proliferation in caCRC models[72]. Metabolomic analysis of the intestinal microbiota may enhance our understanding of its role in colonic carcinogenesis and aid in caCRC prevention.
Several chemopreventive agents, including 5-ASA, thiopurines, and biologic therapies, have demonstrated potential in reducing CRC risk. However, the current evidence remains inconclusive, and further investigation is warranted[73]. Table 2 summarizes the preventive effects of these agents on caCRC. In contrast to the adenoma-carcinoma sequence characteristic of sCRC, caCRC typically progresses through an inflammation-dysplasia-carcinoma pathway. Therefore, regular surveillance of patients with IBD is essential for the early detection of dysplasia and cancer[74]. Evidence suggests that surveillance colonoscopy reduces CRC incidence and mortality in UC[75,76]. The recently updated guidelines from the American Gastroenterological Association recommended initiating endoscopic screening for caCRC 8 years after symptom onset rather than the date of diagnosis[77]. Surveillance intervals should be individualized based on risk factors such as age at diagnosis, disease duration, active endoscopic or histological inflammation, family history of CRC, and the presence of pseudopolyps[78-85]. Patients with concomitant PSC are at a higher risk and should begin annual screening from the time of PSC diagnosis[86,87]. The British Society of Gastroenterology and the European Crohn’s and Colitis Organization (ECCO) stratify patients into low-, intermediate-, and high-risk groups, with recommended surveillance intervals ranging from 1 to 5 years[88,89]. Similarly, the American Society for Gastrointestinal Endoscopy recommends extending surveillance intervals following two consecutive colonoscopies without endoscopic or histological abnormalities[90]. Surveillance strategies proposed by ECCO, American Gastroenterological Association, and the American College of Gastroenterology are presented in Table 3.
| Compound | Reported effect | Recommendation | Ref. |
| 5-ASA | Protective effect for patients with long-standing extensive colitis. Lower risk of developing CRC/dysplasia in UC patients | Mesalamine compounds have a recognized protective role against CRC in UC patients | [99-101] |
| Thiopurines | Protective against HGD and CRC risk in IBD patients | No recommendations due to their carcinogenic effects (i.e., lymphoma, urinary tract cancer, non-melanoma skin cancer) | [102-104] |
| Biologic drugs | Chemopreventive effects of anti-TNF-α treatment in IBD patients | No recommendations | [101,105-107] |
| Statins and folic acid | Controversial chemopreventive role of statins on CRC in IBD patients. Protective effects of folate supplementation against CRC development | No recommendations | [108-110] |
| UDCA | Decrease in colonic dysplasia in patients with UC and PSC. Chemopreventive effects on risk of advanced CRC and HGD | No recommendations | [111] |
| NSAIDs and aspirin | No significant chemoprotective role in CRC risk | No recommendations | [112] |
| Frequency of surveillance | ||
| ECCO 2017 | Every year (high risk) | PSC or stricture or dysplasia detected within past 5 years or extensive colitis with severe active inflammation or family history of CRC in FDR age < 50 |
| Every 2-3 years | Extensive colitis with mild or moderate active inflammation or post-inflammatory polyps or family history of CRC in FDR age > 50 | |
| Every 5 years | Absence of intermediate or high-risk features | |
| ACG 2019 | Every year | PSC |
| Every 1-3 years | UC of any extent beyond the rectum | |
| Adjust intervals | Based on previous colonoscopies and combined risk factors: Duration of disease, younger age at diagnosis, greater extent of inflammation, FDR with CRC | |
| AGA 2021 | Every year | Moderate or severe inflammation (any extent), PSC, family history of CRC in FDR age < 50, dense pseudopolyposis, history of higher-risk visible dysplasia < 5 years ago |
| Every 2-3 years | Mild inflammation (any extent), strong family history of CRC (but no FDR age < 50), features of prior severe colitis (moderate pseudopolyps, extensive mucosal scarring), history of invisible dysplasia or higher-risk visible dysplasia > 5 years ago, history of lower risk visible dysplasia < 5 years ago | |
| Every 5 years | Continuous disease remission since last colonoscopy with mucosal healing on current exam, plus either of: ≥ 2 consecutive exams without dysplasia, minimal historical colitis extent (ulcerative proctitis or < 1/3 of colon in CD) | |
Colonoscopy remains the cornerstone of secondary prevention of CRC in UC, while novel endoscopic techniques have been developed to enhance dysplasia detection (Table 4). Tumor-associated biomarkers, such as aneuploidy, tumor protein p53 mutations, DNA methylation patterns, and microRNA profiles, have been identified in caCRC[91,92]. The evaluation of these biomarkers in colorectal biopsies and other biospecimens (e.g., blood, stool, and urine) offers a promising approach for monitoring carcinogenic progression in UC. Although the clinical utility of these biomarkers requires validation in larger prospective studies, molecular testing is expected to significantly advance the development of high-performance diagnostic and screening tools in the future.
| Type of procedure | Type of biopsies | Strengths | Limitations |
| Standard with light endoscopy | Random biopsies | Increases dysplasia detection rate | Longer procedure times and cost |
| High-definition endoscopy | Targeted biopsies | Images of substantially higher resolution for dysplasia detection | Cost |
| Chromoendoscopy | Targeted biopsies | Best at highlighting irregularities in the architecture of the mucosa thanks to the contrasting dye | Specialized equipment and additional training required, longer procedure time |
| Narrow band imaging | NA | Greater contrast of the mucosal surface | Lower sensitivity in dysplasia detection |
| Fujinon intelligent color enhancement and I scan digital contrast | NA | Perception of subtle changes of the mucosal surface | Limited relevant data |
| Confocal laser endomicroscopy | NA | Real-time microscopy available in vivo during examination | Longer procedure time, extra equipment and training required |
| Full-spectrum endoscopy | NA | Better visualization of the mucosa thanks to increased visual field | Longer withdrawal and total procedure time |
The detection of dysplasia during colonoscopy prompts a critical decision between continued surveillance and surgical intervention[82,83]. Non-polypoid dysplasia, regardless of grade, and endoscopically occult HGD, including flat, random, or non-targeted lesions, are associated with a high risk of synchronous and metachronous carcinoma. Consequently, prompt colectomy is generally recommended for patients with these lesions[84,85]. In contrast, the management of endoscopically invisible LGD remains controversial due to conflicting data regarding its association with synchronous CRC and its progression to HGD and/or CRC[93-101]. A meta-analysis by Thomas et al[6] estimated a 22% positive predictive value for concurrent CRC at colectomy in patients with flat LGD. LGD detected during surveillance was associated with a nine-fold increased risk of progression to CRC and a 12-fold increased risk of developing advanced neoplasia (HGD and CRC). A more recent meta-analysis revealed that synchronous CRC was present in approximately 17% of surgical cases. Among patients with UC-associated LGD under surveillance, the annual progression rate to CRC and advanced neoplasia was 0.8% and 1.8%, respectively[102]. Given these risks, prophylactic proctocolectomy should be offered as an alternative to continued surveillance for patients with LGD in flat mucosa[103].
Given the multifocality and potential for recurrence of neoplastic lesions, partial colectomy is generally considered unsuitable; however, it might be selectively considered in elderly patients[104,105]. Subtotal colectomy with ileorectal anastomosis also poses limitations, as the presence of colonic carcinoma or dysplasia at the time of surgery significantly increases the risk of malignancy in the retained rectal stump[106]. Proctocolectomy is widely accepted as the preferred surgical approach for caCRC; however, the optimal reconstruction method - definitive end ileostomy vs restorative proctocolectomy (RPC) – remains a subject of debate.
Over the past decades, RPC with ileal pouch-anal anastomosis (IPAA) has been increasingly adopted in UC-associated neoplasia. Functional outcomes following IPAA are generally favorable and comparable to those achieved in patients undergoing IPAA for non-neoplastic UC[107,108]. However, the pouch failure rate in patients with UC and CRC is significantly higher than in those without CRC, ranging from 14.2% to 19%[109]. In cases where caCRC involves the rectum, the success of IPAA is largely dependent on tumor stage, with reported failure rates of 16%-28% in locally advanced cancers[110,111]. In contrast to sCRC, where preoperative radiotherapy (RT) or chemoradiotherapy is standard for T3-4 or node-positive tumors of the middle and lower rectum, these modalities are rarely employed in patients with UC and rectal cancer. Current ECCO guidelines recommend RT before pouch surgery and avoiding it after IPAA due to the associated complication risk[103].
The potential for IPAA to increase the risk of pouch dysplasia and carcinoma in patients with caCRC remains debatable. A comprehensive review by Das et al[98] identified 17 confirmed cases of adenocarcinoma arising in the ileal pouch or anorectal mucosa, with most tumors originating from residual rectal mucosa in the anal transitional zone (ATZ). Notably, the interval between RPC and cancer diagnosis generally exceeded 2 years[112]. In a separate study with a median follow-up of 12.9 years, Mark-Christensen et al[99] reported only two cases of pouch carcinoma among 1723 patients with UC who underwent IPAA. Long-term data from large population-based studies conducted by the Cleveland Clinic and the Dutch Pathology Registry estimated the cumulative incidence of pouch neoplasia, including adenocarcinoma, lymphoma, squamous cell carcinoma, and dysplasia, at 5.1% over 25 years and 6.9% over 20 years, respectively[99,100].
PSC represents an additional risk factor for pouch malignancy. Patients with PSC and UC might develop CRC either in the residual colon following subtotal colectomy and ileorectal anastomosis or in the ileal pouch following IPAA[113,114]. In a cohort of 65 patients who underwent IPAA for refractory colitis (40%), dysplasia (48%), or carcinoma (10.8%), Imam et al[115] reported a 5-year cumulative incidence of pouch neoplasia of 5.6%.
An ongoing area of debate concerns whether the type of pouch anastomosis - handsewn at the pectinate line following mucosectomy of the rectal mucosa of the anal canal vs stapled at the level of the distal rectal mucosa - affects the risk of ATZ cancer[116]. Several studies have reported comparable rates of dysplasia and cancer between the two techniques[107,114]. Dysplasia in the ATZ is typically managed through mucosectomy or regular check-ups, depending on the number of positive biopsies and the severity of dysplasia detected[117]. Notably, ATZ neoplasia might originate from pre-existing dysplasia in the rectal mucosa at the time of IPAA[112]. Mucosectomy is generally recommended when dysplasia or cancer is identified in the distal rectum, whereas rigorous endoscopic surveillance with targeted biopsies of the ATZ is advised if mucosectomy is not performed[118,119].
Squamous cell carcinoma after IPAA is exceedingly rare. In a systematic review, Pellino et al[104] identified eight cases of IPAA-related squamous cell carcinoma, six involving the ATZ or rectal cuff and two arising within the pouch body. Notably, no recurrence or disease progression was observed after ATZ mucosectomy with redo-IPAA, suggesting that surgical excision of the ATZ might preserve pouch function while reducing the risk of squamous neoplasia.
Conflicting data exist regarding the prognosis of patients with UC and CRC. While several studies have not identified any significant differences in survival between patients with caCRC and those with sCRC[110,120-124], others have revealed a higher mortality risk in patients with caCRC[120,125,126]. Watanabe et al[109] observed poor overall survival in patients with UC and stage III tumors but found no difference in overall and cancer-specific survival when all cancer stages were considered. In a study by Olén et al[110], patients with UC and a disease duration of ≥ 8 years or a diagnosis of PSC demonstrated an increased risk of CRC-related mortality compared to matched controls, despite presenting with tumors at an earlier stage.
The poor prognosis reported in some caCRC cases might reflect distinct clinico-histological and molecular characteristics compared to sCRC. Mucinous and signet-ring cell carcinomas, which are more prevalent in caCRC, are often diagnosed at advanced stages and carry a higher risk of recurrence[127,128]. These mucin-producing tumors are also less responsive to standard chemotherapy, potentially due to a higher prevalence of microsatellite instability (MSI)[129,130]. Notably, although CRCs in UC share histological features with sporadic MSI-high and Lynch syndrome-associated CRCs, such as mucinous and signet-ring differentiation, they are predominantly microsatellite stable. One hypothesis suggests that inflammation-induced overexpression of microRNAs targeting mismatch repair proteins might serve as an early event in the development of MSI in IBD-associated carcinogenesis. Further research is needed to elucidate the molecular mechanisms underlying the clinical behavior in IBD-associated CRC.
In recent years, the incidence of early-stage caCRC has increased. Contemporary literature reports that 50%-60% of newly diagnosed caCRCs are classified as stage I or II. This shift is likely attributable to increased awareness of cancer risk among patients with UC, along with the implementation of more effective and timely surveillance strategies that promote patient compliance.
CRC remains a significant clinical concern in patients with UC, particularly among those with long-standing disease, elevated inflammatory burden, concomitant PSC, or a history of dysplasia. CI is the principal driver of caCRC, and although its molecular alterations overlap with those in sCRC, their sequence and frequency differ in the inflammatory context. Current surveillance strategies based on colonoscopic monitoring and lesion resection have reduced CRC-related morbidity and mortality in UC. However, the occurrence of interval cancers highlights the limitations of uniform surveillance protocols. To improve clinical outcomes, surveillance paradigms must transition toward risk-adapted strategies guided by molecular and histological biomarkers. Future research should focus on integrating emerging molecular biomarkers, such as DNA methylation signatures and circulating tumor DNA, into routine clinical practice[116,117]. Advances in genomic and immunological profiling offer the potential to transform surveillance from reactive detection to proactive risk stratification, enabling more personalized disease management. Concurrently, incorporating chemopreventive agents[131,132] with established safety and cost-effectiveness profiles, such as 5-ASA, might contribute to modifying disease progression.
Advances in artificial intelligence-assisted endoscopy and confocal laser endomicroscopy might significantly improve dysplasia detection in patients with UC[117]. Prospective studies are warranted to validate liquid biopsy technologies and establish the clinical utility of non-invasive imaging tools for real-time risk stratification. Personalized medicine approaches that integrate molecular, clinical, and environmental risk factors offer a promising avenue for transforming caCRC prevention and early detection paradigms.
| 1. | Bergeron V, Vienne A, Sokol H, Seksik P, Nion-Larmurier I, Ruskone-Fourmestraux A, Svrcek M, Beaugerie L, Cosnes J. Risk factors for neoplasia in inflammatory bowel disease patients with pancolitis. Am J Gastroenterol. 2010;105:2405-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Olén O, Askling J, Sachs MC, Frumento P, Neovius M, Smedby KE, Ekbom A, Malmborg P, Ludvigsson JF. Childhood onset inflammatory bowel disease and risk of cancer: a Swedish nationwide cohort study 1964-2014. BMJ. 2017;358:j3951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 3. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 10886] [Article Influence: 3628.7] [Reference Citation Analysis (2)] |
| 4. | Askling J, Dickman PW, Karlén P, Broström O, Lapidus A, Löfberg R, Ekbom A. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology. 2001;120:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 286] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2124] [Article Influence: 85.0] [Reference Citation Analysis (2)] |
| 6. | Thomas T, Abrams KA, Robinson RJ, Mayberry JF. Meta-analysis: cancer risk of low-grade dysplasia in chronic ulcerative colitis. Aliment Pharmacol Ther. 2007;25:657-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Ullman TA. Patients with low-grade dysplasia should be advised to undergo colectomy. Inflamm Bowel Dis. 2003;9:267-9; discussion 273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 690] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 9. | Castaño-Milla C, Chaparro M, Gisbert JP. Systematic review with meta-analysis: the declining risk of colorectal cancer in ulcerative colitis. Aliment Pharmacol Ther. 2014;39:645-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 205] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 10. | Choi PM, Nugent FW, Schoetz DJ Jr, Silverman ML, Haggitt RC. Colonoscopic surveillance reduces mortality from colorectal cancer in ulcerative colitis. Gastroenterology. 1993;105:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 215] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Söderlund S, Brandt L, Lapidus A, Karlén P, Broström O, Löfberg R, Ekbom A, Askling J. Decreasing time-trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease. Gastroenterology. 2009;136:1561-7; quiz 1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 12. | Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H, Ng S, Maio M, Franke FA, Sundar S, Agarwal N, Bergman AM, Ciuleanu TE, Korbenfeld E, Sengeløv L, Hansen S, Logothetis C, Beer TM, McHenry MB, Gagnier P, Liu D, Gerritsen WR; CA184-043 Investigators. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1157] [Cited by in RCA: 1212] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 13. | Hartman DJ, Binion DG, Regueiro MD, Miller C, Herbst C, Pai RK. Distinct Histopathologic and Molecular Alterations in Inflammatory Bowel Disease-Associated Intestinal Adenocarcinoma: c-MYC Amplification is Common and Associated with Mucinous/Signet Ring Cell Differentiation. Inflamm Bowel Dis. 2018;24:1780-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Kappelman MD, Farkas DK, Long MD, Erichsen R, Sandler RS, Sørensen HT, Baron JA. Risk of cancer in patients with inflammatory bowel diseases: a nationwide population-based cohort study with 30 years of follow-up evaluation. Clin Gastroenterol Hepatol. 2014;12:265-73.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 15. | Herrinton LJ, Liu L, Levin TR, Allison JE, Lewis JD, Velayos F. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology. 2012;143:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 251] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 16. | Nguyen GC, Bressler B. A tale of two cohorts: are we overestimating the risk of colorectal cancer in inflammatory bowel disease? Gastroenterology. 2012;143:288-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Parian AM, Limketkai BN, Chowdhury R, Brewer GG, Salem G, Falloon K, Selaru F, Melia J, Lazarev MG. Serrated Epithelial Change Is Associated With High Rates of Neoplasia in Ulcerative Colitis Patients: A Case-controlled Study and Systematic Review With Meta-analysis. Inflamm Bowel Dis. 2021;27:1475-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Wijnands AM, de Jong ME, Lutgens MWMD, Hoentjen F, Elias SG, Oldenburg B; Dutch Initiative on Crohn and Colitis (ICC). Prognostic Factors for Advanced Colorectal Neoplasia in Inflammatory Bowel Disease: Systematic Review and Meta-analysis. Gastroenterology. 2021;160:1584-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 19. | Brackmann S, Andersen SN, Aamodt G, Langmark F, Clausen OP, Aadland E, Fausa O, Rydning A, Vatn MH. Relationship between clinical parameters and the colitis-colorectal cancer interval in a cohort of patients with colorectal cancer in inflammatory bowel disease. Scand J Gastroenterol. 2009;44:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Baars JE, Kuipers EJ, van Haastert M, Nicolaï JJ, Poen AC, van der Woude CJ. Age at diagnosis of inflammatory bowel disease influences early development of colorectal cancer in inflammatory bowel disease patients: a nationwide, long-term survey. J Gastroenterol. 2012;47:1308-1322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Choi CR, Al Bakir I, Ding NJ, Lee GH, Askari A, Warusavitarne J, Moorghen M, Humphries A, Ignjatovic-Wilson A, Thomas-Gibson S, Saunders BP, Rutter MD, Graham TA, Hart AL. Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: a large single-centre study. Gut. 2019;68:414-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (1)] |
| 22. | Ko HM, Harpaz N, McBride RB, Cui M, Ye F, Zhang D, Ullman TA, Polydorides AD. Serrated colorectal polyps in inflammatory bowel disease. Mod Pathol. 2015;28:1584-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | de Jong ME, Gillis VELM, Derikx LAAP, Hoentjen F. No Increased Risk of Colorectal Neoplasia in Patients With Inflammatory Bowel Disease and Postinflammatory Polyps. Inflamm Bowel Dis. 2020;26:1383-1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Wei HX, Wang B, Li B. IL-10 and IL-22 in Mucosal Immunity: Driving Protection and Pathology. Front Immunol. 2020;11:1315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 25. | Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX, Wang K, Ho SB, Boland BS, Chang JT, Sandborn WJ, Hardiman G, Raz E, Maehara Y, Yoshimura A, Zucman-Rossi J, Guan KL, Karin M. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 538] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 26. | Feagins LA. Role of transforming growth factor-β in inflammatory bowel disease and colitis-associated colon cancer. Inflamm Bowel Dis. 2010;16:1963-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 931] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 28. | Bozec D, Iuga AC, Roda G, Dahan S, Yeretssian G. Critical function of the necroptosis adaptor RIPK3 in protecting from intestinal tumorigenesis. Oncotarget. 2016;7:46384-46400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 29. | Khare V, Paul G, Movadat O, Frick A, Jambrich M, Krnjic A, Marian B, Wrba F, Gasche C. IL10R2 Overexpression Promotes IL22/STAT3 Signaling in Colorectal Carcinogenesis. Cancer Immunol Res. 2015;3:1227-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Ford ML. Gut–Immune Crosstalk: How IL-6 Signaling Links Inflammation to Epithelial Regeneration. Am J Transplant. 2015;15:1451. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Li Y, de Haar C, Peppelenbosch MP, van der Woude CJ. SOCS3 in immune regulation of inflammatory bowel disease and inflammatory bowel disease-related cancer. Cytokine Growth Factor Rev. 2012;23:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Rigby RJ, Simmons JG, Greenhalgh CJ, Alexander WS, Lund PK. Suppressor of cytokine signaling 3 (SOCS3) limits damage-induced crypt hyper-proliferation and inflammation-associated tumorigenesis in the colon. Oncogene. 2007;26:4833-4841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 33. | Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Galle PR, Blessing M, Rose-John S, Neurath MF. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 596] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 34. | Hatamzade Esfahani N, Day AS. The Role of TGF-β, Activin and Follistatin in Inflammatory Bowel Disease. Gastrointest Disord. 2023;5:167-186. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Means AL, Freeman TJ, Zhu J, Woodbury LG, Marincola-Smith P, Wu C, Meyer AR, Weaver CJ, Padmanabhan C, An H, Zi J, Wessinger BC, Chaturvedi R, Brown TD, Deane NG, Coffey RJ, Wilson KT, Smith JJ, Sawyers CL, Goldenring JR, Novitskiy SV, Washington MK, Shi C, Beauchamp RD. Epithelial Smad4 Deletion Up-Regulates Inflammation and Promotes Inflammation-Associated Cancer. Cell Mol Gastroenterol Hepatol. 2018;6:257-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | Ren W, Shen S, Sun Z, Shu P, Shen X, Bu C, Ai F, Zhang X, Tang A, Tian L, Li G, Li X, Ma J. Jak-STAT3 pathway triggers DICER1 for proteasomal degradation by ubiquitin ligase complex of CUL4A(DCAF1) to promote colon cancer development. Cancer Lett. 2016;375:209-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Erreni M, Mantovani A, Allavena P. Tumor-associated Macrophages (TAM) and Inflammation in Colorectal Cancer. Cancer Microenviron. 2011;4:141-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 266] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 38. | Zhang X, Wei L, Wang J, Qin Z, Wang J, Lu Y, Zheng X, Peng Q, Ye Q, Ai F, Liu P, Wang S, Li G, Shen S, Ma J. Suppression Colitis and Colitis-Associated Colon Cancer by Anti-S100a9 Antibody in Mice. Front Immunol. 2017;8:1774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 39. | Zhang X, Ai F, Li X, She X, Li N, Tang A, Qin Z, Ye Q, Tian L, Li G, Shen S, Ma J. Inflammation-induced S100A8 activates Id3 and promotes colorectal tumorigenesis. Int J Cancer. 2015;137:2803-2814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Jain K, Rana R. Role of epigenetics, DNA methylation, histone modification, and microRNA in different cancers. In: Singh AN, Rana R, Nayak S. Advances in Cancer Biomarkers Research. Amsterdam: Elsevier, 2025: 47-63. [DOI] [Full Text] |
| 41. | Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, Zhou Z, Shu G, Yin G. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 1605] [Article Influence: 401.3] [Reference Citation Analysis (0)] |
| 42. | Beck PL, Rosenberg IM, Xavier RJ, Koh T, Wong JF, Podolsky DK. Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol. 2003;162:597-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 156] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 43. | Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;17:79-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 636] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 44. | Goldstone R, Itzkowitz S, Harpaz N, Ullman T. Progression of low-grade dysplasia in ulcerative colitis: effect of colonic location. Gastrointest Endosc. 2011;74:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Tilg H, Adolph TE, Gerner RR, Moschen AR. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell. 2018;33:954-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 582] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 46. | Cui G. T(H)9, T(H)17, and T(H)22 Cell Subsets and Their Main Cytokine Products in the Pathogenesis of Colorectal Cancer. Front Oncol. 2019;9:1002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 47. | Wang C, Gong G, Sheh A, Muthupalani S, Bryant EM, Puglisi DA, Holcombe H, Conaway EA, Parry NAP, Bakthavatchalu V, Short SP, Williams CS, Wogan GN, Tannenbaum SR, Fox JG, Horwitz BH. Interleukin-22 drives nitric oxide-dependent DNA damage and dysplasia in a murine model of colitis-associated cancer. Mucosal Immunol. 2017;10:1504-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | Krzystek-Korpacka M, Zawadzki M, Kapturkiewicz B, Lewandowska P, Bednarz-Misa I, Gorska S, Witkiewicz W, Gamian A. Subsite heterogeneity in the profiles of circulating cytokines in colorectal cancer. Cytokine. 2018;110:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Vyas SP, Goswami R. A Decade of Th9 Cells: Role of Th9 Cells in Inflammatory Bowel Disease. Front Immunol. 2018;9:1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 50. | Hardbower DM, Coburn LA, Asim M, Singh K, Sierra JC, Barry DP, Gobert AP, Piazuelo MB, Washington MK, Wilson KT. EGFR-mediated macrophage activation promotes colitis-associated tumorigenesis. Oncogene. 2017;36:3807-3819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 51. | Singh K, Coburn LA, Asim M, Barry DP, Allaman MM, Shi C, Washington MK, Luis PB, Schneider C, Delgado AG, Piazuelo MB, Cleveland JL, Gobert AP, Wilson KT. Ornithine Decarboxylase in Macrophages Exacerbates Colitis and Promotes Colitis-Associated Colon Carcinogenesis by Impairing M1 Immune Responses. Cancer Res. 2018;78:4303-4315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 52. | Kim C, Lee CK, Chon HJ, Kim JH, Park HS, Heo SJ, Kim HJ, Kim TS, Kwon WS, Chung HC, Rha SY. PTEN loss and level of HER2 amplification is associated with trastuzumab resistance and prognosis in HER2-positive gastric cancer. Oncotarget. 2017;8:113494-113501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 53. | Yang C, Wei C, Wang S, Shi D, Zhang C, Lin X, Dou R, Xiong B. Elevated CD163(+)/CD68(+) Ratio at Tumor Invasive Front is Closely Associated with Aggressive Phenotype and Poor Prognosis in Colorectal Cancer. Int J Biol Sci. 2019;15:984-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 54. | Wu T, Dai Y, Wang W, Teng G, Jiao H, Shuai X, Zhang R, Zhao P, Qiao L. Macrophage targeting contributes to the inhibitory effects of embelin on colitis-associated cancer. Oncotarget. 2016;7:19548-19558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260:102-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 357] [Cited by in RCA: 481] [Article Influence: 43.7] [Reference Citation Analysis (14)] |
| 56. | Kvorjak M, Ahmed Y, Miller ML, Sriram R, Coronnello C, Hashash JG, Hartman DJ, Telmer CA, Miskov-Zivanov N, Finn OJ, Cascio S. Cross-talk between Colon Cells and Macrophages Increases ST6GALNAC1 and MUC1-sTn Expression in Ulcerative Colitis and Colitis-Associated Colon Cancer. Cancer Immunol Res. 2020;8:167-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 57. | Van Elssen CH, Frings PW, Bot FJ, Van de Vijver KK, Huls MB, Meek B, Hupperets P, Germeraad WT, Bos GM. Expression of aberrantly glycosylated Mucin-1 in ovarian cancer. Histopathology. 2010;57:597-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Geremia A, Arancibia-Cárcamo CV. Innate Lymphoid Cells in Intestinal Inflammation. Front Immunol. 2017;8:1296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 59. | Ward-Hartstonge KA, Kemp RA. Regulatory T-cell heterogeneity and the cancer immune response. Clin Transl Immunology. 2017;6:e154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 60. | Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, Maeda Y, Hamaguchi M, Ohkura N, Sato E, Nagase H, Nishimura J, Yamamoto H, Takiguchi S, Tanoue T, Suda W, Morita H, Hattori M, Honda K, Mori M, Doki Y, Sakaguchi S. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 687] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 61. | Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, Ham S, Sandall BP, Khan MW, Mahvi DM, Halverson AL, Stryker SJ, Boller AM, Singal A, Sneed RK, Sarraj B, Ansari MJ, Oft M, Iwakura Y, Zhou L, Bonertz A, Beckhove P, Gounari F, Khazaie K. Expression of RORγt marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med. 2012;4:164ra159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 62. | Rizzo A, Di Giovangiulio M, Stolfi C, Franzè E, Fehling HJ, Carsetti R, Giorda E, Colantoni A, Ortenzi A, Rugge M, Mescoli C, Monteleone G, Fantini MC. RORγt-Expressing Tregs Drive the Growth of Colitis-Associated Colorectal Cancer by Controlling IL6 in Dendritic Cells. Cancer Immunol Res. 2018;6:1082-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 63. | Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 460] [Article Influence: 24.2] [Reference Citation Analysis (1)] |
| 64. | Richard ML, Liguori G, Lamas B, Brandi G, da Costa G, Hoffmann TW, Pierluigi Di Simone M, Calabrese C, Poggioli G, Langella P, Campieri M, Sokol H. Mucosa-associated microbiota dysbiosis in colitis associated cancer. Gut Microbes. 2018;9:131-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 65. | Brennan CA, Garrett WS. Fusobacterium nucleatum - symbiont, opportunist and oncobacterium. Nat Rev Microbiol. 2019;17:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 817] [Article Influence: 136.2] [Reference Citation Analysis (2)] |
| 66. | Yu M, Ye J, Cao S, Liu X, Chen Z. Production and Characterization of Monoclonal Antibodies Against Gp Protein of Ebola Virus. Monoclon Antib Immunodiagn Immunother. 2020;39:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 67. | Arthur JC, Gharaibeh RZ, Mühlbauer M, Perez-Chanona E, Uronis JM, McCafferty J, Fodor AA, Jobin C. Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer. Nat Commun. 2014;5:4724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 68. | Dougherty MW, Jobin C. Shining a Light on Colibactin Biology. Toxins (Basel). 2021;13:346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 69. | Boleij A, Hechenbleikner EM, Goodwin AC, Badani R, Stein EM, Lazarev MG, Ellis B, Carroll KC, Albesiano E, Wick EC, Platz EA, Pardoll DM, Sears CL. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin Infect Dis. 2015;60:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 477] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 70. | Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1113] [Cited by in RCA: 1361] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 71. | Hwang S, Lee CG, Jo M, Park CO, Gwon SY, Hwang S, Yi HC, Lee SY, Eom YB, Karim B, Rhee KJ. Enterotoxigenic Bacteroides fragilis infection exacerbates tumorigenesis in AOM/DSS mouse model. Int J Med Sci. 2020;17:145-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 72. | Lee YK, Mehrabian P, Boyajian S, Wu WL, Selicha J, Vonderfecht S, Mazmanian SK. The Protective Role of Bacteroides fragilis in a Murine Model of Colitis-Associated Colorectal Cancer. mSphere. 2018;3:e00587-e00518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 73. | Tian Y, Xu Q, Sun L, Ye Y, Ji G. Short-chain fatty acids administration is protective in colitis-associated colorectal cancer development. J Nutr Biochem. 2018;57:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 74. | Bezzio C, Festa S, Saibeni S, Papi C. Chemoprevention of colorectal cancer in ulcerative colitis: digging deep in current evidence. Expert Rev Gastroenterol Hepatol. 2017;11:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 75. | Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 926] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 76. | Ananthakrishnan AN, Cagan A, Cai T, Gainer VS, Shaw SY, Churchill S, Karlson EW, Murphy SN, Kohane I, Liao KP. Colonoscopy is associated with a reduced risk for colon cancer and mortality in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2015;13:322-329.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 77. | Suzuki K, Muto T, Shinozaki M, Higuchi Y, Sawada T, Saito Y. Results of cancer surveillance in ulcerative colitis. J Gastroenterol. 1995;30 Suppl 8:40-42. [PubMed] |
| 78. | Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol. 2019;114:384-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 1137] [Article Influence: 162.4] [Reference Citation Analysis (0)] |
| 79. | Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L, Lees C, Ho GT, Satsangi J, Bloom S; IBD Section of the British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1045] [Cited by in RCA: 966] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 80. | American Society for Gastrointestinal Endoscopy Standards of Practice Committee; Shergill AK, Lightdale JR, Bruining DH, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Evans JA, Fanelli RD, Fisher DA, Fonkalsrud L, Foley K, Hwang JH, Jue TL, Khashab MA, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf R, Cash BD, DeWitt JM. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc. 2015;81:1101-21.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 269] [Article Influence: 24.5] [Reference Citation Analysis (1)] |
| 81. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP, Lucassen A, Jenkins P, Fairclough PD, Woodhouse CR; British Society of Gastroenterology; Association of Coloproctology for Great Britain and Ireland. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 817] [Article Influence: 51.1] [Reference Citation Analysis (2)] |
| 82. | Spiceland CM, Lodhia N. Endoscopy in inflammatory bowel disease: Role in diagnosis, management, and treatment. World J Gastroenterol. 2018;24:4014-4020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 83. | Itzkowitz SH, Present DH; Crohn's and Colitis Foundation of America Colon Cancer in IBD Study Group. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 397] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 84. | Bernstein CN, Shanahan F, Weinstein WM. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet. 1994;343:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 364] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 85. | Ullman T, Croog V, Harpaz N, Hossain S, Kornbluth A, Bodian C, Itzkowitz S. Progression to colorectal neoplasia in ulcerative colitis: effect of mesalamine. Clin Gastroenterol Hepatol. 2008;6:1225-30; quiz 1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 86. | Befrits R, Ljung T, Jaramillo E, Rubio C. Low-grade dysplasia in extensive, long-standing inflammatory bowel disease: a follow-up study. Dis Colon Rectum. 2002;45:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 87. | Navaneethan U, Jegadeesan R, Gutierrez NG, Venkatesh PG, Hammel JP, Shen B, Kiran RP. Progression of low-grade dysplasia to advanced neoplasia based on the location and morphology of dysplasia in ulcerative colitis patients with extensive colitis under colonoscopic surveillance. J Crohns Colitis. 2013;7:e684-e691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 88. | Fumery M, Dulai PS, Gupta S, Prokop LJ, Ramamoorthy S, Sandborn WJ, Singh S. Incidence, Risk Factors, and Outcomes of Colorectal Cancer in Patients With Ulcerative Colitis With Low-Grade Dysplasia: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017;15:665-674.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 89. | Mutaguchi M, Naganuma M, Sugimoto S, Fukuda T, Nanki K, Mizuno S, Hosoe N, Shimoda M, Ogata H, Iwao Y, Kanai T. Difference in the clinical characteristic and prognosis of colitis-associated cancer and sporadic neoplasia in ulcerative colitis patients. Dig Liver Dis. 2019;51:1257-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 90. | Kavanagh DO, Carter MC, Keegan D, Doherty G, Smith MJ, Hyland JM, Mulcahy H, Sheahan K, O' Connell PR, O' Donoghue DP, Winter DC. Management of colorectal cancer in patients with inflammatory bowel disease. Tech Coloproctol. 2014;18:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 91. | Abdalla M, Landerholm K, Andersson P, Andersson RE, Myrelid P. Risk of Rectal Cancer After Colectomy for Patients With Ulcerative Colitis: A National Cohort Study. Clin Gastroenterol Hepatol. 2017;15:1055-1060.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 92. | Al-Sukhni W, McLeod RS, MacRae H, O'Connor B, Huang H, Cohen Z. Oncologic outcome in patients with ulcerative colitis associated with dyplasia or cancer who underwent stapled or handsewn ileal pouch-anal anastomosis. Dis Colon Rectum. 2010;53:1495-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Gorfine SR, Harris MT, Bub DS, Bauer JJ. Restorative proctocolectomy for ulcerative colitis complicated by colorectal cancer. Dis Colon Rectum. 2004;47:1377-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 94. | Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S, Tabernero J, Teixeira L, Tortora G, Van Laethem JL, Young R, Penenberg DN, Lu B, Romano A, Von Hoff DD. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107:dju413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 468] [Article Influence: 42.5] [Reference Citation Analysis (4)] |
| 95. | Merchea A, Wolff BG, Dozois EJ, Abdelsattar ZM, Harmsen WS, Larson DW. Clinical features and oncologic outcomes in patients with rectal cancer and ulcerative colitis: a single-institution experience. Dis Colon Rectum. 2012;55:881-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 96. | Remzi FH, Preen M. Rectal cancer and ulcerative colitis: does it change the therapeutic approach? Colorectal Dis. 2003;5:483-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 97. | Spinelli A, Bonovas S, Burisch J, Kucharzik T, Adamina M, Annese V, Bachmann O, Bettenworth D, Chaparro M, Czuber-Dochan W, Eder P, Ellul P, Fidalgo C, Fiorino G, Gionchetti P, Gisbert JP, Gordon H, Hedin C, Holubar S, Iacucci M, Karmiris K, Katsanos K, Kopylov U, Lakatos PL, Lytras T, Lyutakov I, Noor N, Pellino G, Piovani D, Savarino E, Selvaggi F, Verstockt B, Doherty G, Raine T, Panis Y. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Surgical Treatment. J Crohns Colitis. 2022;16:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 190] [Article Influence: 47.5] [Reference Citation Analysis (1)] |
| 98. | Das P, Johnson MW, Tekkis PP, Nicholls RJ. Risk of dysplasia and adenocarcinoma following restorative proctocolectomy for ulcerative colitis. Colorectal Dis. 2007;9:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 99. | Mark-Christensen A, Erichsen R, Brandsborg S, Rosenberg J, Qvist N, Thorlacius-Ussing O, Hillingsø J, Pachler JH, Christiansen EG, Laurberg S. Long-term Risk of Cancer Following Ileal Pouch-anal Anastomosis for Ulcerative Colitis. J Crohns Colitis. 2018;12:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 100. | Kariv R, Remzi FH, Lian L, Bennett AE, Kiran RP, Kariv Y, Fazio VW, Lavery IC, Shen B. Preoperative colorectal neoplasia increases risk for pouch neoplasia in patients with restorative proctocolectomy. Gastroenterology. 2010;139:806-812, 812.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 101. | Derikx LA, Kievit W, Drenth JP, de Jong DJ, Ponsioen CY, Oldenburg B, van der Meulen-de Jong AE, Dijkstra G, Grubben MJ, van Laarhoven CJ, Nagtegaal ID, Hoentjen F; Dutch Initiative on Crohn and Colitis. Prior colorectal neoplasia is associated with increased risk of ileoanal pouch neoplasia in patients with inflammatory bowel disease. Gastroenterology. 2014;146:119-28.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 102. | Silva-Velazco J, Stocchi L, Wu XR, Shen B, Remzi FH. Twenty-year-old stapled pouches for ulcerative colitis without evidence of rectal cancer: implications for surveillance strategy? Dis Colon Rectum. 2014;57:1275-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Selvaggi F, Pellino G, Canonico S, Sciaudone G. Systematic review of cuff and pouch cancer in patients with ileal pelvic pouch for ulcerative colitis. Inflamm Bowel Dis. 2014;20:1296-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 104. | Pellino G, Kontovounisios C, Tait D, Nicholls J, Tekkis PP. Squamous Cell Carcinoma of the Anal Transitional Zone after Ileal Pouch Surgery for Ulcerative Colitis: Systematic Review and Treatment Perspectives. Case Rep Oncol. 2017;10:112-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 105. | Ali RA, Dooley C, Comber H, Newell J, Egan LJ. Clinical features, treatment, and survival of patients with colorectal cancer with or without inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:584-9.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 106. | Thicoïpé A, Laharie D, Smith D, Chabrun E, Rullier A, Poullenot F, Rullier E, Denost Q. Oncological outcomes of IBD-associated versus sporadic colorectal cancer in modern era: a matched case-control study. Int J Colorectal Dis. 2018;33:963-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 107. | Gearhart SL, Nathan H, Pawlik TM, Wick E, Efron J, Shore AD. Outcomes from IBD-associated and non-IBD-associated colorectal cancer: a Surveillance Epidemiology and End Results Medicare study. Dis Colon Rectum. 2012;55:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 108. | Hrabe JE, Byrn JC, Button AM, Zamba GK, Kapadia MR, Mezhir JJ. A matched case-control study of IBD-associated colorectal cancer: IBD portends worse outcome. J Surg Oncol. 2014;109:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 109. | Watanabe T, Konishi T, Kishimoto J, Kotake K, Muto T, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Ulcerative colitis-associated colorectal cancer shows a poorer survival than sporadic colorectal cancer: a nationwide Japanese study. Inflamm Bowel Dis. 2011;17:802-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 110. | Olén O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, Ekbom A, Sørensen HT, Ludvigsson JF. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020;395:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 346] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 111. | Huang A, Yang Y, Shi JY, Li YK, Xu JX, Cheng Y, Gu J. Mucinous adenocarcinoma: A unique clinicopathological subtype in colorectal cancer. World J Gastrointest Surg. 2021;13:1567-1583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (2)] |
| 112. | Alvi MA, Loughrey MB, Dunne P, McQuaid S, Turkington R, Fuchs MA, McGready C, Bingham V, Pang B, Moore W, Maxwell P, Lawler M, James JA, Murray GI, Wilson RH, Salto-Tellez M. Molecular profiling of signet ring cell colorectal cancer provides a strong rationale for genomic targeted and immune checkpoint inhibitor therapies. Br J Cancer. 2017;117:203-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 113. | Wu X, Lin H, Li S. Prognoses of different pathological subtypes of colorectal cancer at different stages: A population-based retrospective cohort study. BMC Gastroenterol. 2019;19:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 114. | Block M, Jørgensen KK, Oresland T, Lindholm E, Grzyb K, Cvancarova M, Vatn MH, Boberg KM, Börjesson L. Colectomy for patients with ulcerative colitis and primary sclerosing cholangitis - what next? J Crohns Colitis. 2014;8:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 115. | Imam MH, Eaton JE, Puckett JS, Loftus EV Jr, Mathis KL, Gossard AA, Talwalkar JA, Lindor KD. Neoplasia in the ileoanal pouch following colectomy in patients with ulcerative colitis and primary sclerosing cholangitis. J Crohns Colitis. 2014;8:1294-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 116. | Passaro A, Al Bakir M, Hamilton EG, Diehn M, André F, Roy-Chowdhuri S, Mountzios G, Wistuba II, Swanton C, Peters S. Cancer biomarkers: Emerging trends and clinical implications for personalized treatment. Cell. 2024;187:1617-1635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 301] [Article Influence: 150.5] [Reference Citation Analysis (0)] |
| 117. | Dakal TC, Dhakar R, Beura A, Moar K, Maurya PK, Sharma NK, Ranga V, Kumar A. Emerging methods and techniques for cancer biomarker discovery. Pathol Res Pract. 2024;262:155567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 118. | Carrat F, Seksik P, Colombel JF, Peyrin-Biroulet L, Beaugerie L; CESAME Study Group. The effects of aminosalicylates or thiopurines on the risk of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 119. | Bonovas S, Fiorino G, Lytras T, Nikolopoulos G, Peyrin-Biroulet L, Danese S. Systematic review with meta-analysis: use of 5-aminosalicylates and risk of colorectal neoplasia in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45:1179-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 120. | Raine T, Bonovas S, Burisch J, Kucharzik T, Adamina M, Annese V, Bachmann O, Bettenworth D, Chaparro M, Czuber-Dochan W, Eder P, Ellul P, Fidalgo C, Fiorino G, Gionchetti P, Gisbert JP, Gordon H, Hedin C, Holubar S, Iacucci M, Karmiris K, Katsanos K, Kopylov U, Lakatos PL, Lytras T, Lyutakov I, Noor N, Pellino G, Piovani D, Savarino E, Selvaggi F, Verstockt B, Spinelli A, Panis Y, Doherty G. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J Crohns Colitis. 2022;16:2-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 665] [Article Influence: 166.3] [Reference Citation Analysis (1)] |
| 121. | Zhu Z, Mei Z, Guo Y, Wang G, Wu T, Cui X, Huang Z, Zhu Y, Wen D, Song J, He H, Xu W, Cui L, Liu C. Reduced Risk of Inflammatory Bowel Disease-associated Colorectal Neoplasia with Use of Thiopurines: a Systematic Review and Meta-analysis. J Crohns Colitis. 2018;12:546-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 122. | Lu MJ, Qiu XY, Mao XQ, Li XT, Zhang HJ. Systematic review with meta-analysis: thiopurines decrease the risk of colorectal neoplasia in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2018;47:318-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 123. | Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, Kucharzik T, Molnár T, Raine T, Sebastian S, de Sousa HT, Dignass A, Carbonnel F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis. 2017;11:769-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 911] [Article Influence: 101.2] [Reference Citation Analysis (1)] |
| 124. | Baars JE, Looman CW, Steyerberg EW, Beukers R, Tan AC, Weusten BL, Kuipers EJ, van der Woude CJ. The risk of inflammatory bowel disease-related colorectal carcinoma is limited: results from a nationwide nested case-control study. Am J Gastroenterol. 2011;106:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 125. | Alkhayyat M, Abureesh M, Gill A, Khoudari G, Abou Saleh M, Mansoor E, Regueiro M. Lower Rates of Colorectal Cancer in Patients With Inflammatory Bowel Disease Using Anti-TNF Therapy. Inflamm Bowel Dis. 2021;27:1052-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 126. | Samadder NJ, Mukherjee B, Huang SC, Ahn J, Rennert HS, Greenson JK, Rennert G, Gruber SB. Risk of colorectal cancer in self-reported inflammatory bowel disease and modification of risk by statin and NSAID use. Cancer. 2011;117:1640-1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 127. | Ananthakrishnan AN, Cagan A, Cai T, Gainer VS, Shaw SY, Churchill S, Karlson EW, Murphy SN, Liao KP, Kohane I. Statin Use Is Associated With Reduced Risk of Colorectal Cancer in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2016;14:973-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 128. | Shah SC, Glass J, Giustino G, Hove JRT, Castaneda D, Torres J, Kumar A, Elman J, Ullman TA, Itzkowitz SH. Statin Exposure Is Not Associated with Reduced Prevalence of Colorectal Neoplasia in Patients with Inflammatory Bowel Disease. Gut Liver. 2019;13:54-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 129. | Potack J, Itzkowitz SH. Colorectal cancer in inflammatory bowel disease. Gut Liver. 2008;2:61-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 130. | Swinson CM, Perry J, Lumb M, Levi AJ. Role of sulphasalazine in the aetiology of folate deficiency in ulcerative colitis. Gut. 1981;22:456-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 131. | Singh S, Khanna S, Pardi DS, Loftus EV Jr, Talwalkar JA. Effect of ursodeoxycholic acid use on the risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2013;19:1631-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 132. | Burr NE, Hull MA, Subramanian V. Does aspirin or non-aspirin non-steroidal anti-inflammatory drug use prevent colorectal cancer in inflammatory bowel disease? World J Gastroenterol. 2016;22:3679-3686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/