Published online Oct 15, 2025. doi: 10.4251/wjgo.v17.i10.108410
Revised: June 19, 2025
Accepted: August 7, 2025
Published online: October 15, 2025
Processing time: 152 Days and 23.2 Hours
Colorectal cancer (CRC) is a malignant tumor characterized by high global inci
To elucidate the mechanism by which modified Yigong San confers therapeutic efficacy against CRC, potentially exerting its effects through apoptosis regulation mediated by the enhancer of zeste homolog 2 (EZH2)/methyltransferase-like 3 (METTL3)/SRY-box transcription factor 4 (SOX4) axis.
In the clinical study, CRC tissues and corresponding adjacent normal samples that fulfilled inclusion criteria were procured. Quantitative reverse transcription polymerase chain reaction was employed to determine the transcriptional expression of EZH2 and METTL3 mRNA. For in vitro experimentation, SW-480 cells were allocated into five experimental conditions: Control, control + serum, control + negative control, control + overexpressing-EZH2, and control + overexpressing-EZH2 + serum. The mRNA expression levels of EZH2, METTL3, SOX4, B-cell lymphoma 2, and Bax across groups were quantified via quantitative reverse transcription polymerase chain reaction, while protein levels were assessed using western blot analysis. The presence of EZH2 binding sites within the METTL3 promoter region was verified through chromatin immunoprecipitation polymerase chain reaction. The optimal concentration of drug-containing serum (5%, 10%, 15%) was determined using the Cell Counting Kit-8 assay. Cell migratory ability was evaluated via scratch assays, and apoptotic activity was quantified by flow cytometry.
The clinical findings demonstrated significantly elevated transcriptional levels of METTL3 and EZH2 mRNA in tumor tissues compared to their adjacent normal counterparts (P < 0.05). In vitro, cells treated with modified Yigong San exhibited a substantial downregulation of EZH2, METTL3, SOX4, B-cell lymphoma 2, and Bax mRNA and protein levels (P < 0.05), relative to the control group. Apoptotic rates were markedly increased, while mi
Modified Yigong San exhibits both preventive and therapeutic potential against CRC, likely mediated through the regulation of apoptosis via the EZH2/METTL3/SOX4 signaling pathway.
Core Tip: In this study, SW-480 cells were used as experimental subjects. Quantitative reverse transcription polymerase chain reaction, western blot, cell scratch assay, flow cytometry and other experimental methods were used to explore the expression of genes such as methyltransferase-like 3 (METTL3), enhancer of zeste homolog 2 (EZH2), SRY-box tran
- Citation: Wang J, Zhang XW, Tang BW, Li Z, Song N. Molecular mechanism of modified Yigong San formula against colorectal cancer via EZH2/METTL3/SOX4 pathway-mediated apoptosis. World J Gastrointest Oncol 2025; 17(10): 108410
- URL: https://www.wjgnet.com/1948-5204/full/v17/i10/108410.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i10.108410
Colorectal cancer (CRC) constitutes one of the most prevalent categories of malignant tumors, marked by elevated global incidence and mortality rates, thereby posing a substantial threat to public health. According to global cancer burden statistics released by the International Agency for Research on Cancer of the World Health Organization in 2020, CRC ranks third in terms of newly diagnosed malignancies and second in cancer-related deaths[1]. The pathogenesis and development of CRC represent a multifactorial and multistage process, shaped by intricate interactions among genetic susceptibility, environmental exposures, lifestyle factors, and other variables. Currently, conventional therapeutic stra
In this context, traditional Chinese medicine (TCM) is recognized for its distinctive therapeutic advantages and introduces novel strategies for the prevention and management of CRC. Although the term “colorectal cancer” does not appear explicitly in classical TCM literature, the condition, based on its clinical presentation, can be subsumed under several disease categories, such as “intestinal masses” (Chang Tan), “accumulations” (Ji Ju), “visceral toxins” (Zang Du), and “locked anus hemorrhoids” (Suo Gang Zhi). Dong-Yuan Li once asserted that “injuries to the spleen and stomach give rise to hundreds of diseases”, implying that spleen qi deficiency constitutes a foundational pathological mechanism in the progression of CRC. Contemporary treatment modalities for CRC, including surgical resection, radiotherapy, chemotherapy, targeted therapy, and immunotherapy, are frequently associated with the depletion of vital qi and da
To further elucidate the specific mechanisms responsible for the therapeutic efficacy of the Yigong San Jiawei formula in CRC treatment, this study was designed to investigate its molecular-level activity. Within CRC-related research, enhancer of zeste homolog 2 (EZH2), methyltransferase-like 3 (METTL3), and SRY-box transcription factor 4 (SOX4) are identified as key epigenetic regulators and transcription factors that exert substantial influence over tumor cell proliferation, metastasis, and therapeutic resistance[10-12]. EZH2 functions as a core component of the polycomb repressive complex 2, primarily suppressing transcription by catalyzing trimethylation of lysine 27 on histone H3. Aberrant overexpression of EZH2 in CRC has been strongly associated with enhanced tumor cell proliferation, invasiveness, and metastatic potential. METTL3, a methyltransferase catalyzing N6-methyladenosine (m6A) modification, plays a fundamental role in the epigenetic regulation of mRNA. Studies have demonstrated that METTL3 promotes tumor cell proliferation by modulating the expression of oncogenes such as MYC and B-cell lymphoma 2 (Bcl2)[13]. Furthermore, METTL3 is involved in the regulation of m6A modifications on long non-coding RNAs and microRNAs, thereby influencing tumor-related gene expression networks[14]. SOX4, as a transcription factor, participates in diverse biological processes including cellular differentiation, developmental programming, and apoptosis. Its capacity to promote malignancy is primarily mediated through the regulation of downstream target genes. In addition, SOX4 has been shown to facilitate epithelial-mesenchymal transition (EMT)[15], a critical process enabling tumor cells to acquire migratory and invasive phenotypes. This study aimed to uncover the underlying mechanism through which the modified Yigong San formula exerts its anti-CRC effects. SW-480 cells were employed as the experimental model, and proteins associated with the EZH2/METTL3/SOX4 axis were analyzed via western blot. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was utilized to quantify gene expression and pathway-related signaling molecules. Apoptotic activity and cell motility were assessed by flow cytometry (FCM) and scratch assays, respectively. By delineating the molecular mechanism through which modified Yigong San confers anti-tumor effects, this study provides a scientific rationale and expanded perspective for its potential application in clinical practice.
Ten pairs of CRC tissues and their corresponding adjacent normal tissues were obtained from Liaoning Cancer Hospital & Institute. The diagnostic criteria for Western medicine were established in accordance with the Guidelines for the Chinese Society of Clinical Oncology, published by People’s Medical Publishing House. The diagnostic standards for TCM were based on Diagnostics of Traditional Chinese Medicine (First Edition, 2018, edited by Tie-Tao Deng, Shanghai Scientific & Technical Publishers) and the Guideline for Diagnosis and Treatment of Tumors in Traditional Chinese Medicine issued by the Chinese Association of Traditional Chinese Medicine. The inclusion criteria comprised the following: (1) A histopathologically confirmed diagnosis of CRC; (2) No prior medical treatment directed at the tumor before surgical resection; and (3) Provision of informed consent and compliance with medical instructions. The exclusion criteria were as follows: (1) Emergency surgical indications, including acute intestinal obstruction, tumor perforation, or diffuse peritonitis; (2) Postoperative pathological confirmation of positive surgical margins; (3) Incomplete clinical documentation; and (4) Comorbidities that may influence complication profiles, such as renal insufficiency, heart failure, hematological disorders, or infectious diseases. The human colon cancer cell line SW-480 (catalog number: CL-0223) was acquired from Pricella Biotechnology Co., Ltd (Wuhan, China). Additionally, twenty Kunming mice (aged 4-6 weeks, weighing 18-20 g) were purchased from Liaoning Changsheng Biotechnology Co., Ltd (Ethical Approval Number for Clinical Trials, No. KY20240341; Ethical Approval Number for Animal Experimentation, No. 2021012125).
The following laboratory instruments and equipment were employed in this study: An HF-90 CO2 incubator (Shanghai Lishen, China); an NW10 LVF ultrapure water system (HealForce, Shanghai); an H-2050R high-speed refrigerated centrifuge (Hunan Xiangyi, China); an AE31 inverted phase contrast microscope (Motic, China); an SW-CJ-2FD clean bench (Sujing Antai, China); a DH36001B electric constant temperature incubator (Tianjin Taisite, China); an ELX-800 microplate reader (BIOTEK, VT, United States); a C6 flow cytometer (BD, NJ, United States); proline micropipettes (BIOHIT, Finland); a DZF-6050 vacuum drying oven (SYSBERY, China); a NANO 2000 UV spectrophotometer (Thermo, MA, United States); a QuantStudio 3 real-time PCR system (Thermo, MA, United States); a Life Express PCR thermal cycler (BIOER, China); a DYY-7C electrophoresis apparatus (Liuyi, China); a WD-9413B gel imaging analysis system (Liuyi, China); a DZKW-4 water bath (Kexi, China); a WD-9405B horizontal shaker (Liuyi, China); a DYCZ-40D transfer tank (Liuyi, China); and a DYCZ-24DN dual vertical protein electrophoresis apparatus (Liuyi, China).
The following reagents and materials were employed in this study: Fetal bovine serum (Hyclone, lot number: SH30084.03, UT, United States); phosphate-buffered saline (PBS) (Double Helix, lot number: P10033, China); trypsin (Beyotime, lot number: C0203, China); Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, lot number: 12100-46, NY, United States); Lipofectamine 2000 (Invitrogen, lot number: 1024993, CA, United States); apoptosis detection kit (Kaiji Biology, catalog number: KGA106, China); TRIzol reagent (Thermo, lot number: 1596-026, MA, United States); super M-MLV reverse transcriptase (BioTeke, lot number: PR6502, China); ribonuclease (RNase) inhibitor (BioTeke, lot number: RP5602, China); 2 × PowerTaq PCR Master Mix (BioTeke, lot number: PR1702, China); SYBR Green dye (Solarbio, lot number: SY1020, China); Cell Counting Kit-8 (CCK-8) (Beyotime, lot number: C0037, China); chromatin immunoprecipitation (ChIP) kit (Millipore, lot number: 17-371, MA, United States); agarose powder (Spain, lot number: 111860); 50 × Tris-acetate-EDTA buffer (Helix, lot number: N10053, China); Goldview nucleic acid stain (Solarbio, lot number: G8142, China); EZH2 antibody (Abcam, lot number: Ab307646, United Kingdom); radioimmunoprecipitation assay lysis buffer (Beyotime, lot number: P0013B, China); phenylmethylsulfonyl fluoride (Beyotime, lot number: ST506, China); bicinchoninic acid protein quantification kit (Beyotime, lot number: P0009, China); primary and secondary antibody stripping buffer (Beyotime, lot number: P0025, China); 30% acrylamide/bis-acrylamide (29:1) solution (Beyotime, lot number: ST003, China); sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) protein loading buffer (Beyotime, lot number: P0015, China); enhanced chemiluminescence reagent (Beyotime, lot number: P0018, China); pre-stained protein molecular weight markers (Fermentas, lot number: 26616, MA, United States); polyvinylidene difluoride membrane (Millipore, lot number: IPVH00010, MA, United States); skim milk powder (Beyotime, lot number: P0216-300 g, China); Bax antibody (Abclonal, lot number: A19684, China); Bcl2 antibody (Abclonal, lot number: A19693, China); goat anti-rabbit immunoglobulin G-horseradish peroxidase conjugate (Thermo, lot number: 31460, MA, United States); internal reference antibody glyceraldehyde 3-phosphate dehydrogenase (Abcam, lot number: Ab128915, United Kingdom); METTL3 antibody (Proteintech, lot number: 15073-1-AP, IL, United States); EZH2 antibody (Proteintech, lot number: 66476-1-Ig, IL, United States); and SOX4 antibody (Proteintech, lot number: 27414-1-AP, IL, United States).
The modified Yigong San formula consisted of accurately proportioned amounts of Ginseng (15 g), Poria (15 g), Atractylodes macrocephala (15 g), prepared Licorice (10 g), Reticulatae Pericarpium (10 g), Smilax glabra (15 g), and Hedyotis diffusa (15 g), all of which were sourced from the outpatient pharmacy of the Affiliated Hospital of Liaoning University of Traditional Chinese Medicine. Each herbal component conformed to the quality standards specified in the 2015 edition of the Chinese Pharmacopoeia. Prior to decoction, the herbs were immersed in warm water for 1 hour, maintaining a water level approximately 5 cm above the herbal materials. The mixture was subsequently subjected to three successive rounds of decoction, each lasting 1 hour. The resulting extract was centrifuged to remove herbal residues and then stored at 4 °C for further use. Following the acquisition, nude mice were randomly assigned to two experimental groups: A control group (n = 5) and a treatment group (n = 15). Oral gavage was administered once daily over a consecutive 7-day period. Two hours after the final administration, anesthesia was induced via intraperitoneal injection of 10% chloral hydrate. Blood samples were collected from the abdominal aorta and centrifuged at 2000 rpm for 10 minutes to achieve serum separation. The resulting serum was sterilized using a 0.22 μm microporous membrane and stored at -20 °C for subse
SW-480 cells were cultured in DMEM supplemented with 10% fetal bovine serum and cultured at 37 °C in a humidified incubator containing 5% CO2. Subculturing was carried out when the cell confluence reached approximately 80%-90%.
Two transfection reagent solutions were prepared separately. Solution 1 consisted of 250 μL of optimized liquid mixed with 10 μL of lipofectamine 2000, while solution 2 contained 250 μL of optimized liquid and 2 μg of plasmid DNA. Each solution was incubated independently for 5 minutes. Following incubation, the two solutions were combined and allowed to stand at room temperature for 20 minutes. The resulting transfection complex was then added to subcultured SW-480 cells, which were maintained at 37 °C in an atmosphere of 5% CO2 for 4 hours. Subsequently, the transfection medium was gently removed and replaced with a complete medium (DMEM supplemented with 10% serum). The cells were further incubated at 37 °C under 5% CO2, and the transfection was deemed complete after 48 hours.
The cultured cells were divided into five experimental groups: The control group (blank control); the control + Chinese medicine group (treated with 10% drug-containing serum); the overexpressing (OE)-NC group (transfected with an empty plasmid); the OE-EZH2 group; and the OE-EZH2 + Chinese medicine group (OE-EZH2 and treated with 10% drug-containing serum). Subsequently, all groups were incubated for 48 hours at 37 °C in a humidified cell culture incubator containing 5% CO2.
The cultured cells were harvested, counted, and adjusted to a concentration of 1 × 104 cells/mL. A 100 μL aliquot of the resulting suspension was added to each well of a 96-well culture plate and incubated at 37 °C in an atmosphere of 5% CO2. After 24 hours, the medium was replaced with serum containing varying concentrations of either control or drug-containing components: Control, 5%, 10%, and 15% control serum, as well as 5%, 10%, and 15% drug-containing serum. The cells were subsequently incubated for an additional 48 hours under the same conditions. Following incubation, 10 μL of CCK-8 solution was added to each well and mixed thoroughly. After a further 1-hour incubation at 37 °C, OD450 values were measured.
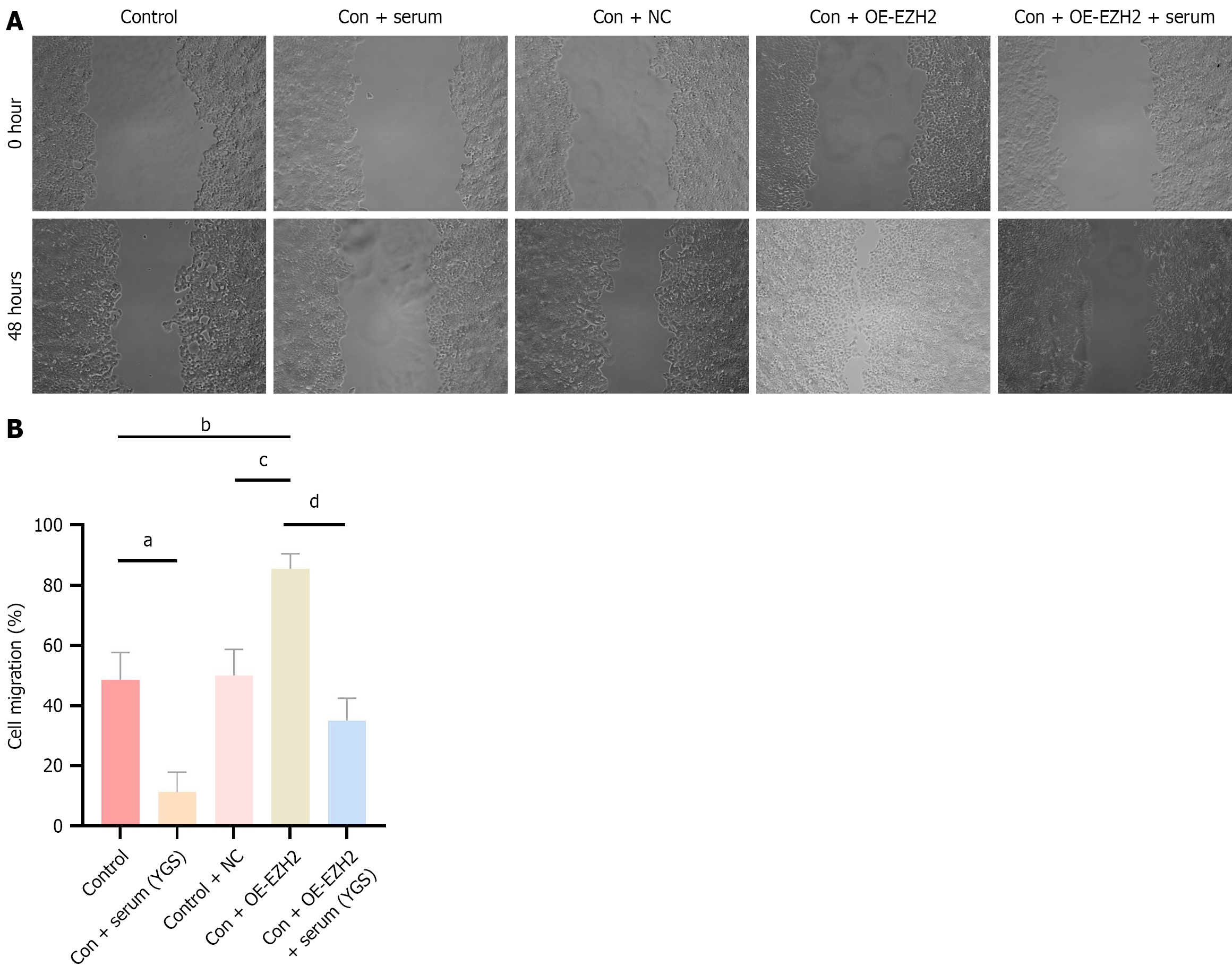
Cells were assigned to the following experimental groups: Control group; 10% control serum group; 10% drug-containing serum group; control group (blank control); control + Chinese medicine group; OE-NC group; OE-EZH2 group; and OE-EZH2 + Chinese medicine group. Transfection procedures were performed according to the corresponding group allocations. After 24 hours, a scratch assay was conducted by removing the culture medium and creating a linear wound across the cell monolayer using a 200 μL pipette tip. The monolayer was then rinsed twice with a serum-free medium to remove cellular debris. Initial cell positions were documented through microscopic imaging. The cells were subsequently incubated in a serum-free medium, and additional images were captured at 0 hour and 48 hours. The migration distance for each group was then measured and analyzed.
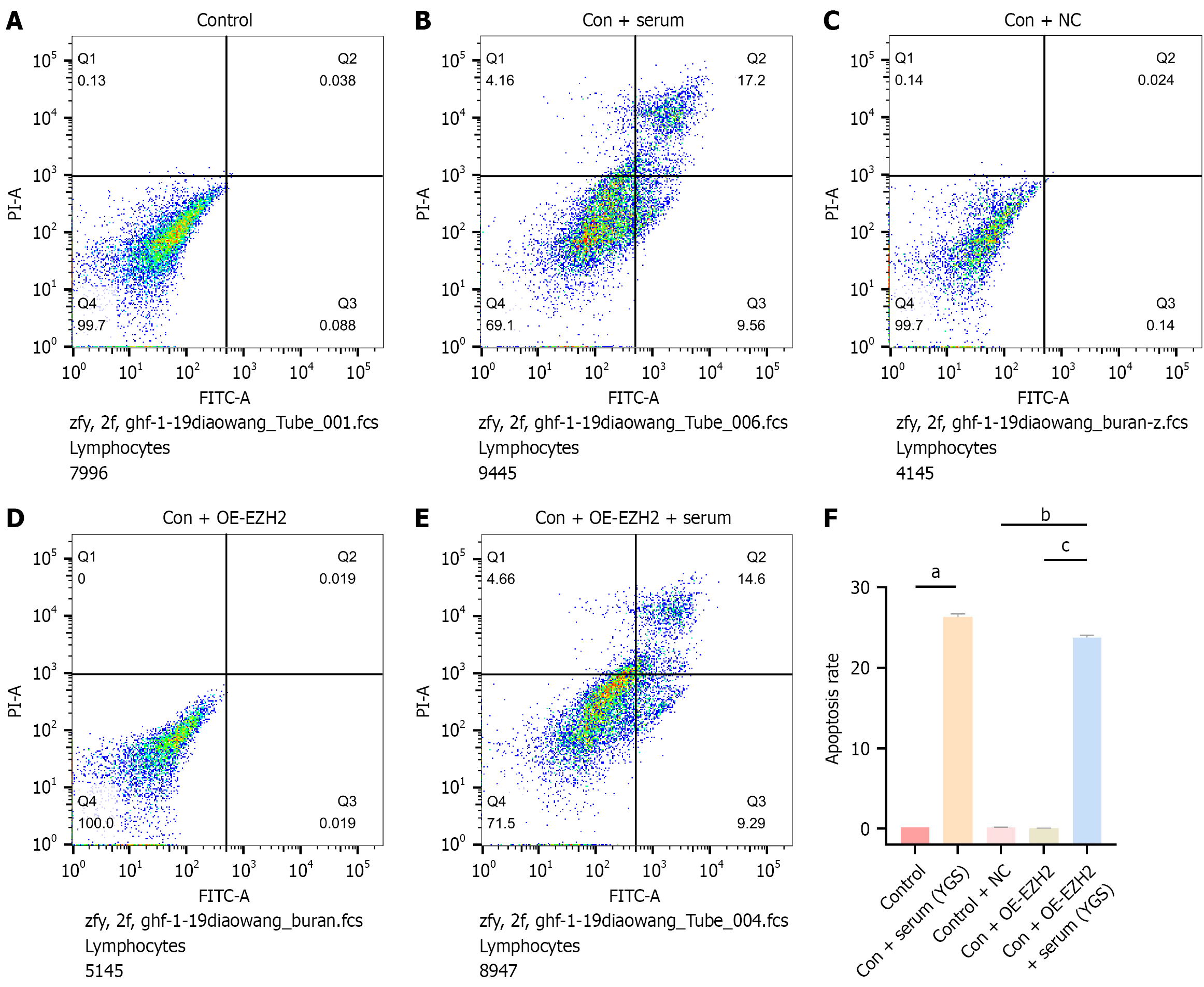
Cells were allocated to experimental groups as described in “Grouping and drug administration”. Specimens were centrifuged at 1000 rpm for 5 minutes, after which cellular pellets were collected and the supernatants were carefully removed by aspiration. The harvested cells were washed twice with PBS and subjected to an additional centrifugation step under identical conditions. Following the removal of the supernatant, approximately 50 μL of PBS was retained in each tube. The resulting cell pellets were gently resuspended in 500 μL of binding buffer per sample. Subsequently, 5 μL of Annexin V-FITC was added and mixed thoroughly, followed by the addition of 5 μL of propidium iodide with gentle agitation. The cell suspensions were incubated in the dark at room temperature for 15 minutes. Apoptotic analysis was conducted using FCM.
Cells were grouped according to the classifications specified in “Grouping and drug administration”. Cell lysis was performed using a protein extraction buffer supplemented with protease and phosphatase inhibitor cocktails. Following complete dissolution, the lysates were centrifuged at 12000 rpm/min for 10 minutes at 4 °C (radius: 30 cm), and the supernatants were transferred to new tubes. Protein concentrations in each group were determined using the bicincho
Cell grouping was performed as described in “Grouping and drug administration”. After 48 hours of incubation, total RNA was extracted from each group. RNA concentrations were quantified using a NANO 2000 UV spectrophotometer. Reverse transcription was carried out according to the manufacturer’s protocol. The reverse transcription conditions included incubation in a 37 °C water bath for 30 minutes, followed by 30 minutes at 42 °C, and a final heating step at 70 °C for 10 minutes. The resulting cDNA was subsequently used as a template, together with gene-specific primers, to amplify EZH2, METTL3, SOX4, Bcl2, and Bax mRNA by PCR. Following amplification, dissociation curve analysis was conducted to confirm the presence of a single amplification product. Relative quantification was then performed. Primer sequences are provided in Table 1.
| Gene name | Primer sequence (5’-3’) | |
| EZH2 | F | 5’-AATCAGAGTACATGCGACTGAGA-3’ |
| R | 5’-GCTGTATCCTTCGCTGTTTCC-3’ | |
| METTL3 | F | 5’-TTGTCTCCAACCTTCCGTAGT-3’ |
| R | 5’-CCAGATCAGAGAGGTGGTGTAG-3’ | |
| SOX4 | F | 5’-AGCGACAAGATCCCTTTCATTC-3’ |
| R | 5’-CGTTGCCGGACTTCACCTT-3’ | |
| Bcl2 | F | 5’-GGTGGGGTCATGTGTGTGG-3’ |
| R | 5’-CGGTTCAGGTACTCAGTCATCC-3’ | |
| Bax | F | 5’-CCCGAGAGGTCTTTTTCCGAG-3’ |
| R | 5’-CCAGCCCATGATGGTTCTGAT-3’ | |
| GAPDH | F | 5’-ACAACTTTGGTATCGTGGAAGG-3’ |
| R | 5’-GCCATCACGCCACAGTTTC-3’ |
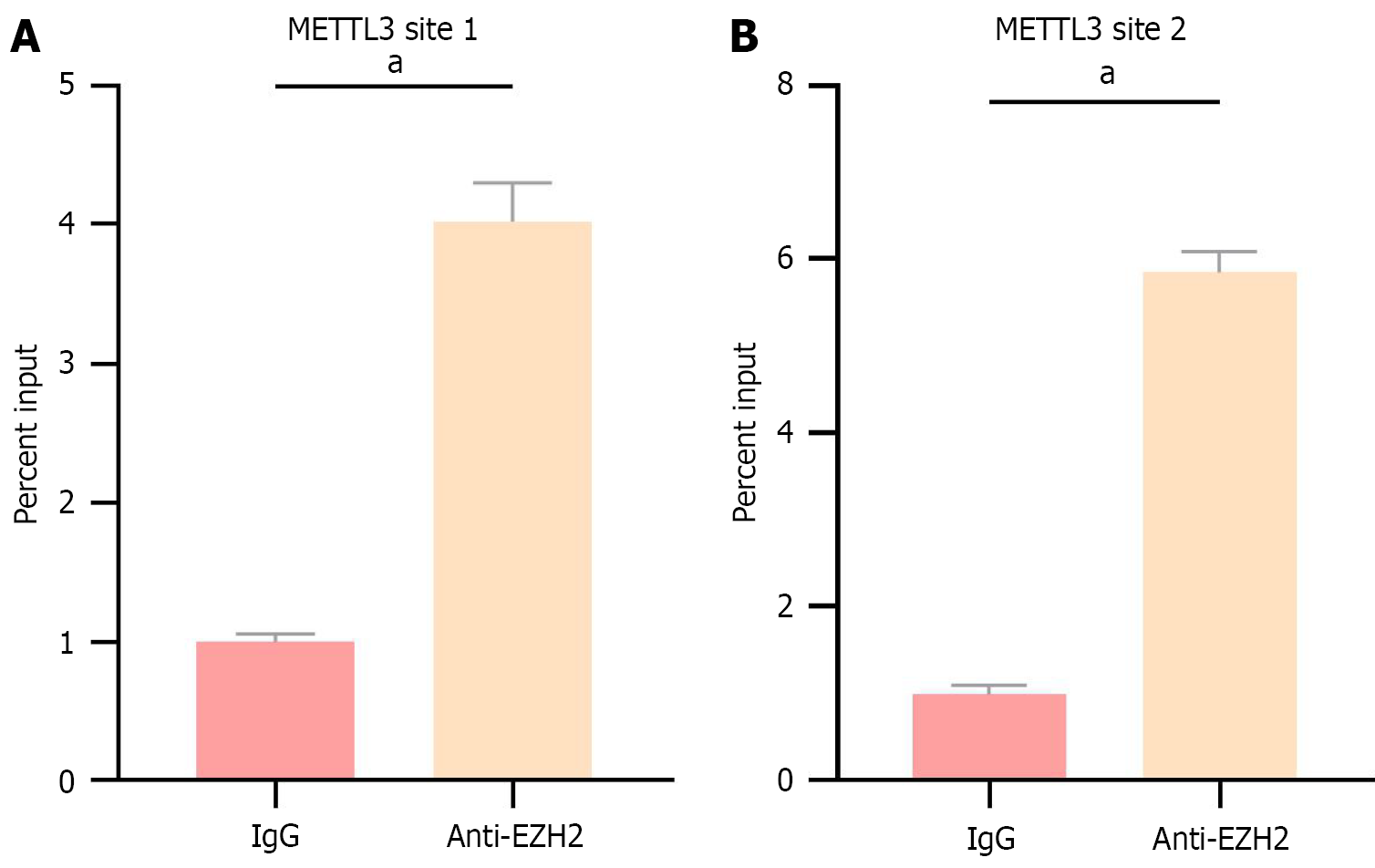
Specimens were fixed by incubation with 1% paraformaldehyde for 10 minutes, followed by the addition of 0.125 mol/L glycine. A 5-minute incubation at room temperature was performed to terminate the DNA-protein crosslinking reaction. SDS lysis buffer supplemented with protease inhibitors was then added, and chromatin was sheared using a sonicator. The resulting lysates were incubated with an EZH2 antibody and protein G magnetic beads to facilitate the formation of DNA-antibody-bead complexes. These complexes were subsequently eluted, purified, and designated as the target. Serum immunoglobulin G antibody was used as a negative control. The final purified DNA fragments were analyzed by qRT-PCR, and the primer sequences are listed in Table 2.
| Primer name | Primer sequence | |
| METTL3 primer 1 | F | AGTCACCAACATGGCAACCA |
| G | AGAGCCACGTTAGGTGTTGG | |
| METTL3 primer 2 | F | ATTAACTGGGCATGGTGGCA |
| G | CCTGGCCTGACACAGAGTTT |
Statistical analysis was conducted using GraphPad Prism 8.4.0 software. Data were expressed as mean ± SD. For comparisons among multiple groups, a one-way analysis of variance (ANOVA) was performed, followed by Tukey’s post hoc multiple comparison test. Statistical significance was defined as P < 0.05.
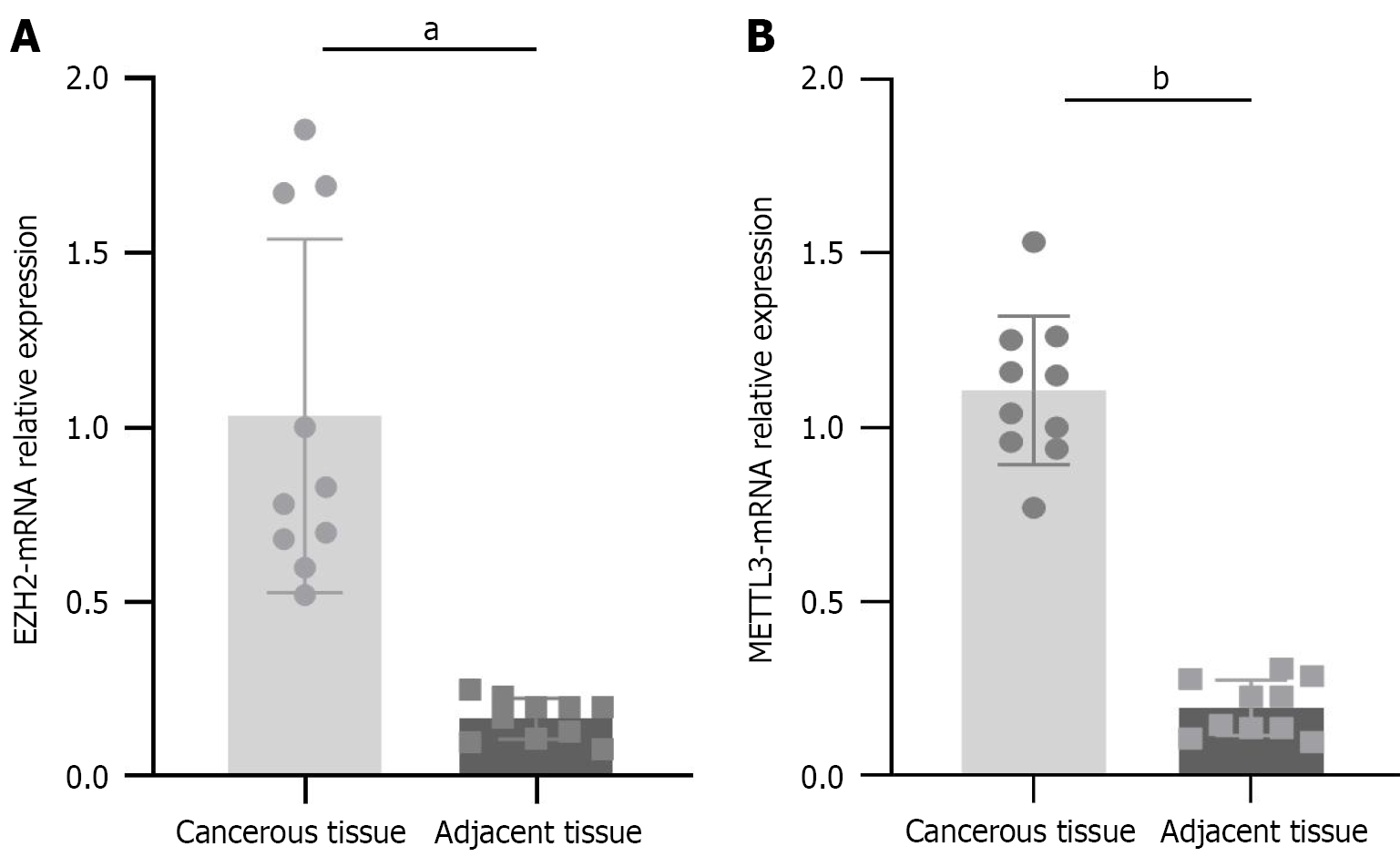
qRT-PCR analysis was performed on qualified cancerous tissues and their corresponding adjacent tissues. As shown in Figure 1, the mRNA expression levels of METTL3 and EZH2 were significantly higher in cancerous tissues compared to adjacent normal tissues (aP < 0.001, bP < 0.001). These results suggest that METTL3 and EZH2 may act as key genetic regulators involved in the initiation and progression of CRC.
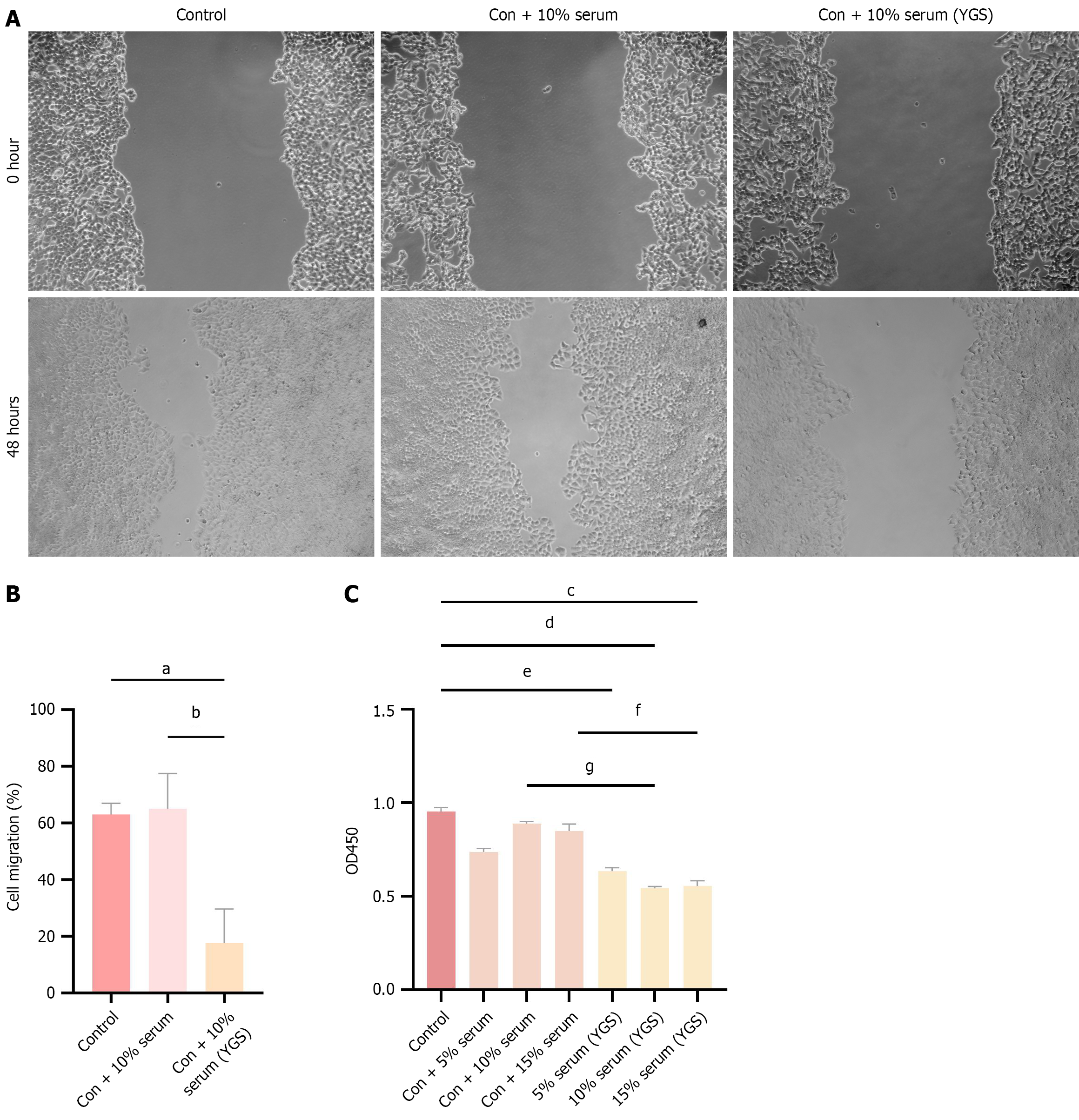
The optimal concentration of the drug was determined using the CCK-8 assay. As shown in Figure 2, OD450 values in the serum-treated group were reduced compared to the control group, with a more pronounced decrease observed in the drug-containing serum group, which exhibited higher inhibition rates. Among all tested concentrations, the 10% drug-containing serum produced the lowest OD450 values and the most significant inhibition of cell viability compared with the 5% and 15% drug-containing serum groups. Results from the cell scratch assay further demonstrated that treatment with 10% drug-containing serum markedly inhibited cell migration. Based on these findings, 10% drug-containing serum was selected as the optimal concentration for subsequent experiments.
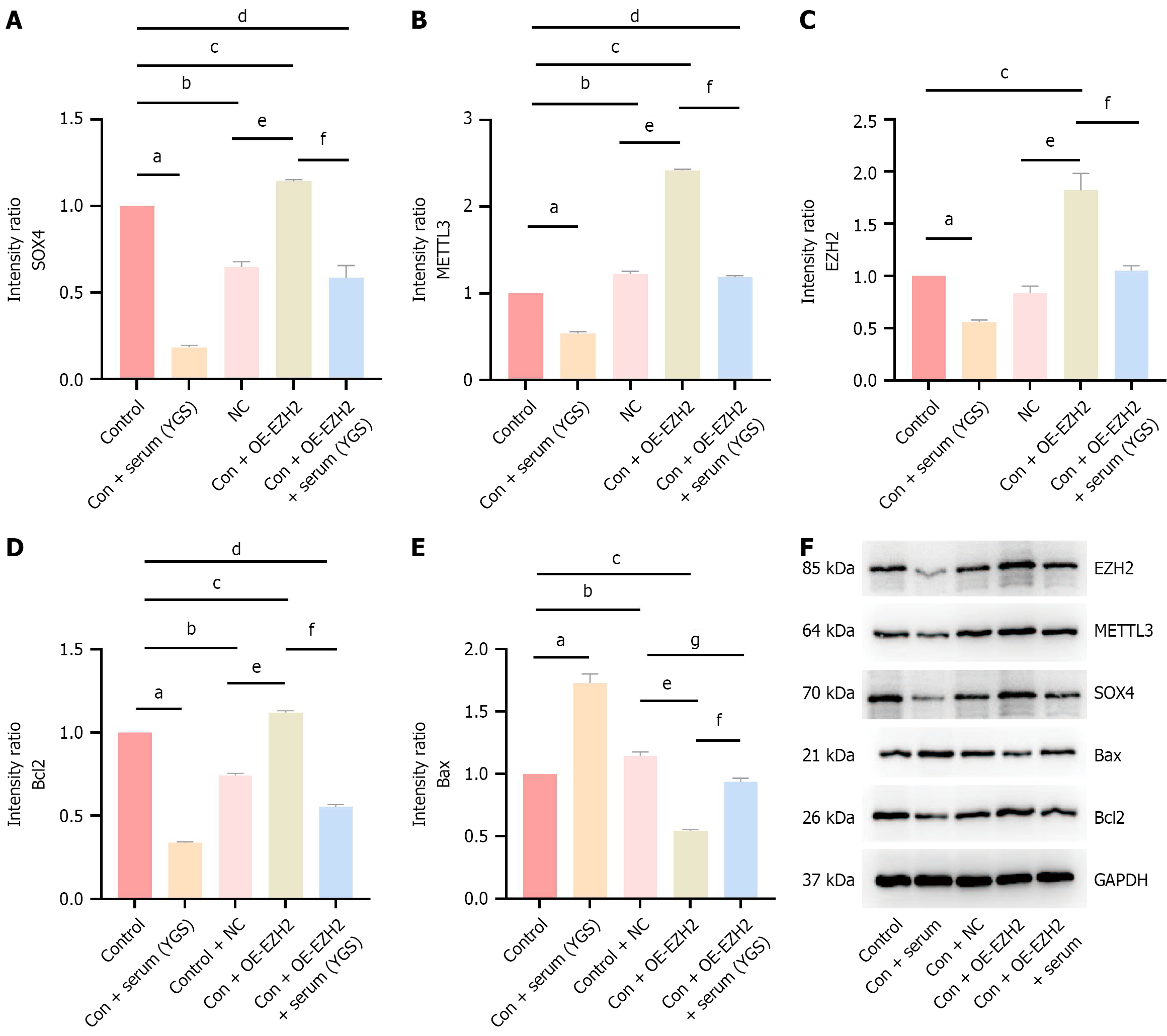
As shown in Figure 3, protein expression levels of EZH2, METTL3, SOX4, and Bcl2 were significantly reduced in the Chinese medicine treatment group compared to the control group, whereas Bax protein expression was markedly increased, with all differences reaching statistical significance. These findings indicate that the modified Yigong San formula is capable of downregulating EZH2, METTL3, and SOX4 protein expression while enhancing apoptosis. Following EZH2 overexpression, a significant increase in the expression levels of EZH2, METTL3, SOX4, and Bcl2 proteins was observed compared to the control group, accompanied by a decrease in Bax expression. In comparison with the control + NC group, the OE-EZH2 group also exhibited elevated EZH2, METTL3, SOX4, and Bcl2 expression, along with reduced Bax levels. However, upon treatment with the modified Yigong San formula, the OE-EZH2 group showed decreased expression of EZH2, METTL3, SOX4, and Bcl2 proteins, whereas Bax expression was upregulated. Collectively, the modified Yigong San formula appears to suppress EZH2, METTL3, and SOX4 expression and promote apoptosis in CRC cells.
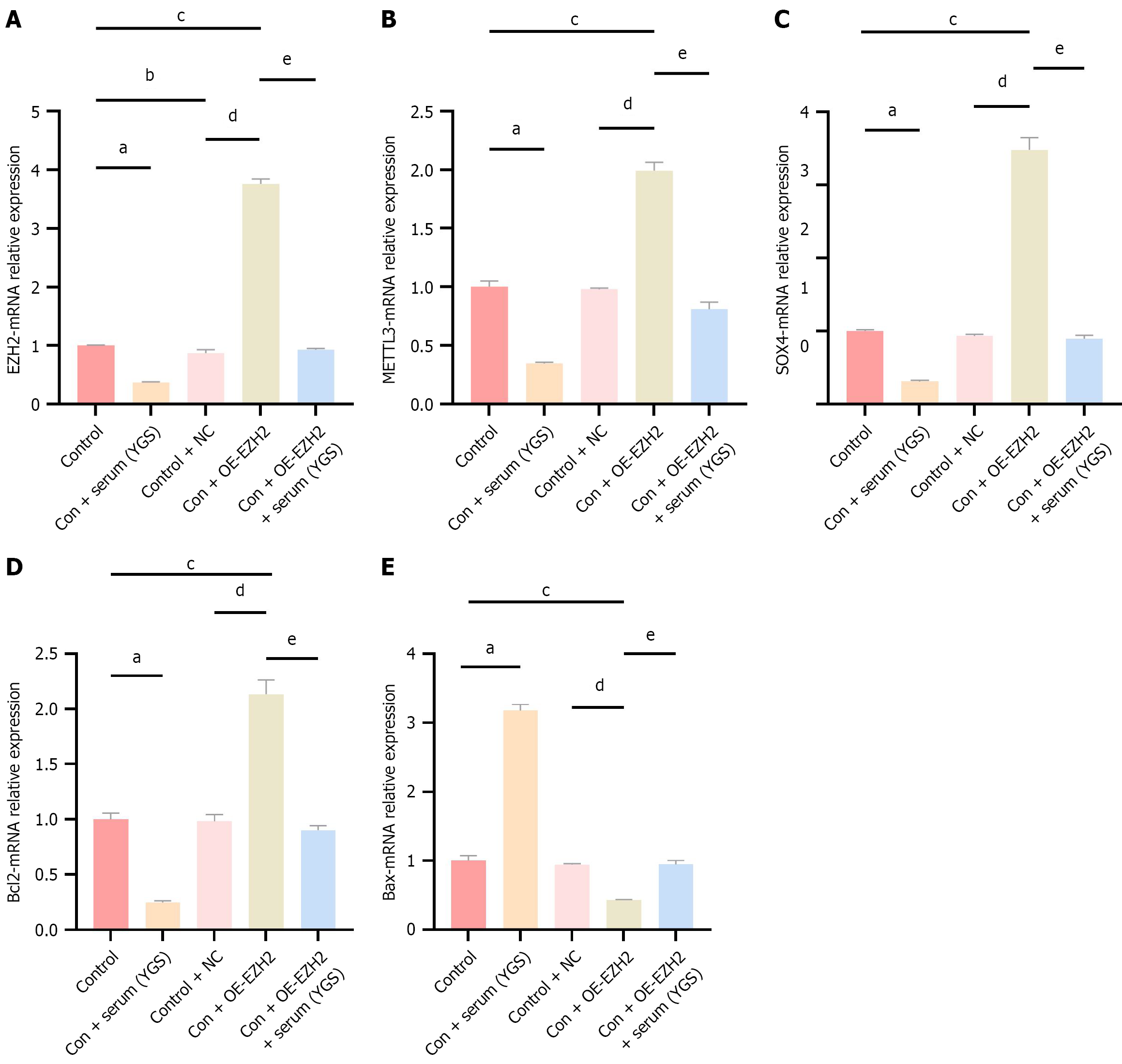
As shown in Figure 4, the mRNA expression levels of EZH2, METTL3, SOX4, Bcl2, and Bax were quantified in each experimental group using qRT-PCR. Compared to the control group, treatment with the modified Yigong San formula resulted in decreased mRNA expression of EZH2, METTL3, SOX4, and Bcl2, alongside a significant increase in Bax mRNA expression. These results suggest that the modified Yigong San formula can downregulate EZH2, METTL3, and SOX4 mRNA expression while promoting apoptosis. In the EZH2 overexpression group, the mRNA expression of EZH2, METTL3, SOX4, and Bcl2 was significantly upregulated, whereas Bax expression was reduced. Similarly, in comparison with the control + NC group, the EZH2 overexpression group showed significantly increased expression of EZH2, METTL3, SOX4, and Bcl2, and decreased Bax expression. Notably, treatment with the modified Yigong San formula in the control + OE-EZH2 group led to reduced mRNA expression of EZH2, METTL3, SOX4, and Bcl2, accompanied by an increase in Bax expression. In conclusion, the modified Yigong San formula appears to suppress EZH2, METTL3, and SOX4 mRNA expression and facilitate apoptosis.
As shown in Figure 5, results from the cell scratch assay revealed that, compared with the control group, the cell migration rate was reduced in the control + Chinese medicine group, elevated in the control + OE-EZH2 group, and unchanged in the control + NC group, with the latter lacking statistical significance. These findings suggest that the modified Yigong San formula may inhibit cell migration. Relative to the control + NC group, the control + OE-EZH2 group exhibited an increased migration rate, indicating that EZH2 may facilitate the migration of CRC cells. Furthermore, in comparison with the control + OE-EZH2 group, the migration rate was significantly decreased in the control + OE-EZH2 + Chinese medicine group.
As demonstrated in Figure 6, FCM analysis revealed a significant increase in the apoptosis rate in the control + Chinese medicine group relative to the control group. Similarly, the apoptosis rate in the control + OE-EZH2 + Chinese medicine group was markedly elevated compared with that in the control + OE-EZH2 group, suggesting that the modified Yigong San formula may enhance cell apoptosis, consistent with the trends observed in the aforementioned apoptotic protein levels. Additionally, no statistically significant differences in apoptosis were detected among the control, control + NC, and control + OE-EZH2 groups, indicating that neither EZH2 overexpression nor blank plasmid transfection induced apoptotic effects.
As shown in Figure 7, CHIP-qPCR analysis revealed that EZH2 binding sites were present in the promoter region of METTL3 in CRC (SW-480) cells.
CRC is considered to originate from spleen qi deficiency, which may result from external pathogenic invasion or internal factors such as irregular dietary habits and emotional disturbances. These pathological conditions are believed to induce the accumulation of internal dampness, heat, blood stasis, and toxins, ultimately compromising intestinal function. As stated in the Suwen·the Inner Classic of the Yellow Emperor, “The spleen is a solitary organ, the central earth that irrigates in all four directions”. The spleen primarily governs transportation and transformation, facilitating the distribution of vital nutrients derived from food and fluids to nourish the five zang and six fu organs, as well as the limbs and the entire body. Additionally, the spleen and stomach play indispensable roles in qi regulation, with the spleen elevating the clear essence and the stomach directing the turbid downward, thereby maintaining the normal conduction function of the large intestine. Moreover, the Suwen·the Secret Classics of Linglan asserts that “The large intestine is the official of conveyance, where transformation occurs”. The large intestine performs its function by enabling the downward transmission of substances. When external pathogens invade and the body’s righteous qi is insufficient to expel them, these pathogenic factors persist and disrupt the transmission mechanism. Persistent impairment, if left untreated, may culminate in the formation of intestinal masses. Based on this pathogenesis, Yigong San, a classical prescription that emphasizes spleen fortification, was selected as the core therapeutic formulation. To potentiate its anti-tumor effects, particularly against malignancies, Poria cocos and Hedyotis diffusa were incorporated, resulting in the development of a modified Yigong San formula. Contemporary pharmacological studies have reported that ginsenoside Rh2, an active compound extracted from ginseng, inhibits CRC cell proliferation through miR-150-3p-mediated pathways[16]. Similarly, atractylenolide I, a key constituent of Atractylodes macrocephala, has been shown to suppress gastric cancer cell proliferation[17]. Beyond its application in gastrointestinal tumors, atractylenolide I has also been implicated in promoting apoptosis, regulating the cell cycle[18], and suppressing ovarian cancer cell proliferation. Furthermore, pachymic acid, the major bioactive compound in Poria cocos, demonstrates anti-tumor activity by inhibiting the proliferation, migration, and invasion of hepatocellular carcinoma cells while enhancing apoptotic processes[19]. In this study, the optimal concentration of the drug was determined, after which SW-480 cells were treated accordingly. Subsequent analyses revealed an increase in apoptosis rates and a concomitant reduction in cell migration (Figures 5 and 6), indicating that the modified Yigong San formula may exhibit preventive and therapeutic efficacy against CRC.
In this study, the mRNA expression levels of EZH2 and METTL3 were markedly elevated in cancerous tissues from CRC patients compared to adjacent non-cancerous tissues (Figure 1). EZH2 has been implicated in tumor aggressiveness and adverse prognosis[20]. Through the silencing of tumor suppressor genes, EZH2 facilitates cell cycle progression and induces EMT, thereby enhancing the invasive and migratory capacities of malignant cells[21]. Additionally, EZH2 has been reported to repress genes involved in anti-tumor immune responses, contributing to tumor immune evasion[22]. The involvement of EZH2 in tumor initiation and progression is thus of considerable significance. METTL3, an RNA methyltransferase responsible for m6A modification, is frequently overexpressed in various malignancies and promotes tumor cell proliferation and metastasis by regulating the expression of downstream targets[23]. With respect to tumor proliferation and invasion, METTL3 has been shown to enhance the translational efficiency of the oncogene SOX2, thereby accelerating CRC cell proliferation[24]. Furthermore, findings from murine xenograft models indicate that knock
The present study revealed that, following EZH2 overexpression, both gene and protein expression levels of METTL3 were significantly upregulated (Figures 3 and 4). ChIP-qPCR analysis demonstrated that EZH2 directly binds to the promoter region of METTL3, indicating that METTL3 expression may be modulated by EZH2 through transcriptional regulation. This finding offers additional insight into the mechanistic interplay between METTL3 and EZH2 in CRC. Previous investigations have shown that EZH2 represses gene expression via histone methylation, and in glioma cells, EZH2 has also been observed to mediate H3K27ac modification, thereby influencing METTL3 expression[27]. Hence, histone modification may constitute a key mediator in the positive feedback loop linking EZH2 and METTL3. METTL3 primarily functions in mediating m6A modification of mRNA. In studies on lung cancer, METTL3 has been identified as the m6A methyltransferase responsible for modifying EZH2, thereby regulating EMT progression[28]. Therefore, it is hypothesized that m6A modification may act as a critical regulatory mechanism in the reciprocal feedback loop between EZH2 and METTL3, although the specific molecular underpinnings have yet to be fully elucidated.
SOX4, acknowledged as a key transcription factor, participates in diverse biological processes, including cellular development, differentiation, and apoptosis. In CRC, SOX4 overexpression has been implicated as a contributing factor to EMT induction and tumor cell migration[29]. The modified Yigong San formula has been demonstrated to regulate the EZH2/METTL3/SOX4 signaling axis, thereby exerting preventive and therapeutic effects against CRC. Elevated expression levels of SOX4 observed in CRC (Figures 3 and 4) coincided with the increased expression of EZH2 and METTL3, suggesting its potential role as a downstream effector of these epigenetic modulators. Further evidence was provided by findings in EZH2-overexpressing cells, where SOX4 Levels were further increased, reinforcing the role of EZH2 in CRC progression through SOX4 upregulation. This supports the identification of SOX4 as a viable therapeutic target in CRC. Notably, even under EZH2-overexpressing conditions, the modified Yigong San formula effectively attenuated the activation of the EZH2/METTL3/SOX4 cascade, indicating its regulatory capability in suppressing CRC advancement via modulation of this pathway.
It was observed that the modified Yigong San formula significantly downregulated the expression of the anti-apoptotic protein Bcl2 while concurrently enhancing the expression of the pro-apoptotic protein Bax (Figures 3 and 4). Subsequent validation via FCM analysis (Figure 6) demonstrated a marked elevation in the apoptotic rate in cells treated with the modified formula compared to the control group. In addition, results from the cell scratch assay (Figure 5) indicated that cell migration in CRC was effectively inhibited following treatment with the modified Yigong San formula. Notably, even under conditions of EZH2 overexpression, the formula maintained its capacity to suppress cell migration and induce apoptosis, suggesting that its preventive and therapeutic efficacy in CRC may be mediated through apoptosis regulation involving the EZH2/METTL3/SOX4 signaling pathway.
We found that METTL3 and EZH2 are highly expressed in CRC patients; the addition of the modified Yigong San for
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68574] [Article Influence: 13714.8] [Reference Citation Analysis (201)] |
| 2. | Wei J. [Advance in clinical treatment of colorectal cancer]. Zhongguo Linchuang Xin Yixue. 2018;11:202-208. [DOI] [Full Text] |
| 3. | Li Y, Zou Y, Gu J, Guo Y. [Observation on the distribution of Chinese medicine syndromes in colorectal cancer patients during adjuvant chemotherapy]. Zhongguo Zhongxiyi Jiehe Zazhi. 2017;37:414-418. [DOI] [Full Text] |
| 4. | Zhang L. [Treating 38 cases of children spleen deficiency resulting from post -infection with Yigongsan]. Hunan Zhongyiyao Daobao. 2002;8:255. [DOI] [Full Text] |
| 5. | Li F, Feng M, Zhang J. [Effect of Jiawei Yigong Powder and Bifid Quadruple Viable Tablets on clinical symptoms, serum gastrointestinal hormones and trace elements of anorexia in children with spleen and stomach weakness]. Linchuang He Shiyan Yixue Zazhi. 2023;22:875-879. [DOI] [Full Text] |
| 6. | Sun D, Zou Y, Song L, Yang H, Chu D, Han S, Liu X, Guo J. [Effect of Ginsenoside Rg3 on Apoptosis of Colorectal Cancer Cells]. Techan Yanjiu. 2021;47:35-40. |
| 7. | Liu S, Ruan J, Wang C, Wang X, Yan J, Wang Y, Liu S. [Research progress on chemical constituents and pharmacological properties of Smilacis Glabrae Rhizoma]. Zhongcaoyao. 2025;56:1064-1077. |
| 8. | Liao D, Zeng J, Lai M, Zhi C, Tang T. [Extract of Baihuasheshecao(Hedyotis diffusa)Improving Apoptosis of Colon Cancer HCT-8 Cells by Down-regulating Hippo-YAP Signaling Pathway]. Zhonghua Zhongyiyao Xuekan. 2022;40:248-251+284. [DOI] [Full Text] |
| 9. | Cai Q, Lin J, Wei L, Zhang L, Wang L, Zhan Y, Zeng J, Xu W, Shen A, Hong Z, Peng J. Hedyotis diffusa Willd inhibits colorectal cancer growth in vivo via inhibition of STAT3 signaling pathway. Int J Mol Sci. 2012;13:6117-6128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Zheng J, Xiao X, Wu C, Huang J, Zhang Y, Xie M, Zhang M, Zhou L. The role of long non-coding RNA HOTAIR in the progression and development of laryngeal squamous cell carcinoma interacting with EZH2. Acta Otolaryngol. 2017;137:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Zhou C, Zhang Z, Zhu X, Qian G, Zhou Y, Sun Y, Yu W, Wang J, Lu H, Lin F, Shen Z, Zheng S. N6-Methyladenosine modification of the TRIM7 positively regulates tumorigenesis and chemoresistance in osteosarcoma through ubiquitination of BRMS1. EBioMedicine. 2020;59:102955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 12. | Shi S, Cao X, Gu M, You B, Shan Y, You Y. Upregulated Expression of SOX4 Is Associated with Tumor Growth and Metastasis in Nasopharyngeal Carcinoma. Dis Markers. 2015;2015:658141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Zhang Y, Liu S, Zhao T, Dang C. METTL3mediated m6A modification of Bcl2 mRNA promotes nonsmall cell lung cancer progression. Oncol Rep. 2021;46:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Yin J, Ding F, Cheng Z, Ge X, Li Y, Zeng A, Zhang J, Yan W, Shi Z, Qian X, You Y, Ding Z, Ji J, Wang X. METTL3-mediated m6A modification of LINC00839 maintains glioma stem cells and radiation resistance by activating Wnt/β-catenin signaling. Cell Death Dis. 2023;14:417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 15. | Zhang R, Zhu W, Ma C, Ai K. Silencing of circRNA circ_0001666 Represses EMT in Pancreatic Cancer Through Upregulating miR-1251 and Downregulating SOX4. Front Mol Biosci. 2021;8:684866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Xu X, Wu Y. [Research Progress in Mechanism of Chinese Herbal Monomer in Treatment of Colorectal Cancer Based on Wnt/β-catenin Signaling Pathway]. Yixue Zongshu. 2021;27:4022-4027. [DOI] [Full Text] |
| 17. | Chen Y. [Mechanism of atractylenolide I inhibiting the proliferation of gastric cancer MGC-803 cells]. Zhongguo Laonianxue Zazhi. 2017;37:2385-2387. [DOI] [Full Text] |
| 18. | Wang M, Huang J, Zhang G, Deng L, Cheng J, Cha X. [Atractylenolide I antagonizes TLR4/MyD88 signaling pathway-mediated IL-6 secretion in ovarian cancer cells and enhances paclitaxel-induced growth inhibition in ovarian cancer cells]. Zhonghua Fuchanke Zazhi. 2011;46. [DOI] [Full Text] |
| 19. | Liao W. [Study on the effect and mechanism of pachymic acid inhibiting hepatocellular carcinoma by upregulating ANKRD1]. Kunming Medical University, 2023. |
| 20. | Lee SH, Li Y, Kim H, Eum S, Park K, Lee CH. The role of EZH1 and EZH2 in development and cancer. BMB Rep. 2022;55:595-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Koyama N, Ishikawa Y, Ohta H, Aoki T, Kyoyama H, Aoshiba K, Uematsu K. miR-4448/Girdin/Akt/AMPK axis inhibits EZH2-mediated EMT and tumorigenesis in small-cell lung cancer. Cancer Med. 2024;13:e70093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Christofides A, Karantanos T, Bardhan K, Boussiotis VA. Epigenetic regulation of cancer biology and anti-tumor immunity by EZH2. Oncotarget. 2016;7:85624-85640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Zeng C, Huang W, Li Y, Weng H. Roles of METTL3 in cancer: mechanisms and therapeutic targeting. J Hematol Oncol. 2020;13:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 471] [Article Influence: 78.5] [Reference Citation Analysis (7)] |
| 24. | Rieger M, Dellenbach C, Vom Berg J, Beil-Wagner J, Maguy A, Rohr S. Enabling comprehensive optogenetic studies of mouse hearts by simultaneous opto-electrical panoramic mapping and stimulation. Nat Commun. 2021;12:5804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Ren H, Wang M, Ma X, An L, Guo Y, Ma H. METTL3 in cancer-associated fibroblasts-derived exosomes promotes the proliferation and metastasis and suppresses ferroptosis in colorectal cancer by eliciting ACSL3 m6A modification. Biol Direct. 2024;19:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 26. | Kang Q, Hu X, Chen Z, Liang X, Xiang S, Wang Z. The METTL3/TRAP1 axis as a key regulator of 5-fluorouracil chemosensitivity in colorectal cancer. Mol Cell Biochem. 2025;480:1865-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Li F, Chen S, Yu J, Gao Z, Sun Z, Yi Y, Long T, Zhang C, Li Y, Pan Y, Qin C, Long W, Liu Q, Zhao W. Interplay of m(6) A and histone modifications contributes to temozolomide resistance in glioblastoma. Clin Transl Med. 2021;11:e553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 28. | Chen WW, Qi JW, Hang Y, Wu JX, Zhou XX, Chen JZ, Wang J, Wang HH. Simvastatin is beneficial to lung cancer progression by inducing METTL3-induced m6A modification on EZH2 mRNA. Eur Rev Med Pharmacol Sci. 2020;24:4263-4270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 29. | Hanieh H, Ahmed EA, Vishnubalaji R, Alajez NM. SOX4: Epigenetic regulation and role in tumorigenesis. Semin Cancer Biol. 2020;67:91-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/