Published online Aug 15, 2023. doi: 10.4251/wjgo.v15.i8.1451
Peer-review started: June 4, 2023
First decision: June 16, 2023
Revised: June 23, 2023
Accepted: July 7, 2023
Article in press: July 7, 2023
Published online: August 15, 2023
Processing time: 66 Days and 22.7 Hours
The incidence of type I gastric neuroendocrine neoplasms (gNENs) has increased significantly over the past 50 years. Although autoimmune gastritis (AIG) increases the likelihood of developing gNENs, the exact incidence and prevalence of this association remain unclear.
To evaluate the incidence and prevalence of type I gNENs in a cohort of patients with a histological diagnosis of AIG.
Patients with a histological diagnosis of AIG were enrolled between October 2020 and May 2022. Circulating levels of CgA and gastrin were assessed at enrollment. Included patients underwent regular endoscopic follow-up to detect gastric neoplastic lesions, enterochromaffin-like (ECL) cell hyperplasia, and the develop
We included 176 patients [142 women (80.7%), median age 64 years, interquartile range (IQR) 53–71 years] diagnosed with AIG between January 1990 and June 2022. At enrollment. One hundred and sixteen patients (65.9%) had ECL hyperplasia, of whom, 29.5% had simple/linear, 30.7% had micronodular, and 5.7% had macronodular type. The median follow-up time was 5 (3–7.5) years. After 1032 person-years, 33 patients developed a total of 50 type I gNENs, with an incidence rate of 0.057 person-years, corresponding to an annual cumulative incidence of 5.7%. Circulating CgA levels did not significantly differ between AIG patients who developed gNENs and those who did not. Conversely, gastrin levels were significantly higher in AIG patients who developed gNENs [median 992 pg/mL IQR = 449–1500 vs 688 pg/mL IQR = 423–1200, P = 0.03]. Calculated gastrin sensitivity and specificity were 90.9% and 1.4%, respectively, with an overall diagnostic accuracy of 30% and a calculated area under the gastrin receiver operating characteristic curve (AUROC or AUC) of 0.53.
Type I gNENs are a significant complication in AIG. Gastrin’s low diagnostic accuracy prevents it from serving as a marker for early diagnosis. Effective strategies for early detection and treatment are needed.
Core tip: Type I gastric neuroendocrine neoplasms (gNENs) in chronic autoimmune gastritis (AIG) are increasingly diagnosed, but no accurate data are available. Noninvasive biomarkers of gNENs in AIG have not yet been identified. According to our results, a non-negligible annual cumulative gNEN incidence of 5.7% was revealed, and among all considered variables, only gastrin proved to have significantly higher median circulating levels in patients who developed gNENs compared to AIG patients without lesions; nevertheless, with low diagnostic accuracy. Further efforts are needed to identify effective strategies for individualizing endoscopic follow-up of AIG patients, to achieve early diagnosis and treat superimposed neuroendocrine lesions.
- Citation: Massironi S, Gallo C, Elvevi A, Stegagnini M, Coltro LA, Invernizzi P. Incidence and prevalence of gastric neuroendocrine tumors in patients with chronic atrophic autoimmune gastritis. World J Gastrointest Oncol 2023; 15(8): 1451-1460
- URL: https://www.wjgnet.com/1948-5204/full/v15/i8/1451.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i8.1451
Type I gastric neuroendocrine neoplasms (gNENs) develop in the neuroendocrine gastric cells[1,2]. Type I gNENs are the most common subtype, and they arise in the context of chronic autoimmune gastritis (AIG)[3,4]. AIG is an organ-specific disease characterized by the immune-mediated destruction of gastric parietal cells, determining inflammation mainly localized in the gastric corpus–fundus[5]. Gastric parietal cell proton pump H+/K+-ATPase) represents the major autoantigen[6-8].
In this condition, the destruction of the oxyntic mucosa and subsequent development of hypo- and achlorhydria and high circulating gastrin levels are the hallmarks of AIG[7,9].
Hypergastrinemia leads to the hyperplasia and neoplasia of enterochromaffin-like (ECL) cells of the gastric corpus, increasing the risk of developing type I gNENs[10-13]. These tumors are usually well-differentiated epithelial lesions originating from ECL cells and growing toward the submucosal layer. They appear as regular lesions covered with a round-shaped pattern of the mucosa[9,14]. Moreover, as demonstrated also through in vivo animal studies, hypergastrinemia represents an influential trophic stimulus for the gastric mucosa. Hypergastrinemia increased cell proliferation in the ulcer margin in rats with induced ulcers in the corpus region[15].
While the spectrum of gastric neuroendocrine hyperplastic and neoplastic proliferation is well documented, the exact prevalence and incidence of type I gNENs have not yet been defined. However, studies have shown that their incidence has increased significantly over the past 50 years[9,16-22]. Determining the exact prevalence and incidence of type I gNENs could help in the development of new strategies for monitoring AIG and more effective surveillance and treatment strategies in these patients[23]. Optimizing surveillance of AIG and identifying associated risk factors could help identify high-risk patients, and early detection and intervention could potentially prevent the development of type I gNENs[24]. In addition, a better understanding of the complex interplay between autoimmune diseases and cancer could lead to the development of more effective surveillance and treatment strategies for type I gNENs in AIG.
The study included patients with a confirmed diagnosis of AIG who were followed from January 2020 to June 2022 in the Department of Gastroenterology of the Fondazione IRCCS San Gerardo dei Tintori, Monza-University of Milan-Bicocca. All subjects gave written informed consent to participate in the study, which was approved by the local ethics committee.
The diagnosis of AIG was based on the presence of atrophic gastritis on the fundus, positivity of antibodies against parietal cells orintrinsic factor (IF), and circulating gastrin levels[1,25,26]. A retrospective analysis was performed on a prospectively collected monocentric database including all consecutive AIG patients from January 1990 to June 2022.
All included patients underwent a baseline examination that included clinical data (sex, age at AIG diagnosis, concomitant diseases, and previous therapies), biometric data [weight, height, and body mass index (BMI)], blood tests, and upper gastrointestinal endoscopy with biopsy collection and histological examination. Unavailable histological data, inadequate biopsy collection, inconsistent laboratory tests, concurrent active neoplasms, or severe organ failure were the study exclusion criteria.
For each included patient, the following clinical information was recorded: Concomitant autoimmune diseases (thyroiditis, celiac disease, type 1 diabetes mellitus, vitiligo, Addison’s disease, myasthenia, oral lichen planus, autoi
For AIG patients who were retrospectively enrolled, clinical data were extracted from medical records and outpatient visits. All data for each patient were anonymized after collection, recorded, evaluated, and finally analyzed.
A standard gastroscope was used for all diagnostic and monitoring EGDs[28]. Patients receiving PPIs were discontinued from medication and the examination was repeated after at least 15 d[29]. During surveillance EGDs, at least five gastric biopsies were obtained according to the updated Sydney protocol[30]. Two biopsies were taken from the antrum, one from the incisura angularis, and two from the gastric corpus and fundus. The collected histological specimens were sent directly to the pathology laboratory for examination. For visible polypoid mucosal lesions, the year of occurrence, location (antrum, body, or fundus), size, treatment, and histological characterization were evaluated[31,32]. All identified lesions were biopsied, or, in the case of multiple polyps, the largest lesion was biopsied and removed, when possible[33]. Lesions were resected with forceps by cold biopsy if their diameter was ≤ 5 mm. For lesions > 5 mm, endoscopic mucosal resection was performed. For lesions > 1 cm, endoscopic ultrasonography was performed to assess the degree of wall invasion before resection and, if necessary, to plan endoscopic submucosal dissection.
Patients with uncomplicated chronic AIG underwent endoscopic surveillance every three years, whereas patients with gNENs underwent endoscopic surveillance annually[34]. For patients with gastric adenocarcinoma, follow-up was performed on a case-by-case basis to determine the most appropriate type of intervention and timing of follow-up[35].
AIG was diagnosed by the presence of corpus–fundus-predominant atrophic gastritis[36]. During EGD, topographical biopsies were taken and collected in separate jars according to the updated Sydney classification[30]. H. pylori infection was investigated in each patient. The Operative Link on Gastritis Assessment (OLGA) score was used to assess the stage of mucosal atrophy and the risk of gastric cancer[37], while the Operative Link on Gastric Intestinal Metaplasia (OLGIM) score was used to estimate the stage of intestinal metaplasia[38,39]. Pseudopyloric metaplasia was also evaluated for its presence and severity[1,13]. Hyperplasia of ECL cells, stained with chromogranin A (CgA), was defined as proliferation > 150 µm in diameter and classified as simple, linear, micronodular, or macronodular according to Solcia et al[40]. Specimens were fixed in formalin and routinely processed. Gastric mucosal sections were stained with hematoxylin–eosin for routine examination and with Alcian blue/periodic acid–Schiff stain to assess intestinal metaplasia. Specimens of gNENs were analyzed for specific markers such as chromogranin A (CgA) and synaptophysin. The MIB-I antibody was used to detect and measure the level of Ki-67, an indicator of neoplastic proliferation. All neuroendocrine tumors were classified according to the 2019 WHO grading system[41] and the European Neuroendocrine Tumor Society guidelines TNM staging system[42].
Morning fasting blood samples were used for all biochemical tests. Tubes containing anticoagulants were used for serum samples, while those with EDTA (1 mg/mL) or heparin were used for plasma samples. Anemia was diagnosed when the hemoglobin level was < 12 g/dL in women and < 13 g/dL in men. Mean corpuscular volume > 99 fL was indicative of macrocytic anemia. In addition, white blood cell count (4 × 103–11 × 103/μL), platelet count (140 × 103–440 × 103/μL), lactate dehydrogenase levels (135–214 U/L), thyroid-stimulating hormone levels (0.27–4.2 μU/mL), vitamin B12 levels (197–771 pg/mL), iron levels (33–193 g/dL), and homocysteine levels (5–12 mmol/L) were also assessed.
Detection of APCAs and anti-IF antibodies was performed by dilution and direct immunofluorescence techniques, and positivity was defined as ≥ 1:80. Blood gastrin levels were measured by the quantitative chemiluminescence immuno
It should be noted that in patients receiving PPI treatment, the medication was discontinued, and the blood test was repeated at least 15 d after discontinuation.
Continuous variables were presented using the median and interquartile range (IQR), whereas categorical variables were presented as numbers (percentage). The Kolmogorov–Smirnoff test was utilized to assess the normality of data distribution. Differences between groups were assessed using the Mann–Whitney and Kruskal–Wallis tests. Fisher’s exact test was used to compare percentages. Spearman’s rank correlation coefficient was used to determine the association between variables of interest, such as sex, age, BMI, use of PPIs, presence of H. pylori infection, positivity for APCAs and/or anti-IF antibodies, OLGA and OLGIM grading, plasma concentration of gastrin, CgA values, and presence of gNENs. A two-sided P < 0.05 was considered statistically significant. GraphPad Prism version 5.0, State Mat version 2 (GraphPad Software, San Diego, CA, USA), and MedCalc software were used for data analysis.
The study included 176 patients, the majority of whom were female (142, 80.7%), had a median BMI of 24.6 kg/m2 (IQR 18.3–52.7), and a median age of 64 years (IQR 53–71). At baseline, 72% of the patients had at least one autoimmune endocrine disease, with autoimmune thyroid disease being the most common (35.8%). Other autoimmune diseases included diabetes mellitus, vitiligo, psoriasis, celiac disease, Addison’s disease, and autoimmune liver diseases such as primary biliary cholangitis, autoimmune hepatitis, and fibromyalgia. H. pylori infection was detected in 20.4% of patients but eradicated in all cases. APCA positivity was found in 83.5% of cases. The median gastrin value of the whole studied population was 668 pg/mL and the median circulating CgA level was 146 ng/mL. APS was diagnosed in 22.1% of patients with at least one autoimmune disease. Further details on the characteristics of the patients, including anthropometric, clinical, biochemical, and baseline histological features, are provided in Table 1.
| Classification | Features |
| Sex, n (%) | Female: 142 (80.7) |
| Age (median) | 64 yr (IQR: 53–71) |
| APCA positivity, n (%) | 147 (83.5) |
| Gastrin | 668 pg/mL (IQR: 340–1200) |
| Chromogranin A (median) | 146 ng/mL (IQR: 106–219) |
| ECL hyperplasia, n (%) | Absent: 60 (34.1) |
| Gastric mucosa atrophy, n (%) | OLGA |
| 0: 58 (32.9) | |
| I: 38 (21.6) | |
| II: 64 (36.4) | |
| III: 13 (7.4) | |
| IV: 3 (1.7) | |
| Gastric mucosa intestinal metaplasia, n (%) | OLGIM |
| 0: 42 (23.9) | |
| I: 63 (35.8) | |
| II: 61 (34.7) | |
| III: 5 (2.8) | |
| IV: 5 (2.8) |
A total of 507 EGDs were performed in 176 patients, with a mean number of three for each patient. All patients showed atrophy of the corpus–fundus, with varying degrees of severity: 16.2% of them had a mild degree of atrophy, 44% had moderate atrophy, and 39.8%had severe atrophy. Intestinal metaplasia was detected in 126 patients (71.5%), with a mild degree in 32.9%, moderate in 28.9%, and severe in 9.6%. Pseudopyloric metaplasia was reported in 27 patients (15.3%). OLGA Stage I, II or III atrophic gastritis was reported in 115 patients, and OLGA IV stage atrophic gastritis was observed in three patients, who had severe gastric atrophy in the corpus–fundus but moderate atrophy in the antrum. ECL hyperplasia was present in 110 patients, but 60 (34.1%) patients did not show ECL hyperplasia.
At enrollment, 116 of 176 patients (65.9%) had ECL hyperplasia, with simple/linear, micronodular, and macronodular patterns reported in 29.5%, 30.7% and 5.7% of cases, respectively.
The median follow-up duration was 5 years, with a range from 3 to 7.5 years. During the follow-up period, after 1032 person-years, 33 patients developed a total of 50 type I gNENs, corresponding to an annual cumulative incidence of 5.7% and an incidence rate of 0.057 person-years.
Patients with and without type I gNEN had similar characteristics (Table 2), both in terms of anthropometric, clinical, and serological features, and according to histological findings (both atrophy and intestinal metaplasia, and ECL hyperplasia). Specifically, with regard to immune-mediated comorbidities, no significant differences were found in the baseline prevalence of thyroiditis, celiac disease, type 1 diabetes mellitus, vitiligo, psoriasis, Addison’s disease, myasthenia, fibromyalgia, oral lichen planus, autoimmune liver disease, autoimmune connective tissue disease, or APS between AIG patients who developed one or more neuroendocrine tumors and AIG patients who did not.
| AIG patients without gNENs | AIG patients with gNENs | P value | |
| Sex (%) | 84.3 (female) | 78 (female) | 0.06 |
| Age (median) | 61 yr (IQR: 50–69) | 66 yr (IQR: 55–73) | 0.68 |
| Autoimmune tyroiditis | 48 (42) | 13 (39) | 0.46 |
| Type 2 diabetes mellitus | 33 (29) | 11 (33) | 0.33 |
| Psoriasis and/or vitiligo | 6 (5) | 3 (9) | 0.12 |
| Auntoimmune polyglandular syndrome | 5 (4) | 2 (6) | 0.23 |
| APCA positivity | 78 (89.7) | 25 (75.8) | 1.00 |
| ECL simple/linear hyperplasia | 29 (33.3) | 7 (21) | 1.01 |
| ECL micronodular hyperplasia | 23 (26.4) | 11(33.3) | 0.73 |
| ECL macronodular hyperplasia | 6 (6.8) | 1 (3) | 0.94 |
| OLGIM II–IV | 34 (39.1) | 17 (51.5) | 0.49 |
| Chromogranin A (median) | 172 ng/mL (IQR: 107–320) | 160 ng/mL (IQR: 115–217) | 0.68 |
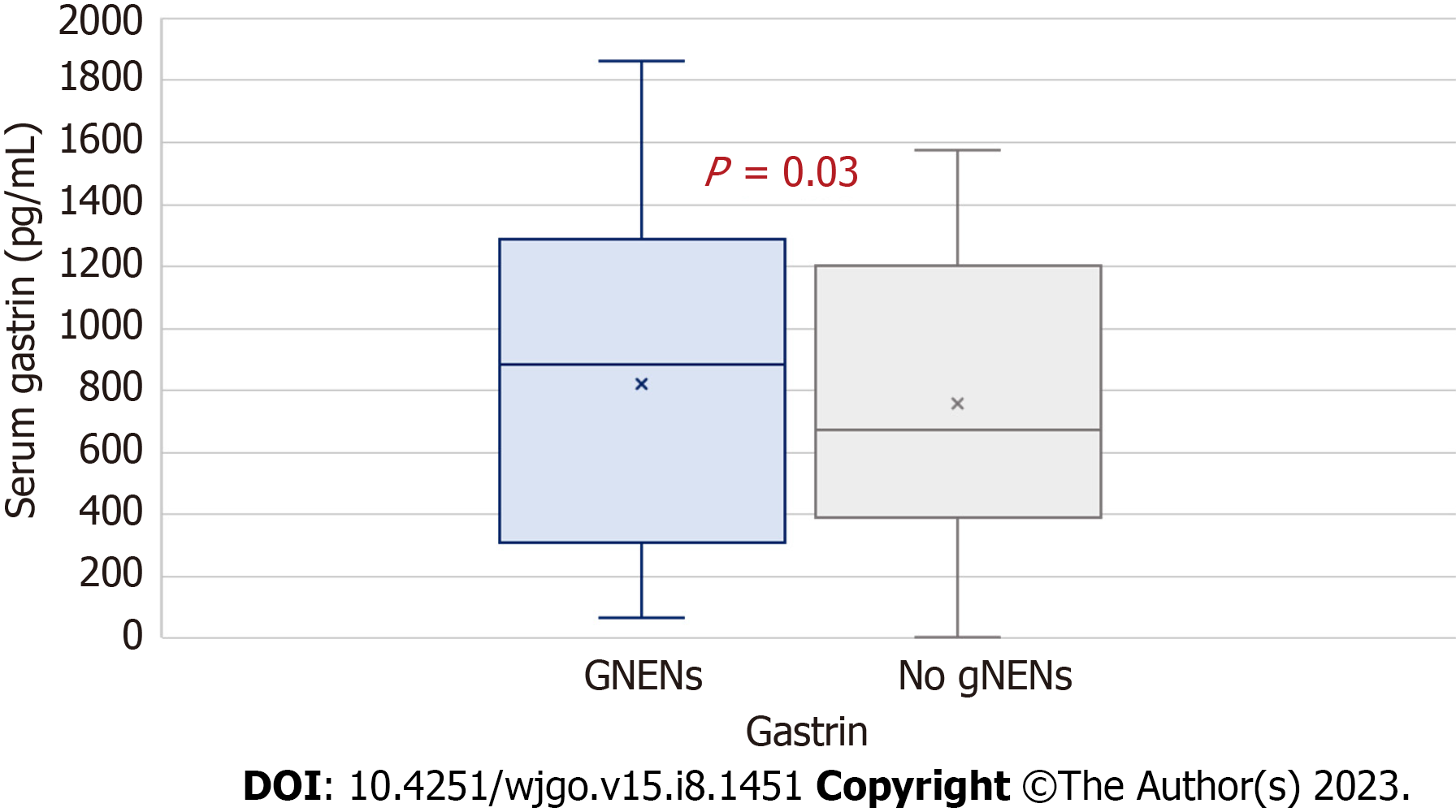
| Gastrin (median) | 688 pg/mL (IQR: 423–1200) | 992 pg/mL (IQR: 449–1500) | 0.03 |
Circulating CgA levels did not significantly differ between the two groups; conversely, the two groups significantly differed in baseline circulating gastrin levels, as AIG patients with gNENs showed higher levels of gastrin (median 992 pg/mL, IQR = 449–1500 vs 688 pg/mL, IQR = 423–1200, P = 0.03) (Figure 1) when compared to AIG patients without type I gNEN.
Calculated gastrin sensitivity and specificity were 90.9% and 1.4%, respectively, with a calculated overall diagnostic accuracy of 30%. A receiver operating characteristic (ROC) curve has been also created to analyze the connection between clinical sensitivity and specificity of gastrin circulating values in terms of gNENs detection, and to identify the best-performing circulating gastrin cutoff value. The calculated area under the gastrin ROC curve (AUROC or AUC) was 0.53 (0.45–0.61), with a Youden index J of 0.14 corresponding to a circulating gastrin cutoff value of 857 pg/mL, with a sensibility of 53.1% and a specificity of 61.2%.
AIG is a chronic autoimmune disease that progressively leads to destruction of the oxyntic gastric mucosa and, consequently, to hypo-/achlorhydria. This results in elevated plasma gastrin levels, hyperplasia, and degeneration of ECL cells, leading to an increased incidence of type I gNENs[6,7,9,10,13].
Our study evaluated the incidence of gNENs among 176 patients diagnosed with AIG between January 1990 and June 2022. Over the course of 1032 person-years, 33 patients developed a total of 50 type I gNENs, resulting in an incidence rate of 5.7 per 100 person-years. The findings of this study provide important insights regarding the incidence of gNENs in AIG. It shows that the incidence rate of gNENs is 5.7% per year, which is higher than that reported in the general population, but consistent with previous studies[21,22,34,45].
The anthropometric, demographic and clinical characteristics of the population included in our study represented the features of previously reported AIG populations[46]. This correspondence emphasizes that type I gNENs represent a neoplastic complication in AIG patients and that they should not be overlooked[1,47-49]. Overall, these findings underscore the importance of vigilance and long-term monitoring in patients diagnosed with AIG.
In addition, at baseline, nearly two-thirds (65.9%) of the AIG patients had ECL hyperplasia, which suggests a heightened susceptibility of AIG patients to early lesions that may progress to gNENs.
The comparison between AIG patients who developed, or not, gNENs during follow-up showed that, while the two populations were comparable in terms of anthropometric and demographic features, the incidence of H. pylori infection, APCA positivity, OLGA and OLGIM scores, and baseline circulating CgA levels, circulating gastrin levels differed significantly.
Currently, the most widely used scores to define the degree of severity of gastric mucosal alteration in AIG are OLGA and OLGIM scores. Our study confirms that, as expected, these scores are not sufficiently accurate in the AIG population, especially with reference to advanced disease stages. In AIG, the antrum is almost always preserved and histologically normal (except for those who have or have had H. pylori infection). In the patients included in our study, OLGA and OLGIM scores did not significantly differ between patients who developed gNEN and those who did not. Therefore, patients with AIG should all be monitored with EGD (with different timing depending on the findings and risk factors), regardless of the OLGA or OLGIM scores[1].
Circulating gastrin levels were significantly more elevated in AIG patients who developed gNENs when compared to AIG patients who did not. Hence, our results confirm that patients who develop gNENs have significantly higher baseline circulating levels of gastrin compared to those who do not, as reported in previous studies[50,51].
Gastrin, a peptide hormone involved in gastric acid secretion, plays a critical role in regulating ECL cell proliferation. In the case of gastric hypo- and achlorhydria, which typically happens in the case of AIG, and which is mainly due to the immune-mediated destruction of oxyntic gastric mucosa, gastric neuroendocrine cells oversecrete gastrin, and elevated gastrin levels promote the growth and proliferation of gastric mucosa, including ECL cells[15]. gNENs derive from the aberrant proliferation of ECL cells[6,49]. The development of neuroendocrine neoplasms, specifically from simple hyper
Despite the multifactorial mechanisms, it is worth noting that the measurement of circulating gastrin levels may serve as a valuable biomarker for indirect assessment of neuroendocrine activity, thus reflecting the risk of developing gNEN in AIG patients. Indeed, in our series, we observed an overall sensitivity of 90.9% for gastrin in detecting gNEN. However, it is important to recognize that elevated gastrin levels also mark neuroendocrine activation resulting from the hypo/anacid condition typical of AIG patients. The calculated gastrin specificity in our series, in fact, only reached 1.4%. Therefore, while elevated gastrin levels may indicate an increased risk of gNEN in AIG patients, this hormone is not able to differentiate the presence of gNEN from gastrin-driven hypergastrinemia associated with AIG.
Overall, the analysis of the gastrin ROC curve yielded an AUC of 0.53, indicating that gastrin had low diagnostic accuracy as a biochemical marker for gNENs, with nearly 50% sensibility and 60% specificity. The identified best-performing cutoff point of 857 pg/mL, which achieved ~50% sensitivity and ~60% specificity, emphasizes the limited discriminatory power of gastrin in diagnosing gNENs, specifically in the population in which its utilization would be appropriate. These findings suggest that while gastrin may be associated with the presence of gNENs, its overall diagnostic performance is modest and may not be reliable as a stand-alone marker for gNEN diagnosis in AIG patients.
The diagnostic accuracy remains low because gastrin levels are consistently elevated in AIG, due to hypochloridria and regardless of the presence of gNENs. In this context, the development of gNENs is only a final epiphenomenon resulting from activation of ECL cells. This further contributes to the challenge of using gastrin as a reliable marker for early detection of gNENs in AIG patients.
Unfortunately, also incorporating CgA levels alongside gastrin levels, the combination of the two markers did not achieve sufficient diagnostic specificity and, thus, accuracy for the detection of gNENs associated with AIG. This is due to the lack of significant difference in circulating levels of CgA between AIG patients who developed at least one gNEN and those who did not develop any gNEN, which can be explained by the nonspecific secretion of CgA by neuroendocrine cells in cases of gastric mucosal atrophy. Unlike gastrin, CgA has no pathogenic role in the proliferation of ECLs and thus in the genesis of gNENs.
It is important to acknowledge the limitations of our study. Firstly, the study was retrospective, which means that the findings should be interpreted with caution. Secondly, it was a single-center study with a small sample size, which may limit generalization of our results. However, the long duration of follow-up and extensive clinical and laboratory investigations provide solid data on the association between AIG and gNEN and its risk magnitude.
Our study confirms that type I gNENs represent a non-negligible complication in patients with AIG and that they are related to hypergastrinemia. Gastrin has only moderate diagnostic accuracy; therefore, it cannot be a noninvasive marker of early diagnosis of gNEN in AIG because of its low specificity. Given these findings, further efforts must be made to identify new noninvasive early markers of aberrant ECL cell proliferation so as to identify effective strategies for individualizing endoscopic follow-up of AIG patients, for early diagnosis and treatment of superimposed neuroendocrine lesions. Further studies are needed to explore the potential clinical implications of these results in managing AIG patients.
Type I gastric neuroendocrine neoplasms (gNENs) in chronic autoimmune gastritis (AIG) are increasingly being diagnosed, but no accurate data are available.
To date, no risk factors for malignant transformation of AIG into NENs have been identified. Their identification may significantly help to optimize endoscopic follow-up of AIG.
The main objective was to evaluate the incidence and prevalence of type I gNENs in a cohort of AIG patients. Secondly, the study aimed to identify potential risk factors for malignant transformation of AIG.
Patients with a histological diagnosis of AIG were enrolled between October 2020 and May 2022. Circulating levels of chromogranin A and gastrin were assessed at enrollment. Patients underwent regular endoscopic follow-up to detect gastric neoplastic lesions, enterochromaffin-like cell hyperplasia, and the development of gNEN.
Among 176 included patients, after 1032 person-years, 33 patients developed a total of 50 gNEN type I, with an incidence rate of 0.057 person-years, corresponding to an annual cumulative incidence of 5.7%. Gastrin levels were significantly higher in AIG patients who developed gNENs [median 992 pg/mL, [interquartile range (IQR) = 449–1500 vs 688 pg/mL, IQR = 423–1200[ (P = 0.03]). Calculated gastrin sensitivity and specificity were 90.9% and 1.4%, respectively, with an overall diagnostic accuracy of 30% and a calculated area under the gastrin receiver operating characteristic curve (AUROC or AUC) of 0.53.
Type I gNENs are a frequent complication of AIG. Hypergastrinemia plays a trophic role in the gastric mucosa, but due to its low specificity, it cannot be a noninvasive marker of early diagnosis of gNEN in AIG.
Further efforts are needed to identify effective strategies for individualizing endoscopic follow-up of AIG patients for early diagnosis and treatment of superimposed neuroendocrine lesions.
| 1. | Shah SC, Piazuelo MB, Kuipers EJ, Li D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology. 2021;161:1325-1332.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 318] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 2. | Köseoğlu H, Duzenli T, Sezikli M. Gastric neuroendocrine neoplasms: A review. World J Clin Cases. 2021;9:7973-7985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Sheikh-Ahmad M, Saiegh L, Shalata A, Bejar J, Kreizman-Shefer H, Sirhan MF, Matter I, Swaid F, Laniado M, Mubariki N, Rainis T, Rosenblatt I, Yovanovich E, Agbarya A. Factors Predicting Type I Gastric Neuroendocrine Neoplasia Recurrence: A Single-Center Study. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Pachuashvili NV, Nagornaya DP, Tertychnyi AS. [Metachronous tumors of the stomach in a patient with autoimmune gastritis]. Arkh Patol. 2023;85:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Neumann WL, Coss E, Rugge M, Genta RM. Autoimmune atrophic gastritis--pathogenesis, pathology and management. Nat Rev Gastroenterol Hepatol. 2013;10:529-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 306] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 6. | Massironi S, Zilli A, Elvevi A, Invernizzi P. The changing face of chronic autoimmune atrophic gastritis: an updated comprehensive perspective. Autoimmun Rev. 2019;18:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 7. | Lenti MV, Rugge M, Lahner E, Miceli E, Toh BH, Genta RM, De Block C, Hershko C, Di Sabatino A. Autoimmune gastritis. Nat Rev Dis Primers. 2020;6:56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 240] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 8. | Gleeson PA, Toh B-H, Alderuccio F, van Driel IR. The Gastric H/K-ATPase: The Principle Target in Autoimmune Gastritis. In: Hirst BH, editor. Molecular and Cellular Mechanisms of H+ Transport. Berlin, Heidelberg: Springer, 1994: 119–126. |
| 9. | Ahmed M. Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol. 2020;12:791-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (20)] |
| 10. | Annibale B, Azzoni C, Corleto VD, di Giulio E, Caruana P, D'Ambra G, Bordi C, Delle Fave G. Atrophic body gastritis patients with enterochromaffin-like cell dysplasia are at increased risk for the development of type I gastric carcinoid. Eur J Gastroenterol Hepatol. 2001;13:1449-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Joo YE, Park HK, Myung DS, Baik GH, Shin JE, Seo GS, Kim GH, Kim HU, Kim HY, Cho SI, Kim N. Prevalence and risk factors of atrophic gastritis and intestinal metaplasia: a nationwide multicenter prospective study in Korea. Gut Liver. 2013;7:303-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Poveda JC, Chahar S, Garcia-Buitrago MT, Montgomery EA, McDonald OG. The Morphologic Spectrum of Gastric Type 1 Enterochromaffin-Like Cell Neuroendocrine Tumors. Mod Pathol. 2023;36:100098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 13. | Rugge M, Bricca L, Guzzinati S, Sacchi D, Pizzi M, Savarino E, Farinati F, Zorzi M, Fassan M, Dei Tos AP, Malfertheiner P, Genta RM, Graham DY. Autoimmune gastritis: long-term natural history in naïve Helicobacter pylori-negative patients. Gut. 2023;72:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 107] [Article Influence: 35.7] [Reference Citation Analysis (1)] |
| 14. | Hirabayashi K, Zamboni G, Nishi T, Tanaka A, Kajiwara H, Nakamura N. Histopathology of gastrointestinal neuroendocrine neoplasms. Front Oncol. 2013;3:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Li H, Helander HF. Hypergastrinemia increases proliferation of gastroduodenal epithelium during gastric ulcer healing in rats. Dig Dis Sci. 1996;41:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Hu H, Li R, Shao L, Zhang Q, Xu R, Zhang S. Gastric lesions in patients with autoimmune metaplastic atrophic gastritis: a retrospective study in a single center. Scand J Gastroenterol. 2022;57:1296-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Rossi RE, Massironi S. The Increasing Incidence of Neuroendocrine Neoplasms Worldwide: Current Knowledge and Open Issues. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Hu P, Bai J, Liu M, Xue J, Chen T, Li R, Kuai X, Zhao H, Li X, Tian Y, Sun W, Xiong Y, Tang Q. Trends of incidence and prognosis of gastric neuroendocrine neoplasms: a study based on SEER and our multicenter research. Gastric Cancer. 2020;23:591-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Chen C, Yang Y, Li P, Hu H. Incidence of Gastric Neoplasms Arising from Autoimmune Metaplastic Atrophic Gastritis: A Systematic Review and Case Reports. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 20. | Cao LL, Lu J, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, Chen QY, Lin M, Tu RH, Huang CM. Incidence and survival trends for gastric neuroendocrine neoplasms: An analysis of 3523 patients in the SEER database. Eur J Surg Oncol. 2018;44:1628-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Miceli E, Vanoli A, Lenti MV, Klersy C, Di Stefano M, Luinetti O, Caccia Dominioni C, Pisati M, Staiani M, Gentile A, Capuano F, Arpa G, Paulli M, Corazza GR, Di Sabatino A. Natural history of autoimmune atrophic gastritis: a prospective, single centre, long-term experience. Aliment Pharmacol Ther. 2019;50:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 22. | Dilaghi E, Baldaro F, Pilozzi E, Conti L, Palumbo A, Esposito G, Annibale B, Lahner E. Pseudopyloric Metaplasia Is Not Associated With the Development of Gastric Cancer. Am J Gastroenterol. 2021;116:1859-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Panzuto F, Campana D, Massironi S, Faggiano A, Rinzivillo M, Lamberti G, Sciola V, Lahner E, Manuzzi L, Colao A, Annibale B. Tumour type and size are prognostic factors in gastric neuroendocrine neoplasia: A multicentre retrospective study. Dig Liver Dis. 2019;51:1456-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Dilaghi E, Bellisario M, Esposito G, Carabotti M, Annibale B, Lahner E. The Impact of Proton Pump Inhibitors on the Development of Gastric Neoplastic Lesions in Patients With Autoimmune Atrophic Gastritis. Front Immunol. 2022;13:910077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Duan S, Rico K, Merchant JL. Gastrin: From Physiology to Gastrointestinal Malignancies. Function (Oxf). 2022;3:zqab062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Rusak E, Chobot A, Krzywicka A, Wenzlau J. Anti-parietal cell antibodies - diagnostic significance. Adv Med Sci. 2016;61:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 27. | Toyoshima O, Nishizawa T, Koike K. Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis. World J Gastroenterol. 2020;26:466-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (2)] |
| 28. | Massironi S, Gallo C, Laffusa A, Ciuffini C, Conti CB, Barbaro F, Boskoski I, Dinelli ME, Invernizzi P. Endoscopic techniques for gastric neuroendocrine tumors: An update. World J Gastrointest Endosc. 2023;15:103-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (3)] |
| 29. | Veysey-Smith R, Moore AR, Murugesan SV, Tiszlavicz L, Dockray GJ, Varro A, Pritchard DM. Effects of Proton Pump Inhibitor Therapy, H. pylori Infection and Gastric Preneoplastic Pathology on Fasting Serum Gastrin Concentrations. Front Endocrinol (Lausanne). 2021;12:741887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3617] [Article Influence: 120.6] [Reference Citation Analysis (6)] |
| 31. | Park DY, Lauwers GY. Gastric polyps: classification and management. Arch Pathol Lab Med. 2008;132:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Yacoub H, Bibani N, Sabbah M, Bellil N, Ouakaa A, Trad D, Gargouri D. Gastric polyps: a 10-year analysis of 18,496 upper endoscopies. BMC Gastroenterol. 2022;22:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Wang W, Zhou ZW. [Surgical treatment of gastric neuroendocrine neoplasms]. Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24:849-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Delle Fave G, O'Toole D, Sundin A, Taal B, Ferolla P, Ramage JK, Ferone D, Ito T, Weber W, Zheng-Pei Z, De Herder WW, Pascher A, Ruszniewski P; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 366] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 35. | Okamoto Y, Kanzaki H, Tanaka T, Sakae H, Abe M, Iwamuro M, Kawano S, Kawahara Y, Okada H. Gastric Adenoma: A High Incidence Rate of Developing Carcinoma and Risk of Metachronous Gastric Cancer according to Long-Term Follow-Up. Digestion. 2021;102:878-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Kishino M, Nonaka K. Endoscopic Features of Autoimmune Gastritis: Focus on Typical Images and Early Images. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Capelle LG, de Vries AC, Haringsma J, Ter Borg F, de Vries RA, Bruno MJ, van Dekken H, Meijer J, van Grieken NC, Kuipers EJ. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71:1150-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 396] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 38. | Yue H, Shan L, Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2018;21:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 39. | Kinoshita H, Hayakawa Y, Koike K. Metaplasia in the Stomach-Precursor of Gastric Cancer? Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 40. | Solcia E, Rindi G, Silini E, Villani L. Enterochromaffin-like (ECL) cells and their growths: relationships to gastrin, reduced acid secretion and gastritis. Baillieres Clin Gastroenterol. 1993;7:149-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2730] [Article Influence: 455.0] [Reference Citation Analysis (3)] |
| 42. | Capurso G, Gaujoux S, Pescatori LC, Panzuto F, Panis Y, Pilozzi E, Terris B, de Mestier L, Prat F, Rinzivillo M, Coriat R, Coulevard A, Delle Fave G, Ruszniewski P. The ENETS TNM staging and grading system accurately predict prognosis in patients with rectal NENs. Dig Liver Dis. 2019;51:1725-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 43. | Díaz Pérez JÁ, Currás Freixes M. [Chromogranin A and neuroendocrine tumors]. Endocrinol Nutr. 2013;60:386-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Louthan O. Chromogranin a in physiology and oncology. Folia Biol (Praha). 2011;57:173-181. [PubMed] |
| 45. | Das S, Dasari A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr Oncol Rep. 2021;23:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 256] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 46. | Adamu MA, Weck MN, Gao L, Brenner H. Incidence of chronic atrophic gastritis: systematic review and meta-analysis of follow-up studies. Eur J Epidemiol. 2010;25:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 47. | Zhang H, Jin Z, Cui R, Ding S, Huang Y, Zhou L. Autoimmune metaplastic atrophic gastritis in chinese: a study of 320 patients at a large tertiary medical center. Scand J Gastroenterol. 2017;52:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Murphy G, Dawsey SM, Engels EA, Ricker W, Parsons R, Etemadi A, Lin SW, Abnet CC, Freedman ND. Cancer Risk After Pernicious Anemia in the US Elderly Population. Clin Gastroenterol Hepatol. 2015;13:2282-9.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 49. | Vannella L, Sbrozzi-Vanni A, Lahner E, Bordi C, Pilozzi E, Corleto VD, Osborn JF, Delle Fave G, Annibale B. Development of type I gastric carcinoid in patients with chronic atrophic gastritis. Aliment Pharmacol Ther. 2011;33:1361-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 50. | Peracchi M, Gebbia C, Basilisco G, Quatrini M, Tarantino C, Vescarelli C, Massironi S, Conte D. Plasma chromogranin A in patients with autoimmune chronic atrophic gastritis, enterochromaffin-like cell lesions and gastric carcinoids. Eur J Endocrinol. 2005;152:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 51. | Campana D, Ravizza D, Ferolla P, Faggiano A, Grimaldi F, Albertelli M, Ricci C, Santini D, Brighi N, Fazio N, Colao A, Ferone D, Tomassetti P. Risk factors of type 1 gastric neuroendocrine neoplasia in patients with chronic atrophic gastritis. A retrospective, multicentre study. Endocrine. 2017;56:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer-reviewed.
Peer-review model: Single-blind
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang YQ, China; Kotelevets SM, Russia; Muguruma N, Japan S-Editor: Fan JR L-Editor: Kerr C P-Editor: Xu ZH