©The Author(s) 2025.
World J Gastrointest Oncol. Dec 15, 2025; 17(12): 112548
Published online Dec 15, 2025. doi: 10.4251/wjgo.v17.i12.112548
Published online Dec 15, 2025. doi: 10.4251/wjgo.v17.i12.112548
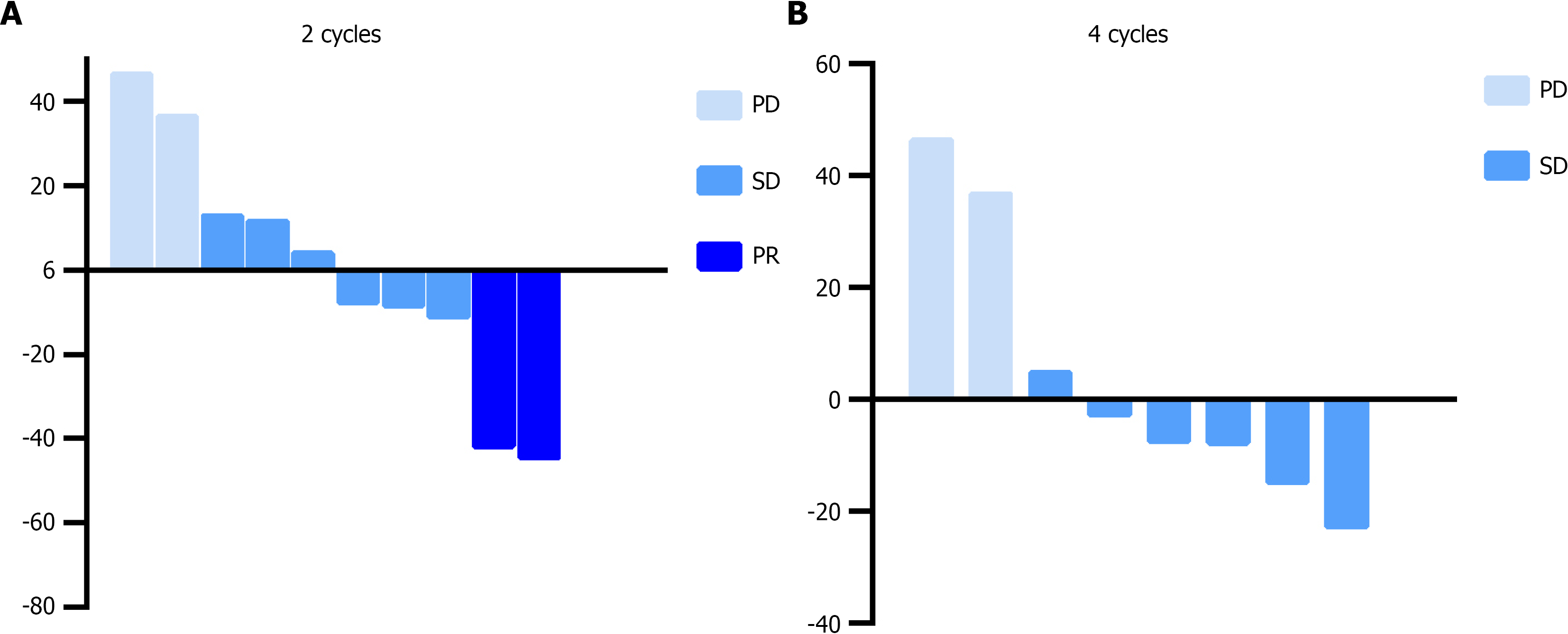
Figure 1 Waterfall plot of maximum percent change in tumor size of per-protocol population received 2 cycles treatment and 4 cycles treatment.
A: Waterfall plot of maximum percent change in tumor size of per-protocol population received 2 cycles treatment; B: Waterfall plot of maximum percent change in tumor size of per-protocol population received 4 cycles treatment. PD: Progressive disease; SD: Stable disease; PR: Partial response.
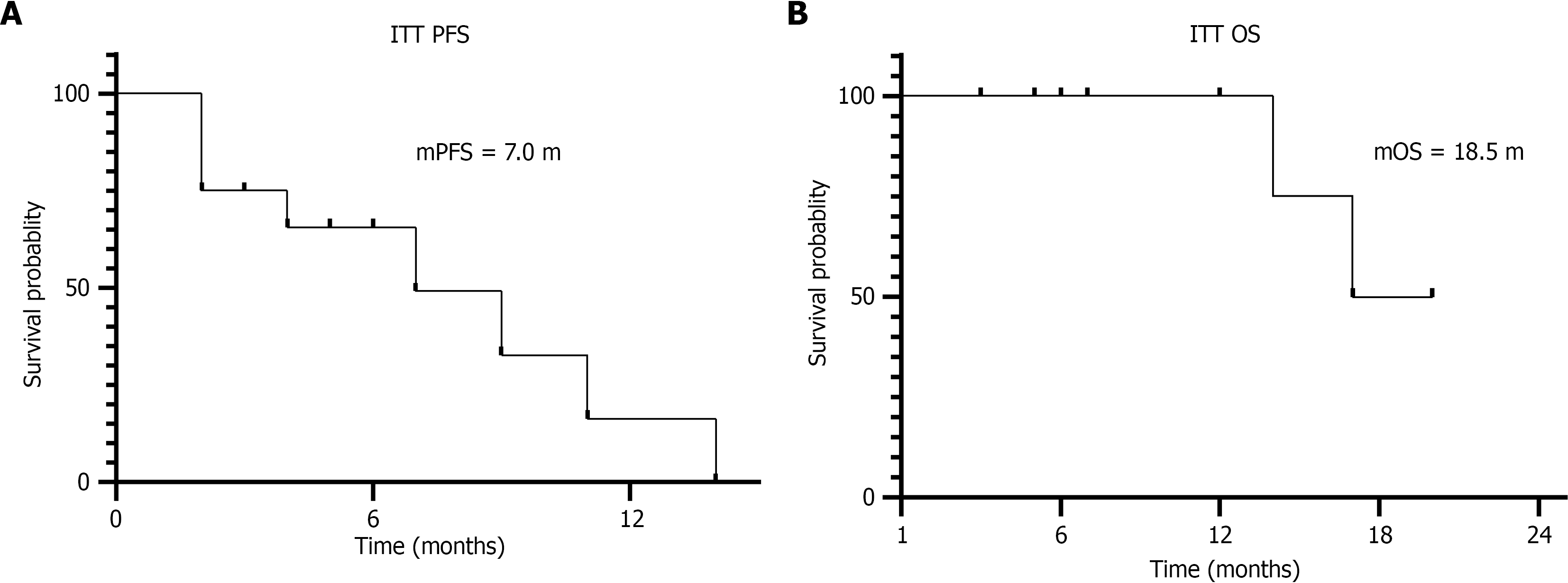
Figure 2 Survival analysis of intention-to-treat population.
A: Progression-free survival in months was analyzed using the Kaplan-Meier method; B: Overall survival in months was analyzed using the Kaplan-Meier method. ITT: Intention-to-treat; PFS: Progression-free survival; OS: Overall survival; mPFS: Median progression-free survival; mOS: Median overall survival.
- Citation: Meng XY, Cai YM, Sun NN, Zhang WH, Cui RX, Zhang L, Zheng CC, Sun Z, Luo WX, Wang FW. Preliminary exploration of programmed death 1 inhibitor combined with fruquintinib and docetaxel for advanced colorectal cancer. World J Gastrointest Oncol 2025; 17(12): 112548
- URL: https://www.wjgnet.com/1948-5204/full/v17/i12/112548.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i12.112548