©The Author(s) 2023.
World J Gastrointest Oncol. Jun 15, 2023; 15(6): 1086-1095
Published online Jun 15, 2023. doi: 10.4251/wjgo.v15.i6.1086
Published online Jun 15, 2023. doi: 10.4251/wjgo.v15.i6.1086
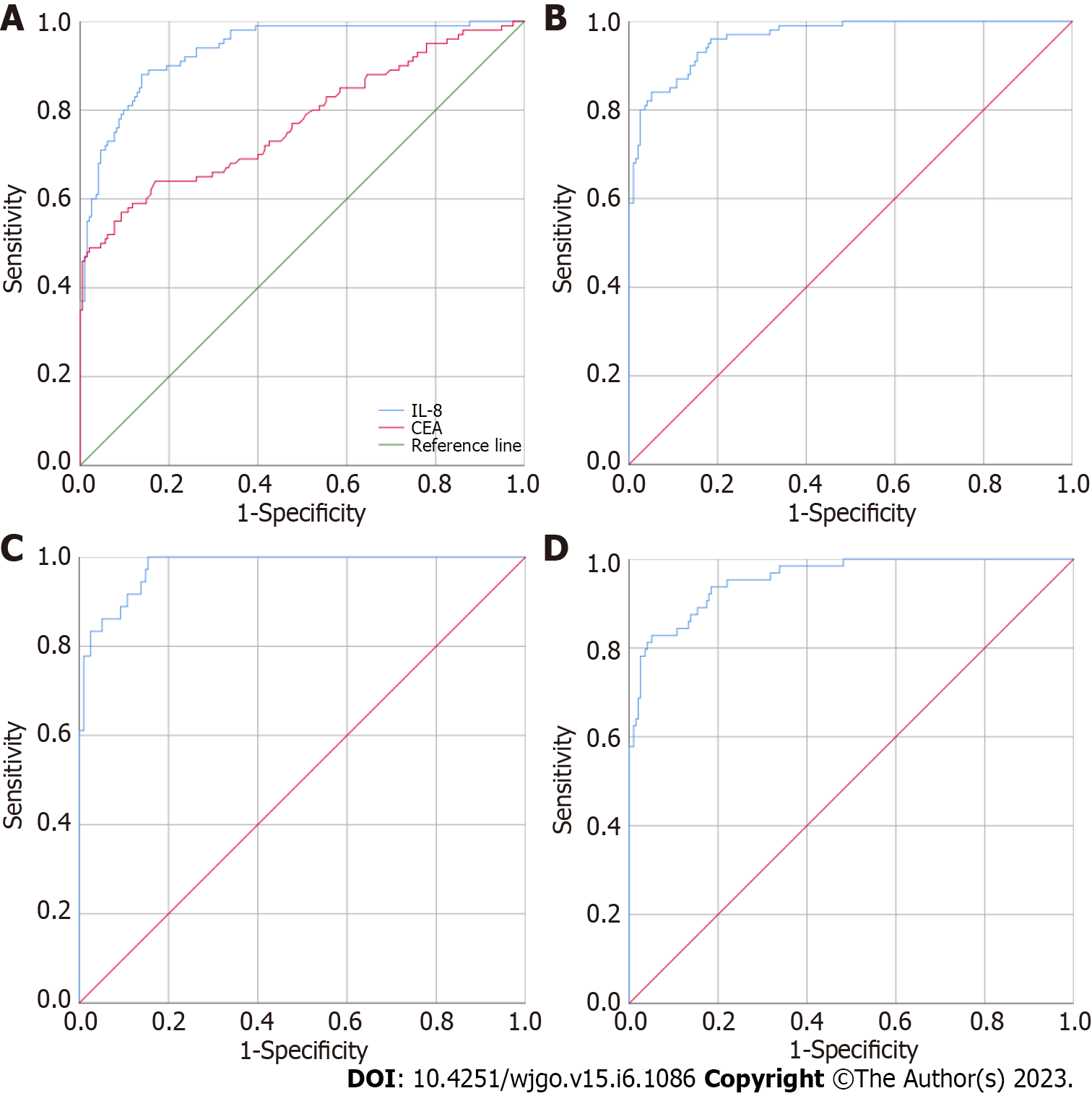
Figure 1 Diagnostic value evaluation of the indicator for differentiation between the healthy control group and colorectal cancer group.
A: CEA and CAMK1D used alone to differentiate between the 195 healthy controls and 101 colorectal cancer patients; B: CEA and CAMK1D joint model for the differentiation of the 195 healthy controls and 101 colorectal cancer patients; C: CEA and CAMK1D joint model for the differentiation of the 195 healthy controls and 38 early colorectal cancer patients; D: CEA and CAMK1D joint model for the differentiation of the 195 healthy controls and 63 advanced colorectal cancer patients.
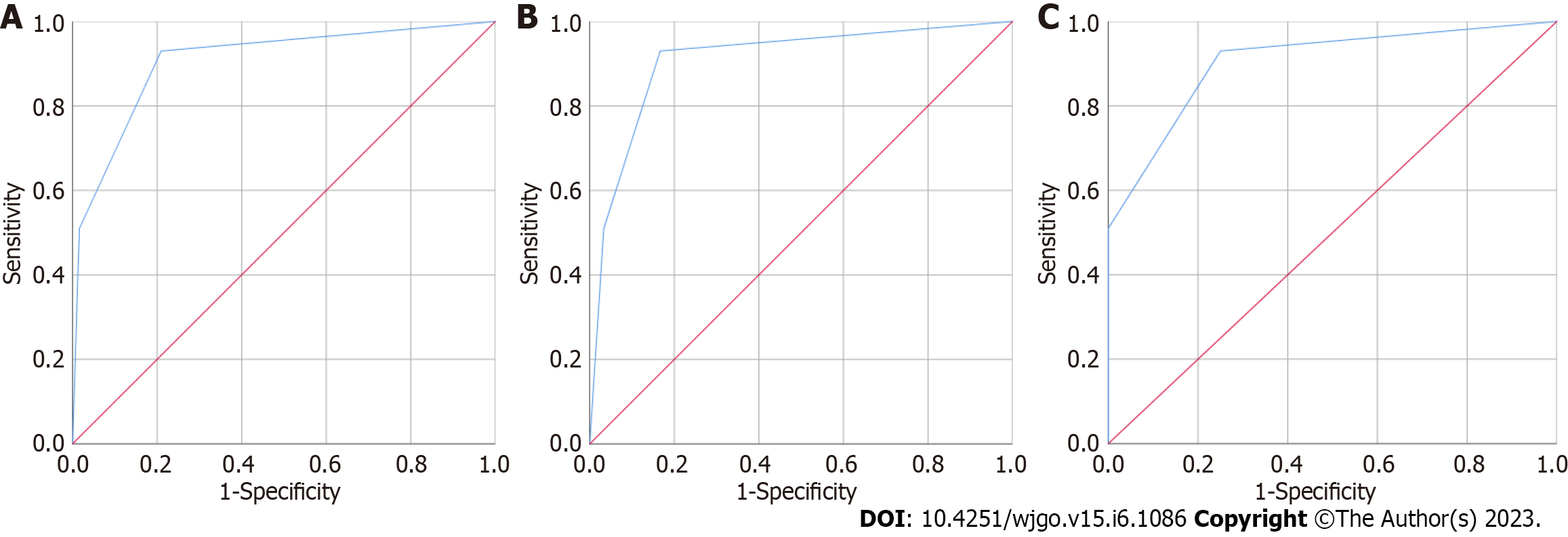
Figure 2 Diagnostic value evaluation of the indicator for the differentiation between the healthy controls and colorectal cancer patients in the validation group.
A: CEA and CAMK1D joint model for the differentiation of 100 healthy controls and 62 patients with colorectal cancer; B: CEA and CAMK1D joint model for the differentiation of 100 healthy controls and 30 patients with early colorectal cancer; C: CEA and CAMK1D joint model for the differentiation of 100 healthy controls and 32 patients with advanced colorectal cancer.
- Citation: Cui Y, Zhang LJ, Li J, Xu YJ, Liu MY. Diagnostic value of circular free DNA for colorectal cancer detection. World J Gastrointest Oncol 2023; 15(6): 1086-1095
- URL: https://www.wjgnet.com/1948-5204/full/v15/i6/1086.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i6.1086