Published online Jun 15, 2023. doi: 10.4251/wjgo.v15.i6.1096
Peer-review started: February 19, 2023
First decision: March 22, 2023
Revised: April 9, 2023
Accepted: April 23, 2023
Article in press: April 23, 2023
Published online: June 15, 2023
Processing time: 116 Days and 1.9 Hours
Currently, chemotherapy combined with immunotherapy is the established first-line standard treatment for advanced gastric cancer (GC). In addition, the combination of radiotherapy and immunotherapy is considered a promising treatment strategy.
In this report, we present a case of achieving nearly complete remission of highly advanced GC with comprehensive therapies. A 67-year-old male patient was referred to the hospital because he presented with dyspepsia and melena for several days. Based on fluorodeoxyglucose positron emission tomography/com
The combination of radiotherapy and immunotherapy for GC is worthy of further exploration.
Core Tip: This case report describes a patient with unresectable advanced gastric cancer who received comprehensive treatment including chemoimmunotherapy and hypofractionated radiotherapy that was applied to treat the primary lesion; satisfactory efficacy was achieved. The combination of radiotherapy and immunotherapy is worthy of further exploration, and the dose division of radiotherapy is an important factor. Hypofractionated radiotherapy, compared to conventional fractionated radiotherapy, may better coordinate with immunotherapy.
- Citation: Zhou ML, Xu RN, Tan C, Zhang Z, Wan JF. Advanced gastric cancer achieving major pathologic regression after chemoimmunotherapy combined with hypofractionated radiotherapy: A case report. World J Gastrointest Oncol 2023; 15(6): 1096-1104
- URL: https://www.wjgnet.com/1948-5204/full/v15/i6/1096.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i6.1096
According to GLOBOCAN 2020, gastric cancer (GC) ranks fifth and fourth in terms of the estimated number of new cases and the number of deaths worldwide, respectively[1]. Of note, the majority of worldwide GC cases and deaths occur annually in China, accounting for 43.9% of the worldwide cases and 48.6% of the worldwide deaths[1]. The median overall survival of patients with advanced GC is only 1 year[1,2]. Such disappointing survival outcomes are mainly the result of the inherent biological aggressiveness of GC and the relatively poor response to currently available therapies.
Cancer immunotherapy has opened a new era of cancer treatment. In 2020, two clinical studies based on KEYNOTE-059 and ATTRACTION-02 established the status of pembrolizumab and nivolumab as third-line treatments for advanced GC[3,4]. While moving from being a third-line treatment to a first-line treatment, immunotherapy for GC has encountered many difficulties and failures. Currently, chemotherapy combined with immunotherapy is the established first-line standard treatment for advanced GC[5].
At present, the focus of tumor immunotherapy has shifted from single-drug therapy to combined immunotherapy, as the combination could potentially lead to increased therapeutic efficacy. Radio
This report presents the case of a patient who was initially diagnosed with unresectable advanced GC and successfully treated with comprehensive therapies including chemotherapy, immunotherapy, and hypofractionated radiotherapy (HFRT). The tumor showed significant regression, and surgery was performed. Eventually, the patient achieved major pathologic regression.
A 67-year-old male patient presenting with dyspepsia and melena for several days was admitted to the Fudan University Shanghai Cancer Center (FUSCC, Shanghai, China) on May 12, 2022.
The patient developed dizziness, poor appetite, epigastrium fullness and discomfort, occasional dull pain, defecation, and no relief after taking omeprazole capsules for five days. Then the patient went to hospital accordingly.
The patient had no significant history of past illness.
The patient had a past history of smoking and alcohol consumption for more than 30 years and had already quit smoking for 2 years. The patient had no significant family history.
Physical examination showed a pale face, indicating anemia (hemoglobin, 97 g/L). An enlarged lymph node was palpated in the left supraclavicular area. No positive signs were observed in abdominal and digital rectal examinations.
The serum levels of carcinoembryonic antigen, alpha-fetoprotein, carbohydrate antigen (CA) 19–9, CA125, CA72-4, CA50, and CA242 were all in the normal ranges.
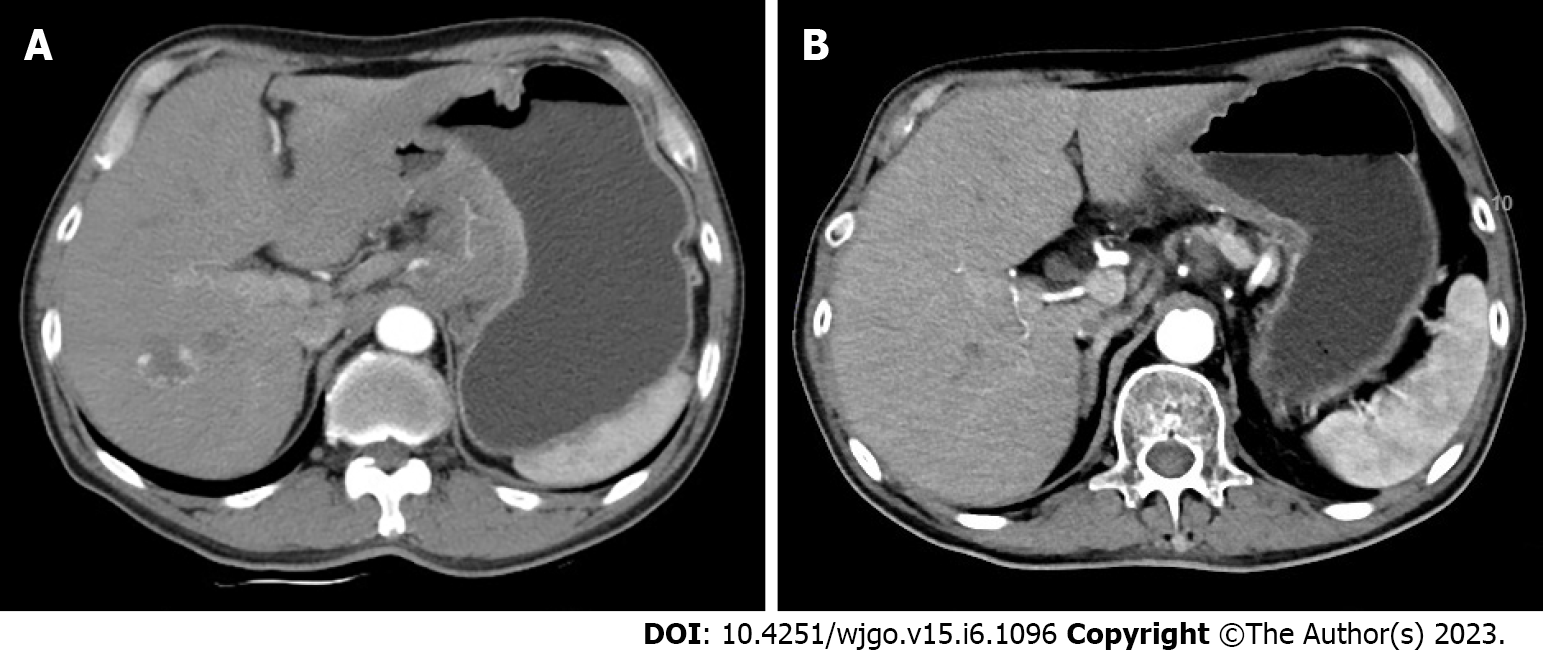
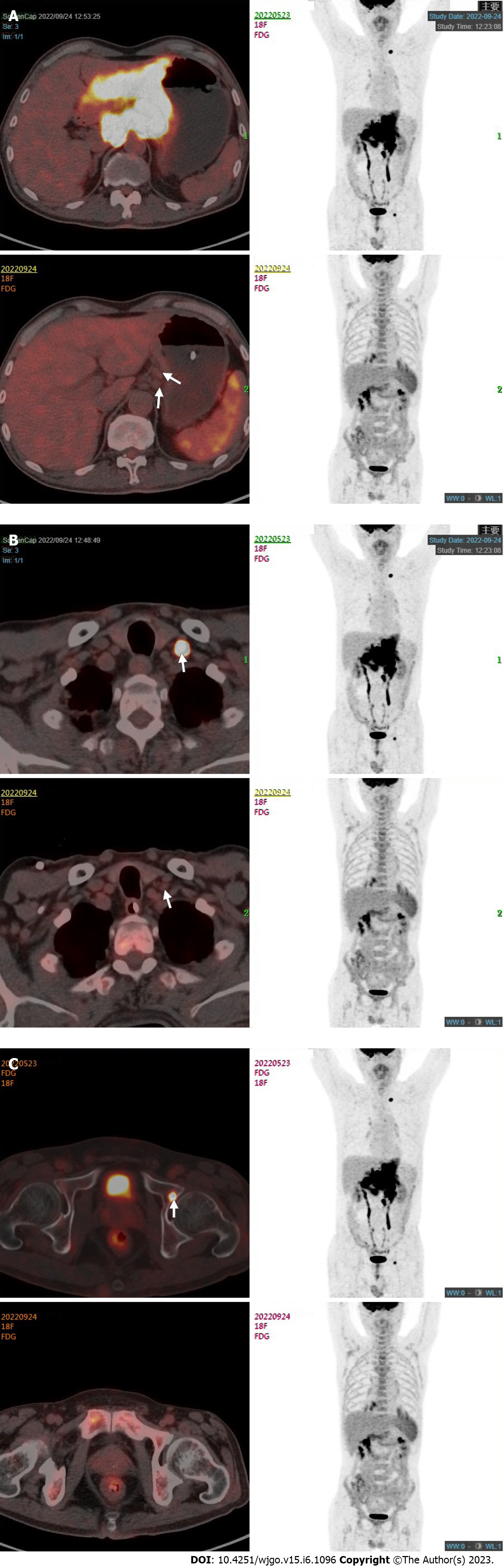
Enhanced computed tomography (CT) scan of the stomach showed thickening of the wall of the gastric body and the antrum with enhancement, and multiple enlarged lymph nodes were detected around the stomach, hepatogastric space, hilar region, and retroperitoneum (Figure 1A). Gastroscopy indicated Borrmann type 3 GC, and pathology examination of gastroscopic biopsy suggested poorly differentiated adenocarcinoma with a proportion of signet ring cell carcinoma and the mixed type according to Lauren’s classification. Immunohistochemistry of biopsy tissue showed proficient mismatch repair (pMMR), HER2 2+ and EBER negativity. Fluorescence in situ hybridization (FISH) showed no HER2 amplification. Next-generation sequencing showed that the tumor mutation burden (TMB) was 5.98 muts/MB. Whole-body fluorodeoxyglucose positron emission tomography/CT (FDG PET/CT) showed the following findings: (1) Diffuse thickening of the gastric wall in the antrum and body with FDG hypermetabolism; (2) perigastric mesenteric turbidity; (3) metastatic lymph nodes visible around the stomach, hepatogastric space, hilar region, retroperitoneum, and left supraclavicular area; (4) left acetabular metastasis; and (5) a small amount of pelvic effusion (Figure 2).
The patient was diagnosed with metastatic GC (cT4N3M1, stage IV) according to the 8th edition of the Union for International Cancer Control TNM classification for GC.
First-line standard treatment was performed, including the mFOLFOX6 regimen and a programmed death-1 (PD-1) inhibitor. The mFOLFOX6 regimen was applied as follows: oxaliplatin (85 mg/m2) was injected intravenously within 2 h on day 1; leucovorin (400 mg/m2) was injected intraveneously within 2 h on day 1; 5-fluorouracil (400 mg/m2) was injected intraveneously and then was continuously infused (2400 mg/m2) within 46 h; chemotherapy was repeated every two weeks. Nivolumab 240 mg was administered every two weeks. Considering that the patient was bleeding from gastric lesions and that the distal gastric tumor induced incomplete obstruction, we decided, after detailed communication with the patient and his family, to add radiotherapy for the primary lesion. After two cycles of chemoimmunotherapy, HFRT targeted to the primary lesion and lymphatic drainage area was performed with a total dose of 24 Gy split into 6 fractions. Then, another four cycles of chemoimmunotherapy were performed.
One month after these treatments, whole-body FDG PET/CT and enhanced abdominal CT were performed to evaluate the treatment effect. The adverse events (AEs) of the treatment were assessed according to the National Cancer Institute Common Terminology Criteria for AEs (CTCAE) version 4.0. AEs included grade 1 gastrointestinal discomfort and grade 2 leukocytopenia. These side effects were resolved after symptomatic treatment, and leukocytopenia was relieved by using granulocyte colony-stimulating factor (G-CSF). The patient’s dyspepsia and melena were relieved remarkably. His tumor markers were still in the normal ranges. Enhanced CT scan of the stomach showed a decrease in the thickness of the gastric wall and the size of the perigastric lymph nodes (Figure 1B). There was an obvious reduction of the gastric lesions and metastatic lymph nodes with a lowered FDG metabolism. The FDG metabolism of the left acetabular metastasis and left supraclavicular lymph nodes tended to be normal (Figure 2). The clinical response was classified as partial response according to the Response Evaluation Criteria in Solid Tumors version 1.1.
Afterward, the case was submitted for multidisciplinary team discussion of GC in FUSCC, and surgery was cautiously recommended. Surgery was performed on October 20, 2022. Laparoscopic exploration found neither ascites nor peritoneal seeding. Therefore, laparoscopic surgery was converted to an open approach, and total gastrectomy with Roux-en-Y reconstruction and D2 lymph node dissection was performed. The histological change was classified as TRG grade 1, according to the National Comprehensive Cancer Network clinical practice guidelines in oncology for GC. Postoperative pathology showed that the tumor bed had ulceration with interstitial fibrosis and inflammatory cell infiltration, which was consistent with the changes after treatment. Combined with the immunohistochemical results, a small number of epithelioid cells, AE1/AE3+, were found within the mucosa, tending to be poorly differentiated adenocarcinoma with changes after treatment. Twenty-six lymph nodes were harvested without tumor metastasis. Thus, the postoperative staging was ypT1aN0Mx. There were no postoperative complications observed. The postoperative treatment plan involved the continuous use of the original regimen and then maintenance with nivolumab until one year after surgery. Chemoimmunotherapy started four weeks after surgery and examinations were performed every three months.
This study describes a patient with oligometastatic GC who received comprehensive treatment, including chemotherapy, radiotherapy, immunotherapy, and surgery. Pronounced remission of the primary lesion was achieved, as shown by FDG PET/CT and validated by postoperative pathology. Meanwhile, the metabolism of bone metastasis and left supraclavicular lymph nodes was also signi
There is a special group of patients with stage IV disease, termed oligometastatic disease, who are in a relatively early and stable state without the tendency of metastasis spreading throughout the body. The number and location of metastatic lesions are limited, and it is believed that long-term survival can be achieved through systemic treatment with local treatment[7,8]. In gastroesophageal (GEJ) tumors, surgery, as a local treatment, is included in systemic treatment and brings survival benefits to patients with oligometastasis, which has been confirmed in the AIO-FLOT3 study[9]. Moreover, a subsequent AIO-FLOT5 study is in progress[10].
With the wide application of immunotherapy, radiotherapy combined with immunotherapy is considered a promising strategy due to its effect on immune activation and tumor microenvironment remodeling. Besides, enhanced mitochondrial metabolism plays an important role in the better treatment response to anti-PD1 agents[11] and radiotherapy[12]. The combination of immunotherapy and radiotherapy can cure patients with oligometastatic tumors, which has been proven in non-small cell lung cancer[13,14], prostate cancer[15], and other tumors[16]. However, the options of radiotherapy, including the sequence, dose, fractionation, and irradiated sites, that exert the best synergies need to be explored and optimized.
The conventional radiotherapy fraction mode is routinely used in GC. Selected studies have attempted HFRT for palliative treatment, especially for curing hemostasis, and several retrospective studies have indicated its good efficacy and safety[17-21]. However, the application of HFRT in GC is still limited.
Li et al[22] reported a prospective phase I study (ClinicalTrials.gov identifier: NCT03427684) of HFRT for the neoadjuvant treatment of GC. This was a dose-escalating study that included three levels of radiotherapy doses: 40.0 Gy/2.5 Gy/16 fractions, 95% isodose line covering the planning target volume (PTV); 95% PTV 41.6 Gy/2.6 Gy/16 fractions; and 95% PTV 43.2 Gy/2.7 Gy/16 fractions. Ultimately, 40.0 Gy/2.5 Gy was determined to be the maximum tolerated dose. Another single-arm prospective study (ClinicalTrials.gov identifier: NCT04162665) from the University of Washington in the United States adopted a short course of HFRT, sequential consolidation chemotherapy, and surgery for locally advanced GC. HFRT adopted a 5 Gy × 5 model with magnetic resonance guidance. The primary endpoint of this study was the pathologic complete regression rate. A single-arm prospective Phase Ib study (ClinicalTrials.gov identifier: NCT04523818) from MD Anderson Cancer Center in the United States explored the efficacy of short-course radiotherapy, sequential consolidation chemotherapy, and surgery in patients with resectable GC. The short course of radiotherapy in this study was divided into 10 fractions and completed within 2 wk. The primary endpoint was the incidence of AEs.
In preclinical models, HFRT has been proven to have better immune activation and less impact on lymphocytes[23,24]. In clinical practice, it seems that HFRT may show advances in certain cancers, and the combination of cancer immunotherapy and HFRT may have more potential[25]. Moreover, HFRT has the advantage of shortening the total treatment duration and saving medical resources. All these findings indicate that HFRT is a direction worth exploring in GC not only in the palliative setting but also in perioperative or treatment for oligometastatic patients.
Based on the aforementioned background, a Phase II clinical trial is being carried out in our center. This study targets patients with gastric/GEJ adenocarcinoma with limited liver metastases or paraaortic lymph node metastases. On the basis of systemic chemotherapy and immunotherapy, combined with HFRT of primary and metastatic lesions, the patients whose lesions can be surgically resected after treatment will receive surgery for primary and metastatic lesions when possible. The primary end point of the study was overall survival.
This study describes a patient with unresectable advanced GC who received comprehensive treatment; satisfactory efficacy was achieved. HFRT was applied to treat the primary lesion. The combination of radiotherapy and immunotherapy is worthy of further exploration. At the same time, the dose division, radiation range, choice of chemotherapy drugs, and arrangement of treatment sequence of radiotherapy need to be explored to better coordinate with immunotherapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kotelevets SM, Russia; Taghizadeh-Hesary F, Iran S-Editor: Chen YL L-Editor: A P-Editor: Yuan YY
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 67855] [Article Influence: 13571.0] [Reference Citation Analysis (192)] |
| 2. | Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 749] [Article Influence: 93.6] [Reference Citation Analysis (0)] |
| 3. | Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1776] [Article Influence: 197.3] [Reference Citation Analysis (0)] |
| 4. | Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1413] [Cited by in RCA: 1501] [Article Influence: 187.6] [Reference Citation Analysis (1)] |
| 5. | Moehler M, Shitara K, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Poulart V, Cullen D, Lei M, Kondo K, Li M, Ajani JA, Janjigian YY. LBA6_PR Nivolumab (nivo) plus chemotherapy (chemo) vs chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann Oncol. 2020;31:S1191. [RCA] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (1)] |
| 6. | Kim TK, Vandsemb EN, Herbst RS, Chen L. Adaptive immune resistance at the tumour site: mechanisms and therapeutic opportunities. Nat Rev Drug Discov. 2022;21:529-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 249] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 7. | Katipally RR, Pitroda SP, Juloori A, Chmura SJ, Weichselbaum RR. The oligometastatic spectrum in the era of improved detection and modern systemic therapy. Nat Rev Clin Oncol. 2022;19:585-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 8. | Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP, Schellenberg D, Ahmad B, Griffioen G, Senthi S, Swaminath A, Kopek N, Liu M, Moore K, Currie S, Bauman GS, Warner A, Senan S. Stereotactic ablative radiotherapy vs standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393:2051-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1019] [Cited by in RCA: 1381] [Article Influence: 197.3] [Reference Citation Analysis (0)] |
| 9. | Al-Batran SE, Homann N, Pauligk C, Illerhaus G, Martens UM, Stoehlmacher J, Schmalenberg H, Luley KB, Prasnikar N, Egger M, Probst S, Messmann H, Moehler M, Fischbach W, Hartmann JT, Mayer F, Höffkes HG, Koenigsmann M, Arnold D, Kraus TW, Grimm K, Berkhoff S, Post S, Jäger E, Bechstein W, Ronellenfitsch U, Mönig S, Hofheinz RD. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol. 2017;3:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 351] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 10. | Al-Batran SE, Goetze TO, Mueller DW, Vogel A, Winkler M, Lorenzen S, Novotny A, Pauligk C, Homann N, Jungbluth T, Reissfelder C, Caca K, Retter S, Horndasch E, Gumpp J, Bolling C, Fuchs KH, Blau W, Padberg W, Pohl M, Wunsch A, Michl P, Mannes F, Schwarzbach M, Schmalenberg H, Hohaus M, Scholz C, Benckert C, Knorrenschild JR, Kanngießer V, Zander T, Alakus H, Hofheinz RD, Roedel C, Shah MA, Sasako M, Lorenz D, Izbicki J, Bechstein WO, Lang H, Moenig SP. The RENAISSANCE (AIO-FLOT5) trial: effect of chemotherapy alone vs. chemotherapy followed by surgical resection on survival and quality of life in patients with limited-metastatic adenocarcinoma of the stomach or esophagogastric junction - a phase III trial of the German AIO/CAO-V/CAOGI. BMC Cancer. 2017;17:893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 11. | Houshyari M, Taghizadeh-Hesary F. Is Mitochondrial Metabolism a New Predictive Biomarker for Antiprogrammed Cell Death Protein-1 Immunotherapy? JCO Oncol Pract. 2023;19:123-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Taghizadeh-Hesary F, Houshyari M, Farhadi M. Mitochondrial metabolism: a predictive biomarker of radiotherapy efficacy and toxicity. J Cancer Res Clin Oncol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M; PACIFIC Investigators. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2750] [Cited by in RCA: 3388] [Article Influence: 376.4] [Reference Citation Analysis (9)] |
| 14. | Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts JGJV, Dumoulin DW, Bahce I, Niemeijer AN, de Langen AJ, Monkhorst K, Baas P. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5:1276-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 740] [Article Influence: 105.7] [Reference Citation Analysis (0)] |
| 15. | Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H, Ng S, Maio M, Franke FA, Sundar S, Agarwal N, Bergman AM, Ciuleanu TE, Korbenfeld E, Sengeløv L, Hansen S, Logothetis C, Beer TM, McHenry MB, Gagnier P, Liu D, Gerritsen WR; CA184-043 Investigators. Ipilimumab vs placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1157] [Cited by in RCA: 1207] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 16. | Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, Sedrak C, Jungbluth AA, Chua R, Yang AS, Roman RA, Rosner S, Benson B, Allison JP, Lesokhin AM, Gnjatic S, Wolchok JD. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1517] [Cited by in RCA: 1663] [Article Influence: 118.8] [Reference Citation Analysis (0)] |
| 17. | Kim MM, Rana V, Janjan NA, Das P, Phan AT, Delclos ME, Mansfield PF, Ajani JA, Crane CH, Krishnan S. Clinical benefit of palliative radiation therapy in advanced gastric cancer. Acta Oncol. 2008;47:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Hashimoto K, Mayahara H, Takashima A, Nakajima TE, Kato K, Hamaguchi T, Ito Y, Yamada Y, Kagami Y, Itami J, Shimada Y. Palliative radiation therapy for hemorrhage of unresectable gastric cancer: a single institute experience. J Cancer Res Clin Oncol. 2009;135:1117-1123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Asakura H, Hashimoto T, Harada H, Mizumoto M, Furutani K, Hasuike N, Matsuoka M, Ono H, Boku N, Nishimura T. Palliative radiotherapy for bleeding from advanced gastric cancer: is a schedule of 30 Gy in 10 fractions adequate? J Cancer Res Clin Oncol. 2011;137:125-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Chaw CL, Niblock PG, Chaw CS, Adamson DJ. The role of palliative radiotherapy for haemostasis in unresectable gastric cancer: a single-institution experience. Ecancermedicalscience. 2014;8:384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Tey J, Choo BA, Leong CN, Loy EY, Wong LC, Lim K, Lu JJ, Koh WY. Clinical outcome of palliative radiotherapy for locally advanced symptomatic gastric cancer in the modern era. Medicine (Baltimore). 2014;93:e118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Li N, Wang X, Tang Y, Zhao D, Chi Y, Yang L, Jiang L, Jiang J, Liu W, Fang H, Liu Y, Song Y, Wang S, Jin J, Li Y. A prospective phase I study of hypo-fractionated neoadjuvant radiotherapy for locally advanced gastric cancer. BMC Cancer. 2018;18:803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Lan J, Li R, Yin LM, Deng L, Gui J, Chen BQ, Zhou L, Meng MB, Huang QR, Mo XM, Wei YQ, Lu B, Dicker A, Xue JX, Lu Y. Targeting Myeloid-derived Suppressor Cells and Programmed Death Ligand 1 Confers Therapeutic Advantage of Ablative Hypofractionated Radiation Therapy Compared With Conventional Fractionated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2018;101:74-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Marciscano AE, Ghasemzadeh A, Nirschl TR, Theodros D, Kochel CM, Francica BJ, Muroyama Y, Anders RA, Sharabi AB, Velarde E, Mao W, Chaudhary KR, Chaimowitz MG, Wong J, Selby MJ, Thudium KB, Korman AJ, Ulmert D, Thorek DLJ, DeWeese TL, Drake CG. Elective Nodal Irradiation Attenuates the Combinatorial Efficacy of Stereotactic Radiation Therapy and Immunotherapy. Clin Cancer Res. 2018;24:5058-5071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 258] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 25. | Zhang Z, Liu X, Chen D, Yu J. Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Signal Transduct Target Ther. 2022;7:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 390] [Reference Citation Analysis (0)] |