Published online Sep 16, 2025. doi: 10.4253/wjge.v17.i9.109144
Accepted: July 17, 2025
Published online: September 16, 2025
Processing time: 135 Days and 10.4 Hours
Endoscopic submucosal dissection (ESD) has emerged as a pivotal therapeutic modality for early gastrointestinal (GI) cancers, providing a minimally invasive approach with curative potential. This technique enables the en bloc resection of neoplastic lesions confined to the mucosa and submucosa, thereby preserving organ function and reducing the need for more radical surgical interventions. ESD provides diagnostic clarity and enhances patient survival rates when performed by skilled practitioners in the early stages of GI cancers such as esophageal, gastric, and colorectal carcinomas. This article examines the indications, procedural advancements, technical considerations, and outcomes associated with ESD in early GI cancers. The challenges and complications that can arise are also highlighted. Additionally, we discuss the evolving role of novel techniques and adjunctive therapies to improve safety and efficacy. As the field progresses, ESD remains a cornerstone in managing early GI cancers, offering patients a promising option for organ preservation and long-term survival.
Core Tip: This article provides a comprehensive overview of endoscopic submucosal dissection for early esophageal, gastric, and colon cancers, detailing technical approaches, common pitfalls, and current evidence. This review also discusses the management of common procedural complications. Gastroenterologists can use this guide to improve outcomes and optimize the application of endoscopic submucosal dissection in managing early gastrointestinal cancer.
- Citation: Pal S, Bhaduri G. Endoscopic submucosal dissection for early gastrointestinal malignancies: Current state and future perspectives. World J Gastrointest Endosc 2025; 17(9): 109144
- URL: https://www.wjgnet.com/1948-5190/full/v17/i9/109144.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i9.109144
Endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR) are the primary endoscopic approaches and have replaced surgery as the standard therapy for early gastrointestinal (GI) cancers. Studies have documented the efficacy and safety of ESD and EMR. However, even with appropriate techniques, en bloc resection in EMR is limited by the snare diameter, necessitating piecemeal removal for larger lesions[1]. This piecemeal approach hinders the accurate assessment of an R0 resection, limiting the use of EMR for treating early-stage malignancies. In addition, some lesions, particularly those with submucosal invasion or scarring from prior procedures, will not lift with submucosal injection, hindering the applicability of EMR[2]. Conversely, ESD involves electrosurgical knives rather than snares for dissecting the tumor at the muscular level. This approach enables the treatment of larger tumors and those with deep submucosal invasion, which are limitations for snare-based methods. At the same time, ESD is a complex and time-consuming procedure, which is only available in tertiary or quaternary referral centers and requires extensive training.
ESD developed as an advanced form of EMR that involves injecting hypertonic saline mixed with epinephrine into the submucosa to aid in endoscopic resection[3]. Eponyms such as exfoliating EMR, cutting EMR, and EMR with cutting incision were initially used to describe specific EMR techniques. ESD was coined in 2003 as a new treatment modality for removing GI lesions, offering an option between EMR and surgery.
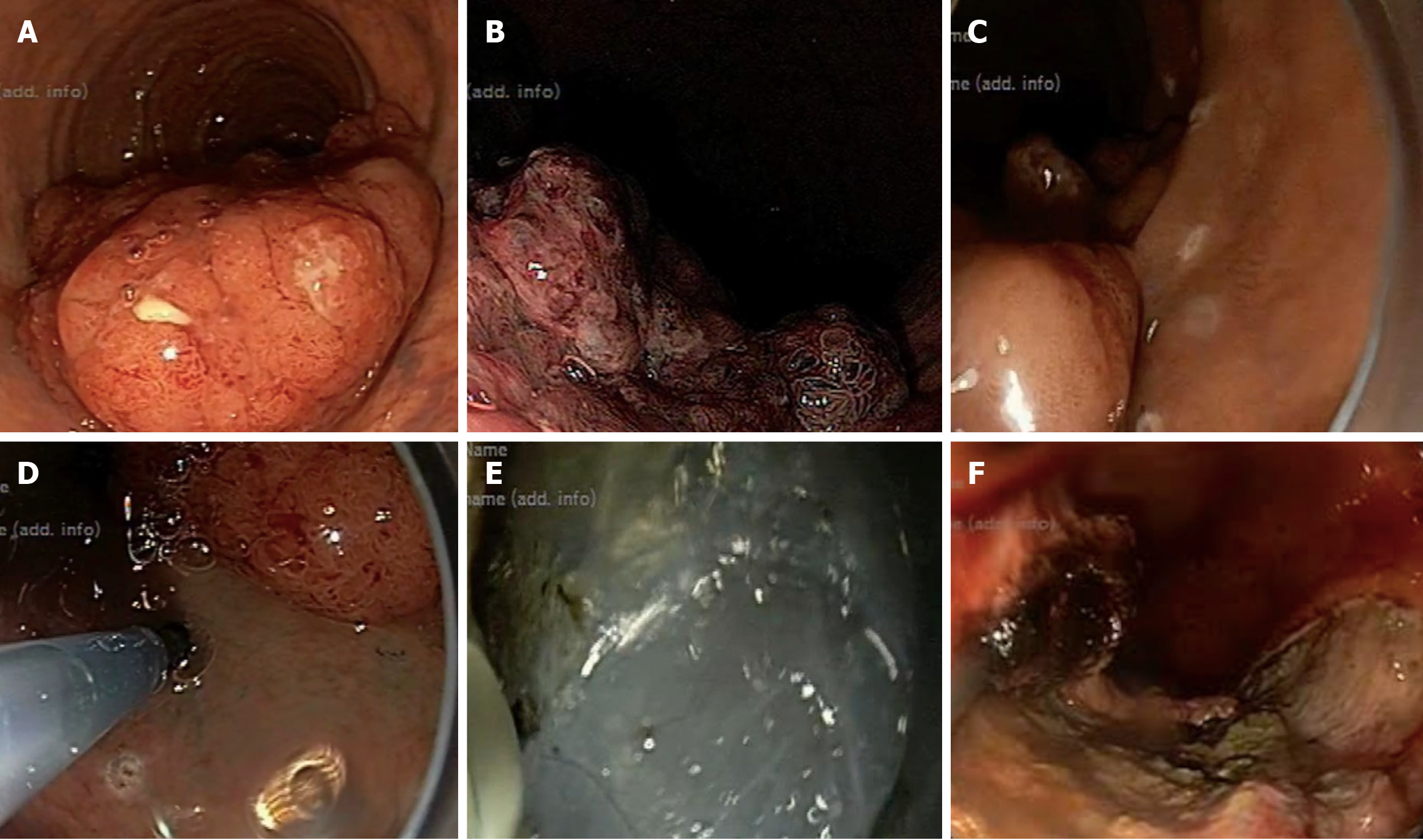
Specialized electrosurgical tools, such as the IT-knife, triangle-tipped knife, and a transparent cap, have been developed to facilitate ESD[4]. ESD comprises three steps: Elevation of lesions from the underlying muscle layer by injecting fluid at the submucosal plane, circumferential cutting of the surrounding mucosa, and subsequent dissection of the connective tissue below the lesion (Figure 1)[5]. It enables complete resection of large lesions as the shape and size of resection can be easily controlled. It also involves submucosal dissection, and is applicable to ulcerative non-lifting neoplasms. These are the salient advantages of ESD over EMR. However, ESD is a time-consuming procedure associated with a higher risk of bleeding and perforation-related complications, and requires the involvement of two or more assistants, unlike EMR. Despite these limitations, ESD has emerged as the standard treatment for early gastric cancer (GC) and esophageal cancer. However, technical challenges hinder its effectiveness for colorectal cancer.
Accurate identification of surface and vascular patterns is essential for distinguishing between adenomatous polyps and carcinoma in situ. There are multiple techniques for detecting lesions with high-grade dysplasia based on endoscopic morphology. The Paris classification was the first system developed for endoscopically classifying neoplastic lesions and remains the most widely used (Table 1). Although initially developed for lesions in the colon, its application has since extended to gastric lesions. The Paris classification of lesions has been shown to correlate with the presence of submucosal invasion (Table 2)[6]. In a study of 479 patients, 0-Is lesions showed a low malignant potential (7.5%), whereas 0-IIc or IIa + IIc lesions had a significantly higher malignant potential (31.8%)[7].
| Endoscopic appearance | Paris class | Description |
| Protruded lesions | Ip | Pedunculated polyps |
| Isp | Semi-pedunculated polyps | |
| Is | Sessile | |
| Flat elevated lesions | 0-IIa | Flat elevation of mucosa |
| 0-IIa/c | Flat elevation with central depression | |
| Flat lesions | 0-IIb | Flat mucosal change |
| 0-IIc | Mucosal depression ≤ 1.2 mm | |
| 0-IIc/IIa | Mucosal depression with raised edge | |
| Excavated lesions | 0-III | Mucosal depressions > 1.2 mm |
| Paris classification | Submucosal invasion, % |
| 0-Ip | 5 |
| 0-Is | 34 |
| 0-IIa | 4 |
| 0-IIb | 0 |
| 0-IIc | 61 |
Narrow band imaging (NBI) is another common endoscopic technique for characterizing colonic lesions. Two NBI classifications, the NBI International Colorectal Endoscopic classification and the Japanese NBI Expert Team (JNET) classification, are currently used to characterize these lesions (Table 3[8] and Table 4). The JNET classification comprises four categories, namely, 1, 2A, 2B, and 3[9]. Distinguishing between type 2B and type 3 Lesions poses a challenge even for experienced endoscopists. As per the existing literature, the JNET classification has a sensitivity of 44.9% to 53.8% for correctly identifying type 2B lesions[10,11].
| Endoscopic appearance | NICE type |
| Lesions with a color similar to the surrounding mucosa may have a net-like vascular pattern or lack a specific vascular pattern | 1 |
| Lesions with a brownish hue with brown vessels surrounding white structures | 2 |
| Lesions with a dark-to-brown appearance, disrupted or missing vessels, and an irregular surface pattern | 3 |
| JNET type | Vessel pattern | Surface pattern | Most likely histology |
| 1 | Invisible | Regular dark or white spots, similar to surrounding normal mucosa | Hyperplastic polyp/sessile serrated polyp |
| 2A | Regular caliber and regular distribution | Regular | Low grade intramucosal neoplasia |
| Tubular/branched/papillary | |||
| 2B | Variable caliber, irregular distribution | Irregular or obscure | High grade intramucosal neoplasia |
| 3 | Loose vessel areas, interruption of thick vessels | Amorphous areas | Deep submucosal invasive cancer |
ESD is an excellent therapeutic option for early-stage esophageal cancer, achieving a high rate of en bloc resection and relatively low local recurrence rate. The outcomes of ESD are significantly better those of EMR. NBI and chromoendoscopy facilitate the detection of early esophageal malignancies. NBI helps detect lesions by highlighting the pattern of small, dot-like vessels and the lesion’s background color compared to the surrounding healthy tissue[12]. The pink color sign, which appears 2-3 minutes after iodine staining, can distinguish between malignant and nonmalignant lesions with a sensitivity and specificity of 88% and 95%, respectively[13].
ESD is a treatment option for esophageal cancers limited to the mucosa’s lamina propria (whether noncircumferential or < 50 μm circumferential), and non-circumferential lesions invading submucosa up to < 200 μm deep[14]. The anatomical characteristics of the esophagus, such as its narrowness, the surrounding structures vertebrae, aorta, and trachea) that can compress it, and its thin muscle layer, make performing a safe ESD quite challenging[15].
In 2005, Oyama et al[16] reported a 95% en bloc resection rate with no perforation or local recurrence and only a 6% incidence of mediastinal emphysema. In a multicenter retrospective cohort study in Japan, ESD led to an en bloc resection rate of 96.7% [95% confidence interval (CI): 94.4%-98.1%] and R0 resection rate of 84.5% (95%CI: 80.5%-87.8%). Perforation, bleeding, esophageal stricture, and postoperative pneumonia occurred in 5.2% (95%CI: 3.3%-7.9%), 0%, 7.1% (95%CI: 4.9%-10.2%), and 1.6% (95%CI: 0.7%-3.5%) of patients, respectively[17].
In a multicenter study, en bloc resection and complete resection rates for esophageal squamous cell carcinoma were 100% and 69.8%, respectively. Postprocedural bleeding and perforation occurred in 4.8% and 1.6% of cases, respectively, whereas stenosis occurred in 23.8% of patients[18]. In another study, ESD achieved a 96.7% R0 resection rate and caused stenosis in 11.5% of patients, with no reports of bleeding or perforation[19].
Perforation: Mediastinal emphysema or mediastinitis can occur from perforation during esophageal ESD, and in severe cases, may rapidly compromise the respiratory and circulatory systems. Intraprocedural perforation during ESD has been reported in about 7% of cases[16,20]. These were successfully managed with clip closure, polyglycolic acid sheets, and fibrin glue. Delayed perforation after esophageal ESD is rare. Five cases of delayed perforation have been reported after esophageal ESD, with a median onset time of 6 days. In these cases, the mucosal defects exceeded half of the esophageal circumference and required invasive treatment including sub-total esophagectomy, empyema drainage, or temporary stent placement[21].
Mediastinal emphysema: Instances of mediastinal emphysema can occur even without perforation and may lead to subcutaneous emphysema in severe cases. Early detection of mediastinal emphysema is possible using imaging techniques, such as X-rays and computed tomography scans within 1 hour of a procedure, with 1.7% and 31% of cases identified, respectively[22]. The majority of these occurrences are minor and do not affect the clinical course. Therefore, routine computed tomography thorax scans are not recommended to detect mediastinal emphysema following uneventful procedures. Additionally, esophageal ESD should always be performed with carbon dioxide, which is quickly absorbed by the body’s tissue, to minimize the risk of mediastinal emphysema[23].
Stricture: Esophageal strictures have been reported in more than 50% of post-ESD defects, especially those involving more than 75% or the entire circumference[24]. A variety of prophylactic approaches for stricture exists, including local steroid injections, oral steroids, temporary stent placement, polyethylene glycolic acid sheets, and cultured oral mucosal epithelial cell sheets[25-27].
Technique: The esophagus has a narrow lumen, making gravity traction difficult. Moreover, ongoing dissection causes the lesion to shift distally, hindering traction and adequate visualization. Thus, improvements in techniques are essential for safe and successful esophageal ESD.
Clip-with-line method: Oyama et al[16] devised the clip-in-line method. Since then, it has been widely used as a simple and effective traction method. In a randomized controlled trial, the median procedure duration for traction-assisted ESD was significantly shorter (45 minutes) compared to conventional ESD (61 minutes) (P < 0.001). Also, traction-assisted ESD was relatively safer with no perforations reported, whereas traditional ESD had a perforation rate of 4.3%[28]. Due to its minimal cost, this method is recommended in Japanese guidelines for the endoscopic treatment of early esophageal cancer.
Tunnel method: For lesions involving more than two-thirds of the circumference, performing conventional ESD is quite difficult and associated with adverse events. The tunnel method offers a more efficient dissection process for large lesions compared to traditional methods, leading to a higher R0 resection rate[29]. In a meta-analysis, the tunnel method was associated with a notable decrease in the amount of time required for dissection during surgery (standardized mean difference = 1.52; 95%CI: 1.09-0.83; P < 0.001), significantly higher R0 rate [odds ratio (OR) = 2.29; 95%CI: 1.54-3.46; P < 0.001] and en bloc resection rate (OR = 3.98; 95%CI: 1.74-9.12; P = 0.001). At the same time, this technique also led to fewer complications such as post-procedure bleeding (OR = 0.38; 95%CI: 0.18-0.83; P = 0.02) and injuries to deeper tissue layers (OR = 0.44; 95%CI: 0.28-0.70; P < 0.001)[30].
Underwater ESD: This technique utilizes a water delivery function to fill the lumen with water via a water jet system. It maintains a good field of view and a wider gap in the mucosal incision space, despite the presence of submucosal fibrosis to some extent. Endotracheal intubation or an overtube is recommended to prevent aspiration[31].
Early GC (EGC) is a type of GC where the tumor invasion is limited to the mucosa or submucosa (classified as T1), regardless of lymph node involvement. The Japanese EGC Association initially proposed ESD as the treatment for nonulcerated well-differentiated EGCs that are confined to the mucosa (T1a) and are smaller than 20 mm, because these lesions have a low risk of spreading to the lymph node[32]. Subsequently, ESD was considered for: (1) Nonulcerated well-differentiated EGCs of any size; (2) Well-differentiated EGCs < 30 mm with superficial submucosal invasion (submucosal first layer; < 500 mm below the muscularis mucosae); or (3) Ulcerated well-differentiated EGCs < 30 mm[33].
A Korean multicenter study reported en bloc resection and complete resection rates of 95.3% and 87.7%, respectively. Incidences of delayed bleeding, significant bleeding, and perforation were 15.6%, 0.6%, and 1.2%, respectively[34]. A Japanese study assessed the outcomes of ESD for patients with EGC with expanded indications, including nonulcerated lesions of any size and ulcerated lesions ≤ 3 cm. The en bloc resection and curative resection rates were 94.9% and 94.7%, respectively. After follow-up for a median period of 30 months (6 months to 89 months), the 5-year overall rate was 94.7% and the disease-specific survival rate was 100%[35].
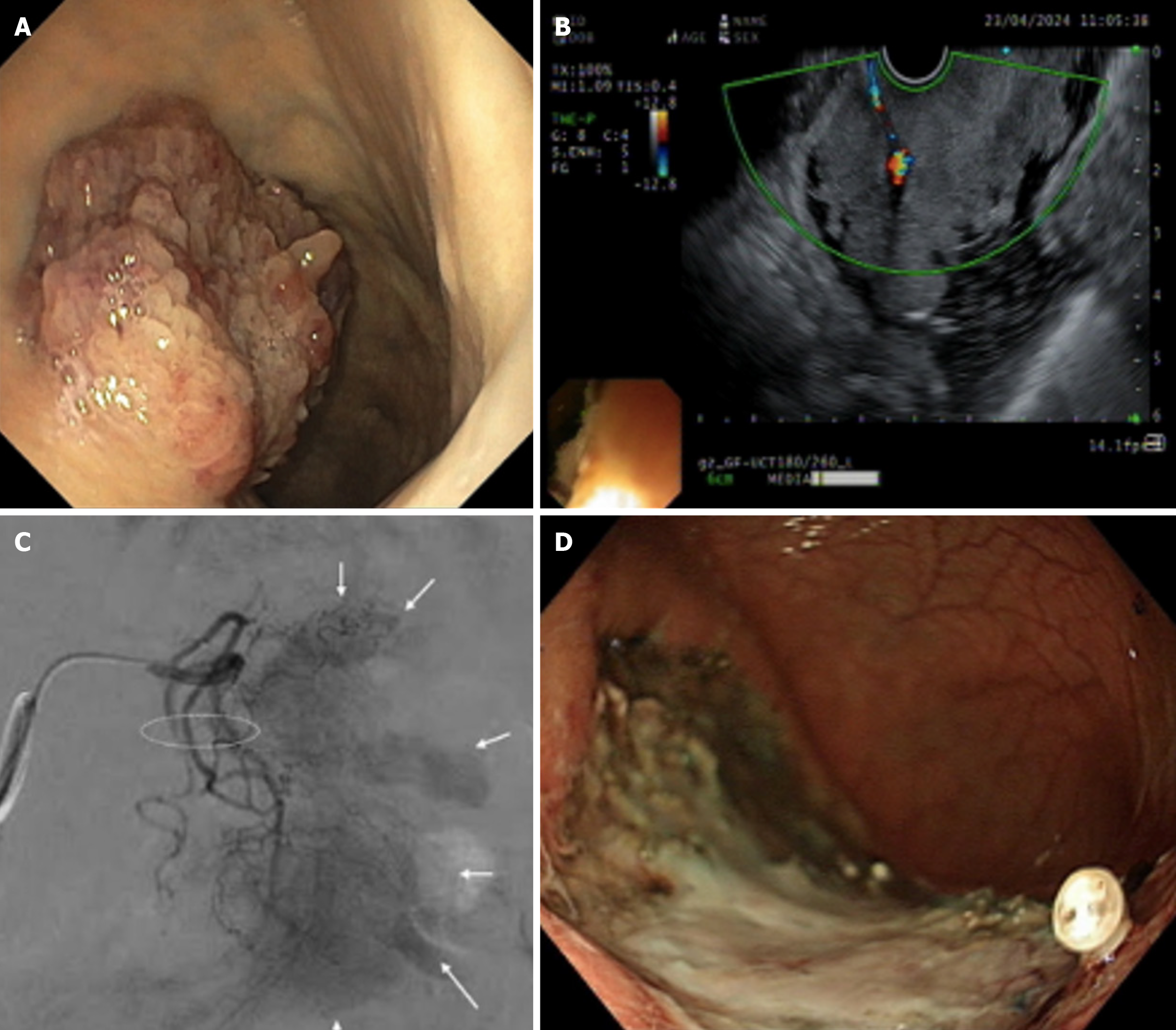
Pain, bleeding, and perforation are some of the common complications associated with ESD[36]. Pain following endoscopic resection is usually mild and well-controlled by antisecretory medications (i.e. proton pump inhibitors and opioids). Bleeding, the most common complication, can be immediate or delayed. Immediate bleeding tends to occur for tumors located in the upper third of the stomach, because many large vessels are located in the vicinity. The rate of immediate bleed ranges from 1.8% to 15.6% according to Korean studies[34,37]. Delayed bleeding is defined as hematemesis or melena within 30 days of procedure and is more likely to occur with lesions in the proximal stomach, those > 40 mm, recurring lesions, and macroscopic flat type lesions[38]. An interesting case report described an elderly woman with a large gastric polypoidal lesion. She underwent pre-procedural superselective embolization of its blood supply, followed by ESD 1 day later. The procedure was performed successfully with no bleeding (Figure 2), and histopathology of the en bloc-resected specimen confirmed intramucosal carcinoma[39], illustrating a novel prophylactic approach for highly vascular lesions.
Minor complications include the development of subsequent strictures and aspiration pneumonia. Strictures are prone to developing at the cardia and pylorus following the resection of lesions that are ≥ 1 cm at the gastroesophageal junction and pylorus, 75% or more circumferential resection, and greater than 5 cm longitudinal dissection. Strictures can be treated by balloon dilatation[40]. Elderly patients are at risk of aspiration pneumonia after ESD. This risk can be minimized by frequently removing gastric fluid and avoiding overdistension of the stomach during the ESD.
Endoscopic screening for adenoma has achieved remarkable success in detecting early-stage colorectal cancer[41]. However, the efficacy of screening depends not only on the adenoma detection rate but also on whether the endoscopic resection is complete. As such, advanced adenomas occur even after polypectomy[42], accounting for 27% of interval cancers at previous polypectomy sites[43]. Moreover, complete polyp resection accounts for up to 19% of interval cancers, which is especially challenging for sessile or flat lesions more than 20 mm. Recurrence rates of about 30% have been reported after piecemeal endoscopic resection[44]. Unlike EMR, ESD is associated with higher en bloc resection and lower recurrence, even for larger, flat, or sessile lesions. ESD is a demanding procedure with several shortcomings, including the need for technical expertise, longer procedure times, and a higher perforation rate.
The 2019 American Gastroenterology Association Clinical Practice Update recommends ESD for colorectal lesions exhibiting: Kudo V-type pit patterns, depressed morphology (Paris 0-IIc), complex architecture (0-Is or 0-IIa + Is), rectosigmoid location, nongranular laterally spreading adenomas up to 20 mm, granular laterally spreading adenomas measuring 30 mm or more, or residual/recurrent colorectal adenomas[45].
A meta-analysis by Fujiya et al[46] showed that ESD was associated with significantly higher odds of achieving curative resection (OR = 4.26) and a lower likelihood of recurrence (OR = 0.08) compared to EMR. The risk of delayed bleeding was similar for both ESD and EMR procedures, with an OR of 0.85; however, the rate of perforation was higher with ESD, showing an OR of 4.96 compared to EMR. In a recent meta-analysis by Zhao et al[47], the en bloc resection rates for ESD and EMR were 95% and 93.2%, respectively. Complete resection rates were much better for ESD than EMR (i.e. 71.9% and 42.8%, respectively), whereas the bleeding rates were similar (4.2% vs 3.5%). Perforation rates were 1.8% and 2.4% for EMR and ESD, respectively. EMR had a significantly higher recurrence rate than ESD (15.9% vs 0.5%)[47]. A retrospective case-controlled study examined 373 colorectal tumors measuring ≥ 20 mm; of these tumors, 145 were treated with ESD and the remaining 228 were treated with EMR. The recurrence rates were 2% and 14% for ESD and EMR, respectively. Perforations occurred in 6.2% of patients undergoing ESD and 1.3% of patients undergoing EMR. Delayed bleeding was reported in 1.4% of patients undergoing ESD and 3.1% of those undergoing EMR[48].
Bleeding and perforation are the two most frequent complications associated with colonic ESD. The risk of intraprocedural bleeding may be minimized by adequately injecting into the submucosal plane, employing meticulous dissection techniques, and proactively coagulating visible vessels. Immediate or delayed bleeding is usually managed with endoscopic hemostasis, and in a few instances, with embolization. Patients with larger lesions (> 40 mm) or proximal colon lesions, those on dual antiplatelet or anticoagulant medications, or those on hemodialysis are at increased risk of post-procedure bleeding. The risk of perforation is higher with associated submucosal fibrosis (OR = 2.9, 95%CI: 1.83-4.59), tumors in the ascending colon and cecum (OR = 2.35, 95%CI: 1.58-3.50), and larger sized tumors (OR = 2.1.7, 95%CI: 1.47-3.21)[49]. In a study by Fujihara et al[50], prophylactic closure of the defect using through-the-scope or over-the-scope clips was performed in 27 of 68 patients who underwent ESD for colorectal tumors. The closure group reported lower incidences of abdominal pain without delayed bleeding or perforation, whereas the nonclosure group had two instances of delayed bleeding and one of perforation.
Esophageal tumors: Performing endoscopic ultrasound (EUS) before ESD is controversial. The overall accuracy for T and N staging by EUS is 90%[51]. A study by Lee et al[52] found EUS to be 86.7% accurate for T1 Lesion determination and 83.3% accurate for lymph node staging. In a study by Pech et al[53] involving 179 patients, EUS accurately identified T stage in 74% of patients, with a sensitivity and specificity of 82% and 91%, respectively. According to Puli et al[54], EUS had 81.6% sensitivity and 99.4% specificity for staging T1 tumors, 81.4% and 96.3% for T2 tumors, 91.4% and 94.4% for T3 tumors, and 92.4% and 97.4% for T4 tumors, respectively, suggesting higher accuracy in staging advanced cancer than early cancer. Initial studies highlighted the crucial role of EUS in selecting suitable candidates for endoscopic resection; however, subsequent research revealed the shortcomings of EUS. Bergeron et al[55] demonstrated that EUS correctly staged tumor depth in 39% of T1a and 51% of T1b tumors. Amid these conflicting reports, the European Society for GI Endoscopy conditionally recommends that EUS be considered for superficial esophageal carcinomas with suspicion of submucosal invasion or lymph node metastases[56].
Conventional endoscopy can accurately evaluate the depth of invasion (mucosa vs submucosa) in early EGC in 72%-84% of cases[57]. There have been inconsistent reports comparing the accuracy of EUS with conventional endoscopy. In a study by Mouri et al[58], the accuracy of EUS in assessing the depth of invasion was 99% for mucosal lesions and 87% for lesions in the mucosa-submucosa junction and extending to the proximal submucosa (submucosal first layer). Lee et al[59] retrospectively studied 393 patients with well-differentiated EGC and concluded that the proportion of patients receiving appropriate treatment was similar between the endoscopy- and EUS-based groups (75.3% vs 71.5%; P = 0.184).
EUS can detect submucosal fibrosis, a condition that often hinders successful ESD. Hirasawa et al[60] analyzed 26 cases of incomplete resection due to severe scarring. The authors considered surgery preferable to ESD for EGC that penetrated > 5 mm into the submucosa. In a study by Kikuchi et al[61], 110 patients with EGC were divided into two groups based on EUS findings: Group P, with few blood vessels in the submucosa or < 4 small vessels per field of view; and group R comprising the remaining patients. Although EUS did not predict the incidence of perforation or exacerbation of anemia, the R group had a significantly longer mean procedure time (105.4 minutes vs 65.5 minutes; P < 0.001) and significantly increased incidence of deeper layer injury and hemoclip usage. Thus, in the absence of a unanimous consensus, the endoscopist can perform a diagnostic EUS before ESD.
EUS has shown varying degrees of success in accurately staging early colorectal cancer, with studies demonstrating a sensitivity range of 57%-91% over the years[62]. One meta-analysis in 2009 revealed that EUS demonstrated a pooled sensitivity and specificity of 87.8% and 98.3% respectively, for detecting T1 rectal lesions[63].
Urban et al[64] concluded that high-frequency ultrasound probes guided the treatment of early colorectal cancers depending upon the endoscopic morphology. Lesions with low-risk morphology could be treated based on their endoscopic appearance. High-frequency probe ultrasonography changed the treatment approach in about 42% of high-risk lesions, specifically those that were depressed or had an invasive pit pattern. However, this technique sometimes led to unnecessary surgery for lesions that could have been handled endoscopically. Thus, a pre-procedure EUS should only be performed when there is suspicion of submucosal fibrosis. According to a study by Makino et al[65], interruption of the third layer by more than 5 mm predicts incomplete ESD, suggesting that surgery is a more appropriate treatment option. Also, EUS is an unreliable tool for nodal staging[51]. Thus, for rectal malignancies, magnetic resonance imaging of the pelvis is routinely conducted before endoscopic resection to rule out lymph node involvement that might necessitate neoadjuvant chemotherapy and surgical resection.
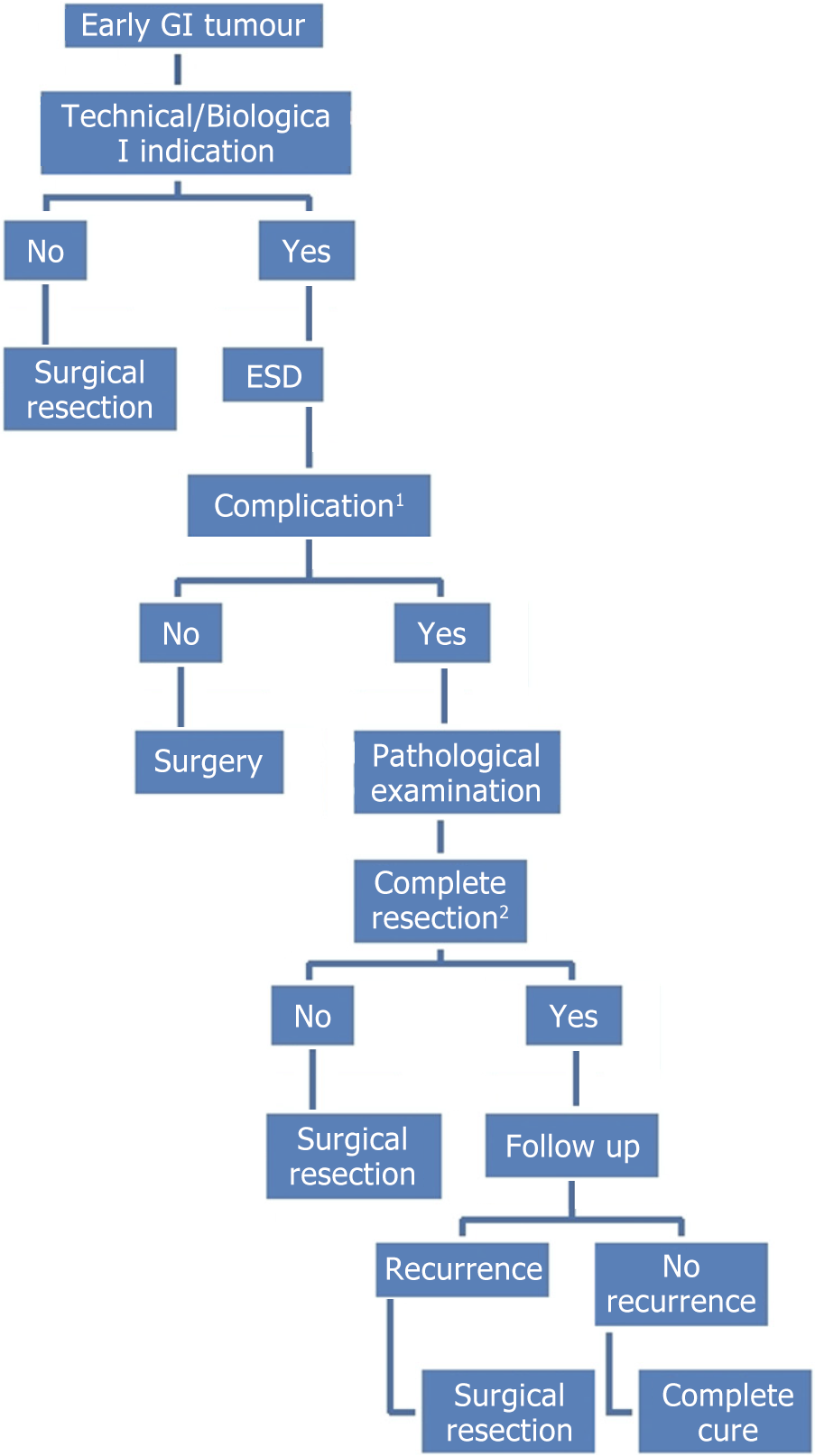
The main advantages of ESD compared to surgery are that it is less invasive, less expensive, and preserves physiological function; however, complete resection is not possible in certain complex cases. Thus, the complete resection rate is not 100%, even in high-volume centers. Subsequent surgery is inevitable in these cases, so a systematic approach is needed to carefully select patients with early GI cancers who can benefit from an endoscopic approach. Additional surgery, including lymph node dissection, is recommended if endoscopic resection of the tumor is not found to be curative after microscopic examination. An ESD is considered noncurative if cancer cells are found at the lateral or deep margins, if the cancer has invaded the deep layer, if lymphatic vessels are invaded, if the cell type is undifferentiated, or if any combination of these factors is present (Figure 3 and Table 5)[66].
| Esophagus | Stomach | Colon and rectum |
| Tumors in contact with or invading the muscularis mucosa | Positive lateral margins | Positive vertical margins at the site of submucosal invasion |
| Tumors invading the submucosal layer | Deep submucosal invasion, regardless of positive vertical margins (> 500 μm) | Vascular or lymphatic invasion |
| Vascular or lymphatic invasion | Depth of submucosal invasion greater than 1000 μm | |
| Diffuse type histology | Vascular or lymphatic invasion |
For endoscopists with sufficient experience, ESD is technically feasible and safe for the resection of early GI cancers. Given the excellent technical and oncologic outcomes, ESD should be considered for lesions meeting the absolute criteria for endoscopic resection. Additionally, ESD may be a decent treatment modality for lesions, fulfilling the expanded number of candidates unfit for extensive surgery. However, most of the publications on ESD outcomes and safety are from Eastern countries, especially Japan. As ESD is an established endoscopic resection in Western countries, additional research will be valuable to explore differences in tumor behavior among patients and risk factors for lymph nodal metastases specific to Western patients.
| 1. | Ferlitsch M, Hassan C, Bisschops R, Bhandari P, Dinis-Ribeiro M, Risio M, Paspatis GA, Moss A, Libânio D, Lorenzo-Zúñiga V, Voiosu AM, Rutter MD, Pellisé M, Moons LMG, Probst A, Awadie H, Amato A, Takeuchi Y, Repici A, Rahmi G, Koecklin HU, Albéniz E, Rockenbauer LM, Waldmann E, Messmann H, Triantafyllou K, Jover R, Gralnek IM, Dekker E, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2024. Endoscopy. 2024;56:516-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 145] [Article Influence: 72.5] [Reference Citation Analysis (1)] |
| 2. | Takenaka R, Kawahara Y, Okada H, Hori K, Inoue M, Kawano S, Tanioka D, Tsuzuki T, Yagi S, Kato J, Uemura M, Ohara N, Yoshino T, Imagawa A, Fujiki S, Takata R, Yamamoto K. Risk factors associated with local recurrence of early gastric cancers after endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 267] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K, Sugano K. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 300] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 5. | Kakushima N, Fujishiro M. Endoscopic submucosal dissection for gastrointestinal neoplasms. World J Gastroenterol. 2008;14:2962-2967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1379] [Article Influence: 60.0] [Reference Citation Analysis (13)] |
| 7. | Moss A, Bourke MJ, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Chen RY, Byth K. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011;140:1909-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 450] [Article Influence: 30.0] [Reference Citation Analysis (1)] |
| 8. | Hayashi N, Tanaka S, Hewett DG, Kaltenbach TR, Sano Y, Ponchon T, Saunders BP, Rex DK, Soetikno RM. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc. 2013;78:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 326] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 9. | Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Kaneko K, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Iwatate M, Ishikawa H, Murakami Y, Yoshida S, Saito Y. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016;28:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 430] [Article Influence: 43.0] [Reference Citation Analysis (1)] |
| 10. | Iwatate M, Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Ishikawa H, Murakami Y, Yoshida S, Saito Y; Japan NBI Expert Team (JNET). Validation study for development of the Japan NBI Expert Team classification of colorectal lesions. Dig Endosc. 2018;30:642-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 11. | Wang Y, Li WK, Wang YD, Liu KL, Wu J. Diagnostic performance of narrow-band imaging international colorectal endoscopic and Japanese narrow-band imaging expert team classification systems for colorectal cancer and precancerous lesions. World J Gastrointest Oncol. 2021;13:58-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Ishihara R, Inoue T, Uedo N, Yamamoto S, Kawada N, Tsujii Y, Kanzaki H, Hanafusa M, Hanaoka N, Takeuchi Y, Higashino K, Iishi H, Tatsuta M, Tomita Y, Ishiguro S. Significance of each narrow-band imaging finding in diagnosing squamous mucosal high-grade neoplasia of the esophagus. J Gastroenterol Hepatol. 2010;25:1410-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Ishihara R, Yamada T, Iishi H, Kato M, Yamamoto S, Yamamoto S, Masuda E, Tatsumi K, Takeuchi Y, Higashino K, Uedo N, Tatsuta M, Ishiguro S. Quantitative analysis of the color change after iodine staining for diagnosing esophageal high-grade intraepithelial neoplasia and invasive cancer. Gastrointest Endosc. 2009;69:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Ishihara R, Arima M, Iizuka T, Oyama T, Katada C, Kato M, Goda K, Goto O, Tanaka K, Yano T, Yoshinaga S, Muto M, Kawakubo H, Fujishiro M, Yoshida M, Fujimoto K, Tajiri H, Inoue H; Japan Gastroenterological Endoscopy Society Guidelines Committee of ESD/EMR for Esophageal Cancer. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig Endosc. 2020;32:452-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 307] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 15. | Okubo Y, Ishihara R. Endoscopic Submucosal Dissection for Esophageal Cancer: Current and Future. Life (Basel). 2023;13:892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 16. | Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 461] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 17. | Tsujii Y, Nishida T, Nishiyama O, Yamamoto K, Kawai N, Yamaguchi S, Yamada T, Yoshio T, Kitamura S, Nakamura T, Nishihara A, Ogiyama H, Nakahara M, Komori M, Kato M, Hayashi Y, Shinzaki S, Iijima H, Michida T, Tsujii M, Takehara T. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal neoplasms: a multicenter retrospective cohort study. Endoscopy. 2015;47:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 18. | Stephant S, Jacques J, Brochard C, Legros R, Lepetit H, Barret M, Lupu A, Rostain F, Rivory J, Ponchon T, Pioche M, Wallenhorst T. High proficiency of esophageal endoscopic submucosal dissection with a "tunnel + clip traction" strategy: a large French multicentric study. Surg Endosc. 2023;37:2359-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Probst A, Ebigbo A, Eser S, Fleischmann C, Schaller T, Märkl B, Schiele S, Geissler B, Müller G, Messmann H. Endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma: long-term follow-up in a Western center. Clin Endosc. 2023;56:55-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 20. | Takahashi H, Arimura Y, Masao H, Okahara S, Tanuma T, Kodaira J, Kagaya H, Shimizu Y, Hokari K, Tsukagoshi H, Shinomura Y, Fujita M. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc. 2010;72:255-264, 264.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 248] [Article Influence: 15.5] [Reference Citation Analysis (4)] |
| 21. | Iwatsubo T, Takeuchi T, Lee SW, Kawaguchi S, Ota K, Kojima Y, Higuchi K. Very Delayed Perforation after Esophageal Endoscopic Submucosal Dissection and Intralesional Triamcinolone Injection. Case Rep Gastroenterol. 2022;16:462-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Tamiya Y, Nakahara K, Kominato K, Serikawa O, Watanabe Y, Tateishi H, Takedatsu H, Toyonaga A, Sata M. Pneumomediastinum is a frequent but minor complication during esophageal endoscopic submucosal dissection. Endoscopy. 2010;42:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Nonaka S, Saito Y, Takisawa H, Kim Y, Kikuchi T, Oda I. Safety of carbon dioxide insufflation for upper gastrointestinal tract endoscopic treatment of patients under deep sedation. Surg Endosc. 2010;24:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy. 2009;41:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 25. | Wang W, Ma Z. Steroid Administration is Effective to Prevent Strictures After Endoscopic Esophageal Submucosal Dissection: A Network Meta-Analysis. Medicine (Baltimore). 2015;94:e1664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Ohki T, Yamato M, Ota M, Takagi R, Murakami D, Kondo M, Sasaki R, Namiki H, Okano T, Yamamoto M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143:582-588.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 362] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 27. | Ye LP, Zheng HH, Mao XL, Zhang Y, Zhou XB, Zhu LH. Complete circular endoscopic resection using submucosal tunnel technique combined with esophageal stent placement for circumferential superficial esophageal lesions. Surg Endosc. 2016;30:1078-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 28. | Koike Y, Hirasawa D, Fujita N, Maeda Y, Ohira T, Harada Y, Suzuki K, Yamagata T, Tanaka M. Usefulness of the thread-traction method in esophageal endoscopic submucosal dissection: randomized controlled trial. Dig Endosc. 2015;27:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Zhai YQ, Li HK, Linghu EQ. Endoscopic submucosal tunnel dissection for large superficial esophageal squamous cell neoplasms. World J Gastroenterol. 2016;22:435-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Zhang T, Zhang H, Zhong F, Wang X. Efficacy of endoscopic submucosal tunnel dissection versus endoscopic submucosal dissection for superficial esophageal neoplastic lesions: a systematic review and meta-analysis. Surg Endosc. 2021;35:52-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Masunaga T, Kato M, Yahagi N. Successful endoscopic submucosal dissection using the water pressure method for cervical esophageal cancer. Dig Endosc. 2021;33:e93-e94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1951] [Article Influence: 216.8] [Reference Citation Analysis (1)] |
| 33. | Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 360] [Article Influence: 72.0] [Reference Citation Analysis (1)] |
| 34. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ, Kim HJ, Kim JJ, Ji SR, Seol SY. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 483] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 35. | Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 528] [Article Influence: 31.1] [Reference Citation Analysis (1)] |
| 36. | Saito I, Tsuji Y, Sakaguchi Y, Niimi K, Ono S, Kodashima S, Yamamichi N, Fujishiro M, Koike K. Complications related to gastric endoscopic submucosal dissection and their managements. Clin Endosc. 2014;47:398-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 37. | Tsuji Y, Ohata K, Ito T, Chiba H, Ohya T, Gunji T, Matsuhashi N. Risk factors for bleeding after endoscopic submucosal dissection for gastric lesions. World J Gastroenterol. 2010;16:2913-2917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Mannen K, Tsunada S, Hara M, Yamaguchi K, Sakata Y, Fujise T, Noda T, Shimoda R, Sakata H, Ogata S, Iwakiri R, Fujimoto K. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J Gastroenterol. 2010;45:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | Pal S, Mohta S, Choudhury SR. Preprocedural arterial embolization as a novel proactive approach for safe endoscopic submucosal dissection. Gastrointest Endosc. 2025;101:466-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy. 2009;41:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Pox CP, Altenhofen L, Brenner H, Theilmeier A, Von Stillfried D, Schmiegel W. Efficacy of a nationwide screening colonoscopy program for colorectal cancer. Gastroenterology. 2012;142:1460-7.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 42. | Martínez ME, Baron JA, Lieberman DA, Schatzkin A, Lanza E, Winawer SJ, Zauber AG, Jiang R, Ahnen DJ, Bond JH, Church TR, Robertson DJ, Smith-Warner SA, Jacobs ET, Alberts DS, Greenberg ER. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 436] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 43. | Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol. 2006;4:1259-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 243] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 44. | Woodward TA, Heckman MG, Cleveland P, De Melo S, Raimondo M, Wallace M. Predictors of complete endoscopic mucosal resection of flat and depressed gastrointestinal neoplasia of the colon. Am J Gastroenterol. 2012;107:650-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Draganov PV, Wang AY, Othman MO, Fukami N. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol. 2019;17:16-25.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 352] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 46. | Fujiya M, Tanaka K, Dokoshi T, Tominaga M, Ueno N, Inaba Y, Ito T, Moriichi K, Kohgo Y. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:583-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 279] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 47. | Zhao HJ, Yin J, Ji CY, Wang X, Wang N. Endoscopic mucosal resection versus endoscopic submucosal dissection for colorectal laterally spreading tumors: a meta-analysis. Rev Esp Enferm Dig. 2020;112:941-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T, Fujii T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 438] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 49. | Kataoka Y, Tsuji Y, Sakaguchi Y, Minatsuki C, Asada-Hirayama I, Niimi K, Ono S, Kodashima S, Yamamichi N, Fujishiro M, Koike K. Bleeding after endoscopic submucosal dissection: Risk factors and preventive methods. World J Gastroenterol. 2016;22:5927-5935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 50. | Fujihara S, Mori H, Kobara H, Nishiyama N, Kobayashi M, Rafiq K, Masaki T. The efficacy and safety of prophylactic closure for a large mucosal defect after colorectal endoscopic submucosal dissection. Oncol Rep. 2013;30:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 51. | Han Y, Sun S, Guo J, Ge N, Wang S, Liu X, Wang G, Hu J, Wang S. Is endoscopic ultrasonography useful for endoscopic submucosal dissection? Endosc Ultrasound. 2016;5:284-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Lee G, I H, Kim SJ, Jeong YJ, Kim IJ, Pak K, Park DY, Kim GH. Clinical implication of PET/MR imaging in preoperative esophageal cancer staging: comparison with PET/CT, endoscopic ultrasonography, and CT. J Nucl Med. 2014;55:1242-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 53. | Pech O, Günter E, Dusemund F, Origer J, Lorenz D, Ell C. Accuracy of endoscopic ultrasound in preoperative staging of esophageal cancer: results from a referral center for early esophageal cancer. Endoscopy. 2010;42:456-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 54. | Puli SR, Reddy JB, Bechtold ML, Antillon D, Ibdah JA, Antillon MR. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol. 2008;14:1479-1490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 243] [Cited by in RCA: 253] [Article Influence: 14.1] [Reference Citation Analysis (2)] |
| 55. | Bergeron EJ, Lin J, Chang AC, Orringer MB, Reddy RM. Endoscopic ultrasound is inadequate to determine which T1/T2 esophageal tumors are candidates for endoluminal therapies. J Thorac Cardiovasc Surg. 2014;147 765-71:Discussion 771-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 56. | Pimentel-Nunes P, Libânio D, Bastiaansen BAJ, Bhandari P, Bisschops R, Bourke MJ, Esposito G, Lemmers A, Maselli R, Messmann H, Pech O, Pioche M, Vieth M, Weusten BLAM, van Hooft JE, Deprez PH, Dinis-Ribeiro M. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2022. Endoscopy. 2022;54:591-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 476] [Article Influence: 119.0] [Reference Citation Analysis (0)] |
| 57. | Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Endoscopic prediction of tumor invasion depth in early gastric cancer. Gastrointest Endosc. 2011;73:917-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 58. | Mouri R, Yoshida S, Tanaka S, Oka S, Yoshihara M, Chayama K. Usefulness of endoscopic ultrasonography in determining the depth of invasion and indication for endoscopic treatment of early gastric cancer. J Clin Gastroenterol. 2009;43:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 59. | Lee JY, Choi IJ, Kim CG, Cho SJ, Kook MC, Ryu KW, Kim YW. Therapeutic Decision-Making Using Endoscopic Ultrasonography in Endoscopic Treatment of Early Gastric Cancer. Gut Liver. 2016;10:42-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Hirasawa D, Maeda Y. Submucosal fibrosis detected by endoscopic ultrasonography may predict incomplete endoscopic submucosal dissection. Dig Endosc. 2015;27 Suppl 1:24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Kikuchi D, Iizuka T, Hoteya S, Yamada A, Furuhata T, Yamashita S, Domon K, Nakamura M, Matsui A, Mitani T, Ogawa O, Kaise M. Prospective Study about the Utility of Endoscopic Ultrasound for Predicting the Safety of Endoscopic Submucosal Dissection in Early Gastric Cancer (T-HOPE 0801). Gastroenterol Res Pract. 2013;2013:329385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 62. | Keihanian T, Othman MO. Colorectal Endoscopic Submucosal Dissection: An Update on Best Practice. Clin Exp Gastroenterol. 2021;14:317-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 63. | Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16:254-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 64. | Urban O, Kliment M, Fojtik P, Falt P, Orhalmi J, Vitek P, Holeczy P. High-frequency ultrasound probe sonography staging for colorectal neoplasia with superficial morphology: its utility and impact on patient management. Surg Endosc. 2011;25:3393-3399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 65. | Makino T, Kanmura S, Sasaki F, Nasu Y, Funakawa K, Tanaka A, Arima S, Nakazawa J, Taguchi H, Hashimoto S, Numata M, Uto H, Tsubouchi H, Ido A. Preoperative classification of submucosal fibrosis in colorectal laterally spreading tumors by endoscopic ultrasonography. Endosc Int Open. 2015;3:E363-E367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Asano M. Endoscopic submucosal dissection and surgical treatment for gastrointestinal cancer. World J Gastrointest Endosc. 2012;4:438-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/