Published online Sep 16, 2025. doi: 10.4253/wjge.v17.i9.109396
Revised: May 30, 2025
Accepted: August 20, 2025
Published online: September 16, 2025
Processing time: 125 Days and 12 Hours
In endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), submucosal lifting agents such as crystalloid-oil emulsion solution (COES) are used for improved effect. Starch-based polysaccharide solution (SPS), which in powder form acts as effective hemostatic agent, are now available as an alter
To compare SPS to COES outcomes as lifting agents in colonic EMR and ESD.
This is a retrospective study of patients who underwent colonic EMR or ESD and received submucosal injection of either SPS or COES at a single academic center from March 2021 to November 2023. A total of 79 patients were included in the COES group and 99 patients in the SPS group from chart review. Intraprocedural bleeding was defined as bleeding during a procedure requiring hemostatic in
Successful resection was achieved in all 178 patients. Average lesion size in SPS group was 2.6 cm vs 2.4 cm in COES group. Average procedure time was 22 minutes shorter in the SPS group (P < 0.05). Intraprocedural bleeding was 24.1% more frequent in COES group (P < 0.01). The 30-day adverse events were 9.37% more frequent in the COES group (P < 0.01). En bloc resection was achieved 22.2% more frequently in patients receiving SPS submucosal injection (P < 0.01).
SPS colonic submucosal injection appears to be beneficial over COES, as it is associated with lower intraprocedural bleeding, less adverse events, shorter procedures, and more frequent en bloc resections.
Core Tip: This retrospective study compares starch-based polysaccharide solution (SPS) and crystalloid-oil emulsion solution (COES) as submucosal lifting agents in colonic endoscopic mucosal resection and endoscopic submucosal dissection. SPS use was associated with shorter procedure times, significantly less intraprocedural bleeding, fewer adverse events, and higher en bloc resection rates compared to COES. These findings support the potential dual role of SPS as both an effective lifting and hemostatic agent.
- Citation: Kahlon S, Alsamman A, Desai J, Urayama S. Unsuspected efficacy of starch-based polysaccharide vs crystalloid-oil emulsion for lifting in colonic endoscopic resections. World J Gastrointest Endosc 2025; 17(9): 109396
- URL: https://www.wjgnet.com/1948-5190/full/v17/i9/109396.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i9.109396
Gastrointestinal (GI) endoscopic resection interventions such as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) commonly utilize submucosal lifting agents to optimize excision of adenomas, superficial cancers, or other GI mucosal lesions[1]. The submucosal cushion allows safe and complete removal of target lesion, decreasing the risk of adverse events such as bleeding or perforation[2]. Traditional application of normal saline and other volume-expanding agents as submucosal injection solutions have been found to dissipate within short periods, which may result in a higher risk for adverse events for prolonged procedures[2]. More recently, crystalloid-oil emulsion solution (COES) such as Eleview® has been shown to produce longer lifts[3]. Randomized control trials comparing Eleview® to normal saline demonstrated that Eleview® required less volume, led to shorter procedure time, and had fewer resection pieces during EMR[4].
Starch-based polysaccharide solution (SPS) such as EndoClot® has now become available as an alternative submucosal lifting agent[3]. Preliminary data has shown that it led to longer lasting and higher lift to improve mucosal separation for easier dissection[5]. While no human studies have compared SPS to other lifting agents, in vivo studies in pig tissue have shown that submucosal injection with SPS results in prolonged lift and reduced incidence of bleeding or perforation of the tissue[5]. A recent clinical trial by Lee et al[6] has demonstrated that a novel lifting agent MC-003 which is a sodium alginate solution, a polysaccharide derived from brown algae, has outperformed normal saline for gastric ESD procedures[7]. As novel lifting agents continue to emerge, it is important to clarify the efficacy of SPS amongst other readily available lifting agents.
Compared to other submucosal injection solutions, SPS may also lead to reducing the risks of bleeding along with thermal injury and perforation while facilitating en bloc resection[1,5]. Starch-based polysaccharide (SP) in powder form had been developed initially as a hemostatic spray, readily absorbing water from blood and concentrating clotting components to promote coagulation, functioning as a procoagulant scaffold when exposed to blood[7,8]. Although the powder form is easily washed away without exposure to blood, locally contained SP in gel form, when utilized as a submucosal lifting agent, has a potential for further hemostatic effect from procoagulant scaffolding activity[9]. In this regard, data comparing the potential coagulating effect of SPS to other lifting agents, including COES, is lacking as the previous studies focus on the applicability and the lifting characteristics of different agents in vivo and ex vivo animal studies[5]. Current study focuses on such a gap in the pertinent comparison between commonly utilized SPS and COES amongst a cohort of patients.
Thus, in the current study, we hypothesized that SPS submucosal injection is associated with less intraprocedural bleeding and thus less overall adverse events, and shorter procedural duration in comparison to COES in colonic EMR and ESD. Outcomes of patients who received SPS submucosal lifting agent during a EMR or ESD procedure for polypectomy during colonoscopy are compared to outcomes of patients who received a COES agent.
This is a retrospective study of consecutive patients who underwent EMR or ESD using SPS or COES for submucosal lift between March 2021 to November 2023 at a tertiary academic medical center. This study was approved by the Institutional Review Board of the University of California, Davis, in accordance with its ethical standards, No. 1982596-1.
One hundred eighty-four patients were identified via electronic medical record to have undergone EMR or ESD procedure during colonoscopy with SPS or COES for submucosal lift during the study period. Patients without electronic documentation of EMR or ESD procedure reports were excluded from the study. Endoscopic procedures for all included patients were performed: By one advanced fellow at a tertiary medical center under the supervision and operation of single attending physician, an experienced endoscopist. From this population, patients were ascertained who met the inclusion criteria of age over 18 years, have at least one nonpedunculated polyp on index endoscopy, have at least one nonpedunculated polyp on endoscopic procedure for resection or dissection, received submucosal injection solution intra-procedurally for polypectomy, underwent successful resection or dissection with pathologic specimen, and received a pathologic diagnosis. Polyp size was classified with a cutoff of 2 cm in diameter[10]. Exclusion criteria were, no polyp identified on endoscopy for resection or dissection, submucosal injection not performed, unsuccessful resection consisting of no pathologic specimen sent for analysis, and no pathologic diagnosis determined. One hundred and seventy-eight patients were included in this study and segregated into two groups based on submucosal injection solution utilized. There were 79 patients included in the COES group and 99 patients in the SPS group.
Patient characteristics and clinical data were extracted from chart review including age, sex, endoscopic procedures performed (colonoscopy or flexible sigmoidoscopy). Written procedure reports were used to extract data for the following characteristics: (1) Resection technique performed (EMR or ESD); (2) Polyp location and size; (3) Submucosal lifting agent used (SPS or COES); (4) Quality of lift; (5) Polypectomy technique; (6) Presence of intraprocedural bleeding requiring intervention; (7) Intervention performed for bleeding control; (8) Post-polypectomy defect closure required; (9) Post-polypectomy mucosal findings; (10) Duration of procedure; and (11) Procedural complications. Interventions for intraprocedural bleeding included the use of snare tip coagulation, argon plasma coagulation, and/or coagulation graspers. Pathologic diagnosis was extracted from the final pathology report. Adverse events within 30 days of procedure were extracted from the follow-up chart review.
IBM Statistical Package for the Social Sciences Statistics (version 26) was used for all statistical analysis. Frequencies and percentages were calculated for all nominal and ordinal variables. Differences in optimal procedural techniques, procedural complications, and adverse events were compared and tested for significance by χ2 test for comparing proportions. Subgroup analysis was also conducted for differences across the same outcomes. P < 0.05 was considered si
Ninety-nine patients were included in the SPS group and 79 patients in the COES group. Table 1 summarizes the demographics and clinical outcomes of all patients. Study subjects included 52.5% and 56.9% males in the SPS and COES group, respectively. The average age of subjects was 66 in the SPS and 68 in the COES group. In both groups, pathology mostly showed tubular, tubulovillous adenomas, or sessile serrated adenomas.
| Outcome | Submucosal injection group | |||
| Starch-based polysaccharide solution group | Crystalloid-oil emulsion solution group | t-test/χ2 statistic | P value | |
| Gender | ||||
| Males | 52/99 (52.5) | 45/79 (56.9) | 0.34 | 0.55 |
| Females | 47/99 (47.4) | 34/79 (43.0) | ||
| Age | ||||
| Mean age | 66 | 68 | 2.69 (t-test) | 0.32 |
| Pathologic diagnosis | ||||
| Adenoma | 44/99 (44.4) | 43/79 (54.4) | 1.75 | 0.41 |
| Sessile serrated adenoma/hyperplastic polyp | 46/99 (46.4) | 30/79 (37.9) | ||
| Adenocarcinoma | 9/99 (9.1) | 6/79 (7.6) | ||
In the SPS group, 39.4% of polyps were in the ascending colon and 16.2% were in the transverse colon. In the COES group, 22.7% of polyps were in the descending colon while 19.0% were in the ascending colon. For both groups, more polyps were similarly resected in the right-sided colon: 63.6% in the SPS group and 53.1% in the COES group (P = 0.16).
EMR was conducted more frequently compared to ESD in both groups (75.7% in SPS group and 92.4% in COES group, P < 0.01). The quality of lift was most frequently described as “adequate” in both groups; however, this outcome was less consistently reported. The 32.3% and 10.1% of polyps were resected en bloc in the SPS and COES groups, respectively (P < 0.01). Intraprocedural bleeding requiring intervention occurred more commonly in the COES group (44.3% of cases) compared to the SPS group (20.2% of cases) (P < 0.01) (Table 2).
| Outcome | Submucosal injection group | |||
| Starch-based polysaccharide solution group | Crystalloid-oil emulsion solution group | χ2 statistic | P value | |
| Polypectomy technique | ||||
| Endoscopic mucosal resection | 75/99 (75.7) | 73/79 (92.4) | 8.69 | < 0.01 |
| Endoscopic submucosal dissection | 24/99 (24.2) | 6/79 (7.6) | ||
| Polyp location | ||||
| Right colon | 63/99 (63.6) | 42/79 (53.2) | 1.99 | 0.16 |
| Left colon | 36/99 (36.3) | 37/79 (46.8) | ||
| Polyp size | ||||
| < 2 cm | 28/99 (28.2) | 26/79 (32.9) | 3.21 | 0.20 |
| 2-4 cm | 45/99 (45.5) | 41/79 (51.9) | ||
| ≥ 4 cm | 26/99 (26.2) | 12/79 (15.2) | ||
| Quality of lift | ||||
| Adequate | 71/99 (71.7) | 57/79 (72.2) | 1.30 | 0.52 |
| Not adequate | 12/99 (12.1) | 13/79 (16.5) | ||
| Not reported | 16/99 (16.6) | 9/79 (11.4) | ||
| Polypectomy resection | ||||
| En bloc | 32/99 (32.3) | 8/79 (10.1) | 12.43 | < 0.01 |
| Piecemeal | 67/99 (67.7) | 71/79 (89.9) | ||
| Intraprocedural bleeding requiring intervention | ||||
| Yes | 20/99 (20.2) | 35/79 (44.3) | 11.95 | < 0.01 |
| No | 79/99 (79.8) | 44/79 (55.7) | ||
| 30-day adverse events | ||||
| Rectal bleeding | 2/99 (2.0) | 7/79 (8.9) | 6.65 | < 0.01 |
| Bowel perforation | 0/99 (0.0) | 2/79 (2.5) | ||
In assessing 30-day adverse events post-polypectomy, 11.4% of cases in the COES group had adverse events while these only occurred 2.0% of cases in the SPS group (P < 0.01). Two 30-day adverse events occurred in the SPS group. Both were incidental episodes of rectal bleeding that did not warrant admission. In the COES group, there were nine adverse events, with six delayed bleeding and three bowel perforations.
The average duration of procedures in the SPS group were significantly shorter at 96 minutes compared to 117 minutes in the COES group (t = 2.76, P < 0.01). This comparison is summarized in Table 3 below.
| Outcome | Starch-based polysaccharide solution group | Crystalloid-oil emulsion solution group | t-test/χ2 statistic | P value |
| Average duration of procedure (minute) | 96 ± 45.18 | 117 ± 57.57 | 2.76 (t-test) | < 0.01 |
| Intraprocedural bleeding requiring intervention | 10/99 (10.1) | 35/79 (44.3) | 27.21 | < 0.01 |
| En bloc resection | 32/99 (32.3) | 8/79 (10.1) | 12.43 | < 0.01 |
| 30-day adverse events | 2/99 (2.0) | 9/79 (11.4) | 6.66 | 0.01 |
For contiguous data normality testing using the Shapiro-Wilk method was conducted. For a sample size of 178 age values, the W statistic was 0.982, corresponding to P > 0.05 and consistent with a normal distribution. Thus, average ages for the study population were compared using t-test. For a sample size of 178 procedure duration measurements, the W statistic was 0.993, corresponding to P > 0.05 and consistent with a normal distribution. Thus, average duration of procedures between SPS and COES groups were compared using t-test.
Table 4 shows the results of χ2 tests to compare the differences in intraprocedural bleeding, en bloc resection, and 30-day adverse effects between the two groups. Intraprocedural bleeding occurred significantly more in COES group during EMR and ESD (P < 0.01). En bloc resection was achieved significantly more in SPS group (P < 0.01). The 30-day adverse events were more frequent in the COES group at a significant rate (P = 0.01).
| Occurrences and frequencies of measured outcomes | Submucosal injection group: Right-sided colon | |||
| Starch-based polysaccharide solution | Crystalloid-oil emulsion solution | χ2 statistic | P value | |
| Any size | ||||
| Intraprocedural bleeding requiring intervention | 8/63 (12.7) | 15/42 (35.7) | 7.80 | < 0.01 |
| En bloc resection | 21/63 (33.3) | 6/42 (14.3) | 4.79 | 0.02 |
| Adverse events | 0/63 (0.0) | 3/42 (7.1) | NS | NS |
| < 2 cm | ||||
| Intraprocedural bleeding requiring intervention | 0/16 (0.0) | 0/17 (0.00) | NS | NS |
| En bloc resection | 1/16 (6.3) | 4/17 (23.5) | 1.91 | 0.16 |
| Adverse events | 0/16 (0.0) | 1/17 (5.9) | NS | NS |
| 2-4 cm | ||||
| Intraprocedural bleeding requiring intervention | 8/47 (17.0) | 15/24 (62.5) | 15.00 | < 0.01 |
| En bloc resection | 20/47 (42.6) | 2/24 (8.3) | 8.69 | < 0.01 |
| Adverse events | 0/47 (0.0) | 2/24 (8.3) | NS | NS |
| ≥ 4 cm | ||||
| Intraprocedural bleeding requiring intervention | 3/17 (17.7) | 4/5 (80.0) | 6.92 | < 0.01 |
| En bloc resection | 10/17 (58.8) | 1/5 (20.0) | 2.33 | 0.13 |
| Adverse events | 0/17 (0.0) | 0/5 (0.0) | NS | NS |
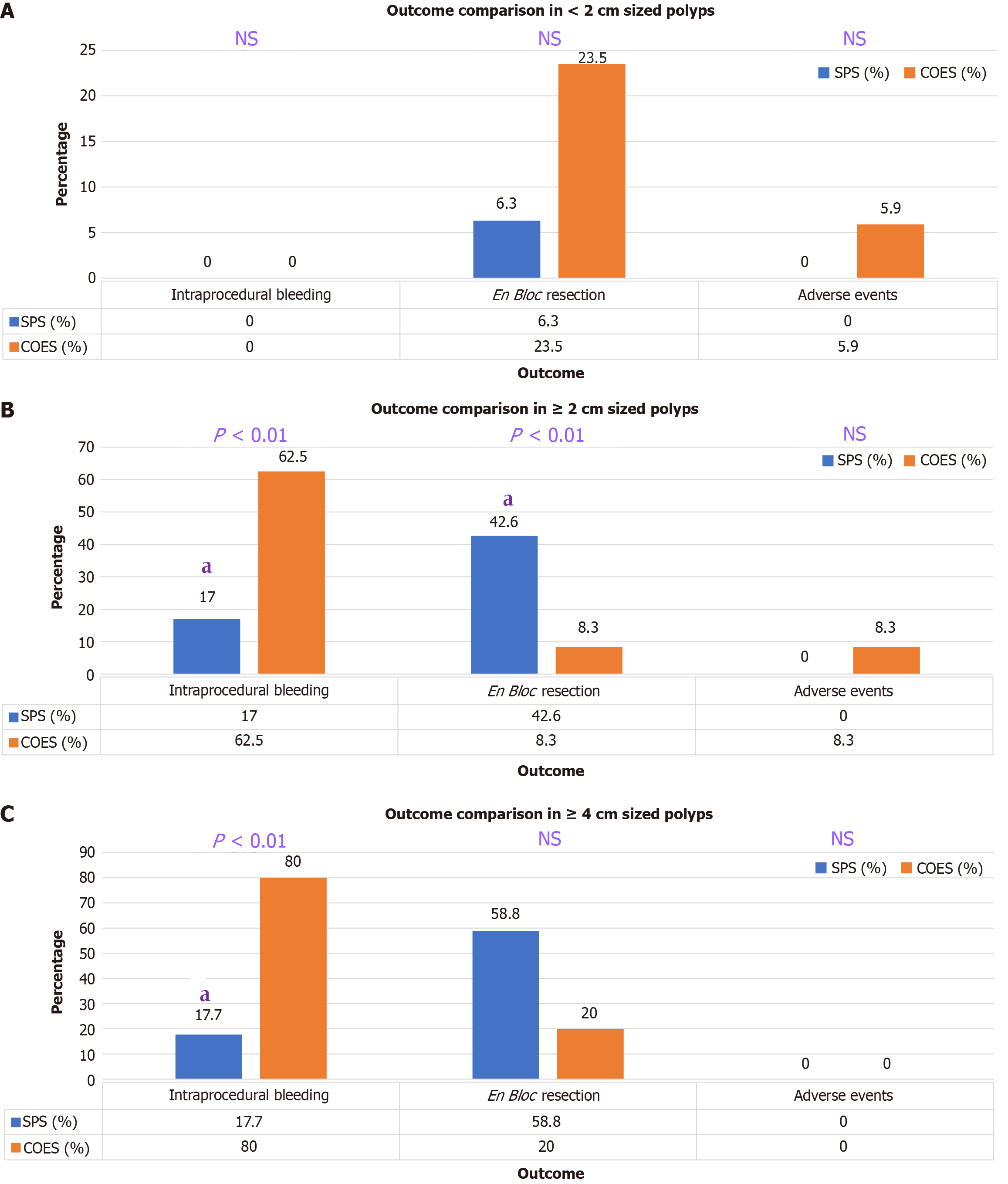
Table 5 and Figure 1 show results from subgroup analysis for polyps limited to the right-sided colon (proximal to splenic flexure) stratified by polyp size. For all polyps in the right colon, 35.7% of patients in the COES group experienced intraprocedural bleeding compared to 12.7% in the SPS group (P < 0.01). All lesions in the right colon were resected via en bloc resection 19% more often with SPS (P = 0.02). Large polyps (2 cm) in the right colon also had 45.5% more intraprocedural bleeding with COES injection compared to SPS (P < 0.01). In the right colon, lesions 2 cm were significantly more often resected en bloc with SPS injection by 34.3% (P < 0.01). All 30-day adverse events in the right-sided colon procedures occurred in the COES group (7.1% of COES cases) while no cases in the SPS had any adverse events.
| Occurrences and frequencies of measured outcomes | Submucosal injection group: Right-sided colon | |||
| Starch-based polysaccharide solution | Crystalloid-oil emulsion solution | χ2 statistic | P value | |
| Any size | ||||
| Intraprocedural bleeding requiring intervention | 8/63 (12.7) | 15/42 (35.7) | 7.80 | < 0.01 |
| En bloc resection | 21/63 (33.3) | 6/42 (14.3) | 4.79 | 0.02 |
| Adverse events | 0/63 (0.0) | 3/42 (7.1) | NS | NS |
| < 2 cm | ||||
| Intraprocedural bleeding requiring intervention | 0/16 (0.0) | 0/17 (0.00) | NS | NS |
| En bloc resection | 1/16 (6.3) | 4/17 (23.5) | 1.91 | 0.16 |
| Adverse events | 0/16 (0.0) | 1/17 (5.9) | NS | NS |
| 2-4 cm | ||||
| Intraprocedural bleeding requiring intervention | 8/47 (17.0) | 15/24 (62.5) | 15.00 | < 0.01 |
| En bloc resection | 20/47 (42.6) | 2/24 (8.3) | 8.69 | < 0.01 |
| Adverse events | 0/47 (0.0) | 2/24 (8.3) | NS | NS |
| ≥ 4 cm | ||||
| Intraprocedural bleeding requiring intervention | 3/17 (17.7) | 4/5 (80.0) | 6.92 | < 0.01 |
| En bloc resection | 10/17 (58.8) | 1/5 (20.0) | 2.33 | 0.13 |
| Adverse events | 0/17 (0.0) | 0/5 (0.0) | NS | NS |
Both in vitro and in vivo studies have validated a SPS as an effective lifting agent with a potentially safer profile compared to crystalloid-oil emulsion injection solution[5]. These benefits may be attributable to sustained lift given SPSs’ higher viscosity[8,11]. The SPS injection contrasts with SP hemostatic spray, which have the same chemical composition and strong pro-coagulant properties in presence of blood but are nonaggregating and typically washed away in its absence. SPS for submucosal injection exists as a gel, which once injected into a submucosal space, it remains in the confined place even in the absence of blood. Thus, SPS is readily available for potentially promoting coagulation in the locale by the gel polymer matrix which could continue to absorb water and/or act as scaffolding substance for aggregating coagulation components[12].
Initially, intraprocedural bleeding requiring intervention was examined in our study and compared between the two injectable solutions. Injection with a SPS yielded intraprocedural bleeding 34.2% less often (P < 0.01) when examining all cases in the study. In further subgroup analysis, lesions 2 cm and 4 cm had significantly reduced rates of intraprocedural bleeding with the SPS submucosal injection used. All right-sided colon lesions, as well as the right colon lesions 2 cm subset, and lesions 4 cm also had significantly reduced rates of intraprocedural bleeding in SPS group. The 30-day adverse event, we found, was significantly reduced in SPS group.
While intraprocedural bleeding may be seen as a technical interference to the procedure rather than a true compli
Amongst all procedures, the SPS group had a significantly shorter average length of procedure time compared to the COES group (96 minutes compared to 117 minutes) (P < 0.01). This difference was still observed despite the increased number of ESD procedures in the SPS group. In general, ESD procedures are more time-intensive when compared to EMR given the breadth of dissection and resources required[16]. And from a practical standpoint, prolonged procedures may influence the final management of the endoscopic approach taken for each case. The faster procedure time with SPS may reflect the ability of sustained lifting and the benefit of reduced intraprocedural complications when using the solution[5].
We observed that rates of en bloc 2 cm and 4 cm, there was additional significantly increased frequencies of en bloc 2 cm in the right-sided colon. In certain cases, en bloc resection is preferred to piecemeal resection given the increased risk of recurrence with the latter technique, especially in large polyps[17]. Metanalysis has revealed local recurrence after EMR of nonpedunculated colorectal lesions occurs in 3% of en bloc resections and in 20% of piecemeal resections[18]. Recent comparative studies have shown recurrence after piecemeal resection to range from 15% to 30%[17,19]. Adverse events occurred more frequently in the COES group across all procedures (P = 0.01). There were only two 30-day adverse events in the SPS group; both were incidental episodes of rectal bleeding that did not warrant admission. However, the COES group had nine adverse events, among those six delayed bleeding; and three bowel perforations that led to admission and surgery. Both EMR and ESD run the risk of bowel perforation depending on a multitude of factors, with ESD being particularly more prone to the complication given the procedural difficulty and deeper tissue involvement[14]. Other studies have found that the risk factors for perforation included lesions 2 cm, piecemeal resection, and ESD[19,20]. Thus, it is further relevant that SPS injection led to significantly less adverse events in all lesions 2 cm.
Some of the limitations of the study include the small sample size and this study being a retrospective one with potential introduction of selection bias. Quality of lift was an outcome measured in this study; however, procedure reports detailed the difficulty with obtaining adequate lifting due to prior resections, scar tissue, or residual polyp. Given the variability, no objective measurement of lift quality was made in this study.
While SPS is positioned as a viable option, its relative cost must be considered. No studies to date have assessed differences in procedural cost based on choice of submucosal lifting agent. Although the current SPS option is lower in cost compared to COES at our institution, future studies analyzing the cost differences of the different agents would be beneficial, particularly if the upfront cost of a certain lifting agent may be offset by shortened procedure duration and improved outcomes.
Further areas of research would assess the outcomes discussed in a prospective, high-powered study to further compare these submucosal lifting agents. Randomized control studies assigning patients to different submucosal injection solutions would improve the generalizability of any findings.
Submucosal injection with a SPS is a promising intraprocedural tool that may lead to reduced intraprocedural complications via its submucosal lifting characteristics as well as its potential hemostatic effect. Additionally, these solutions may bolster complete resection and reduces the risk of recurrence of nonpedunculated colonic lesions. Continued investigations are warranted to determine the ideal submucosal injection solution that is inexpensive, with adequate density, ease in administration, long-lasting lift, and reduces the likelihood of adverse events. The search for an ideal submucosal injection solution is in its evolution. However, SPS is a promising substrate in the current tool set.
| 1. | Castro R, Libânio D, Pita I, Dinis-Ribeiro M. Solutions for submucosal injection: What to choose and how to do it. World J Gastroenterol. 2019;25:777-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (5)] |
| 2. | Uraoka T, Saito Y, Yamamoto K, Fujii T. Submucosal injection solution for gastrointestinal tract endoscopic mucosal resection and endoscopic submucosal dissection. Drug Des Devel Ther. 2009;2:131-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Giannino V, Salandin L, Macelloni C, Longo LM. Evaluation of Eleview(®) Bioadhesive Properties and Cushion-Forming Ability. Polymers (Basel). 2020;12:346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 4. | Repici A, Wallace M, Sharma P, Bhandari P, Lollo G, Maselli R, Hassan C, Rex DK. A novel submucosal injection solution for endoscopic resection of large colorectal lesions: a randomized, double-blind trial. Gastrointest Endosc. 2018;88:527-535.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Dai MS, Hu KW, Wu W, Yin GJ, Hu DM. EndoClot(®)SIS Polysaccharide Injection as a Submucosal Fluid Cushion for Endoscopic Mucosal Therapies: Results of Ex Vivo and In Vivo Studies. Dig Dis Sci. 2019;64:2955-2964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Lee AY, Jang JY, Seo JY, Kim SH, Choi JM, Cho JY. Efficacy and safety of MC-003 solution for endoscopic mucosal or submucosal resection: a prospective, multicenter, randomized, triple-blinded, parallel-group, phase III study. Gastrointest Endosc. 2024;100:36-45.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Coronel E. A new kid on the "bloc"? A case for another submucosal injection agent for endoscopic resection of early gastric neoplasms. Gastrointest Endosc. 2024;100:46-48. [PubMed] [DOI] [Full Text] |
| 8. | Chandrasinghe PC, De Silva A, Deen KI. Novel use of Absorbable Modified Polymer (AMP®); EndoClot™ as an adjunct in the management of bleeding from the liver bed during laparoscopic cholecystectomy. Springerplus. 2015;4:249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Bustamante-Balén M, Plumé G. Role of hemostatic powders in the endoscopic management of gastrointestinal bleeding. World J Gastrointest Pathophysiol. 2014;5:284-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 10. | Markarian E, Fung BM, Girotra M, Tabibian JH. Large polyps: Pearls for the referring and receiving endoscopist. World J Gastrointest Endosc. 2021;13:638-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 11. | Gostout CJ. Ode to the submucosal fluid cushion. Endoscopy. 2004;36:638-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Beg S, Al-Bakir I, Bhuva M, Patel J, Fullard M, Leahy A. Early clinical experience of the safety and efficacy of EndoClot in the management of non-variceal upper gastrointestinal bleeding. Endosc Int Open. 2015;3:E605-E609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Burgess NG, Metz AJ, Williams SJ, Singh R, Tam W, Hourigan LF, Zanati SA, Brown GJ, Sonson R, Bourke MJ. Risk factors for intraprocedural and clinically significant delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol. 2014;12:651-61.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 14. | Bahin FF, Rasouli KN, Byth K, Hourigan LF, Singh R, Brown GJ, Zanati SA, Moss A, Raftopoulos S, Williams SJ, Bourke MJ. Prediction of Clinically Significant Bleeding Following Wide-Field Endoscopic Resection of Large Sessile and Laterally Spreading Colorectal Lesions: A Clinical Risk Score. Am J Gastroenterol. 2016;111:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 15. | Liu C, Wu R, Sun X, Tao C, Liu Z. Risk factors for delayed hemorrhage after colonoscopic postpolypectomy: Polyp size and operative modality. JGH Open. 2019;3:61-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Rashid MU, Alomari M, Afraz S, Erim T. EMR and ESD: Indications, techniques and results. Surg Oncol. 2022;43:101742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Knabe M, Pohl J, Gerges C, Ell C, Neuhaus H, Schumacher B. Standardized long-term follow-up after endoscopic resection of large, nonpedunculated colorectal lesions: a prospective two-center study. Am J Gastroenterol. 2014;109:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy. 2014;46:388-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 292] [Article Influence: 24.3] [Reference Citation Analysis (2)] |
| 19. | Briedigkeit A, Sultanie O, Sido B, Dumoulin FL. Endoscopic mucosal resection of colorectal adenomas > 20 mm: Risk factors for recurrence. World J Gastrointest Endosc. 2016;8:276-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Arezzo A, Passera R, Marchese N, Galloro G, Manta R, Cirocchi R. Systematic review and meta-analysis of endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal lesions. United European Gastroenterol J. 2016;4:18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/