Published online Oct 16, 2025. doi: 10.4253/wjge.v17.i10.110116
Revised: June 17, 2025
Accepted: September 18, 2025
Published online: October 16, 2025
Processing time: 140 Days and 4.5 Hours
The incidence and mortality of colorectal cancer continue to rise. For early-stage colorectal cancer, endoscopic resection has become a preferred or important treatment option due to its significant advantages in operative time, extent of trauma, and medical costs. However, increasing lesion diameter significantly elevates the technical difficulty of endoscopic resection. Currently, robust evi
To evaluate the efficacy and safety of endoscopic resection for colorectal lesions ≥ 30 mm in diameter.
This retrospective study reviewed data from 102 patients who underwent en
Among 102 patients who underwent endoscopic resection, 99 received endoscopic submucosal dissection and 3 underwent endoscopic full-thickness resection. Four patients (3.9%) required conversion to surgical radical resection postoperatively. All patients exhibited favorable wound healing at the resection sites, and no long-term complications were observed during the 3-month postoperative colonoscopy follow-up. The primary perioperative complication was post-endoscopic submucosal dissection electrocoagulation syndrome (PEECS) (24/102, 23.5%). Multivariate analysis identified lesion location in the transverse colon as an independent risk factor for PEECS occurrence (odds ratio = 6.734, 95% confidence interval: 1.623-27.945, P = 0.009).
Large colorectal lesion diameter does not constitute an absolute contraindication to endoscopic resection. Experienced endoscopic centers can achieve complete resection with a favorable efficacy and safety profile. Notably, lesion location in the transverse colon is identified as an independent risk factor for PEECS.
Core Tip: This single-center retrospective study evaluated the efficacy and safety of endoscopic resection for large colorectal lesions. Results demonstrated that lesion diameter does not constitute an absolute contraindication to endoscopic therapy, with complete resection achievable in experienced centers. Critically, lesion location in the transverse colon was identified as an independent risk factor for post-endoscopic submucosal dissection electrocoagulation syndrome.
- Citation: Zhu WW, Yang X, Yang Z, Liu J, Jia W, Chen XL, Tian Y, Gao TJ, Sun GY, Zhang M, Liu CH, Yu JY, Huo JF, Zhao HN. Endoscopic treatment of large colorectal lesions: A retrospective analysis of efficacy and safety. World J Gastrointest Endosc 2025; 17(10): 110116
- URL: https://www.wjgnet.com/1948-5190/full/v17/i10/110116.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i10.110116
Advancements in endoscopic diagnostic and therapeutic techniques have significantly improved the detection rates of colorectal cancer and its precancerous lesions. As a prevalent malignancy in China, colorectal cancer exhibits persistently increasing incidence and mortality rates. According to the latest data from the National Cancer Center of China, there were 517100 new colorectal cancer cases in 2022, ranking second among all malignancies (first among gastrointestinal tumors). Correspondingly, 240000 deaths were recorded, positioning it as the fourth leading cause of cancer-related mortality[1]. The selection of endoscopic treatment strategies for colorectal lesions is determined by lesion diameter. According to the latest endoscopic management consensus: For diminutive lesions with a diameter of less than 5 mm, snare resection or biopsy forceps removal is recommended; for small lesions with a diameter of 6-9 mm, snarectomy, especially cold snare resection, is recommended; for elevated lesions > 10 mm in diameter, we recommend using appropriate loopers according to the characteristics of the tip; for lesions > 20 mm in diameter, we recommend using endoscopic submucosal dissection (ESD)[2].
A cardinal advantage of ESD is the achievement of complete resection (R0). This margin-negative resection enables precise histopathological evaluation and significantly reduces postoperative recurrence risk[3]. A meta-analysis in
Lesion diameter is positively correlated with the technical difficulty of endoscopic resection. Current domestic and international guidelines have not established a definitive size threshold for endoscopic treatment of gastrointestinal lesions. Evidence regarding endoscopic management of large colorectal lesions remains limited, with existing studies primarily focusing on perioperative outcomes of ESD. Research indicates that ESD provides short-term safety and efficacy for large laterally spreading tumors (LSTs) in the colorectum[7,8]. Several studies define large colorectal lesions as those exceeding 20 mm, 30 mm, or 40 mm in diameter, demonstrating the clinical applicability of endoscopic resection for such lesions[7-9]. However, increasing lesion diameter may elevate complication risks due to prolonged procedure duration and expanded resection area. Intraoperative complications predominantly include perforation and hemorrhage; postoperative adverse events encompass infection, abdominal pain, fever, and post-ESD electrocoagulation syndrome (PEECS), with severe cases potentially developing delayed perforation or delayed bleeding[10].
Consequently, endoscopic resection of large colorectal lesions necessitates advanced operator expertise and meticulous perioperative management. This study defined large colorectal lesions as those exceeding 30 mm in diameter. We conducted a retrospective analysis of patients with such lesions who underwent ESD or EFTR at General Hospital of Northern Theater Command between January 2023 and July 2024. Technical efficacy, safety, and recurrence rates at 3-month follow-up were evaluated to assess the clinical value of endoscopic management for large colorectal lesions.
This retrospective study reviewed data from 102 patients who underwent endoscopic resection for colorectal lesions measuring ≥ 30 mm in diameter at General Hospital of Northern Theater Command between January 2023 and July 2024. The inclusion criteria were: (1) Age 18-80 years; (2) Undergoing ESD or EFTR with documented informed consent; (3) Lesion diameter ≥ 30 mm confirmed by colonoscopy or imaging studies; and (4) Complete clinical and follow-up data available. The exclusion criteria were: (1) Incomplete follow-up data or death from non-procedural causes; (2) Use of anticoagulants within 7 days prior to the procedure; and (3) Special populations.
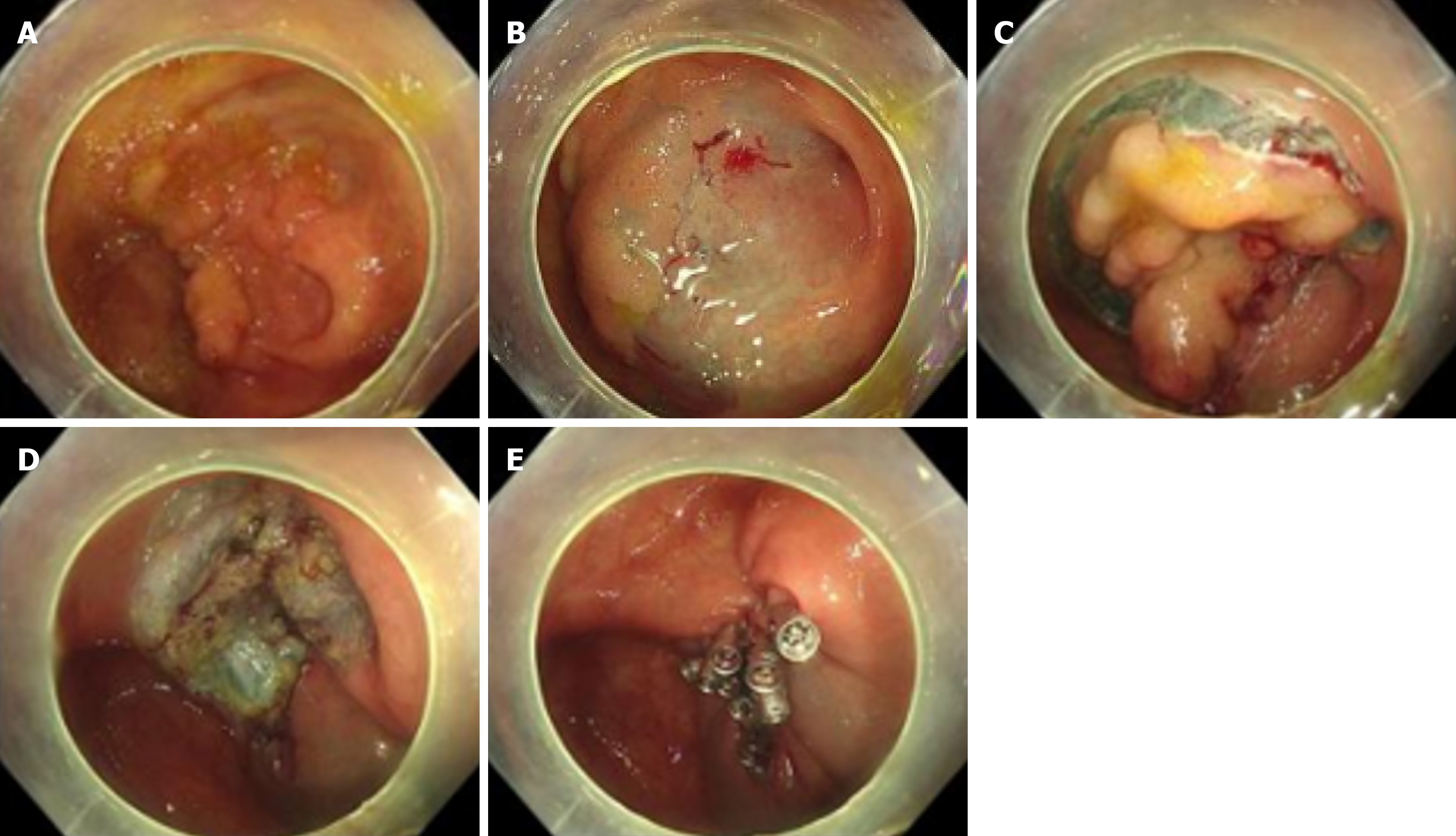
ESD or EFTR procedures were performed according to standardized protocols: (1) Injection: Submucosal injection of methylene blue-supplemented succinylated gelatin solution was administered at multiple points around the lesion periphery using an injection needle; (2) Incision and dissection: Circumferential mucosal incision was performed, followed by submucosal dissection using an electrosurgical knife; (3) Hemostasis: Prophylactic coagulation was applied to all visible vessels, including soft coagulation, hemostatic forceps coagulation and clip deployment; and (4) Specimen retrieval: Resected specimens were pinned flat on calibrated boards, fixed in 10% neutral-buffered formalin, and histopathologically assessed for margin status. Immediate needle decompression via right lower quadrant puncture was performed if a rigid abdomen developed post-full-thickness resection (Figure 1).
Single-channel electrogastroscope (Olympus, No. GIF-Q260J, Japan), distal attachment cap (Olympus, No. D-201-11804, Japan), CO2 injection vapor pump, 200D system hydrogen fluoride generator, MFK knife, disposable hypodermic needle (Olympus, No. NM-4 L-1, Japan), disposable hook loopers, high-frequency hemostatic forceps (Olympus, No. FD-410 LR, Japan), disposable metal hemostatic clips (harmony clip), nylon cord coils, dental floss, 0.9% sodium chloride injection, methylene blue and succinyl gel.
Postoperative routine according to the patient’s intraoperative condition includes fasting and water fasting for 3-6 days, de-pillowed lying down for 6 hours, and giving intravenous nutrition. Symptomatic supportive treatments such as anti-infection, hemostasis, and rehydration were given to observe the occurrence of postoperative bleeding, perforation, infection, and other complications. And follow up on the patients’ long-term healing, record the occurrence of recurrence and other long-term complications, and track whether the patients with deeper tumor infiltration have additional postoperative radical surgery.
Primary outcome measures: Whether the patient proceeded to radical surgical resection post-procedure, whether the patient recurred in postoperative follow-up colonoscopy, and wound healing. Secondary outcome measures: Incidence of perioperative complications, complete resection rate (R0). En bloc resection means that the endoscopic removal of the lesion is performed in a single piece, resulting in one intact specimen. Piecemeal resection means that the endoscopic removal of the lesion is performed in multiple fragments, resulting in more than one specimen piece. The rate of complete resection (R0) means that the proportion of cases where histopathological examination of the en bloc resection specimen confirmed negative horizontal and vertical margins. PEECS means that the occurrence of post-procedural fever
Statistical analysis was performed using SPSS 27.0 software. All statistical tests were two-sided, and a P-value < 0.05 would be considered statistically significant for the differences tested. Continuous variables were expressed as mean ± SD, and discrete variables were expressed as frequency (%). The number of patients experiencing adverse events and the total frequency of adverse events were calculated separately. Univariate logistic regression analysis was used to preliminarily screen for factors potentially associated with the occurrence of postoperative PEECS. Multivariate logistic regression analysis was then employed to identify independent risk factors for postoperative PEECS.
A total of 102 patients were enrolled in this study. The cohort comprised 52 male patients (51.0%) and 50 female patients (49.0%). The mean age was 63.25 ± 10.7 years. The general data of the patients included body mass index, age, gender, family history, past medical history, preoperative gastrointestinal symptoms, pre- and post-operative complete blood count, coagulation parameters, fecal occult blood test, serum albumin level, and body temperature (Table 1).
| Characteristic | n = 102 |
| Age (years) | 63.25 ± 10.7 |
| Sex | |
| Male | 52 (51) |
| Female | 50 (49) |
| Antecedent history | |
| History of smoking | 36 (35.35) |
| History of alcohol | 32 (31.4) |
| Family history | 6 (5.9) |
| Medical history | |
| Hypertension | 33 (32.4) |
| Diabetes | 16 (15.7) |
| Coronary artery disease | 14 (13.7) |
| History of cerebrovascular | 8 (7.8) |
| Disease other | 6 (5.9) |
| Medication history | |
| Antiplatelet drugs | 10 (9.8) |
| Anticoagulants | 0 |
The lesions of 102 patients collected from our center ranged from 30 mm to 70 mm in diameter, with a mean diameter of approximately 38.4 ± 11.5 mm. Favorable healing was observed in 98 patients (96.1%). Four patients (3.9%) were referred for radical surgery based on histopathological findings indicating tumor infiltration depth > 1000 μm, consistent with guideline recommendations. No recurrence or long-term stenosis was detected in any patient during follow-up co
| Lesions and perioperative period | Cases |
| Lesion site | |
| Cecum | 6 (5.9) |
| Ascending colon | 14 (13.7) |
| Transverse colon | 12 (11.8) |
| Descending colon | 3 (2.9) |
| Sigmoid colon | 24 (23.5) |
| Rectum | 43 (42.2) |
| Morphology of lesions | |
| Polyp-like | 26 (25.5) |
| LST | 72 (70.6) |
| Mucosal bulge | 5 (4.9) |
| Histology | |
| Polyp | 2 (2) |
| Low-grade intraepithelial neoplasia | 1 (1) |
| High-grade intraepithelial neoplasia | 28 (27.5) |
| Adenoma | 4 (3.9) |
| Lipoma | 4 (3.9) |
| Carcinoma | 62 (60.8) |
| Else | 1 (1) |
| Type of excision | |
| Complete resection (R0) | 98 (96.1) |
| En bloc resection | 100 (98) |
| Fractional resection | 2 (2) |
| Horizontal cutting edge | |
| Positive | 2 (2) |
| Negatives | 100 (98) |
| Vertical cutting edge | |
| Positive | 0 (0) |
| Negatives | 102 (100) |
| Placement of intestinal decompression tubes | |
| Yes | 46 (45.1) |
| No | 56 (54.9) |
| Perioperative complications | |
| PEECS | 24 (23.5) |
| Postoperative bleeding | 2 (2) |
| Perforation | 0 (0) |
| Malaise | 10 (9.8) |
| Bloody stool | 2 (2) |
Univariate logistic regression analysis identified the following factors as significantly associated with the occurrence of postoperative intraperitoneal PEECS: Lesion located in the transverse colon, lesion diameter, wound area, and LST morphology (Table 3).
| β | SE | P value | OR value | 95%CI | |
| Age (years) | -0.150 | 0.022 | 0.481 | 0.985 | 0.944-1.028 |
| Sex [M (0); F (1)] | 0.270 | 0.468 | 0.565 | 1.310 | 0.523-3.279 |
| History of smoking [N (0); Y (1)] | -0.629 | 0.526 | 0.232 | 0.533 | 0.190-1.495 |
| History of alcohol [N (0); Y (1)] | -1.030 | 0.596 | 0.084 | 0.357 | 0.111-1.150 |
| BMI > 24 kg/m2 [N (0); Y (1)] | 0.657 | 0.481 | 0.172 | 1.929 | 0.752-4.947 |
| Cerebrovascular disease [N (0); Y (1)] | 0.087 | 0.852 | 0.919 | 1.091 | 0.205-5.795 |
| Coronary artery disease [N (0); Y (1)] | 0.307 | 0.644 | 0.633 | 1.060 | 0.385-4.805 |
| Hypertension [N (0); Y (1)] | 0.300 | 0.488 | 0.538 | 1.350 | 0.519-3.512 |
| Diabetes [N (0); Y (1)] | 0.095 | 0.631 | 0.880 | 1.100 | 0.319-3.791 |
| Taking anti-platelet medication [N (0); Y (1)] | 0.371 | 0.733 | 0.613 | 1.449 | 0.344-6.100 |
| Taking anticoagulants [N (0); Y (1)] | 0.103 | 0.467 | 0.826 | 1.108 | 0.444-2.768 |
| Hemoglobin (g/L) | 0.004 | 0.015 | 0.807 | 1.004 | 0.975-1.033 |
| Blood platelet (× 109/L) | 0.003 | 0.004 | 0.446 | 1.003 | 0.995-1.012 |
| Leucocyte (× 109/L) | 0.191 | 0.150 | 0.204 | 1.210 | 0.902-1.625 |
| PT (seconds) | -0.168 | 0.215 | 0.435 | 0.845 | 0.554-1.289 |
| APTT (seconds) | -0.027 | 0.032 | 0.400 | 0.974 | 0.915-1.036 |
| INR | -1.779 | 3.577 | 0.619 | 0.169 | 0.000-187.008 |
| Serum albumin (g/L) | 0.018 | 0.050 | 0.717 | 1.018 | 0.923-1.123 |
| Cecum [N (0); Y (1)] | 0.520 | 0.899 | 0.563 | 1.682 | 0.289-9.803 |
| Ascending colon [N (0); Y (1)] | 0.307 | 0.644 | 0.633 | 1.360 | 0.385-4.805 |
| Transverse colon [N (0); Y (1)] | 1.386 | 0.635 | 0.029 | 4.000 | 1.153-13.876 |
| Descending colon [N (0); Y (1)] | -20.063 | 23205.422 | 0.999 | 0.000 | 0.000-0.000 |
| Sigmoid colon [N (0); Y (1)] | -0.545 | 0.606 | 0.369 | 0.580 | 0.177-1.902 |
| Rectum [N (0); Y (1)] | -0.487 | 0.489 | 0.319 | 0.614 | 0.235-1.602 |
| Diameter (cm) | 0.567 | 0.197 | 0.004 | 1.763 | 1.199-2.593 |
| Area (cm2) | 0.064 | 0.021 | 0.003 | 1.066 | 1.022-1.112 |
| Polyp-like [N (0); Y (1)] | -1.050 | 0.652 | 0.107 | 0.350 | 0.097-1.256 |
| LST [N (0); Y (1)] | 1.310 | 0.662 | 0.048 | 3.706 | 1.013-13.551 |
| Mucosal bulge [N (0); Y (1)] | -20.090 | 17974.843 | 0.999 | 0.000 | 0.000-0.000 |
| High-grade intraepithelial neoplasia [N (0); Y (1)] | 0.111 | 0.517 | 0.830 | 1.118 | 0.406-3.076 |
| Carcinoma [N (0); Y (1)] | 0.524 | 0.505 | 0.299 | 1.689 | 0.628-4.543 |
| No intestinal decompression tubes placed [N (0); Y (1)] | 0.013 | 0.468 | 0.978 | 1.013 | 0.404-2.537 |
Variables demonstrating statistical significance (P < 0.05) in univariate logistic regression were subjected to multivariate logistic regression analysis. This analysis identified lesion location in the transverse colon as an independent risk factor for postoperative PEECS (Table 4).
| β | SE | P value | OR value | 95%CI | |
| Transverse colon [N (0); Y (1)] | 1.907 | 0.726 | 0.009 | 6.734 | 1.623-27.945 |
| Diameter (cm) | 0.504 | 0.281 | 0.073 | 1.656 | 0.954-2.873 |
| Area (cm2) | 0.363 | 0.785 | 0.644 | 1.438 | 0.309-6.697 |
| LST [N (0); Y (1)] | 1.800 | 0.936 | 0.055 | 6.048 | 0.965-37.903 |
Currently, the main endoscopic techniques employed for the treatment of large gastrointestinal lesions include ESD, hybrid ESD, EFTR, submucosal tunneling endoscopic resection, and combined endoscopic-laparoscopic surgery. Selection among these modalities is determined by factors such as lesion size, morphology, location, depth of submucosal invasion, and the endoscopist’s level of expertise. For lesions not amenable to complete endoscopic removal or demonstrating deep submucosal invasion, radical surgery is the recommended treatment. Expert consensus on endoscopic diagnosis and treatment for colorectal cancer and precancerous lesions in China indicates the criteria for curative resection: (1) Negative vertical margins; (2) Pathological diagnosis of papillary adenocarcinoma, tubular adenocarcinoma, or medullary carcinoma; (3) Submucosal invasion depth < 1000 μm; (4) No lymphovascular invasion; and (5) Low-grade (grade 1) tumor budding[2]. Failure to satisfy any of these criteria necessitates radical surgery. Anastomotic leakage, fistula formation, stenosis, and incisional hernia represent the most frequent postoperative complications of surgical intervention[11]. Compared to surgical resection, endoscopic therapy offers advantages including minimal invasiveness, procedural simplicity, lower cost, higher patient acceptance, and faster postoperative recovery. Consequently, patients and their families often prefer endoscopic resection. This is particularly true for patients with ultra-low rectal lesions, where preservation of anal function is a paramount concern. ESD is a commonly employed technique for the treatment of gastrointestinal neoplasms. However, the inherent anatomical characteristics of the colorectum - such as thin walls and narrow lumens - significantly increase the technical difficulty of resecting larger lesions endoscopically. This necessitates heightened operator expertise, prolongs procedure time, and elevates the risk of complications. Currently, with ongoing advancements in endoscopic technology and instrumentation, experienced endoscopists are progressively tackling lesions of larger diameters, with success rates showing steady improvement. Nevertheless, the critical diameter threshold for choosing between surgical resection and endoscopic removal for large colorectal lesions remains an area requiring further investigation to establish clear guidelines.
Lesions with deeper invasion and larger diameters are associated with higher recurrence rates, regardless of treatment via surgical resection or endoscopic excision. A study from Denmark said that for surgical radical surgery for colon cancer, the 5-year recurrence rate was 6.8% for stage I, 11.6% for stage II, and 24.6% for stage III, and for surgical radical surgery for rectal cancer, the 5-year recurrence rate was 9.5% for stage I, 18.4% for stage II, and 28.8% for stage III[12]. The paramount objective in endoscopic resection is the achievement of histologically negative margins. In a retrospective cohort study by Boda et al[13] involving 1259 patients undergoing colorectal ESD with a mean lesion diameter of 33 mm, the rates of en bloc resection, complete resection, and R0 resection were 92.6%, 87.4%, and 83.7%, respectively. In the present case series, only 2 patients (2%) exhibited positive horizontal margins, and piecemeal resection was performed in 2 cases (2%). The negative margin rates were 98% for horizontal margins and 100% for vertical margins. The R0 resection rate was 96.1%, with en bloc resection achieved in 98% of cases. All patients remained recurrence-free during endoscopic surveillance. All procedures were performed by two experienced endoscopists, each having completed over 1500 ESD procedures. This high-volume operator experience contributed to satisfactory R0 outcomes, with postoperative surveillance showing complete mucosal healing and no recurrence. Despite negative margins in 4 patients, referral to surgical resection was mandated per guidelines due to submucosal invasion depth exceeding 1000 μm.
The major complications following ESD include delayed perforation, delayed bleeding, and stricture formation, with a significantly higher incidence observed in patients with larger lesion diameters[14]. A meta-analysis incorporating 97 studies revealed that the rate of ESD perforation in Asian countries was 4.5%, compared with a global average of 5.2%, and the rate of delayed bleeding in Asian countries was 2.4%, compared with a global average of 2.7%[15]. During endoscopic resection, operators may perform intentional full-thickness resection to ensure histologically negative margins when deep tumor invasion is suspected, resulting in controlled perforation. Immediate closure of such perforations with through-the-scope clips mitigates adverse outcomes. Postoperative delayed bleeding also requires vigilant management. In a multivariate analysis evaluating delayed bleeding within 30 days after colorectal ESD, rectal location, size > 50 mm, American Society of Anesthesiologists score III/IV, antithrombotic medication, and age > 75 years were shown to be risk factors for delayed bleeding[16]. Therefore, lesions of larger diameter warrant increased vigilance for delayed bleeding. In our cohort, delayed bleeding occurred on postoperative day 8 in two patients. Both lesions were located in the rectum and featured larger mucosal defects that were not closed with hemostatic clips. Emergency endoscopic hemostasis was successfully performed in both cases, followed by uneventful recovery and discharge. Regarding postoperative stenosis, routine endoscopic follow-up in all patients to date has revealed no instances of stenosis.
The most frequent perioperative complication following endoscopic resection of large colorectal lesions at our center was PEECS, occurring in 24 cases (23.5%). We conducted a risk factor analysis for this outcome. Univariate logistic regression identified lesion diameter, wound surface area, transverse colon location, and LST morphology as factors associated with PEECS development. Subsequent multivariate logistic regression revealed that transverse colon location was identified as the independent risk factor for PEECS. This finding suggests clinicians should remain vigilant for postoperative PEECS in patients presenting with these risk factors. Current studies indicate that the incidence of PEECS following ESD ranges from 9.5% to 40.2%, with lesion diameter identified as an independent risk factor in reports[14,17]. Notably, PEECS demonstrates a relatively high incidence rate. Compared to perforation and delayed bleeding, its clinical presentation is less specific and more likely to be overlooked. We defined PEECS as cases meeting the following criteria: The occurrence of post-procedural fever (≥ 37.8 °C) accompanied by either localized rebound tenderness or leukocytosis (≥ 10.8 × 109/L), in the absence of perforation as confirmed by postoperative imaging. The studies confirmed that PEECS occurred significantly more frequently after ESD than after polypectomy[18]. The underlying mechanism of PEECS development remains incompletely understood. Multiple studies propose that PEECS results from intraoperative thermal injury to the muscularis propria and serosal layers during endoscopic procedures[14,17]. Electrocoagulation-induced thermal damage triggers a transmural inflammatory response in the bowel wall, releasing inflammatory mediators such as interleukin-6 and tumor necrosis factor alpha. This cascade induces local edema and serositis. Notably, lesions exhibiting submucosal fibrosis or large diameters during ESD pose an increased risk for muscularis propria injury. Such deep thermal damage to the colonic wall may initiate PEECS and prolong procedure duration. Omori et al[19] investigated the relationship between the extent of muscularis propria injury and PEECS occurrence. Their analysis identified muscular layer exposure or tearing as an independent risk factor, while large specimen size and prolonged procedure duration were also associated with increased PEECS incidence. In our center, to reduce the incidence of PEECS, prophylactic antibiotics are administered to high-risk patients, and the operator selectively places an intestinal decompression tube according to the patient’s intraoperative condition; a total of 46 (45.1%) patients had an intestinal decompression tube placed, and the average number of days for placing the tube was 4.84 ± 1.29 days. Two previous studies in our center have indicated that prophylactic placement of intestinal decompression tubes after ESD can reduce the incidence of PEECS[20,21].
Current evidence confirms that post-ESD ulcer closure significantly reduces the incidence of PEECS[22]. Nevertheless, large wound surfaces present technical challenges for complete closure. Recent advances in novel suturing techniques now facilitate secure closure of extensive defects, promoting deeper tissue healing and reducing complication risks beyond superficial approximation. Masunaga et al[23] developed a novel endoscopic suturing technique termed the origami method, a modified double-layer closure. This approach achieves secure wound closure through direct muscularis propria-to-muscularis propria apposition, enhancing mechanical stability. Concurrently, emerging evidence reports the efficacy of line-assisted complete clip closure and adhesive-free double-loop clips in achieving complete wound closure. Both techniques significantly reduce bacterial exposure of the resection surface, thereby lowering PEECS incidence[22,24]. Advancements in endoscopic imaging and device innovation now enable precise preoperative assessment of lesion extent and invasion depth[25,26]. Concurrently, evolving ESD techniques and emerging ele
This retrospective study has the following limitations: First, this study was a single-center retrospective study, and only 102 patients were included, which is a small number, and this may produce a certain bias. Second, the follow-up time was 3 months postoperatively to review the colonoscopy, which is a relatively short period. Third, the operator was not the same person, and the level of the operator’s skill may also have an impact on the results. Fourth, to prevent postoperative complications, our surgeons placed intestinal decompression tubes in the high-risk group and not in the low-risk group based on their experience, which may cause some selection bias.
In conclusion, although endoscopic resection of large colorectal lesions poses significant technical challenges, carries a high risk of complications, and demands considerable operator expertise, it can achieve complete resection with favorable safety and efficacy profiles in high-volume endoscopy centers. Our findings demonstrate that PEECS constitutes the primary complication, which is effectively manageable through prophylactic measures. Lesion location in the transverse colon was identified as an independent risk factor. No recurrence or long-term complications were observed during follow-up. Consequently, lesion size alone should not preclude endoscopic resection as a therapeutic option.
We sincerely thank Dr. Yang for participating in the revision of the article.
| 1. | Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. 2024;4:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 1200] [Article Influence: 600.0] [Reference Citation Analysis (0)] |
| 2. | Expert Group on Early Diagnosis and Treatment of Cancer; Chinese Society of Oncology; Chinese Medical Association. [Expert consensus on the early diagnosis and treatment of colorectal cancer in China (2023 edition)]. Zhonghua Yi Xue Za Zhi. 2023;103:3896-3908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 3. | Fuccio L, Ponchon T. Colorectal endoscopic submucosal dissection (ESD). Best Pract Res Clin Gastroenterol. 2017;31:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Yeh JH, Tseng CH, Huang RY, Lin CW, Lee CT, Hsiao PJ, Wu TC, Kuo LT, Wang WL. Long-term Outcomes of Primary Endoscopic Resection vs Surgery for T1 Colorectal Cancer: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2020;18:2813-2823.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 5. | Zhou PH, Cai MY, Yao LQ. [Expert consensus on endoscopic submucosal dissection for digestive tract mucosal lesions]. Zhenduanxue Lilun Yu Shijian. 2012;11:531-535. [DOI] [Full Text] |
| 6. | Dolan RD, Bazarbashi AN, McCarty TR, Thompson CC, Aihara H. Endoscopic full-thickness resection of colorectal lesions: a systematic review and meta-analysis. Gastrointest Endosc. 2022;95:216-224.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Wang HX, Lian JJ, Chen SY, Zhou PH, Xu MD, Zhong YS, Zhang YQ, Chen WF. [Outcomes of endoscopic submucosal dissection for colorectal large laterally spreading tumors]. Zhongguo Neijing Zazhi. 2017;23:80-84. [DOI] [Full Text] |
| 8. | Lu FY, Cheng CE, Li R, Lu ZP, Huang GJ, Wang B, Huang X. [Safety of ESD in the treatment of large colorectal laterally spreading tumors of the colorectum with diameters > 3 cm in the elderly]. Xuzhou Yike Daxue Xuebao. 2020;40:570-574. [DOI] [Full Text] |
| 9. | Dos Santos CEO, Pereira-Lima JC, Onófrio FQ. Large Colorectal Lesions: Evaluation and Management. GE Port J Gastroenterol. 2016;23:197-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Deprez PH. Endoscopic diagnosis and treatment of upper gastrointestinal tumors. Endoscopy. 2011;43:966-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Pallan A, Dedelaite M, Mirajkar N, Newman PA, Plowright J, Ashraf S. Postoperative complications of colorectal cancer. Clin Radiol. 2021;76:896-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Nors J, Iversen LH, Erichsen R, Gotschalck KA, Andersen CL. Incidence of Recurrence and Time to Recurrence in Stage I to III Colorectal Cancer: A Nationwide Danish Cohort Study. JAMA Oncol. 2024;10:54-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 100] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 13. | Boda K, Oka S, Tanaka S, Nagata S, Kunihiro M, Kuwai T, Hiraga Y, Furudoi A, Terasaki M, Nakadoi K, Higashiyama M, Okanobu H, Akagi M, Chayama K. Clinical outcomes of endoscopic submucosal dissection for colorectal tumors: a large multicenter retrospective study from the Hiroshima GI Endoscopy Research Group. Gastrointest Endosc. 2018;87:714-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Jung D, Youn YH, Jahng J, Kim JH, Park H. Risk of electrocoagulation syndrome after endoscopic submucosal dissection in the colon and rectum. Endoscopy. 2013;45:714-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Fuccio L, Hassan C, Ponchon T, Mandolesi D, Farioli A, Cucchetti A, Frazzoni L, Bhandari P, Bellisario C, Bazzoli F, Repici A. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86:74-86.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 16. | Albouys J, Montori Pina S, Boukechiche S, Albéniz E, Vidal G, Legros R, Dahan M, Lepetit H, Pioche M, Schaefer M, Geyl S, Carrier P, Loustaud-Ratti V, Valgueblasse V, Brule C, Rodrigues R, Enguita German M, Jacques J. Risk of delayed bleeding after colorectal endoscopic submucosal dissection: the Limoges Bleeding Score. Endoscopy. 2024;56:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Yamashina T, Takeuchi Y, Uedo N, Hamada K, Aoi K, Yamasaki Y, Matsuura N, Kanesaka T, Akasaka T, Yamamoto S, Hanaoka N, Higashino K, Ishihara R, Iishi H. Features of electrocoagulation syndrome after endoscopic submucosal dissection for colorectal neoplasm. J Gastroenterol Hepatol. 2016;31:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Kim SJ, Kim SY, Lee J. Prognosis and risk factors of electrocoagulation syndrome after endoscopic submucosal dissection in the colon and rectum. Large cohort study. Surg Endosc. 2022;36:6243-6249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 19. | Omori T, Funasaka K, Horiguchi N, Kamano T, Nagasaka M, Nakagawa Y, Miyahara R, Hashimoto S, Shibata T, Ohmiya N, Hirooka Y. Injury to the muscle layer, increasing the risk of post-colorectal endoscopic submucosal dissection electrocoagulation syndrome. J Gastroenterol Hepatol. 2023;38:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 20. | Dong YP. [Efficacy Analysis of Intestinal Decompression and Drainage in Preventing Electrocoagulation Syndrome after ESD for Colorectal Mucosal Lesions: A Prospective Study]. Dalian Medical University, 2023. |
| 21. | Jia W. [Analysis of the efficacy of intestinal decompression drains in preventing postoperative complications of ESD for colorectal mucosal lesions]. Jinzhou Medical University, 2020. |
| 22. | Yamasaki Y, Takeuchi Y, Iwatsubo T, Kato M, Hamada K, Tonai Y, Matsuura N, Kanesaka T, Yamashina T, Arao M, Suzuki S, Shichijo S, Nakahira H, Akasaka T, Hanaoka N, Higashino K, Uedo N, Ishihara R, Okada H, Iishi H. Line-assisted complete closure for a large mucosal defect after colorectal endoscopic submucosal dissection decreased post-electrocoagulation syndrome. Dig Endosc. 2018;30:633-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Masunaga T, Kato M, Sasaki M, Iwata K, Miyazaki K, Kubosawa Y, Mizutani M, Takatori Y, Matsuura N, Nakayama A, Takabayashi K, Yahagi N. Modified double-layered suturing for a mucosal defect after colorectal endoscopic submucosal dissection (Origami method) (with video). Gastrointest Endosc. 2023;97:962-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | Abiko S, Yoshida S, Yoshikawa A, Harada K, Kawagishi N, Sano I, Oda H, Miyagishima T. Feasibility of a new ligation using the double-loop clips technique without an adhesive agent for ulceration after endoscopic submucosal dissection of the colon (with video). Gastrointest Endosc. 2020;92:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Meier B, Elsayed I, Seitz N, Wannhoff A, Caca K. Efficacy and safety of combined EMR and endoscopic full-thickness resection (hybrid EFTR) for large nonlifting colorectal adenomas. Gastrointest Endosc. 2023;98:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Yoshida M, Takizawa K, Nonaka S, Shichijo S, Suzuki S, Sato C, Komori H, Minagawa T, Oda I, Uedo N, Hirasawa K, Matsumoto K, Sumiyoshi T, Mori K, Gotoda T, Ono H; CONNECT-E Study Group. Conventional versus traction-assisted endoscopic submucosal dissection for large esophageal cancers: a multicenter, randomized controlled trial (with video). Gastrointest Endosc. 2020;91:55-65.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/