INTRODUCTION
Gallstone disease
Cholelithiasis, another name for gallstones, is a disorder where hardened, concentrated structures of bile are stuck in the gallbladder and bile ducts, clogging the biliary system[1]. These pieces of bile are composed of many substances which paved the pathway for classification. Bile stones come in three primary varieties[2]. The most frequent cause of gallstones is by far cholesterol stones[2]. Most frequently, cholesterol stones are linked to metabolic dysfunctions such as diabetes or hyperlipidemia[2,3]. Then there are black-pigmented stones and brown-pigmented stones. Black stones are composed of calcium bilirubin ate resulting from the breakdown of hemoglobin[2,4]. These can occur in inflammatory conditions such as Crohn’s disease or disease-causing extensive hemolysis[2]. Rarely are brown pigment stones seen; this may result from bacterial or parasite diseases as well as biliary structures[3].
METHODOLOGY
A narrative literature review was conducted to explore the relationship between melatonin and gallstone disease, with a focus on the prevalence, pathophysiology, potential therapeutic effects, and clinical implications of melatonin in gallstone formation and management. The objective was to synthesize current evidence from experimental studies, clinical trials, reviews, and clinical guidelines relevant to this topic. Relevant literature was identified through a comprehensive search of electronic databases including PubMed, Google Scholar, ScienceDirect, and Scopus. The search strategy incorporated combinations of keywords such as “gallstones”, “cholelithiasis”, “melatonin”, “bile composition”, “gallbladder motility”, “oxidative stress”, and “cholesterol metabolism”, using Boolean operators such as “AND” and “OR” to optimize results. The search was restricted to articles published in English between 2000 and 2024. Studies were included if they involved human adult participants (≥ 18 years) or animal models relevant to gallstone disease, and if they investigated any aspect of melatonin’s role in the development, prevention, or treatment of gallstones, including its effect on bile composition, gallbladder function, antioxidant properties, or lipid metabolism. Exclusion criteria comprised editorials, case reports, conference abstracts without accessible full texts, non-English publications, studies focusing on unrelated hepatobiliary disorders, or those not assessing melatonin in the context of gallstones. From the included studies, key data were extracted, including study design, population characteristics or animal models used, dosage and mode of melatonin administration, biological mechanisms evaluated, and observed outcomes related to gallstone formation or prevention. The selected findings were thematically organized into categories including epidemiology, experimental mechanisms, clinical applications, and therapeutic insights. A qualitative synthesis was then performed to highlight recurring trends, research gaps, and the translational potential of melatonin in gallstone disease management.
Epidemiology
According to a study, gallstones are thought to affect 6 million males and 14 million women between the ages of 20 and 74[3]. Another study conducted by gastroenterology clinics in North America explains that North American Indians have been shown to have the greatest rate of cholelithiasis, accounting for (64.1%) of women and (29.5%) of men[5]. Among them, 75% are cholesterol stones, which are associated with diabetes, dyslipidemia, insulin resistance, obesity, and intake of diets containing high amounts of saturated fats and sugar and low fibre[3,5].
Risk factors
Extensive research has been done on the risk factors of this disease which has led us to the 4Fs of gallbladder risk factors which are: (1) Female; (2) Fat; (3) Forty; and (4) Fertile[6,7]. Gallstones are also associated with age[6]. According to estimates, 20% of persons over 40 and 30% of adults over 70 have biliary calculi[8].
PATHOPHYSIOLOGY
Metabolic imbalances
The pathophysiology of gallstones is extensively researched. When the chemicals in bile are more soluble than they should be, gallstones form thus making the bile supersaturated. This supersaturation of the bile leads to the precipitation of small crystals that get stuck in the mucus membrane of the gallbladder, resulting in gallbladder sludge[3,9]. These crystals then ultimately coalesce to form large stones. These stones may migrate into the biliary tracts and cause obstruction leading to cholangitis and pancreatitis[3]. The presence of cholesterol stones may indicate increased bile cholesterol due to hepatic secretions, quick changes in the bile's cholesterol phase, or decreased motility combined with excessive secretion and accumulation of mucin in the gallbladder causing inflammation[3,10]. Moreover, dietary intake with less fiber percentage decreases colonic motility and increases the formation of stones due to stasis[11].
Hormonal impacts
Increased amounts of glycogen in the liver can also cause gallstones. This is due to the mechanism that glycogen intermediates are used for triglyceride synthesis and increased levels of triglycerides can cause decreased gallbladder motility and impaired contractility due to sensitivity to cholecystokinin[3,11]. Resistance to insulin works through the mechanism of 3-hydroxy-3-methylglutaryl coenzyme upregulation a reductase, which, independent of adiposity, increases cholesterol release and decreases intestinal absorption of cholesterol. Lack of gallbladder contractions may also be due to the phenomenon of reduced levels of cholecystokinin released by the duodenum, which sets a positive feedback mechanism and promotes more gallstone formation[12]. Many hormones also impact the incidence of gallstones. Hormones such as vasoactive intestinal peptide and human fibroblast growth factor regulate the emptying and refilling phase of the gallbladder in the postprandial period[13,14]. These hormones are regulated when bile acids reach the terminal ileum. Another such hormone is estrogen, which upregulates the synthesis of cholesterol by activating estrogen receptor alpha[15].
Neutrophil-mediated stone formation
The phospholipid lamella also functions as a transporter of excess cholesterol and plays a role in the crystallisation of cholesterol. These crystals attract neutrophils by chemotaxis, which attracts similar crystals leading to the formation of larger stones[16].
Altered gut microbiota
The gut flora can also be a cause of gallbladder stones. The gut microbiota is mutated by increased levels of toxins such as pesticides and heavy metals, increasing the risk of gallstones[17]. Another study states that gut bacteria that produce biofilms have an association with gallstones. Some gram-positive anaerobes are linked to greater amounts of the stone forming[17]. Metabolic imbalances greatly contribute to and promote the formation of gallstones[3].
Obesity
Additionally, obesity is a major factor in the development of gallstones. Obesity leads to hypertrophy of hepatocytes, leading to the activation of transcription factors that, some research shows, promote gallstone formation[18].
Genetic mutations
There is also some weightage of genetics in the occurrence of gallstones. Mutations in certain genes pose a high risk of developing gallstones. One such gene is the mucin-like protocadherin gene which results in biliary cholesterol secretion and high hydrophilic bile salts[19].
Secondary causes
Infection is one of the major causes of secondary gallstone formation. Bacterial infiltration takes place via the sphincter of Oddi and acts as stone nucleators, initializing the crystallization of cholesterol stones in conjunction with prostaglandins and lecithin[3]. Medications such as ezetimibe and other selective cholesterol absorption inhibitors cause increased cholesterol production and decreased cholesterol absorption by the intestines which typically results in high blood and bile cholesterol levels[20,21].
Clinical presentation and complications
Gallstones are not generally diagnosed until they obstruct the biliary tree[1,3]. Once there is a blockage, the most common presenting complaint is periodic discomfort in the upper right quadrant of the abdomen, anatomically known as the right hypochondriac region, which often develops in the post-prandial period and can also radiate to the back along with nausea and emesis[8]. Other symptoms may include pyrexia, rapid heartbeat, icterus in case of liver damage, pruritus, diarrhea, chills, and a loss of appetite[8]. If left untreated, it may worsen and present as an inflammatory disease such as cholangitis, pancreatitis, and cholecystitis[1]. Cholecystitis will occur if the biliary tree is completely and permanently blocked, leading to infection (cholangitis) and inflammation. Pancreatitis may present due to the migration of gallstones blocking the pancreatic duct and causing inflammation[1].
CONVENTIONAL MANAGEMENT STRATEGIES FOR GALLSTONES
The treatment options for gallstone disease include active observation as well as medications and surgical procedures. Physicians treat asymptomatic gallstones by simply monitoring the patient because the risk for symptom development or complications remains minimal according to research[22]. Yet risk remains since up to 20% of asymptomatic patients will eventually need medical intervention because of symptom or complication development[23].
Medical management
Medical treatment implements ursodeoxycholic acid (UDCA), which functions as a hydrophilic bile acid that decreases bile cholesterol saturation and enables slow stone breakdown in cholesterol-rich stone compositions. The strategy only works for patients who have small, non-calcified, radiolucent stones but need continued treatment between 6 months and 24 months for successful results[24]. After medical treatment stops, many patients experience repeated gallstone formation because UDCA therapy does not work against pigment stones or when gallbladder function becomes impaired[25].
Surgical management
Healthcare professionals use laparoscopic cholecystectomy as their primary treatment for people who have symptomatic gallstones and complicated cholelithiasis. Laparoscopy has become the preferred procedure over open cholecystectomy since it delivers both minimal invasiveness and short patient recovery periods and abbreviated hospitalization[26]. The underlying invasiveness of surgery produces notable risks which include hemorrhage alongside bile duct injury as well as infections together with complications related to anesthesia. Injury to the bile duct occurs rarely at a rate of 0.3 to 0.5 percent yet it leads to serious persistent complications that require strictures and cholangitis treatment as well as reconstructive surgical operations[27]. The surgery achieves a definitive cure through gallbladder removal while ending symptoms that lead to future occurrences[28]. The symptoms that make up post-cholecystectomy syndrome (PCS) are abdominal bloating with flatulence accompanied by diarrhea and right upper quadrant pain that arises after surgery in (10%–30%) of patients[29]. Medical specialists identify biliary dyskinesia along with sphincter of Oddi dysfunction and altered enterohepatic bile circulation as possible causes of PCS. A significant number of patients experience deteriorated quality of life following cholecystectomy mainly when their original symptoms persist or reappear after surgery. Extracorporeal shock wave lithotripsy (ESWL) presents itself as a suitable non-surgical treatment option for particular patient groups. ESWL treatment needs multiple sessions to work properly, yet it can only treat certain patients who have solitary radiolucent stones, while the stones often return after therapy[30]. Weight control along with dietary adjustments are the preventive measures for gallstones, yet they prove inadequate for healing formed stones[31].
Lifestyle modifications
It functions as a basic pillar in preventing gallstone formation. The recommendation for gallstone prevention includes weight reduction in combination with dietary modifications which should include saturated fat reduction with increased fibre intake along with regular exercise. Quick weight reduction creates a higher hazard of gallstone formation because it leads to biliary stasis and heightens cholesterol circulation[31]. The effectiveness of lifestyle modifications for decreasing gallstones is established but these techniques fail to break up existing gallstones so patients use other treatment options instead.
Limitations to conventional treatments
Multiple barriers exist to the conventional management methods that healthcare providers widely use and validate in clinical practice. Medical interventional approaches and drug-based treatments require improvement because of their invasive nature alongside long treatment periods recurrent risks and side effects and minimal therapeutic flexibility. Scientists are actively searching for treatment agents with dual capability to stall gallstone development alongside resolving the fundamental pathophysiological factors of cholesterol supersaturation together with gallbladder dysmotility inflammation and oxidative stress. Research has revealed that melatonin functions as a pleiotropic hormone that controls daily rhythms but also shows significant antioxidant activity and anti-inflammatory properties and helps decrease cholesterol levels during preclinical examinations. Clinical evidence indicates that melatonin demonstrates therapeutic potential for gallstone disease because it controls lipid activity along with improving bile drainage while conserving gallbladder epithelial cells from oxidizing damage. Standard doses of melatonin provide excellent safety alongside human-friendly tolerability therefore it shows promise to assist surgery-resistant patients or those at high risk of recurrence. Its favourable characteristics establish melatonin as an intriguing research subject for treating gallstone disease despite shortcomings of current treatment approaches.
PHYSIOLOGICAL OVERVIEW OF MELATONIN
Melatonin is a prevalent biological molecule, produced and secreted by the pineal gland in response to darkness, that acts as a regulator that aligns the physiology of the organism according to the day/night and seasonal changes of the external environment[32]. This whole pattern of melatonin production and secretion is under the control of suprachiasmatic nuclei, is programmed according to the schedule of light exposure, and adjusts according to the length of night[33]. Evidence suggests that melatonin stabilizes the circadian rhythms, more specifically of the sleep-wake cycle and core temperature of the body[33]. Studies indicate that behind sleep and circadian disorders, melatonin dysregulation is considered to be one of the key factors[34]. Recently, exogenous melatonin supplementation has proven to be of great use in improving the sleep quality of individuals suffering from insomnia or poor sleep[35,36].
Adverse effects of exogenous melatonin supplements appear to be limited to headache, dizziness, and drowsiness that may mislead the patients to believe that melatonin supplements are generally safe, whereas it has been found that those carrying copies of the G allele where pancreatic expression of MTNR1B is increased may show impaired postprandial glucose disposal under the impact of melatonin. Therefore, users of melatonin supplements should undergo periodic glycated hemoglobin monitoring[37].
CLINICAL EVIDENCE OF MELATONIN IN GALLSTONE DISEASE
Melatonin, with strong antioxidant and anti-inflammatory properties, plays a crucial role in maintaining gastrointestinal (GI) and hepatobiliary health. Its influence extends beyond circadian rhythm regulation, impacting lipid metabolism, smooth muscle motility, and oxidative balance. It has also proven to show improvement in symptoms when given to patients of irritable bowel syndrome (IBS) in a clinical trial[38]. Moreover, another study upon females experiencing IBS found melatonin supplementation as treatment to be quite effective[36]. Recent studies have highlighted melatonin’s protective effects against gallstone formation by reducing biliary cholesterol levels and improving gallbladder motility. Additionally, it modulates hepatic functions and demonstrates therapeutic potential in various GI disorders and cancers.
Antioxidant and anti-inflammatory functions
Melatonin, chemically known as N-acetyl-5-methoxytryptamine, is a hormone derived from indoleamine that performs a wide range of physiological and biological functions. It acts as a powerful antioxidant, modulates inflammation, neutralizes free radicals, regulates the body’s internal clock, and promotes sleep[39]. Evidence suggests that in primitive unicellular organisms, melatonin’s original function was primarily to combat oxidative stress by scavenging free radicals[40]. As organisms evolved, melatonin was adapted to serve additional roles, including acting as a messenger that translates changes in light exposure into hormonal signals in more complex life forms. One of the distinguishing characteristics of melatonin as an antioxidant is its ability to neutralize multiple free radicals through a cascade mechanism. Furthermore, it can be naturally upregulated in response to moderate oxidative stress. These unique attributes make melatonin a highly effective endogenous antioxidant that plays a critical role in protecting cells from oxidative damage[40]. A study from Russia shows that combined treatment of hepatitis along with melatonin has proven to be beneficial and has shown no side effects[41].
Influence on lipid metabolism, biliary system, and smooth muscle motility
Melatonin, while primarily synthesized in the pineal gland, is also abundantly produced in various other tissues such as the GI tract, retina, testes, lymphocytes, and Harder’s glands. It may also play a role in lipid metabolism by influencing processes like fat cell formation (adipogenesis) and the breakdown of fats (lipolysis)[42]. The effects of melatonin supplementation on lipid metabolism, body fat accumulation, and obesity were investigated in a study using ovariectomized rats as a menopause model. The rats that received melatonin supplements for eight weeks showed decreased body weight gain, perirenal fat mass, and gonad fat mass in addition to a significant decrease in the messenger RNA levels of the fatty acid synthesis enzymes[43]. Melatonin has been found to significantly reduce the activities of hepatic lipogenic enzymes such as fatty acid synthase, stearoyl-CoA desaturase 1, acetyl-CoA carboxylase, peroxisome proliferator-activated receptor-γ, and Sterol regulatory element-binding protein 1c[44]. It also increases the relative expression of hepatic carnitine palmitoyltransferase-1α in high-fat diet-induced hyperlipidemia[45]. Recently, widespread interest has grown regarding the protective effects of melatonin on smooth muscle dysfunction. Release of GI melatonin from serotonin-rich enterochromaffin cells of the GI mucosa suggests a close antagonistic relationship with serotonin and seems to be related to the periodicity of food intake. Evidence suggests that melatonin released in response to lipid infusion exerts a modulatory influence that decreases the inhibitory effects of the ileal brake on gastric emptying. On isolated bowel, melatonin reduced the tone but not the amplitude or frequency of contraction of GI smooth muscles[44]. By directly activating melatonin receptors, scavenging free radicals, or increasing the availability of nitric oxide in the central nervous system, melatonin also reduces sympathetic tone. Melatonin stops cholecystitis's harmful effects on smooth muscle and the enteric nervous system by reducing oxidative stress. By normalizing Ca2+ dependent and independent contraction, melatonin can also help detrusor muscles regain their reduced contractility[46]. With all this evidence, melatonin is seen as a promising candidate for modulating smooth muscle motility.
Potential for modulating gallbladder function
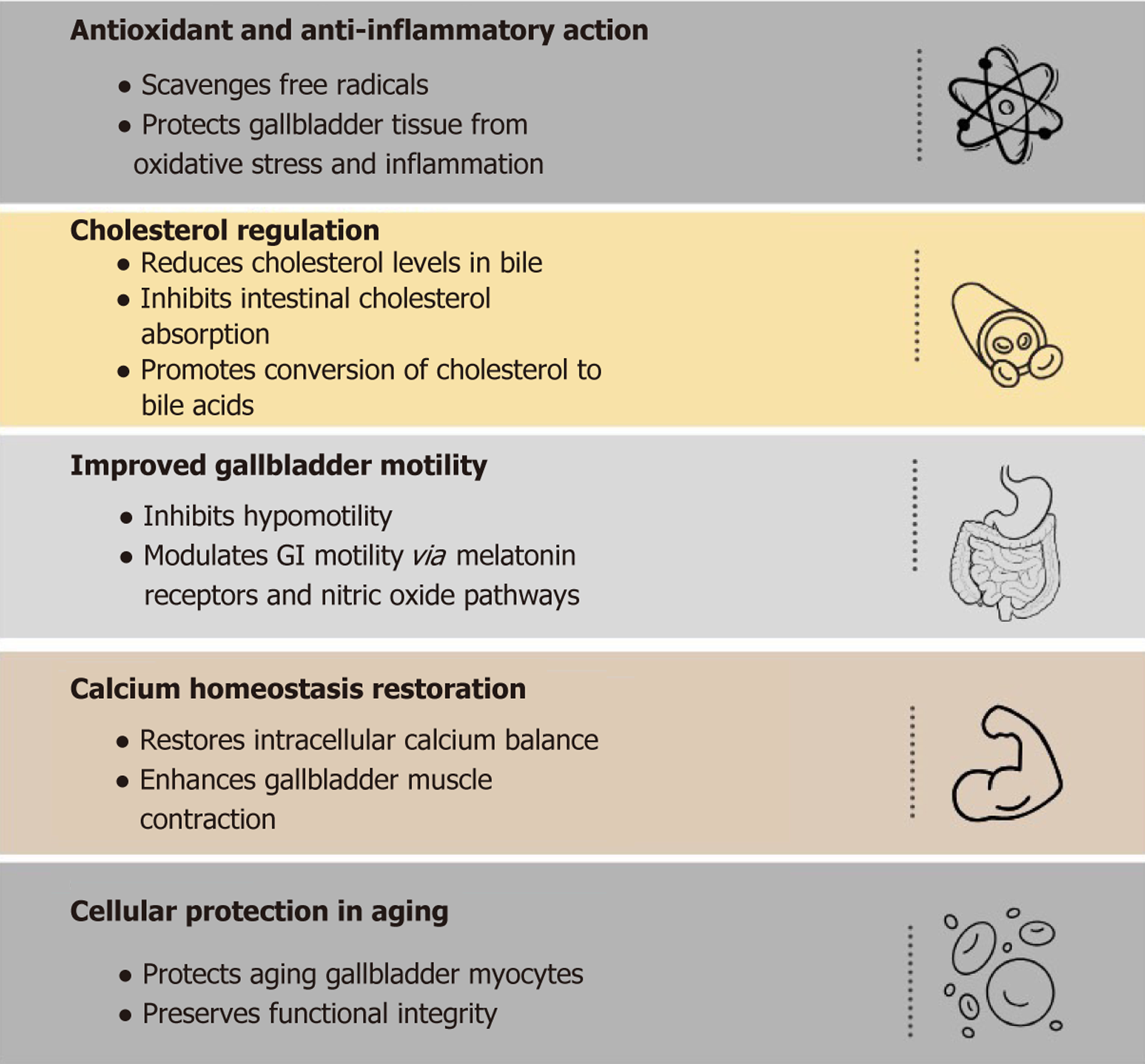
A lot of studies suggest that human bile, particularly gallbladder bile, contains high physiological levels of the antioxidant melatonin[47]. Melatonin is of great significance in the management of different pathologies and cancers of different parts of the body and those of GI including the management of acute cholecystitis by restoring Ca2+ homeostasis and resolving inflammation[48], some studies have emphasized the functions of melatonin against gallstone formation, because of its effects on aging gall bladder myocytes and its antioxidant qualities, melatonin has been shown to prevent gallstones from forming and lower biliary cholesterol levels by increasing the conversion of cholesterol to bile acids and preventing cholesterol absorption through the intestinal epithelium, which lowers the risk of cholelithiasis[49]. Reactive oxygen species and hypo motility of the gall bladder are to be set as targets for the treatment of gallstones with melatonin[49]. A detailed summary of the effect of melatonin on gallstone disease is shown in Figure 1.
Figure 1 Therapeutic potential of melatonin in gallstone disease.
Melatonin contributes to gallstone prevention and management through its antioxidant and anti-inflammatory action, cholesterol regulation, improved gallbladder motility, calcium homeostasis restoration, and cellular protection in ageing. Each mechanism is associated with a specific physiological target involved in gallstone pathogenesis.
Anti-cancer potential for gall bladder and other GI cancers
Melatonin has shown potential in the treatment of several cancers, including cholangiocarcinoma, esophageal, gastric, hepatocellular, colorectal, and pancreatic cancers[50,51]. It also plays a significant role in protecting the liver from damage[50]. A study upon patients with inoperable advanced hepatocellular carcinoma included melatonin in the treatment plan and found out it to be enhancing the immunological potential of patients and improving the effect of trans catheter arterial chemoembolization[52]. It’s anticancer and chemo preventive effects are attributed to several mechanisms. Melatonin inhibits cancer cell growth, movement, and invasion by influencing various transcription factors and associated signaling pathways. In hepatocellular carcinoma, it overcomes resistance to apoptosis by activating both intrinsic and extrinsic apoptotic pathways, induces endoplasmic reticulum-related apoptosis, and helps reverse tumor-induced immune suppression by regulating tumor-derived exosomes[53]. Studies also indicate that melatonin suppresses both mitochondrial respiration and glycolysis in liver cancer cells, contributing to its anticancer action[54]. Furthermore, melatonin reduces the growth of doxorubicin-resistant HepG2 cells and lowers the expression of drug resistance-related genes[55]. It also affects pyruvate and lactate metabolism, thereby counteracting the Warburg effect, which influences cellular structure and energy dynamics[56]. It has proven to contribute in control of tumor growth as a natural anti-angiogenic molecule and a study has proven its role in opposition of angiogenesis dependent proliferation of cancer cells[57]. These findings support melatonin’s role as a valuable adjuvant in cancer therapy.
CONCLUSION
Gallstone disease remains a prevalent and clinically significant condition influenced by a complex interplay of metabolic, hormonal, genetic, and lifestyle factors. Conventional management approaches, while effective for symptom relief and complication control, are limited by invasiveness, recurrence risks, and their inability to reverse underlying pathophysiological mechanisms. This highlights the urgent need for safer, non-invasive alternatives. Emerging evidence suggests that melatonin, owing to its antioxidant, anti-inflammatory, lipid-regulating, and smooth muscle modulating properties, offers a promising adjunct or alternative therapy. By reducing biliary cholesterol saturation, improving gallbladder motility, and mitigating oxidative stress, melatonin could potentially alter the course of gallstone disease and prevent recurrence, particularly in high-risk or surgery-resistant populations. Furthermore, its anticancer properties enhance its relevance in hepatobiliary and GI health. Future clinical trials are warranted to validate melatonin’s therapeutic efficacy and establish standardized treatment protocols for its integration into gallstone disease management.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Afghanistan
Peer-review report’s classification
Scientific Quality: Grade A, Grade A
Novelty: Grade A, Grade B
Creativity or Innovation: Grade B, Grade B
Scientific Significance: Grade B, Grade A
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Xu M, PhD, China S-Editor: Luo ML L-Editor: A P-Editor: Zhang YL