Published online Sep 27, 2025. doi: 10.4254/wjh.v17.i9.111126
Revised: July 10, 2025
Accepted: August 21, 2025
Published online: September 27, 2025
Processing time: 94 Days and 1.5 Hours
Liver transplantation (LT) is the preferred curative treatment for early-stage hepatocellular carcinoma (HCC). However, approximately 17% of patients exp
To assess which explant-based prognostic model best predicts HCC recurrence after LT.
A systematic search was performed in PubMed, EMBASE, Web of Science, and the Cochrane Library from inception to January 30, 2025. Nine retrospective stu
All studies were retrospective and included validation cohorts from North America, Europe, and Asia. The overall recurrence rate was 7%. For high-risk thresholds, pooled sensitivity and specificity were Risk Estimation of Tumor Recurrence after Transplant (RETREAT) ≥ 5 (0.381/0.953), Decaens ≥ 4 (0.676/0.817), and PCRS ≥ 3 (0.217/0.987). Among high-risk patients, recurrence reached 45% (95%CI: 35.1-57.0). Area under the curve comparisons showed no statistically significant differences among models. Thus, no model demonstrated clear superiority.
Although several explant-based models exist, their limited sensitivity suggests that many patients at risk of recurrence remain unidentified. The RETREAT score, developed in a large cohort, remains the most extensively va
Core Tip: This systematic review and meta-analysis assessed the prognostic accuracy of three explant-based models-Risk Estimation of Tumor Recurrence after Transplant (RETREAT), Decaens, and Predicting Cancer Recurrence Score-in predicting hepatocellular carcinoma recurrence after liver transplantation. Analyzing nine retrospective studies with 5348 patients, recurrence was 7%, with no significant differences in area under the curve among models. While RETREAT is the most validated, all models demonstrated limited sensitivity, risking underestimation of recurrence risk. The findings highlight the need for improved prognostic tools, potentially through incorporating larger, prospective datasets and artificial intelligence, to enhance risk stratification, guide post-transplant surveillance, and optimize patient outcomes.
- Citation: Christofoli de Barros I, Vanzin Fernandes M, Rodríguez Villafuerte S, Brandão ABM. Explant-based prognostic models for hepatocellular carcinoma recurrence after liver transplantation: A systematic review and meta-analysis. World J Hepatol 2025; 17(9): 111126
- URL: https://www.wjgnet.com/1948-5182/full/v17/i9/111126.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i9.111126
Liver transplantation (LT) is the treatment of choice for patients with early-stage hepatocellular carcinoma (HCC) and decompensated cirrhosis and/or clinically significant portal hypertension, offering the possibility of curing both the tumor and the underlying liver disease[1,2]. However, HCC recurs in approximately 17% of patients after LT and remains the leading cause of death in this population[3,4]. Risk factors associated with recurrence include vascular invasion, tumor size > 5 cm, multifocal disease, and poor differentiation. These histopathologic and clinical characteristics are essential components in developing post-LT predictive models. Moreover, extrahepatic recurrence and early recurrence (within one year after LT) are associated with worse post-recurrence survival[5].
Several prognostic scoring systems have been proposed to guide surveillance after LT in patients transplanted for HCC. These models incorporate pre-transplant and post-transplant variables such as tumor size and number, micro
However, there are currently no evidence-based, standardized criteria for risk stratification that can be formally recommended in clinical practice. Therefore, the objective of this study was to identify which explant-based risk pre
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews of Diagnostic Test Accuracy Studies guidelines[7]. The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO), No. CRD42024569977[8].
Studies were included based on the following predefined criteria: (1) Published in a peer-reviewed journal; (2) Included patients who underwent LT after the publication of the Milan criteria as listing criteria for HCC[9]; (3) Confirmed pathological diagnosis of HCC post-transplant; (4) Identified risk factors for HCC recurrence and developed or validated individual-level risk prediction models; (5) Reported at least one outcome of interest: Post-LT HCC recurrence rate or the predictive performance of models over a five-year follow-up; and (6) Provided data to calculate true positive (TP), false positive (FP), false negative (FN), and true negative (TN) rates. No language restrictions were applied. Studies were excluded if they assessed only pre-transplant data for recurrence prediction, or were in the form of abstracts, editorials, letters, narrative reviews, systematic reviews, or meta-analyses. The literature search covered all studies published until January 30, 2025.
A comprehensive literature search was conducted in PubMed, EMBASE, Web of Science, and the Cochrane Library using the following terms: ("liver transplantation" OR "hepatic transplantation") AND (HCC OR "hepatocellular carcinoma" OR hepatoma OR "liver cancer" OR "hepatic cancer" OR "liver neoplasia" OR "hepatic neoplasia") AND (recurrence OR relapse) AND ("predictive model" OR "predictive models" OR "AFP-R3" OR "R3-AFP" OR "UCLA nomogram" OR UCLA OR AFP OR "AFP score" OR RETREAT OR "Combo-MORAL" OR "Post-MORAL" OR MORAL).
The reference lists of eligible studies, prior systematic reviews, and meta-analyses were also manually screened for additional studies. Two reviewers (Christofoli de Barros I, Rodríguez Villafuerte S) independently conducted the search, imported citations into Zotero software (version 6.0.26, Vienna, Austria), and screened titles and abstracts. Full texts of potentially eligible studies were assessed independently. Discrepancies were resolved through discussion with a third reviewer (Vanzin Fernandes M).
Data extracted from each study included: (1) Study characteristics: First author, publication year, country, sample size, and follow-up duration; (2) Patient characteristics: Number of patients, age, liver disease etiology, prior HCC therapies, and transplant listing criteria; and (3) Outcomes: HCC recurrence rates and data on TP, TN, FP, and FN. For studies that assessed multiple models or thresholds, data were extracted separately for each model.
Two investigators (Christofoli de Barros I, Rodríguez Villafuerte S) independently assessed the methodological quality of the included studies using the revised Quality Assessment of Diagnostic Accuracy Studies tool (QUADAS-2)[10]. Disagreements were resolved with a third investigator (Vanzin Fernandes M). The QUADAS-2 tool evaluates four domains: (1) Patient selection; (2) Index test; (3) Reference standard; and (4) Flow/timing. Each domain was rated as having low, high, or unclear risk of bias. Results are summarized in Supplementary material.
Pooled sensitivity and specificity for each prognostic model were calculated using a random-effects model, along with 95%CI. Forest plots and summary receiver operating characteristic (ROC) curves were constructed using the bivariate method, and the area under the ROC curve (AUROC) was determined. When applicable, multiple thresholds were analyzed separately. Comparative analysis of area under the curve (AUC) across models was conducted using both direct and indirect bivariate approaches. Heterogeneity was assessed using the inconsistency index (I²). All statistical analyses were performed using R software version 4.3.2. A two-sided P < 0.05 was considered statistically significant.
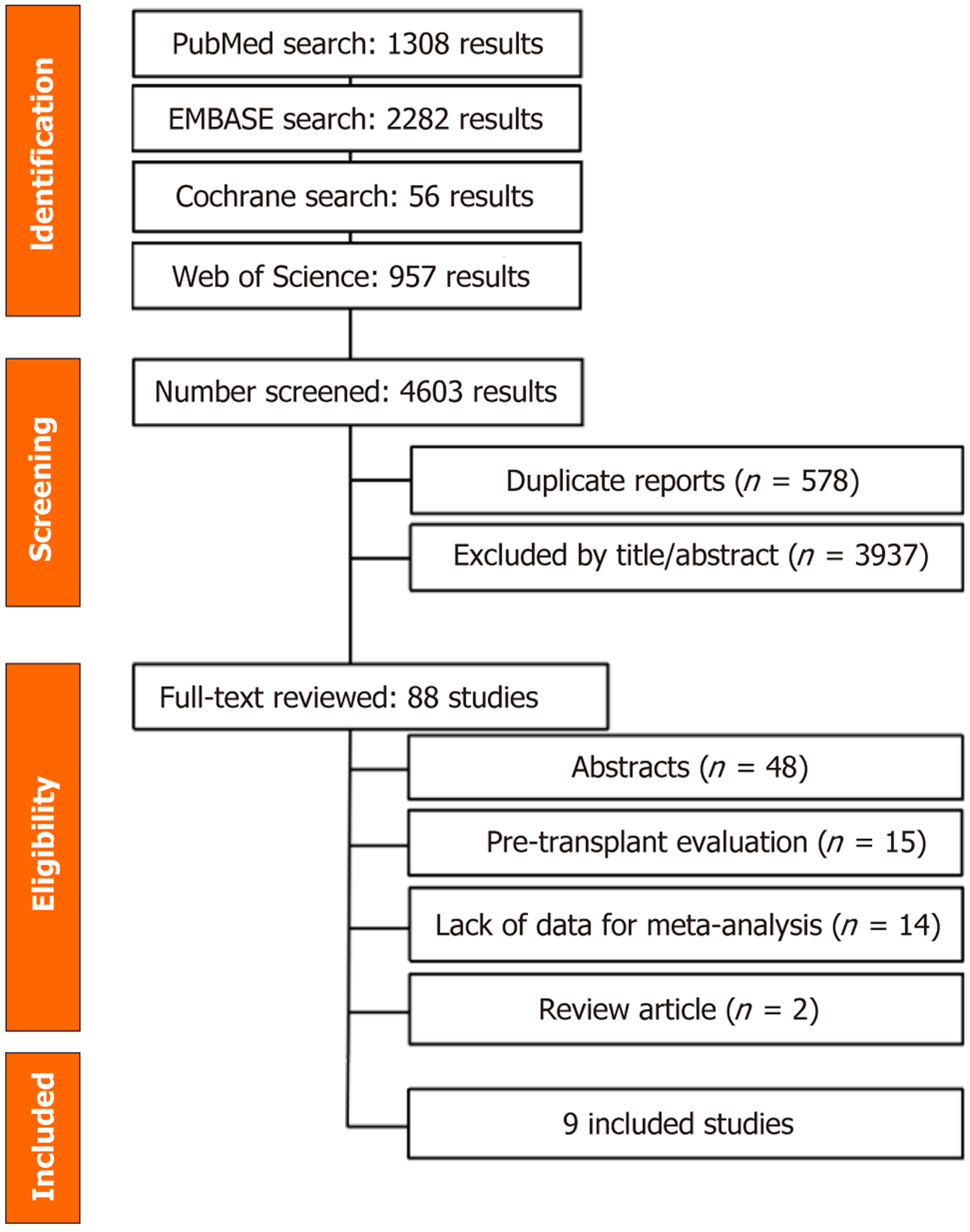
The initial search retrieved 4603 records (Figure 1). After removing duplicates and screening titles and abstracts, 68 full-text articles were reviewed. Nine retrospective studies, encompassing 5348 liver transplant recipients and evaluating three predictive scores, met the eligibility criteria for inclusion[11-19]. One study by Abdelfattah et al[20] was excluded due to overlapping population data with a previously published study from 2021[11].
All included studies focused on patients with confirmed HCC on explant analysis following LT. Patient characteristics are summarized in Table 1[11-19,21-32]. Most study participants were male, with a mean age of approximately 60 years. The leading etiologies of cirrhosis were chronic viral hepatitis (particularly hepatitis C virus) and alcohol-related liver disease. Six studies reported locoregional therapy use while patients were on the transplant waiting list. All transplant procedures used deceased donor grafts. The Milan criteria were the most applied for waitlist eligibility, although one center also used the University of California, San Francisco criteria, and another exclusively applied the revised United Kingdom criteria. All included studies were retrospective in design; six were conducted in Europe, two in North America, and one in Asia. Study-specific characteristics are detailed in Supplementary Table 1[11-19,21-32].
| Ref. | Age (years) | Male (%) | Etiology of liver disease (%) | Hepatocellular carcinoma allocation criteria | Within Milan criteria (%) | Loco regional therapy1 (%) |
| Parfitt et al[24], 2007 | 54 | 84 | HCV (42.7%), alcohol (21.3%), HBV (17.3%) | N/A | 66.72 | N/A |
| Chan et al[19], 2008 | 56.2 | 89.7 | HCV (58%), HBV (33%), alcohol (17%) | N/A | N/A | N/A |
| Agopian et al[21], 2015 | 59.5 | 73.3 | HCV (58%), HBV (16%), alcohol (9%) | Milan/UCSF | 84 | 60 |
| Mehta et al[12], 2017 | 58.2 | 75.2 | HCV (58.8%), HBV (16.1%), MAFLD (8.0%) | Milan | 50.32 | 91.5 |
| Halazun et al[25], 2017 | 57.8 | 79.9 | HCV (69.3%), HBV (15.3%), alcohol (11.8%) | Milan | 66.6 | 81.7 |
| Costentin et al[13], 2017 | 52.7 | 85.2 | HBV or HCV (61.8%), alcohol (28.8%), others (9.4%) | Milan | 64.22 | 67.7 |
| Mehta et al[14], 2018 | 60 | 76.7 | HCV (62.8%), MAFLD (8.0%), alcohol (7.3%) | Milan | 852 | 91.1 |
| Mirón Fernández et al[15], 2019 | 56.2 | 76.2 | HCV (39%), mixed (alcohol/HCV): (22.9%), HBV (16.2%) | N/A | 59.4 | N/A |
| Feng et al[26], 2019 | 59 | 82.2 | HBV (88.1%), HCV (5.9%), alcohol (2%) | Milan | 79.22 | 75 |
| Sánchez Segura et al[27], 2020 | 59 | 82.8 | HCV (38.4%), alcohol (25.3%), mixed (alcohol/HCV): (23.2%) | N/A | 62.6 | 94.9 |
| Hasan et al[28], 2021 | 65.5 | 75 | HCV (55.8%), MAFLD (25%), alcohol (5.8%) | Milan | 100 | 58 |
| Ma et al[29], 2021 | 51.98 | 92.1 | HCV (54.9%), alcohol (23.5%), HBV (7.8%) | N/A | 37.12 | N/A |
| Abdelfattah et al[11], 2021 | 57.1 | 68.5 | HCV (60.3%), HBV (26%), others (13.7%) | Milan | 100 | N/A |
| Åberg et al[16], 2021 | 57.4 | 79.3 | HCV (59.2%), alcohol (28.4%), HBV (17.9%) | N/A | 61.52 | 35.7 |
| Aziz et al[30], 2021 | 62 | 89 | HCV (46%), alcohol (11%), HBV (5%) | Milan/UCSF/total tumor volume < 15 cm3 | 49.3 | 68.5 |
| Costentin et al[22], 2022 | 58 | 82.7 | HBV or HCV (57.8%), alcohol (31.3%), others (10.8%) | Milan/alpha-fetoprotein score | 62.32 | 68.5 |
| Reddy et al[17], 2022 | 59 | 73.8 | HCV (41.9%), alcohol (18.5%), HBV (11.2%) | Revised United Kingdom criteria | 84.3 | 76.4 |
| Van Hooff et al[18], 2022 | 61.2 | 79.8 | HCV (20.7%), alcohol (16.3%), HBV (13.8%) | N/A | 79.32 | 75.4 |
| Cuadrado et al[31], 2023 | 60 | 87.9 | Alcohol (34.8%), HCV (31.8%), mixed (alcohol/HCV): (19.7%) | N/A | 98.5 | 77.3 |
| Tran et al[23], 2023 | 58.0 | 77.9 | HCV (61.6%), alcohol (10.7%), HBV (10.6%) | Milan | 71.9 | 86.2 |
| Brandão et al[32], 2024 | 60.5 | 71.9 | HCV (87.5%), alcohol (9.4%), HBV (3.1%) | Brazilian Milan criteria | 71.6 | 65.2 |
Three risk models for post-transplant HCC recurrence were assessed across multiple studies and included in the meta-analysis: (1) Risk Estimation of Tumor Recurrence after Transplant (RETREAT), which incorporates AFP at LT, microvascular invasion, the diameter of the largest viable tumor, and the number of viable tumors[12]; (2) Decaens score, based on largest tumor size, number of nodules, and histologic differentiation grade[33]; and (3) Predicting Cancer Re
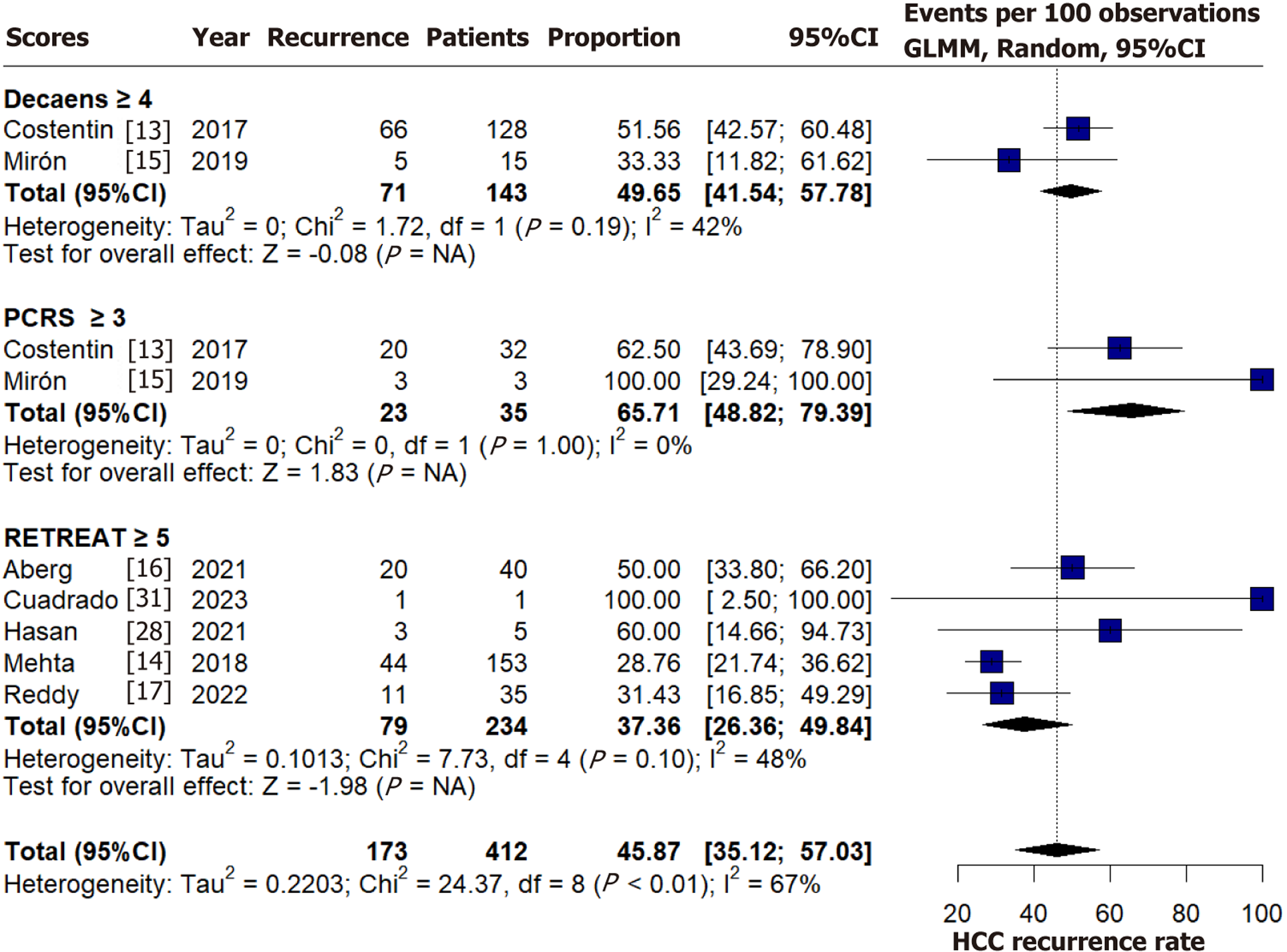
The overall pooled recurrence rate of HCC post-LT was approximately 7% (347/4976), excluding the study by Costentin et al[13], for which recurrence data were unavailable. Among patients classified as high-risk according to predictive models, the pooled recurrence rate was 45.87% (95%CI: 35.15-57.03, I² = 67%), as illustrated in Figure 2[13-17,28,31]. Score-specific recurrence rates for high-risk categories are also presented in Figure 2[13-17,28,31]. The bivariate analyses of sensitivity, specificity, and AUC for each model are presented in Table 2. Positive and negative predictive values are shown in Supplementary Table 3[11-32].
| Bivariate analysis | ||||||
| Score | Sensibility | Sensibility 95%CI | Specificity | Specificity 95%CI | AUC | AUC 95%CI |
| RETREAT ≥ 5 | 0.386 | 0.252-0.540 | 0.950 | 0.897-0.976 | 0.793 | 0.406-0.945 |
| RETREAT ≥ 4 | 0.601 | 0.443-0.740 | 0.861 | 0.759-0.924 | 0.807 | 0.611-0.880 |
| RETREAT ≥ 3 | 0.686 | 0.560-0.790 | 0.736 | 0.647-0.810 | 0.771 | 0.704-0.809 |
| PCRS ≥ 1 | 0.582 | 0.306-0.815 | 0.851 | 0.797-0.893 | 0.852 | 0.521-0.905 |
| PCRS ≥ 3 | 0.223 | 0.154-0.311 | 0.950 | 0.915-0.971 | 0.431 | 0.246-0.952 |
| Decaens ≥ 4 | 0.591 | 0.329-0.810 | 0.821 | 0.597-0.934 | 0.772 | 0.518-0.889 |
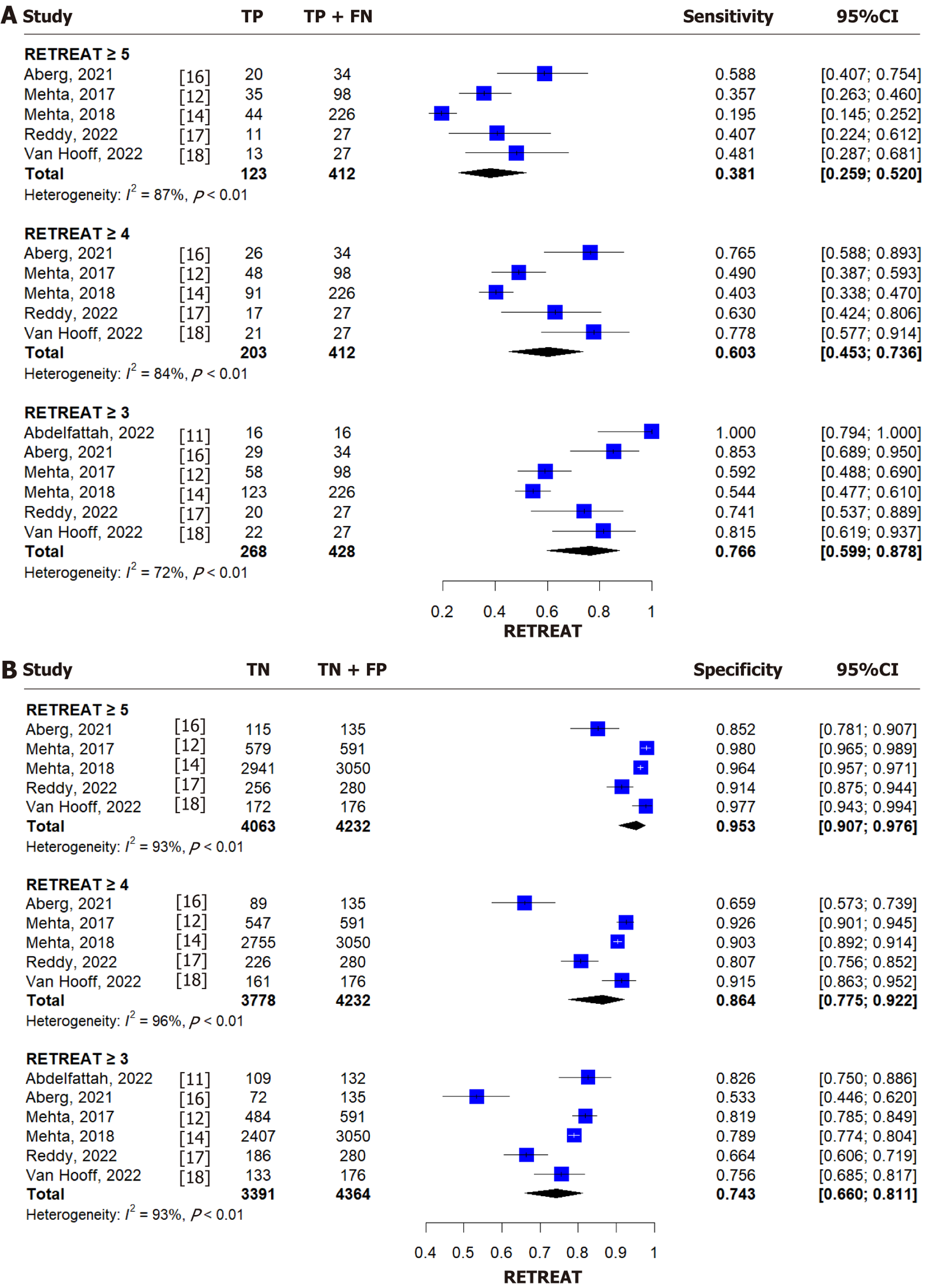
For a RETREAT score ≥ 5, the pooled sensitivity and specificity were 0.381 (95%CI: 0.259-0.520, I² = 87%) and 0.953 (95%CI: 0.907-0.976, I² = 93%), respectively (Figure 3)[11,12,14,16-18]. For a score ≥ 4, the sensitivity and specificity were 0.603 (95%CI: 0.453-0.736, I² = 84%) and 0.864 (95%CI: 0.775-0.922, I² = 96%) (Figure 3)[11,12,14,16-18]. The corresponding AUC based on summary ROC curve analysis was 0.807 (95%CI: 0.611-0.880). For a score ≥ 3, pooled sensitivity and specificity were 0.766 (95%CI: 0.599-0.878, I² = 72%) and 0.743 (95%CI: 0.660-0.811, I² = 93%) (Figure 3)[11,12,14,16-18]. Diagnostic performance (AUC and 95%CI) for all RETREAT thresholds is summarized in Tables 2 and 3.
| Score | RETREAT ≥ 5 | RETREAT ≥ 4 | RETREAT ≥ 3 | PCRS ≥ 1 | PCRS ≥ 3 | Decaens ≥ 4 |
| RETREAT ≥ 5 | - | 0.963 | 0.860 | 0.809 | 0.941 | 0.913 |
| RETREAT ≥ 4 | 0.963 | - | 0.632 | 0.697 | 0.956 | 0.779 |
| RETREAT ≥ 3 | 0.860 | 0.632 | - | 0.382 | 0.931 | 0.948 |
| PCRS ≥ 1 | 0.809 | 0.697 | 0.382 | - | 0.903 | 0.545 |
| PCRS ≥ 3 | 0.941 | 0.956 | 0.931 | 0.903 | - | 0.940 |
| Decaens ≥ 4 | 0.913 | 0.779 | 0.948 | 0.545 | 0.940 | - |
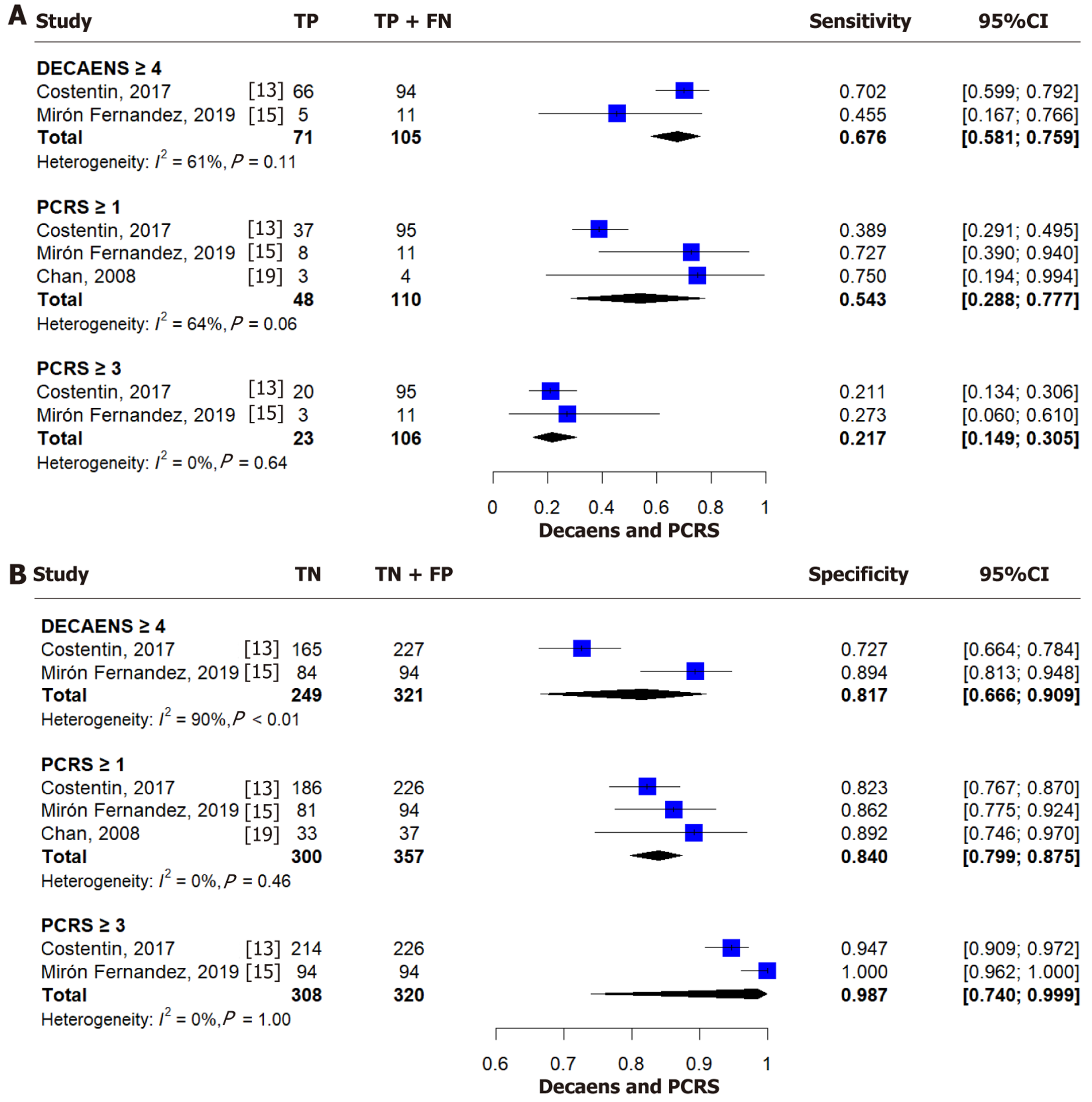
For a Decaens score ≥ 4, the pooled sensitivity and specificity were 0.676 (95%CI: 0.581-0.759, I² = 61%) and 0.817 (95%CI: 0.666-0.909, I² = 90%), respectively (Figure 4)[13,15,19]. AUROC values are provided in Tables 2 and 3.
For a PCRS score ≥ 1, the pooled sensitivity was 0.543 (95%CI: 0.288-0.777, I² = 64%) and specificity was 0.840 (95%CI: 0.799-0.875, I² = 0%) (Figure 4)[13,15,19]. For a score ≥ 3, sensitivity dropped to 0.217 (95%CI: 0.149-0.305, I² = 0%) while specificity increased to 0.987 (95%CI: 0.740-0.999, I² = 0%) (Figure 4B)[13,15,19]. Corresponding AUROC values are summarized in Tables 2 and 3.
The risk of bias across individual studies, as evaluated using the QUADAS-2 tool, is presented in Supplementary Table 2.
To the best of our knowledge, this is the first systematic review and meta-analysis to evaluate the prognostic performance of predictive models based on explant pathology data for estimating HCC recurrence following LT. A total of nine studies and three predictive scores-RETREAT, Decaens, and PCRS-were included[12,19,33]. These models were generally constructed using multivariable analyses, incorporating variables with significant associations (P < 0.05) in univariable analysis. The most frequently used predictors included AFP, tumor size and number, histological differentiation, and vascular invasion.
Across the included studies (excluding Costentin et al[13], which did not report recurrence rates), the pooled HCC recurrence rate post-LT was approximately 7% (347/4976). Recurrence rates varied significantly, from 4.4% in a large United States cohort of 3276 patients[14] to 20.1% in a smaller Swedish cohort of 169 patients[15].
The PCRS (or Chan score), developed in 2008, was derived from a cohort of 116 United States patients who underwent LT with HCC found on explant-18.9% of which were incidental tumors. This relatively small and heterogeneous cohort may limit generalizability. The score includes four explant variables: (1) Diameter of the largest nodule; (2) Bilobar involvement; (3) Macrovascular invasion; and (4) Tumor differentiation. The score ranges from -3 to 6, stratifying patients into low (≤ 0), intermediate (1-2), and high (≥ 3) risk categories. In the original cohort, no recurrences occurred in the low-risk group, while recurrence rates were 19.3% and 66.7% in the intermediate-risk and high-risk groups, respectively[19]. In our meta-analysis, patients with PCRS ≥ 3 had a recurrence probability of 66% within five years. The sensitivity and specificity for PCRS > 3 were 22.3% (95%CI: 15.4%-31.1%) and 95.0% (95%CI: 91.5%-97.1%), respectively, indicating high specificity but poor sensitivity. The AUROC for this cutoff was 0.431 (95%CI: 0.246-0.952), suggesting limited overall performance and considerable uncertainty due to a wide confidence interval. The limited sample size and heterogeneity of the included cohort, combined with the scarcity of external validation studies, may undermine the reliability of the pooled estimates. The broad confidence intervals and suboptimal sensitivity suggest that PCRS should not be used in isolation for clinical decision-making. Rigorous prospective validation is necessary prior to its routine clinical imp
The Decaens score, published in 2011, was developed to improve LT candidate selection and was intended for pre-transplant use. It was derived from 373 patients transplanted in France between 1988 and 1998. The score incorporates the number of nodules, the maximum tumor diameter (based on imaging at listing), and tumor differentiation-requiring biopsy, which was performed in only 11% of the cohort. Thus, tumor grading was ultimately based on explant pathology. Patients with scores > 4 were at higher risk of recurrence. In the original cohort, 5-year recurrence was 46% for scores ≥ 4 and 20.8% for scores < 4[33]. In our meta-analysis, high-risk patients (score ≥ 4) had a 50% recurrence rate within five years. Sensitivity and specificity were 0.591 (95%CI: 0.329-0.810) and 0.821 (95%CI: 0.597-0.934), respectively, with an AUC of 0.772-indicating acceptable discriminative ability. However, the wide confidence interval for sensitivity warrants caution. Moreover, this score is not applicable to patients with nonviable tumors, limiting its clinical utility.
The RETREAT score, developed from a cohort of 721 United States patients who underwent LT within the Milan criteria between 2002 and 2012, incorporates AFP at transplant, microvascular invasion, and the sum of the largest viable tumor diameter and number of viable tumors. It stratifies recurrence risk across six score levels. In the development cohort, 5-year recurrence increased from 2.9% (score 0) to 75.2% (score ≥ 5), outperforming the Milan criteria[12]. Validation using the UNOS database (3276 patients) confirmed its superior classification performance, as measured by the Net Reclassification Index (NRI) (NRI: 0.28, P < 0.001)[14]. However, the NRI has recently been criticized[34]. In this validation cohort, recurrence at three years ranged from 1.6% (score 0) to 29.0% (score > 5). It is worth noting that the median follow-up in the validation cohort was only 1.9 months, potentially underestimating recurrence compared to the development cohort (11.6% vs 4.4%).
Our analysis revealed that the RETREAT score's sensitivity and specificity varied by cutoff level (Supplementary Table 1)[11-19,21-32]. For RETREAT ≥ 5, specificity was 95.3% (95%CI: 90.7%-98.5%) but sensitivity was low. Lowering the cutoff to ≥ 4 increased sensitivity to 57.3% and reduced specificity to 84.3%; at ≥ 3, sensitivity rose to 77.4%, while specificity declined further to 66.4%. This trade-off highlights the model's high negative predictive value, supporting its utility in identifying patients at low risk who may benefit from less intensive surveillance.
All three scores demonstrated high specificity (≥ 74%), particularly RETREAT, supporting their value in excluding low-risk individuals. Positive predictive value (PPV) increased with higher score thresholds, reinforcing their utility in risk stratification. For instance, RETREAT’s PPV rose from 27.3% (≥ 3) to 51.4% (≥ 5), and PCRS ≥ 3 achieved the highest PPV at 65.7% (95%CI: 48.8%-79.4%).
Although the models show limited sensitivity at higher thresholds, their clinical utility remains evident, especially in identifying high-risk individuals. The Decaens score (≥ 4) provides a balance of sensitivity and specificity, making it a robust tool among those evaluated. These models-despite relying primarily on morphometric and surrogate biological markers (e.g., AFP)-can guide targeted post-transplant strategies.
There remains debate over whether surveillance should be universal or limited to high-risk patients. Current guidelines-including the American Association for the Study of Liver Diseases 2023 Practice Guidance and the International Liver Cancer Association-International Liver Transplantation Society (ILTS) 2024 consensus-recommend sur
Despite a lack of evidence-based protocols, existing data suggest that closer surveillance improves outcomes and access to curative therapies[36]. The ILTS recommends abdominal/chest imaging and AFP every 3 months for the first 3 years[35], while the National Comprehensive Cancer Network suggests imaging and AFP every 3-6 months for 2 years, then every 6-12 months[37].
Importantly, patients transplanted for HCC may have distinct immunologic profiles, influencing both rejection risk and recurrence[38]. Gene expression signatures hold promise for personalized post-LT management[38,39].
This meta-analysis has limitations. All included studies were retrospective in nature, introducing inherent risks of selection and reporting biases-particularly with respect to patient inclusion criteria, data completeness, and follow-up duration. These limitations constrain the generalizability of our findings and underscore the need for prospective, multicenter validation cohorts to confirm the performance of these models under standardized conditions. Differences in patient characteristics, inclusion criteria, and outcome definitions may have contributed to heterogeneity. The overall recurrence rate (7%) is lower than in other large cohorts, potentially due to underreporting or short follow-up. None of the studies included patients undergoing living donor LT; however, a systematic review found no significant recurrence rate differences based on donor type[40]. We could not include promising models such as COMBO-MORAL[21], R3-AFP[22], and RELAPSE[23] due to insufficient data, which limits the scope of our conclusions and highlights the need for future validation in diverse populations.
Despite these limitations, consistent methodology strengthens the validity of our findings. This review provides cli
Patients with higher scores based on explant data exhibit a markedly elevated risk of HCC recurrence within five years post-transplant. Although current guidelines do not endorse a specific model for surveillance, our findings support their utility in guiding follow-up. Deep learning model has been applied in this context. While AUROC comparisons do not establish the clear superiority of one model, high-risk thresholds-RETREAT ≥ 5 (37% recurrence), Decaens ≥ 4 (50%), and PCRS ≥ 3 (66%)-clearly identify patients who require intensive surveillance. These scores may enable better resource allocation, enhancing cost-effectiveness and early recurrence detection. Low-risk patients may benefit from less frequent follow-up. Our review also highlights the need to integrate histopathological, immunological, and treatment-related variables into future predictive models, ideally in large prospective studies using artificial intelligence to improve stratification and personalize care.
The authors thank Hospital Vozandes Quito-HVQ, Quito, Ecuador, for their support in the publication of this article.
| 1. | Mehta N, Bhangui P, Yao FY, Mazzaferro V, Toso C, Akamatsu N, Durand F, Ijzermans J, Polak W, Zheng S, Roberts JP, Sapisochin G, Hibi T, Kwan NM, Ghobrial M, Soin A. Liver Transplantation for Hepatocellular Carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1136-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 2. | Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA, Mendiratta-Lala M, Brown DB, Rilling WS, Goyal L, Wei AC, Taddei TH. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922-1965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 900] [Cited by in RCA: 1161] [Article Influence: 387.0] [Reference Citation Analysis (23)] |
| 3. | Bzeizi KI, Abdullah M, Vidyasagar K, Alqahthani SA, Broering D. Hepatocellular Carcinoma Recurrence and Mortality Rate Post Liver Transplantation: Meta-Analysis and Systematic Review of Real-World Evidence. Cancers (Basel). 2022;14:5114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 4. | Marrone G, Leone MS, Biolato M, Liguori A, Bianco G, Spoletini G, Gasbarrini A, Miele L, Pompili M. Therapeutic Approach to Post-Transplant Recurrence of Hepatocellular Carcinoma: Certainties and Open Issues. Cancers (Basel). 2023;15:5593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Al-Ameri A, Yu X, Zheng S. Predictors of post-recurrence survival in hepatocellular carcinoma patients following liver transplantation: Systematic review and meta-analysis. Transplant Rev (Orlando). 2022;36:100676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Hoffman D, Mehta N. Recurrence of hepatocellular carcinoma following liver transplantation. Expert Rev Gastroenterol Hepatol. 2021;15:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM; and the PRISMA-DTA Group, Clifford T, Cohen JF, Deeks JJ, Gatsonis C, Hooft L, Hunt HA, Hyde CJ, Korevaar DA, Leeflang MMG, Macaskill P, Reitsma JB, Rodin R, Rutjes AWS, Salameh JP, Stevens A, Takwoingi Y, Tonelli M, Weeks L, Whiting P, Willis BH. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA. 2018;319:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1683] [Cited by in RCA: 2332] [Article Influence: 291.5] [Reference Citation Analysis (0)] |
| 8. | Barros I, Fernandes M, Brandao A. Performance of predictive models for hepatocellular carcinoma recurrence after liver transplantation: A systematic review and meta-analysis. PROSPERO. 2024;. |
| 9. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5393] [Article Influence: 179.8] [Reference Citation Analysis (7)] |
| 10. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 10396] [Article Influence: 693.1] [Reference Citation Analysis (3)] |
| 11. | Abdelfattah MR, El-Haddad HM, Elsiesy H. Validation of Risk Estimation of Tumor Recurrence After Transplant Score in Patients With Hepatocellular Carcinoma Treated by Liver Transplant. Exp Clin Transplant. 2021;19:1298-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, Burns JM, Sanchez W, Greig PD, Grant DR, Roberts JP, Yao FY. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017;3:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 310] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 13. | Costentin CE, Amaddeo G, Decaens T, Boudjema K, Bachellier P, Muscari F, Salamé E, Bernard PH, Francoz C, Dharancy S, Vanlemmens C, Radenne S, Dumortier J, Hilleret MN, Chazouillères O, Pageaux GP, Calderaro J, Laurent A, Roudot-Thoraval F, Duvoux C; Liver Transplantation French Study Group. Prediction of hepatocellular carcinoma recurrence after liver transplantation: Comparison of four explant-based prognostic models. Liver Int. 2017;37:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Mehta N, Dodge JL, Roberts JP, Yao FY. Validation of the prognostic power of the RETREAT score for hepatocellular carcinoma recurrence using the UNOS database. Am J Transplant. 2018;18:1206-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 15. | Mirón Fernández I, León Díaz FJ, Sánchez Segura J, Sánchez Pérez B, Pérez Daga JA, Fernández Aguilar JL, Montiel Casado MC, Santoyo Santoyo J. Comparison of 3 Explant-Based Prognostic Models as Predictors of Hepatocellular Carcinoma Recurrence After Liver Transplantation: Analysis of Our Experience. Transplant Proc. 2019;51:80-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Åberg F, Abrahamsson J, Schult A, Bennet W, Rizell M, Sternby-Eilard M. The RETREAT score provides valid predictions regarding hepatocellular carcinoma recurrence after liver transplantation. Transpl Int. 2021;34:2869-2874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Reddy SHS, Mehta N, Dodge JL, Hakeem AR, Khorsandi SE, Jassem W, Vilca-Melendez H, Cortes-Cerisuelo M, Srinivasan P, Prachalias A, Heneghan MA, Aluvihare V, Suddle A, Miquel R, Rela M, Heaton ND, Menon KV. Liver transplantation for HCC: validation of prognostic power of the RETREAT score for recurrence in a UK cohort. HPB (Oxford). 2022;24:596-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | van Hooff MC, Sonneveld MJ, Ijzermans JN, Doukas M, Sprengers D, Metselaar HJ, den Hoed CM, de Man RA. External Validation of the RETREAT Score for Prediction of Hepatocellular Carcinoma Recurrence after Liver Transplantation. Cancers (Basel). 2022;14:630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Chan EY, Larson AM, Fix OK, Yeh MM, Levy AE, Bakthavatsalam R, Halldorson JB, Reyes JD, Perkins JD. Identifying risk for recurrent hepatocellular carcinoma after liver transplantation: implications for surveillance studies and new adjuvant therapies. Liver Transpl. 2008;14:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Abdelfattah MR, Elsiesy H. Post-liver transplant HCC recurrence: Patterns, treatment, and outcome. Cell Ther Transplant. 2022;11:39-44. [DOI] [Full Text] |
| 21. | Agopian VG, Harlander-Locke M, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, Finn RS, Tong M, Hiatt JR, Busuttil RW. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 22. | Costentin C, Piñero F, Degroote H, Notarpaolo A, Boin IF, Boudjema K, Baccaro C, Podestá LG, Bachellier P, Ettorre GM, Poniachik J, Muscari F, Dibenedetto F, Duque SH, Salame E, Cillo U, Marciano S, Vanlemmens C, Fagiuoli S, Burra P, Van Vlierberghe H, Cherqui D, Lai Q, Silva M, Rubinstein F, Duvoux C; French-Italian-Belgium and Latin American collaborative group for HCC and liver transplantation. R3-AFP score is a new composite tool to refine prediction of hepatocellular carcinoma recurrence after liver transplantation. JHEP Rep. 2022;4:100445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 23. | Tran BV, Moris D, Markovic D, Zaribafzadeh H, Henao R, Lai Q, Florman SS, Tabrizian P, Haydel B, Ruiz RM, Klintmalm GB, Lee DD, Taner CB, Hoteit M, Levine MH, Cillo U, Vitale A, Verna EC, Halazun KJ, Tevar AD, Humar A, Chapman WC, Vachharajani N, Aucejo F, Lerut J, Ciccarelli O, Nguyen MH, Melcher ML, Viveiros A, Schaefer B, Hoppe-Lotichius M, Mittler J, Nydam TL, Markmann JF, Rossi M, Mobley C, Ghobrial M, Langnas AN, Carney CA, Berumen J, Schnickel GT, Sudan DL, Hong JC, Rana A, Jones CM, Fishbein TM, Busuttil RW, Barbas AS, Agopian VG. Development and validation of a REcurrent Liver cAncer Prediction ScorE (RELAPSE) following liver transplantation in patients with hepatocellular carcinoma: Analysis of the US Multicenter HCC Transplant Consortium. Liver Transpl. 2023;29:683-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Parfitt JR, Marotta P, Alghamdi M, Wall W, Khakhar A, Suskin NG, Quan D, McAllister V, Ghent C, Levstik M, McLean C, Chakrabarti S, Garcia B, Driman DK. Recurrent hepatocellular carcinoma after transplantation: use of a pathological score on explanted livers to predict recurrence. Liver Transpl. 2007;13:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Halazun KJ, Najjar M, Abdelmessih RM, Samstein B, Griesemer AD, Guarrera JV, Kato T, Verna EC, Emond JC, Brown RS Jr. Recurrence After Liver Transplantation for Hepatocellular Carcinoma: A New MORAL to the Story. Ann Surg. 2017;265:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 227] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 26. | Feng J, Zhu R, Feng D, Yu L, Zhao D, Wu J, Yuan C, Chen J, Zhang Y, Zheng X. Prediction of Early Recurrence of Solitary Hepatocellular Carcinoma after Orthotopic Liver Transplantation. Sci Rep. 2019;9:15855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Sánchez Segura J, León Díaz FJ, Pérez Reyes M, Cabañó Muñoz D, Sánchez Pérez B, Pérez Daga JA, Montiel Casado C, Santoyo Santoyo J. Predictive Models of Hepatocellular Carcinoma Recurrence After Liver Transplantation. Transplant Proc. 2020;52:546-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Hasan B, Colak Y, Khalid RA, Castillo M, Castaneda D, Tandon K, Shaw JJ, Erim T, Zervos XB, Castro FJ, Al-Khalloufi K. Early Detection of Hepatocellular Carcinoma Recurrence in the Posttransplant Population: A Comparison of RETREAT and Cleveland Clinic Florida Scoring System. Transplant Proc. 2021;53:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Ma E, Li J, Xing H, Li R, Shen C, Zhang Q, Ma Z, Tao Y, Qin L, Zhao J, Wang Z. Development of a predictive nomogram for early recurrence of hepatocellular carcinoma in patients undergoing liver transplantation. Ann Transl Med. 2021;9:468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Aziz S, Sey M, Marotta P, Driman D, Parfitt J, Teriaky A, Brahmania M, Skaro A, Qumosani K. Recurrent Hepatocellular Carcinoma After Liver Transplantation: Validation of a Pathologic Risk Score on Explanted Livers to Predict Recurrence. Transplant Proc. 2021;53:1975-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Cuadrado A, Fortea JI, Rodríguez-Lope C, Puente Á, Fernández-Vilchez V, Echavarria VJ, Castillo Suescun FJ, Fernández R, Echeverri JA, Achalandabaso M, Toledo E, Pellón R, Rodríguez Sanjuan JC, Crespo J, Fábrega E. Risk of Recurrence of Hepatocarcinoma after Liver Transplantation: Performance of Recurrence Predictive Models in a Cohort of Transplant Patients. J Clin Med. 2023;12:5457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | Brandão ABM, Rodriguez S, Marroni CA, Junior AMF, Fernandes MV, Mucenic M. Performance of eight predictive models for hepatocellular carcinoma recurrence after liver transplantation: A comparative study. Ann Hepatol. 2024;29:101184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 33. | Decaens T, Roudot-Thoraval F, Badran H, Wolf P, Durand F, Adam R, Boillot O, Vanlemmens C, Gugenheim J, Dharancy S, Bernard PH, Boudjema K, Calmus Y, Hardwigsen J, Ducerf C, Pageaux GP, Hilleret MN, Chazouillères O, Cherqui D, Mallat A, Duvoux C. Impact of tumour differentiation to select patients before liver transplantation for hepatocellular carcinoma. Liver Int. 2011;31:792-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Kerr KF. Net Reclassification Index Statistics Do Not Help Assess New Risk Models. Radiology. 2023;306:e222343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Berenguer M, Burra P, Ghobrial M, Hibi T, Metselaar H, Sapisochin G, Bhoori S, Kwan Man N, Mas V, Ohira M, Sangro B, van der Laan LJW. Posttransplant Management of Recipients Undergoing Liver Transplantation for Hepatocellular Carcinoma. Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1143-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 36. | Verna EC, Patel YA, Aggarwal A, Desai AP, Frenette C, Pillai AA, Salgia R, Seetharam A, Sharma P, Sherman C, Tsoulfas G, Yao FY. Liver transplantation for hepatocellular carcinoma: Management after the transplant. Am J Transplant. 2020;20:333-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 37. | Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, Chahal P, Chang DT, Cloyd J, Covey AM, Glazer ES, Goyal L, Hawkins WG, Iyer R, Jacob R, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Sahai V, Schefter T, Singh G, Stein S, Vauthey JN, Venook AP, Yopp A, McMillian NR, Hochstetler C, Darlow SD. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 647] [Article Influence: 129.4] [Reference Citation Analysis (2)] |
| 38. | Li CX, Ling CC, Shao Y, Xu A, Li XC, Ng KT, Liu XB, Ma YY, Qi X, Liu H, Liu J, Yeung OW, Yang XX, Liu QS, Lam YF, Zhai Y, Lo CM, Man K. CXCL10/CXCR3 signaling mobilized-regulatory T cells promote liver tumor recurrence after transplantation. J Hepatol. 2016;65:944-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 39. | Chiang CC, Yeh H, Lim SN, Lin WR. Transcriptome analysis creates a new era of precision medicine for managing recurrent hepatocellular carcinoma. World J Gastroenterol. 2023;29:780-799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Elkomos BE, Abdo M, Mamdouh R, Abdelaal A. Can living donor liver transplantation provide similar outcomes to deceased-donor liver transplantation for hepatocellular carcinoma? A systematic review and meta-analysis. Hepatol Int. 2023;17:18-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/