Published online Sep 27, 2025. doi: 10.4254/wjh.v17.i9.109340
Revised: June 21, 2025
Accepted: September 2, 2025
Published online: September 27, 2025
Processing time: 140 Days and 22.6 Hours
Occult hepatitis B virus infection (OBI) is defined by the detection of replication-competent hepatitis B virus (HBV) DNA in the liver and/or blood despite the ab
A 33-year-old Chinese male was diagnosed with HBV infection in 2001. The patient first presented in 2012 with abnormal liver function tests and received initial treatment with conventional interferon therapy, which failed to achieve a virological response. Antiviral therapy was subsequently switched to entecavir monotherapy. By August 2019, the patient exhibited an HBsAg level of 29.93 IU/mL with undetectable HBV DNA (< 25 IU/mL). At this point, combination therapy with entecavir and pegylated interferon α (PEG-IFN α) was initiated. Remarkably, while HBsAg declined to 0.42 IU/mL by April 2020, a paradoxical HBV DNA rebound to 173 IU/mL was observed. The regimen was consequently modified to tenofovir alafenamide and PEG-IFN α. By October 2020, the patient achieved HBsAg seroconversion (HBsAg 0.01 IU/mL, hepatitis B surface antibody 52.18 mIU/mL) for the first time, while maintaining low-level viremia (37 IU/mL), consistent with transition to OBI. The patient was then switched to PEG-IFN α monotherapy. In November 2021, he discontinued PEG-IFN α therapy, and one month later, both HBV DNA (< 10 IU/mL) and HBsAg (< 0.05 IU/mL) were negative. This response has been sustained through follow-up.
This case study illustrates the efficacy of sequential combination therapy in achieving functional cure in CHB patients, including those with a prolonged infection history. It highlights OBI as a transitional yet underrecognized phase during sequential antiviral therapy. While the patient ultimately achieved functional cure, the transient persistence of HBV DNA despite HBsAg clearance suggests the need for continued monitoring. This case provides new insights into OBI development during treatment and underscores the importance of further research into its long-term implications.
Core Tip: This case illustrates the emergence of occult hepatitis B infection (OBI) as a distinct transitional phase during sequential combination therapy for chronic hepatitis B. Unlike previously reported cases where OBI develops in individuals with resolved infection, this case suggests that antiviral therapy itself may induce an OBI-like state before achieving functional cure. As OBI has been associated with potential reactivation risks. Understanding the mechanisms behind treatment-induced OBI will be crucial in refining therapeutic strategies and improving long-term hepatitis B virus management.
- Citation: Wang L, Liang H, Wang C, Liang MY, Zeng QL, Zhu PF, Lv J. Functional cure in an occult hepatitis B virus infection patient on sequential therapy: A case report. World J Hepatol 2025; 17(9): 109340
- URL: https://www.wjgnet.com/1948-5182/full/v17/i9/109340.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i9.109340
Hepatitis B virus (HBV) infection is a significant global health problem. Worldwide, an estimated 254 million people are chronic carriers of hepatitis B surface antigen (HBsAg), of which approximately 20%-40% require antiviral therapy based on current guideline recommendations[1]. Approved anti-HBV therapies include interferon (IFN) and nucleos(t)ide ana
The current therapeutic goal is a functional cure, defined as sustained HBsAg loss (< 0.05 IU/mL) and undetectable HBV DNA at the end of treatment. Combination therapy is frequently employed to achieve this goal, as numerous studies have demonstrated its effectiveness in restoring the host's antiviral immune response and eliminating the virus[5].
Occult hepatitis B virus infection (OBI) is characterized by the persistence of replication-competent HBV DNA in the liver and/or blood despite the absence of detectable serum HBsAg (< 0.05 IU/mL). It has been observed in individuals with resolved HBV infection, those receiving immunosuppressive therapy, and patients undergoing antiviral treatment. While OBI is generally considered a low-replication state, its clinical implications remain uncertain, particularly regarding the risk of viral reactivation, transmission, and liver disease progression[6]. The occurrence of OBI during antiviral therapy is not well understood, and its role as a transitional phase in HBV treatment has not been extensively studied.
Here, we present a case of CHB that transitioned through OBI during sequential combination therapy before ultimately achieving functional cure. This case provides new insights into the significance of OBI during treatment and underscores the need for continued monitoring.
A 33-year-old Chinese male presented to the Department of Infectious Diseases of the First Affiliated Hospital of Zhengzhou University in August 2019 with 18 years of CHB and recurrent liver function abnormalities for 3 months.
The patient was diagnosed with HBV infection in 2001, presenting as HBsAg-positive, hepatitis B e antigen (HBeAg)-positive, and hepatitis B c antibodies-positive, with normal liver function tests, and was not treated. In 2012, he developed abnormal liver function and was treated with short-acting IFN, but the response was poor, leading to a switch to en
The patient did not have a history of diabetes, hypertension, heart disease, tuberculosis, chronic hepatitis C virus in
The patient did not have a history of smoking or alcohol consumption, and there was no familial history of similar diseases.
The patient’s body temperature was 36.9 °C, pulse was 79 beats per minute, respiratory rate was 18 breaths per minute, and blood pressure was 129/79 mmHg. The patient exhibited no liver palms or spider angiomas, and jaundice was not observed in either the skin or sclera. The abdomen appeared flat, with no visible abdominal wall varicosities and no tenderness or rebound tenderness noted. The liver and the spleen were not palpable. The patient had no ascites and no edema in both lower limbs.
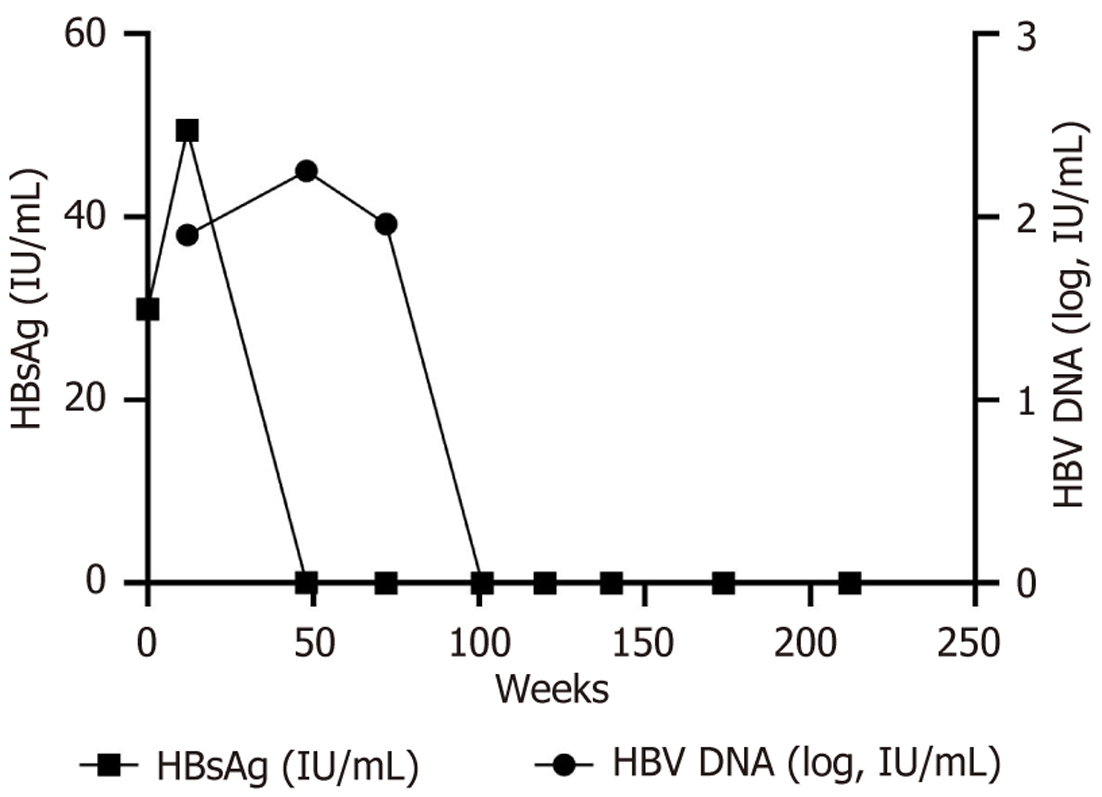
Laboratory examinations are shown in Table 1. Changes in serum HBsAg and HBV DNA are shown in Figure 1. Test results and changes throughout treatment were tallied using GraphPad Prism 9 software.
| Weeks | Treatment regimen | HBV-DNA (IU/mL) | HBsAg (0-0.05 IU/mL) | HBsAb (0-10 mIU/mL) | HBeAg (0-0.18 PEIU/mL) | HBeAb (1-999 S/CO) | HBcAb (0-1 S/CO) | ALT (0-40 U/L) | AST (0-40 U/L) | GGT (0-58 U/L) | CAP (< 238 dB/m) | LSM (2.5-7kPa) | AST/ALT | |
| 0 | ETV | < 251 | 29.93 | 0.029 | 0.029 | 1.093 | 0.029 | 29 | 37 | 35 | 269 | 6.3 | 1.28 | |
| 4 | ETV + PEG | — | 445.65 | 0.68 | 0.11 | 1.14 | 8.94 | 71 | 75 | 53 | - | - | 1.06 | |
| 8 | ETV + PEG | — | 337.45 | 0.24 | 0.09 | 1.13 | 8.75 | 216 | 217 | 95 | - | - | 1.00 | |
| 12 | ETV + PEG | 8.0E + 011 | 49.48 | 0.31 | 0.11 | 1.16 | 8.92 | 126 | 129 | 105 | - | - | 1.02 | |
| 16 | ETV + PEG | 1.40E + 021 | 5.47 | 0.28 | 0.1 | 1.15 | 8.03 | 109 | 116 | 113 | 230 | 7.1 | 1.06 | |
| 33 | ETV + PEG | 1.73E + 021 | 0.42 | 1.96 | 0.11 | 1.1 | 6.99 | 122 | 119 | 120 | 278 | 7.9 | 0.98 | |
| 48 | ETV + PEG | 1.77E + 022 | 0.06 | 6 | 0.11 | 1.13 | 9.37 | 61 | 73 | 86 | 294 | 11.7 | 1.19 | |
| OBI phase | 61 | ETV + PEG | 3.70E + 012 | 0.01 | 52.18 | 0.14 | 1.2 | 8.55 | 91 | 101 | 119 | - | - | 1.11 |
| 72 | ETV + PEG | 9.20E + 012 | 0.01 | 142.41 | 0.14 | 1.3 | 9.32 | 98 | 119 | 117 | 253 | 9.8 | 1.21 | |
| 82 | PEG | 1.90E + 012 | 0 | 134.59 | 0.14 | 1.55 | 8.44 | 122 | 157 | 112 | - | - | 1.29 | |
| 91 | PEG | 1.10E + 012 | 0.01 | 82.28 | 0.13 | 1.3 | 7.12 | 73 | 90 | 119 | - | - | 1.23 | |
| 101 | PEG | < 102 | 0.02 | 49.55 | 0.15 | 1.32 | 7.51 | 65 | 80 | 94 | - | - | 1.23 | |
| 115 | PEG | 4.10E + 012 | 0.02 | 58.37 | 0.18 | 1.54 | 7.53 | 30 | 72 | 120 | - | - | 2.40 | |
| 120 | D/C PEG for 1 months | < 102 | 0 | 89.18 | 0.22 | 1.43 | 7.64 | - | - | - | - | - | - | |
| 129 | D/C PEG for 3 months | < 102 | 0 | 91.6 | 0.16 | 1.36 | 6.37 | 26 | 29 | 34 | - | - | 1.12 | |
| 140 | D/C PEG for 6 months | < 102 | 0 | 112.26 | 0.14 | 1.32 | 8.73 | 23 | 25 | 33 | - | - | 1.09 | |
| 154 | D/C PEG for 9 months | - | 0 | 123.62 | 0.16 | 1.34 | 7.05 | 18 | 26 | 28 | - | - | 1.44 | |
| 174 | D/C PEG for 13 months | < 102 | 0 | 44.04 | 0.15 | 1.42 | 6.49 | 20 | 26 | 26 | 176 | 7 | 1.30 | |
| 188 | D/C PEG for 17 months | < 102 | 0.03 | 30.84 | 0.18 | 1.46 | 6.61 | 20 | 30 | 30 | - | - | 1.60 | |
| 212 | D/C PEG for 23 months | < 251 | 0 | 8.11 | 0.17 | 1.02 | 6.31 | 20 | 29 | 30 | 239 | 5.9 | 1.45 | |
Abdominal ultrasonography in the patient revealed a liver of normal size with a regular, homogeneous parenchymal structure and no focal lesions.
Based on the clinical presentation, laboratory findings, and exclusion of other causes, a diagnosis of CHB was established.
In August 2019, laboratory tests showed HBsAg level of 29.93 IU/mL, HBeAg-negative (< 0.18 PEIU/mL), HBV DNA below the lower limit of detection (< 25 IU/mL), controlled attenuation parameter 269 dB/m and liver stiffness measurement 6.3 kPa. At this time, HBV DNA was negative (< 25 IU/mL), and HBsAg was at a low level, because ETV can potently inhibit HBV DNA replication but has a low clearance of HBsAg. Pegylated interferon α (PEG-IFN α) can promote HBsAg serological conversion by activating the immune system and breaking immune tolerance. Therefore, the combination of ETV and PEG-IFN α was given.
In November 2019, the patient presented with a biochemical episode of hepatitis B (ALT 126 U/L, aspartate aminotransferase 129 U/L). At this time, HBV DNA 80 IU/mL, HBsAg 49.48 IU/mL. The patient had no history of other medications (anti-tuberculosis medications, herbal medications, etc.) or recent alcohol consumption. His relevant examinations: Total bilirubin 11.4 μmol/L, albumin 47.1 g/L, for the time being, liver injury caused by HBV DNA virological breakthrough, fatty liver, and alcoholic liver disease were not considered. PEG-IFN α can cause hepatocellular injury and ALT elevation, most often occurring at 4-12 weeks of treatment, with ALT elevations typically ranging from 2-10 times the upper limit of normal. As the patient was in good general condition (no jaundice, normal coagulation), the com
Several test data points between weeks 16 and 33 of the combined treatment were missing due to unforeseen circumstances. In December 2019, the patient returned to his hometown of Wuhan, which was subsequently affected by the outbreak of coronavirus disease 2019 and placed under lockdown. As a result, the patient was unable to undergo the required tests during this period. However, the patient ensured that the treatment continued as scheduled by purchasing necessary medications in advance or having them mailed to maintain the course of treatment.
By April 2020, HBV DNA increased to 173 IU/mL while HBsAg decreased to 0.42 IU/mL. At this time, controlled attenuation parameter 278 dB/m and liver stiffness measurement 7.9 kPa. Given tenofovir alafenamide (TAF)’s superior efficacy in HBV DNA suppression and fibrosis progression delay, the treatment was adjusted to a combination of TAF and PEG-IFN α.
By October 2020, the patient’s HBsAg turned negative (< 0.05 IU/mL) for the first time, with hepatitis B surface antibody at 52.18 IU/mL, and HBV DNA remained detectable (37 IU/mL). The patient had transitioned to OBI, characterized by undetectable HBsAg despite persistent low-level HBV DNA. Given this development, therapy was continued with PEG-IFN α monotherapy to promote further viral suppression.
By November 2021, HBV DNA levels were still detectable at 41 IU/mL, while HBsAg remained negative (< 0.05 IU/mL). The patient subsequently discontinued PEG-IFN α therapy.
One month after the end of treatment, follow-up tests confirmed that both HBV DNA (< 10 IU/mL) and HBsAg (< 0.05 IU/mL) were negative, with hepatitis B surface antibody of 89.18 IU/mL and normalized liver function. Subsequent follow-up assessments showed sustained HBsAg loss (< 0.05 IU/mL), undetectable HBV DNA, and normal liver function, indicating achievement of functional cure.
Recent advancements in CHB treatment have led to a clearer understanding and wider use of NAs and PEG-IFN α in achieving functional cure[7]. A meta-analysis showed that sequential combination therapy performed better in HBsAg regression compared to initial combination therapy[8]. “The expert consensus on clinical cure (functional cure) of chronic hepatitis B” from China outlines a roadmap for achieving functional cure of CHB[9]. According to this roadmap, patients with low baseline HBsAg levels, HBeAg negativity, and sequential PEG-IFN α treatment are more likely to achieve fun
OBI is commonly observed in individuals with resolved HBV infection or those undergoing immunosuppressive therapy[12,13], but its emergence during active antiviral treatment has not been extensively studied. In this case, the patient transitioned into an OBI state following sequential therapy with ETV, TAF, and PEG-IFN α, characterized by HBsAg clearance but persistent low-level HBV DNA before achieving functional cure.
The emergence of OBI during sequential combination therapy may be attributed to several mechanisms. One possible explanation is the suppression of HBsAg expression. Both PEG-IFN α and NAs effectively inhibit HBV replication and viral protein synthesis[14]. PEG-IFN α enhances immune-mediated clearance of HBsAg-producing hepatocytes[15], while NAs suppress HBV DNA replication[16]. As a result, HBsAg levels may decline below the detection limit while residual HBV DNA persists, leading to an OBI state. In addition to direct antiviral effects, host immune responses may also play a critical role in the development of OBI. Studies suggest that individuals with OBI exhibit stronger HBV-specific T-cell responses compared to those with overt infection[17]. This shift in the immune landscape could contribute to the sup
Although the patient ultimately achieved functional cure, the transient occurrence of OBI during antiviral therapy raises important clinical considerations. One of the primary concerns is the risk of HBV reactivation, particularly in individuals who become immunosuppressed or discontinue antiviral treatment. Previous studies have shown that OBI can serve as a potential reservoir for HBV reactivation, leading to progressive liver disease or fulminant hepatitis in high-risk populations[19,20]. Therefore, long-term monitoring of HBV DNA levels is crucial, even in patients who achieve HBsAg clearance. Another important consideration is the potential underestimation of OBI in clinical practice. Current monitoring strategies primarily focus on detecting HBsAg and HBV DNA, which may not be sufficient to identify OBI cases. During the OBI phase, the HBV genome exists as closed circular DNA and exhibits low replicative activity. As a result, HBV DNA can be detected intermittently in serum or plasma. Detection of HBV DNA using current routine methods may result in missed OBI. This highlights the need for revised clinical guidelines for more sensitive screening strategies, particularly in patients receiving antiviral therapy, especially those exhibiting signs of functional cure.
Additionally, the occurrence of OBI during sequential combination therapy raises questions about its role in the bro
To conclude, this case illustrates the emergence of OBI as a distinct phase during sequential combination therapy for chronic hepatitis B. Unlike previously reported cases where OBI develops in individuals with resolved infection, this case suggests that antiviral therapy itself may induce an OBI-like state before achieving functional cure. The persistence of low-level HBV DNA despite HBsAg clearance underscores the need for long-term monitoring, as OBI has been associated with potential reactivation risks. Understanding the mechanisms behind treatment-induced OBI will be crucial in refining therapeutic strategies and improving long-term HBV management.
We thank the patient for their participation and consent.
| 1. | Guidelines for the prevention, diagnosis, care and treatment for people with chronic hepatitis B infection [Internet]. Geneva: World Health Organization; 2024 Mar- . [PubMed] |
| 2. | Lok AS, McMahon BJ, Brown RS Jr, Wong JB, Ahmed AT, Farah W, Almasri J, Alahdab F, Benkhadra K, Mouchli MA, Singh S, Mohamed EA, Abu Dabrh AM, Prokop LJ, Wang Z, Murad MH, Mohammed K. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology. 2016;63:284-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 446] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 3. | Hermann A, Wennmann DO, Gromnitza S, Edeling M, Van Marck V, Sudol M, Schaefer L, Duning K, Weide T, Pavenstädt H, Kremerskothen J. WW and C2 domain-containing proteins regulate hepatic cell differentiation and tumorigenesis through the hippo signaling pathway. Hepatology. 2018;67:1546-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Woo ASJ, Kwok R, Ahmed T. Alpha-interferon treatment in hepatitis B. Ann Transl Med. 2017;5:159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Chen L, Lin L, Zhou H, Tang W, Wang H, Cai W, Bao S, Guo S, Xie Q. Peginterferon and Entecavir Combination Therapy Improves Outcome of Non-Early Response Hepatitis B e Antigen-Positive Patients. Open Forum Infect Dis. 2020;7:ofaa462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Raimondo G, Locarnini S, Pollicino T, Levrero M, Zoulim F, Lok AS; Taormina Workshop on Occult HBV Infection Faculty Members. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. 2019;71:397-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 400] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 7. | Wong GLH, Gane E, Lok ASF. How to achieve functional cure of HBV: Stopping NUCs, adding interferon or new drug development? J Hepatol. 2022;76:1249-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 200] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 8. | Qiu K, Liu B, Li SY, Li H, Chen ZW, Luo AR, Peng ML, Ren H, Hu P. Systematic review with meta-analysis: combination treatment of regimens based on pegylated interferon for chronic hepatitis B focusing on hepatitis B surface antigen clearance. Aliment Pharmacol Ther. 2018;47:1340-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Chinese Society of Infectious Disease Chinese Society of Hepatology; Chinese Medical Association. [The expert consensus on clinical cure (functional cure) of chronic hepatitis B]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:594-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 10. | Lim SG, Yang WL, Ngu JH, Chang J, Tan J, Ahmed T, Dan YY, Lim K, Lee YM, Lee GH, Tan PS, Wai KL, Phyo WW, Khine HHTW, Lee C, Tay A, Chan E. Switching to or Add-on Peginterferon in Patients on Nucleos(t)ide Analogues for Chronic Hepatitis B: The SWAP RCT. Clin Gastroenterol Hepatol. 2022;20:e228-e250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Kao JH, Jeng WJ, Ning Q, Su TH, Tseng TC, Ueno Y, Yuen MF. APASL guidance on stopping nucleos(t)ide analogues in chronic hepatitis B patients. Hepatol Int. 2021;15:833-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 12. | Michalak TI, Pardoe IU, Coffin CS, Churchill ND, Freake DS, Smith P, Trelegan CL. Occult lifelong persistence of infectious hepadnavirus and residual liver inflammation in woodchucks convalescent from acute viral hepatitis. Hepatology. 1999;29:928-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Saitta C, Pollicino T, Raimondo G. Occult Hepatitis B Virus Infection: An Update. Viruses. 2022;14:1504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 86] [Reference Citation Analysis (0)] |
| 14. | Martinez MG, Testoni B, Zoulim F. Biological basis for functional cure of chronic hepatitis B. J Viral Hepat. 2019;26:786-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Li Y, Yang S, Li C, Ma Z, Zhang M, Zou W, Wu Z, Hou H, Wang W, Zhu L. Efficacy of short-term Peg-IFN α-2b treatment in chronic hepatitis B patients with ultra-low HBsAg levels: a retrospective cohort study. Virol J. 2024;21:231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Broquetas T, Carrión JA. Current Perspectives on Nucleos(t)ide Analogue Therapy for the Long-Term Treatment of Hepatitis B Virus. Hepat Med. 2022;14:87-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 17. | Samal J, Kandpal M, Vivekanandan P. Molecular mechanisms underlying occult hepatitis B virus infection. Clin Microbiol Rev. 2012;25:142-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Mak LY, Wong DK, Pollicino T, Raimondo G, Hollinger FB, Yuen MF. Occult hepatitis B infection and hepatocellular carcinoma: Epidemiology, virology, hepatocarcinogenesis and clinical significance. J Hepatol. 2020;73:952-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 19. | Kwak MS, Kim YJ. Occult hepatitis B virus infection. World J Hepatol. 2014;6:860-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Lee BT, Perumalswami PV. Diagnosis and Management of Occult Hepatitis B Infection. Curr Hepatology Rep. 2020;19:354-361. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/