Published online Sep 27, 2025. doi: 10.4254/wjh.v17.i9.109179
Revised: June 28, 2025
Accepted: August 21, 2025
Published online: September 27, 2025
Processing time: 141 Days and 8.5 Hours
Cirrhosis is a progressive condition characterized by fibrosis that can lead to severe complications and increased mortality. The mac-2 binding protein glyco
To investigate thresholds of M2BPGi associated with HCC, EV, and decomp
This was a prospective study. A total of 153 patients with cirrhosis who met the inclusion criteria were enrolled. The patients were diagnosed with HCC and EV according to the Baveno VII and European Association for the Study of the Liver guidelines. Baseline serum M2BPGi levels were assessed along with other routine tests. The data analysis aimed to determine the cutoff values of M2BPGi for pre
In the study 85.6% of patients were Child-Pugh B and C. M2BPGi mean cutoff index was 7.1 ± 3.7, showing no significant etiological differences. However, M2BPGi levels varied significantly among Child-Pugh classes, EV classifications, and between patients with and without HCC (P < 0.01). M2BPGi cutoff values for predicting HCC, EV, and decompensated cirrhosis were 6.50, 6.64, and 5.25, respectively. Mul
Serum M2BPGi predicted cirrhosis complications, including decompensation and varices, especially in HCC. Combined with AFP, it enhanced HCC detection. Future liver biopsy studies are needed for tissue confirmation.
Core Tip: This study introduces serum mac-2 binding protein glycosylation isomer (M2BPGi) as a novel biomarker for predicting complications in cirrhotic patients, particularly hepatocellular carcinoma (HCC), liver decompensation, and esophageal varices. The findings show that combining M2BPGi with alpha-fetoprotein significantly enhances diagnostic accuracy, achieving an impressive sensitivity of 92.8% for HCC detection. Importantly, M2BPGi levels correlate with disease severity as measured by the Child-Pugh classification, highlighting its potential role in clinical risk stratification.
- Citation: Doan TH, Nguyen KM, Nguyen XV, Pham ATN, Le ND. Evaluating thresholds of Mac-2 binding protein glycosylation isomer in association with clinical outcomes in patients with cirrhosis. World J Hepatol 2025; 17(9): 109179
- URL: https://www.wjgnet.com/1948-5182/full/v17/i9/109179.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i9.109179
Cirrhosis represents an advanced stage in most chronic liver diseases. The 1-year mortality rate associated with cirrhosis exhibits significant variability, ranging from 1% to 57%, contingent on the degree of decompensation. Globally, it is the 14th leading cause of death and ranks 4th in Central Europe[1]. According to a Global Burden of Disease study conducted in 2020, cirrhosis accounted for approximately 1.32 million deaths in 2017, with about 0.44 million occurring in females and 0.88 million in males[2]. The mortality rate due to cirrhosis is particularly high in low-income regions such as sub-Saharan Africa, Central Asia, and Southeast Asia[2]. Notably, in Viet Nam, statistics from 2018 indicated that cirrhosis was the seventh leading cause of death among the top ten causes[3].
Cirrhosis can result in severe complications. Hepatocellular carcinoma (HCC), esophageal varices (EV), and liver decompensation are the most significant concerns in such patients. Indeed, 80%-90% of patients with HCC are diagnosed with cirrhosis[4]. Given the high prevalence of cirrhosis in patients with HCC, international guidelines strongly re
More specifically, previous studies demonstrated that the combination of AFP and ultrasound exhibited only 63% sensitivity for the early detection of HCC[5]. The limitation of the deployment of AFP in conjunction with ultrasound for HCC surveillance may lead to more than 40% of patients being misdiagnosed with early cases. Consequently, researchers are actively exploring new and more specific markers for the early detection of HCC in patients with chronic hepatitis, regardless of cirrhosis status. Protein induced by vitamin K absence or antagonist-II, AFP-L3, and particularly the GAAD score, have recently attracted the attention of numerous researchers and clinicians because the combination of individual parameters has improved HCC diagnostic performance[6]. Despite their superior attributes the high-cost individual parameters contributing to the GAAD score may constrain their usage among patients.
While effective treatment of the underlying causes of cirrhosis is important, identifying factors that can predict future complications, such as EV and liver decompensation, is crucial for triggering strict follow-up. Traditionally, an invasive method, esophagogastroduodenoscopy-retrieved parameters, consisting of size and the presence of red signs, was the gold standard for determining the existence of EV. Recent advancements in scientific and technical knowledge have led to the investigation of noninvasive methods aimed at ruling out the presence of EV in patients with cirrhosis, both in general and in specific contexts. These indicators, including platelet count and liver stiffness, accurately exclude low-risk EV in patients with cirrhosis[7]. Nevertheless, FibroScan has been found to be affected by obesity and acute inflammation, which may result in a false elevation. In addition, limited installation and well-served personnel requirements limit acc
Mac-2 binding protein (M2BP) is a secreted glycoprotein (about 90 kDa) that contains seven N-glycans per monomer. M2BP exhibits high polymerization in serum, forming a large doughnut-like structure[8]. It is secreted by hepatic stellate cells (HSCs) in the liver and by macrophages from other organs. M2BP interacts with collagens IV, V, and VI, fibronectin, and nidogens[9]. Alterations in the glycan structure of M2BP reflect the degree of cell differentiation and carcinogenesis[10]. For instance, the N-glycosylation of M2BP changes during liver disease progression, with altered M2BP levels increasing in advanced fibrosis. Lectin Wisteria floribunda agglutinin specifically recognizes the altered M2BP produced by HSCs [M2BP glycosylation isomer (M2BPGi)][8]. HSCs are the primary producers of M2BPGi, which acts as a jux
Recent studies have highlighted the potential of M2BPGi as a biological marker for evaluating liver fibrosis. M2BPGi has been found to correlate with fibrosis stages in various etiologies, including viral hepatitis, metabolic dysfunction-associated steatotic liver disease, autoimmune hepatitis[11], and the prognosis of HCC in patients with chronic hepatitis B infection and chronic hepatitis C, regardless of treatment status[12,13]. Moreover, several studies have shown that M2BPGi is a promising short-term predictor of recurrent HCC in patients undergoing transarterial chemoembolization and hepatectomy[14]. Recently, M2BPGi has been found to be a potential marker to detect other cirrhosis-induced com
Building on the existing literature that has explored the value of M2BPGi in liver fibrosis among patients with chronic hepatitis, this study further investigated its association with clinical outcomes in patients with cirrhosis. To address this concern we conducted a study titled “Assessing thresholds of M2BPGi in association with clinical outcomes in patients with cirrhosis”. This study aimed to investigate M2BPGi concentration in the association with HCC, EV, and deco
This cross-sectional, prospective study was conducted at the Gastroenterology Department of Da Nang Hospital in Da Nang City, Viet Nam, from March 1, 2022 to September 31, 2024. A total of 153 patients with cirrhosis receiving treatment at the inpatient gastroenterology department were enrolled in the study and evaluated for HCC and EV. Blood sampling was performed on the same day as imaging diagnosis.
The diagnosis of cirrhosis was based on a comprehensive examination comprising the following: (1) Clinical signs and radiological findings, including morphological alterations in the liver (coarse liver echotexture with irregular margins), ascites, splenomegaly, and jaundice; (2) Laboratory test results: Aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio > 1, total bilirubin > 17.1 μmol/L, serum albumin < 35 g/L, international normalized ratio > 1.3, or prolonged prothrombin time; and (3) FibroScan of ≥ 11 kPa and/or an AST to platelet ratio index ≥ 2 for patients with ambiguous clinical findings[16]. The exclusion criteria were as follows: (1) Acute decompensation; (2) End-stage deco
HCC was defined according to the 2018 American Association for the Study of Liver Diseases Guidelines: (1) Lesion > 1 cm and AFP > 20 ng/mL; and/or (2) Criteria on multiphase imaging by multiphase CT and magnetic resonance imaging[17]. The presence of EV was determined according to the Japan Gastroenterological Endoscopy Society classification: F0, no varicose appearance; F1, straight, small-caliber varices; F2, moderately enlarged, beady varices; and F3, markedly enlarged, nodular or tumor-shaped varices[18]. High-risk EVs were defined as medium or large varices, small varices with red signs, or Child–Pugh C in accordance with the European Association for the Study of Liver guidelines[19].
Blood samples were collected from all patients on the same day as the imaging diagnosis. Routine laboratory data were collected, including hemoglobin, WBC, platelet count, AST, ALT, albumin, total bilirubin, ammonia, and total bilirubin. AFP was measured by a Cobas C6000 analyzer (Roche Diagnostics, Indianapolis, IN, United States), and viral tests inc
Liver stiffness was measured using transient elastography (FibroScan; Echosens, Paris, France) by an experienced operator following established recommendations[20]. The results of at least 10 valid shots obtained in the session were expressed in kPa. A success rate of < 60% or an interquartile range/median value of > 30% was considered unreliable.
To confirm HCC cases all patients underwent computed tomography (CT) and/or magnetic resonance imaging (MRI) examinations on the same day as their clinical examination and blood sampling. A GE Optima 128-slice CT scanner (GE Healthcare, Chicago, IL, United States) and Philips 1.5T Multiva MRI system (Philips Healthcare, Best, Netherlands) with multiphase contrast enhancement were employed to detect HCC in patients with cirrhosis.
Endoscopic procedures were conducted using the Olympus Evis X1, 170, and 190 systems (Olympus, Tokyo, Japan). The classification of variceal size was as follows: (1) Small, < 5 mm diameter; (2) Medium, 5-10 mm; and (3) Large, > 10 mm. Red signs were identified using narrow-band imaging technology, which enhances the contrast between blood vessels and surrounding tissues by employing different light wavelengths. Narrow-band imaging facilitates the visualization of red spots by increasing the visibility of vascular structures. Patients who did not exhibit these features were considered to be at low risk for variceal bleeding.
Serum M2BPGi was measured in stored serum by employing a lectin-antibody sandwich immunoassay on an automated immunoassay system, HISCL-5000 (Sysmex), as previously reported[8]. Venous blood was collected prior to the en
Continuous variables were expressed as mean ± SD or median (interquartile range), and categorical data were reported as number (percentage). Differences between groups were evaluated using Student’s t-test or the χ2 statistic, as appropriate. The predictability of M2BPGi for complications, including liver decompensation, the presence of EV, high-risk EV, and HCC, was expressed as the area under the receiver operating characteristic curve (AUROC). Multivariate logistic reg
Table 1 shows the basic characteristics of the patients. The study population consisted predominantly of males (80.4%), with a mean age of 56.3 years. Liver function markers such as AST, ALT, and bilirubin levels indicated a significant degree of liver dysfunction, with notably elevated AST and bilirubin levels. Albumin levels decreased, consistent with impaired liver function. The platelet count also decreased, likely reflecting advanced liver disease or portal hypertension. A significant proportion of patients were classified as Child-Pugh class B (41.8%) or C (43.8%), indicating moderate-to-severe liver dysfunction. The mean FibroScan value of 36.4 kPa further corroborates the presence of significant liver fib
| Characteristic, n = 153 | Finding |
| Male | 123 (80.4) |
| Age (years) | 56.3 ± 10.9 |
| AST (U/L) | 93.2 (49.4-149.5) |
| ALT (U/L) | 48.0 (30.2-82.9) |
| Total bilirubin (μmol/L) | 41.8 (20.7-69.8) |
| Albumin (g/L) | 27.1 (22.0-33.1) |
| Hemoglobin (g/L) | 111.0 (94.0-124.0) |
| Platelet as × 109/L | 94.0 (59.0-145.0) |
| AFP (ng/Ml) | 4.66 (2.4-13.0) |
| Child-Pugh | |
| A | 22 (14.4) |
| B | 64 (41.8) |
| C | 67 (43.8) |
| M2BPGi C.O.I. | 7.1 ± 3.7 |
| FibroScan in kPa, n = 77 | 36.4 ± 20.5 |
As shown in Table 2, serum M2BPGi levels significantly increased with the degree of cirrhosis (Child-Pugh) (P < 0.01), model for end-stage liver disease score (P = 0.0018), and severity of EV (P < 0.01). Notably in the group of patients with HCC, M2BPGi levels were also significantly higher than those in the group with cirrhosis without HCC (P < 0.01).
| Characteristic | n | M2BPGi C.O.I. | P value1 |
| Child-Pugh | |||
| A | 22 | 5.20 ± 2.65 | < 0.001 |
| B | 64 | 6.14 ± 2.89 | |
| C | 67 | 8.63 ± 4.08 | |
| MELD score | |||
| ≤ 9 | 38 | 6.09 | 0.003 |
| 10-19 | 53 | 6.43 | |
| 20-29 | 39 | 7.85 | |
| 30–39 | 10 | 10.02 | |
| ≥ 40 | 7 | 11.11 | |
| Esophageal varices | |||
| F0 | 22 | 5.3 ± 4.3 | < 0.05 |
| F1 | 10 | 5.0 ± 3.8 | |
| FII | 21 | 7.6 ± 3.4 | |
| FIII | 57 | 8.1 ± 3.8 | |
| HCC | |||
| Yes | 28 | 8.8 ± 2.9 | < 0.05 |
| No | 125 | 6.7 ± 3.8 | |
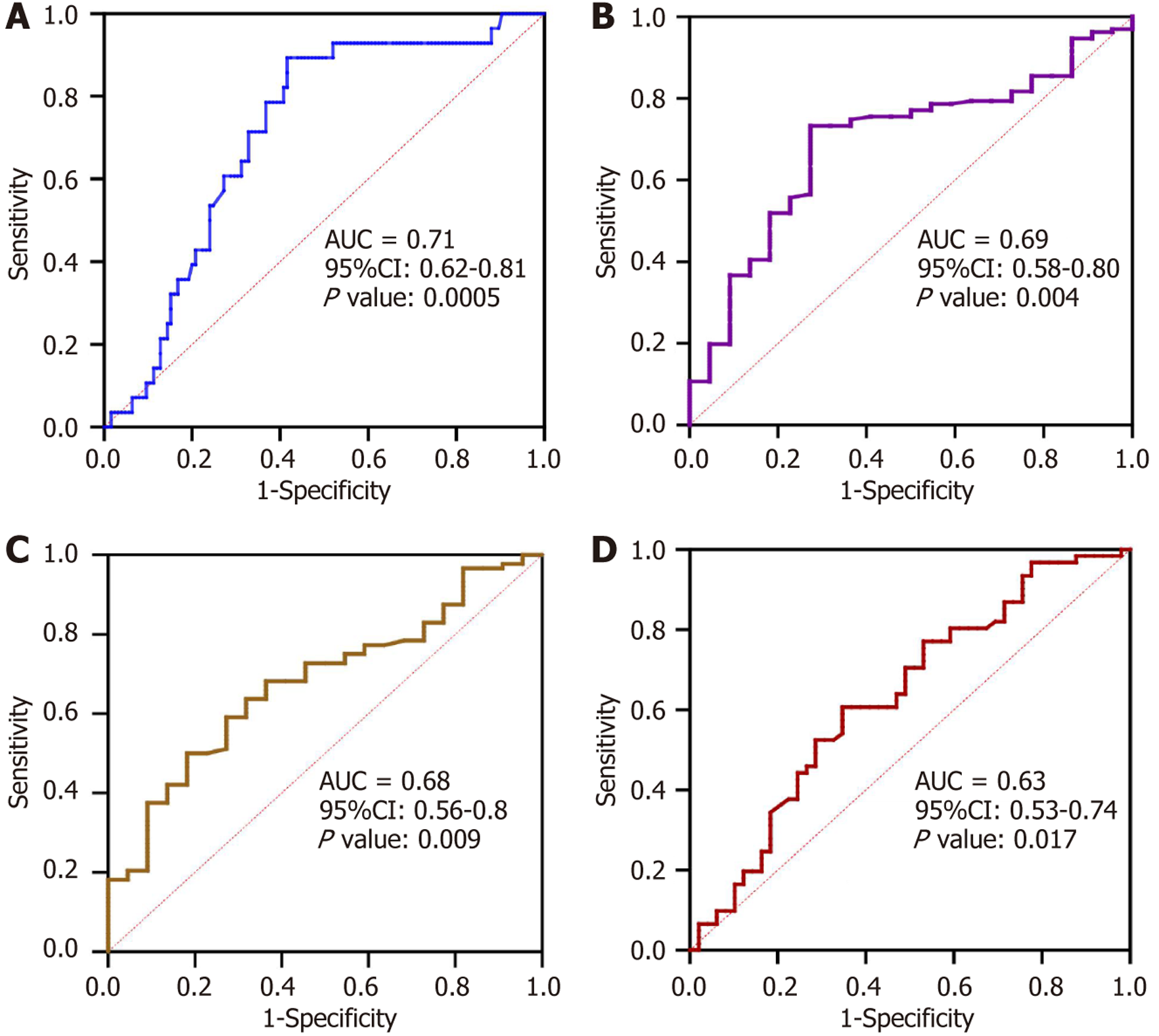
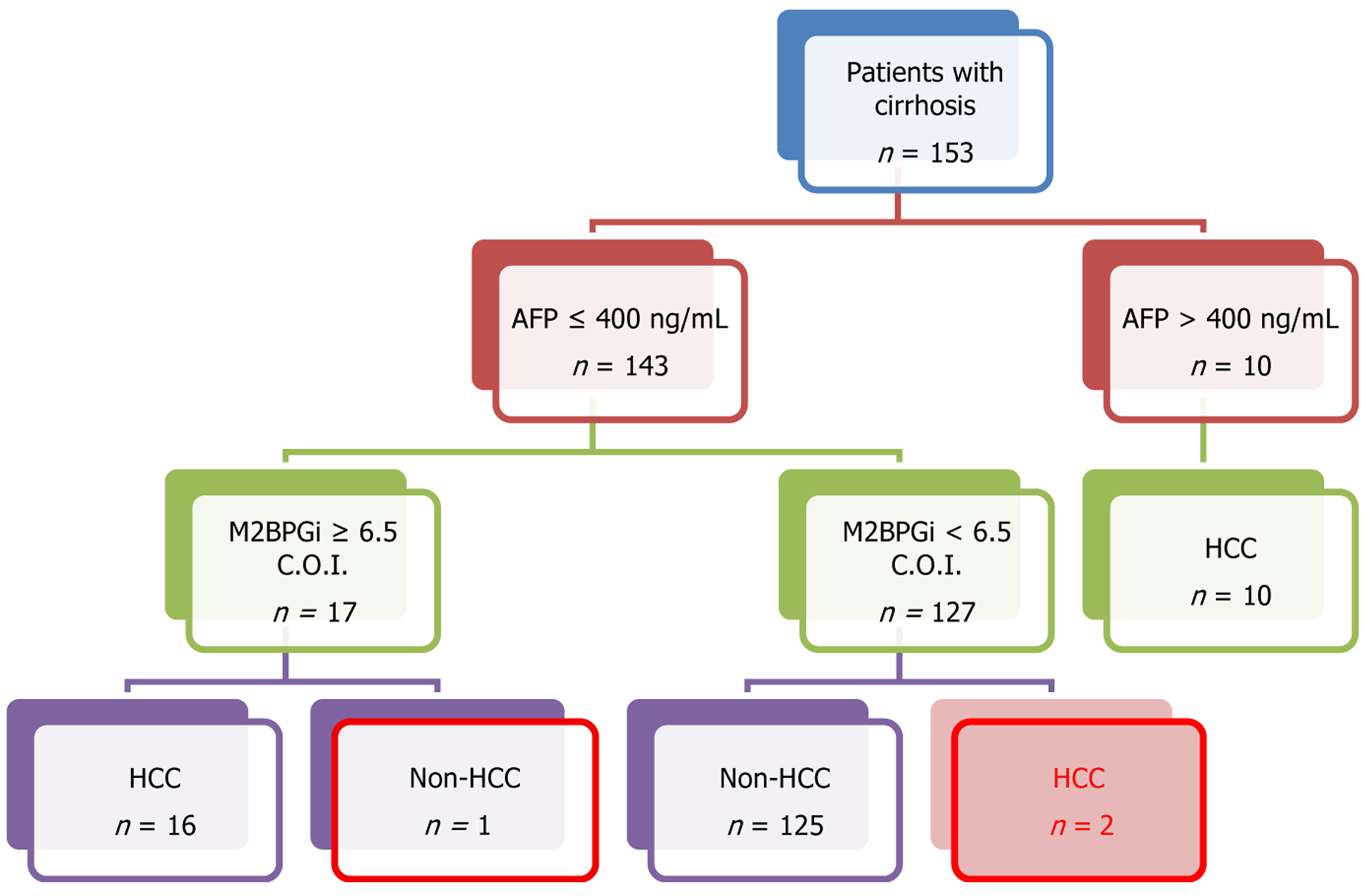
Analysis of the ROC curve was conducted to determine the cutoff value of M2BPGi levels for diagnosing HCC, liver decompensation, and the presence of EV (Figure 1). For HCC the ROC curve demonstrated an AUROC of 0.71 at the cutoff of 6.5 C.O.I., with sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) of 89.3%, 58.4%, 96.1%, and 32.5%, respectively (Figure 1A). Further investigation was performed using an algorithm combining M2BPGi (> 6.5 C.O.I.) and AFP (> 400 IU/L) to detect HCC cases. The algorithm resulted in a significant improvement in HCC detection with an accuracy of 98.0%. This algorithm, illustrated in Figure 2, yielded a sensitivity of 92.8%, a specificity of 99.2%, an NPV of 98.4%, and a PPV of 96.3%.
The results demonstrated that M2BPGi can distinguish liver decompensation at 5.25 C.O.I. with an AUROC of 0.69, balanced sensitivity (73.3%) and specificity (72.7%), and a high NPV of 94.1%, thus rendering it reliable for excluding decompensation (Figure 1B). Similarly, the model demonstrated moderate accuracy, with a specificity of 72.7% and a relatively low PPV of 30.8% for the presence of EV (cut-off: 6.64, AUROC: 0.68), indicating limited reliability in confirming positive cases (Figure 1C). For high-risk EV (cutoff: 6.86, AUROC: 0.63), the discriminatory power was weak, with fair sensitivity (60.7%) and specificity (65.3%), suggesting limited clinical utility (Figure 1D).
As shown in Table 3, the multivariate logistic regression analysis revealed that M2BPGi was an independent risk factor associated with decompensation with an adjusted odds ratio (aOR) of 2.11, 95%CI: 1.37-3.83 and the presence of EV (aOR: 1.3, 95%CI: 1.08-1.64).
| Variable | Univariate analysis | Multivariate analysis | ||
| OR | 95%CI | aOR | 95%CI | |
| Liver decompensation | ||||
| MELD score | 1.19 | 1.10-1.32 | 1.18 | 1.04-1.41 |
| M2BPGi | 1.23 | 1.06-1.47 | 2.11 | 1.37-3.83 |
| FibroScan | 1.06 | 1.02-1.12 | 1.05 | 0.98-1.15 |
| AAR | 1.80 | 1.06-3.48 | 4.12 | 0.84-35.8 |
| Esophageal varices | ||||
| Platelets | 0.99 | 0.99-1.00 | 1.00 | 0.99-1.02 |
| FibroScan | 0.99 | 0.97-1.02 | 0.98 | 0.95-1.00 |
| M2BPGi | 1.10 | 1.01-1.22 | 1.30 | 1.08-1.64 |
| AAR | 1.02 | 0.78-1.36 | 1.04 | 0.62-1.69 |
| Child-Pugh | ||||
| A | Reference | Reference | ||
| B | 4.75 | 1.70-14.80 | 4.65 | 1.06-24.11 |
| C | 4.21 | 1.52-13.01 | 1.90 | 0.32-11.90 |
Our study demonstrated that M2BPGi is associated with HCC, liver compensation, and the presence of EV in patients with cirrhosis. M2BPGi has been identified as a fibrosis biomarker in patients with various etiologies including HBV, hepatitis C virus, metabolic dysfunction-associated steatotic liver disease, and autoimmune hepatology[8,13]. Furth
As shown in Table 2, serum M2BPGi levels significantly increased with cirrhosis severity (Child-Pugh score). These findings align with those of previous studies, such as that by Eso et al[24], who reported M2BPGi levels in Child-Pugh A, B, and C cirrhosis of 0.94, 4.775, and 11.37, respectively. Similarly, Hanai et al[25] observed serum Wisteria floribunda agglutinin positive-M2BP levels in Child-Pugh classes A, B, and C with C.O.I.s of 2.90, 6.15, and 9.45, respectively.
The present study demonstrated that an M2BPGi concentration of 6.5 C.O.I. can diagnose HCC in patients with cirrhosis, achieving an AUROC of 0.71 and a significant NPV of 96.1% (P < 0.001). These findings align with the research conducted by Liu et al[22] in which M2BPGi was identified as a substantial short-term predictor of HCC in chronic hepatitis B patients, particularly within 1 year to 2 years before diagnosis, with AUROCs exceeding 0.80. Tseng et al[26] reported that elevated levels of M2BPGi independently predicted HCC risk and enhanced existing models such as PAGE-B. Moreover, Jun et al[21] demonstrated that M2BPGi outperformed AFP in predicting HCC risk, particularly in patients with HBV without advanced fibrosis. Su et al[9] noted that M2BPGi levels declined significantly in patients without HCC but remained elevated in HCC cases. These findings further support the use of M2BPGi as a dynamic surveillance tool.
This study also revealed that M2BPGi levels of ≥ 6.5 C.O.I. considerably increased the risk of HCC, underscoring its clinical significance for early detection. Despite the high NPV for ruling out HCC, M2BPGi exhibited an inferior PPV. While M2BPGi demonstrated high sensitivity and low specificity, AFP showed a contrasting pattern (data not shown). To enhance the diagnosis of HCC, the algorithm combining M2BPGi and AFP showed superior performance compared to either individual M2BPGi or AFP. Specifically, this algorithm significantly improved the sensitivity, specificity, and PPV for diagnosing HCC and mitigated the limitations of these assays. Indeed, 16 out of 28 HCC cases with AFP levels lower than 400 ng/L were greater than 6.5 C.O.I. Collectively, this algorithm suggests a novel approach combining these assays to improve the early detection of HCC and reduce misdiagnoses caused by the low sensitivity of AFP. However, this model requires validation in a larger population, owing to the small sample size.
Regarding liver decompensation, our study identified a C.O.I. of 5.25 for M2BPGi in predicting liver decompensation, yielding an AUROC of 0.69, sensitivity of 73.3%, and specificity of 72.7%. Multivariate regression analysis demonstrated that M2BPGi was an independent risk factor for liver decompensation (aOR: 2.11; 95%CI: 1.37-3.83). Previous studies have indicated that elevated M2BPGi levels are associated with worsening of fibrosis and decompensation. For instance, M2BPGi was identified as an independent predictor of clinical decompensation in patients with compensated cirrhosis with an OR of 11.958 (95%CI: 1.876-76.226, P = 0.009)[27]. Uojima et al[28] observed that patients with decompensated cirrhosis exhibited significantly higher M2BPGi levels (6.91 ± 5.04) than patients with compensated cirrhosis (2.22 ± 1.61, P < 0.0001), with a C.O.I. of 3.37 predicting decompensated cirrhosis with 77.8% sensitivity and 86.7% specificity. These findings demonstrated a relationship between M2BPGi levels and liver decompensation progression, although a uni
The final clinical outcome examined in this study was the presence of EV. The M2BPGi demonstrated a fair discriminative ability. The sensitivity was moderate (59.1%), while the specificity was higher (72.7%) for identifying patients without EV. The PPV was 89.7%, indicating a strong correlation between positive test results and the presence of EV. However, its low NPV of 30.8% limits its efficacy in ruling out EV. Wu et al[29] demonstrated a positive correlation between M2BPGi and hepatic venous pressure gradient (HVPG) in patients with cirrhosis, reporting M2BPGi levels of 2.33 C.O.I. for clinically significant portal hypertension (HVPG > 10 mmHg) and 5.93 C.O.I. for severe portal hyper
Kikukawa et al[15] also reported higher M2BPGi levels in patients with esophagogastric varices compared with those without esophagogastric varices. Furthermore, this study indicated that the M2BPGi value had predictive ability for high-risk EV, with an AUROC of 0.63 (P < 0.05). A previous study found higher M2BPGi levels in patients with high-risk EV than in those without (C.O.I. of 11.4 vs 3.7, P < 0.001), using a C.O.I. of 5; the sensitivity, specificity, PPV, and NPV were 92.6%, 70.1%, 55.6%, and 95.9%, respectively[23]. Our study demonstrated lower sensitivity and NPV (60.7% and 56.8%, respectively), which may indicate the limitation of this test in excluding patients with high-risk EV. Another factor con
This study had several limitations. First, as this was a single-center study with a small sample size, the findings may not be generalizable to the entire target population. Second, the use of FibroScan for staging fibrosis may introduce bias owing to its inherent limitations. Additionally, the lack of liver histology deferred us from HCC confirmation. Hence, MRI/CT was used as a reference method due to its non-invasive nature. Third, given the cross-sectional nature of this study and the collection of data solely at baseline, the absence of longitudinal measurements constrained our ability to observe temporal changes in M2BPGi. Fourth, our algorithm combining AFP and M2BPGi was investigated in a small sample size as HCC cases diagnosed in our patient population may have resulted in biased data. Thus, we suggest a larger population to investigate this algorithm in an establishment and validation cohort before its implementation in practical procedures. Furthermore, the predominance of males and treatment history may contribute to biased cutoffs. Finally, the limitation of the subset sample sizes (e.g., EV, high-risk EV, and liver decompensation) precluded comprehensive data analysis. Future prospective multicenter studies should address and uncover the barriers. Nevertheless, the study results may provide a novel approach for diagnosing the aforementioned cirrhosis-induced complications in limited-resource settings.
Serum M2BPGi appears to be a valuable and accessible non-invasive biomarker for predicting clinically significant com
| 1. | Gu W, Hortlik H, Erasmus HP, Schaaf L, Zeleke Y, Uschner FE, Ferstl P, Schulz M, Peiffer KH, Queck A, Sauerbruch T, Brol MJ, Rohde G, Sanchez C, Moreau R, Arroyo V, Zeuzem S, Welsch C, Trebicka J. Trends and the course of liver cirrhosis and its complications in Germany: Nationwide population-based study (2005 to 2018). Lancet Reg Health Eur. 2022;12:100240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (2)] |
| 2. | GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1184] [Cited by in RCA: 1117] [Article Influence: 186.2] [Reference Citation Analysis (5)] |
| 3. | Ta H, Lin B, Palaniappan L. Vietnamese and Vietnamese-American health statistics, 2003-2019. Journal of Asian Health. 2020. [cited 12 August, 2025]. Available from: https://med.stanford.edu/content/dam/sm/care/VNDataBrief.pdf. |
| 4. | Simonetti RG, Cammà C, Fiorello F, Politi F, D'Amico G, Pagliaro L. Hepatocellular carcinoma. A worldwide problem and the major risk factors. Dig Dis Sci. 1991;36:962-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 260] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, Waljee AK, Singal AG. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154:1706-1718.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 894] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 6. | Piratvisuth T, Hou J, Tanwandee T, Berg T, Vogel A, Trojan J, De Toni EN, Kudo M, Eiblmaier A, Klein HG, Hegel JK, Madin K, Kroeniger K, Sharma A, Chan HLY. Development and clinical validation of a novel algorithmic score (GAAD) for detecting HCC in prospective cohort studies. Hepatol Commun. 2023;7:e0317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 7. | Ding NS, Nguyen T, Iser DM, Hong T, Flanagan E, Wong A, Luiz L, Tan JY, Fulforth J, Holmes J, Ryan M, Bell SJ, Desmond PV, Roberts SK, Lubel J, Kemp W, Thompson AJ. Liver stiffness plus platelet count can be used to exclude high-risk oesophageal varices. Liver Int. 2016;36:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, Hige S, Sakamoto M, Kage M, Mizokami M, Narimatsu H. A serum "sweet-doughnut" protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep. 2013;3:1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 9. | Su TH, Peng CY, Tseng TC, Yang HC, Liu CJ, Liu CH, Chen PJ, Chen DS, Kao JH. Serum Mac-2-Binding Protein Glycosylation Isomer at Virological Remission Predicts Hepatocellular Carcinoma and Death in Chronic Hepatitis B-Related Cirrhosis. J Infect Dis. 2020;221:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Narimatsu H. Development of M2BPGi: a novel fibrosis serum glyco-biomarker for chronic hepatitis/cirrhosis diagnostics. Expert Rev Proteomics. 2015;12:683-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Shirabe K, Bekki Y, Gantumur D, Araki K, Ishii N, Kuno A, Narimatsu H, Mizokami M. Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: more than a biomarker of liver fibrosis. J Gastroenterol. 2018;53:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 12. | Bui HH, Nguyen ST, Phan ST, Nguyen KM, Nguyen CD. Evaluating M2BPGi as a Marker for Liver Fibrosis in Patients with Chronic Hepatitis B. Dig Dis Sci. 2023;68:4407-4417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Tamaki N, Kurosaki M, Loomba R, Izumi N. Clinical Utility of Mac-2 Binding Protein Glycosylation Isomer in Chronic Liver Diseases. Ann Lab Med. 2021;41:16-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Lee IC, Lei HJ, Wang LC, Yeh YC, Chau GY, Hsia CY, Chou SC, Luo JC, Hou MC, Huang YH. M2BPGi Correlated with Immunological Biomarkers and Further Stratified Recurrence Risk in Patients with Hepatocellular Carcinoma. Liver Cancer. 2025;14:68-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Kikukawa K, Uchida-Kobayashi S, Tamori A, Yoshida K, Kotani K, Motoyama H, Kozuka R, Hagihara A, Fujii H, Morikawa H, Enomoto M, Murakami Y, Kawada N. Serum Mac-2-binding protein glycosylation isomer predicts esophagogastric varices in cirrhotic patients with chronic hepatitis C virus infection treated with IFN-free direct-acting antiviral agent: M2BPGi levels predict varices in SVR patients. Ann Hepatol. 2020;19:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | World Health Organization. Guidelines for the prevention care and treatment of persons with chronic hepatitis B infection. 2015. [cited 12 August, 2025]. Available from: https://www.who.int/publications/i/item/9789241549059. |
| 17. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3435] [Article Influence: 429.4] [Reference Citation Analysis (3)] |
| 18. | Tajiri T, Yoshida H, Obara K, Onji M, Kage M, Kitano S, Kokudo N, Kokubu S, Sakaida I, Sata M, Tajiri H, Tsukada K, Nonami T, Hashizume M, Hirota S, Murashima N, Moriyasu F, Saigenji K, Makuuchi H, Oho K, Yoshida T, Suzuki H, Hasumi A, Okita K, Futagawa S, Idezuki Y. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc. 2010;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 253] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 19. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1983] [Article Influence: 247.9] [Reference Citation Analysis (2)] |
| 20. | Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, Castera L, Choi BI, Chou YH, Cosgrove D, Dietrich CF, Ding H, Amy D, Farrokh A, Ferraioli G, Filice C, Friedrich-Rust M, Nakashima K, Schafer F, Sporea I, Suzuki S, Wilson S, Kudo M. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41:1126-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 680] [Article Influence: 61.8] [Reference Citation Analysis (5)] |
| 21. | Jun T, Hsu YC, Ogawa S, Huang YT, Yeh ML, Tseng CH, Huang CF, Tai CM, Dai CY, Huang JF, Chuang WL, Yu ML, Tanaka Y, Nguyen MH. Mac-2 Binding Protein Glycosylation Isomer as a Hepatocellular Carcinoma Marker in Patients With Chronic Hepatitis B or C Infection. Hepatol Commun. 2019;3:493-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Liu J, Hu HH, Lee MH, Korenaga M, Jen CL, Batrla-Utermann R, Lu SN, Wang LY, Mizokami M, Chen CJ, Yang HI. Serum Levels of M2BPGi as Short-Term Predictors of Hepatocellular Carcinoma in Untreated Chronic Hepatitis B Patients. Sci Rep. 2017;7:14352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 23. | Nababan SHH, Kalista KF, Jasirwan CO, Kurniawan J, Lesmana CRA, Sulaiman AS, Hasan I, Gani RA. Mac-2 Binding Protein Glycosylation Isomer for Screening High-Risk Esophageal Varices in Liver Cirrhotic Patient. Livers. 2021;1:60-67. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Eso Y, Takai A, Taura K, Takahashi K, Ueda Y, Marusawa H, Seno H. Association of Mac-2-binding protein glycosylation isomer level with nutritional status in chronic liver disease. J Gastroenterol Hepatol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Hanai T, Shiraki M, Ohnishi S, Miyazaki T, Ideta T, Kochi T, Imai K, Suetsugu A, Takai K, Shimizu M, Moriwaki H. Impact of serum glycosylated Wisteria floribunda agglutinin positive Mac-2 binding protein levels on liver functional reserves and mortality in patients with liver cirrhosis. Hepatol Res. 2015;45:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Tseng TC, Peng CY, Hsu YC, Su TH, Wang CC, Liu CJ, Yang HC, Yang WT, Lin CH, Yu ML, Lai HC, Tanaka Y, Nguyen MH, Liu CH, Chen PJ, Chen DS, Kao JH. Baseline Mac-2 Binding Protein Glycosylation Isomer Level Stratifies Risks of Hepatocellular Carcinoma in Chronic Hepatitis B Patients with Oral Antiviral Therapy. Liver Cancer. 2020;9:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Xu WP, Wang ZR, Zou X, Zhao C, Wang R, Shi PM, Yuan ZL, Yang F, Zeng X, Wang PQ, Sultan S, Zhang Y, Xie WF. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein evaluates liver function and predicts prognosis in liver cirrhosis. J Dig Dis. 2018;19:242-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Uojima H, Hidaka H, Tanaka Y, Inoue T, Onoue M, Wada N, Kubota K, Nakazawa T, Shibuya A, Fujikawa T, Nakayama T, Yamanoue H, Sung JH, Kako M, Koizumi W. Wisteria floribunda agglutinin-positive human Mac-2 binding protein in decompensated cirrhosis. J Gastroenterol Hepatol. 2018;33:1889-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Wu PS, Hsieh YC, Lee KC, Huang YH, Hou MC, Lin HC. Mac-2 binding protein glycosylation isomer is a potential biomarker to predict portal hypertension and bacterial infection in cirrhotic patients. PLoS One. 2021;16:e0258589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/