Published online Sep 27, 2025. doi: 10.4254/wjh.v17.i9.109118
Revised: May 21, 2025
Accepted: August 8, 2025
Published online: September 27, 2025
Processing time: 149 Days and 2.5 Hours
Severe alcoholic hepatitis (SAH) is associated with high short-term mortality. The SAH population exhibits extreme heterogeneity in disease severity, clinical presentation, decompensations, and outcomes. Nonetheless, improving outcomes and preventing adverse events is a major challenge when selecting an appropriate treatment for alcoholic hepatitis. Currently, steroids are the standard of care for SAH with Maddrey’s discriminant function > 32 and model for end stage liver disease > 20; however, they have limited usage due to ineligibility in approximately two-third of such patients. Approximately 25% of patients do not respond to steroids and require alternative therapies. An array of evolving therapies, such as granulocyte colony-stimulating factors, plasma exchange, fecal microbiota transplantation, antibiotics, anti-cytokine therapies, and N-acetylcysteine, showing variable success, are emerging. Hence, it is also crucial to select appro
Core Tip: Clinically, severe alcoholic hepatitis (SAH) presents with jaundice, with or without ascites or encephalopathy, and it progresses rapidly. The patient population is heterogeneous and needs to be stratified according to their eligibility for specific therapies. Emerging alternatives or rescue therapies are available for SAH. The decision for the preferred therapy is based on patient profile, clinical experiences, and its side effects profile. This comprehensive review provides insight into the stratification of patients who need specific therapies, with the primary focus on the therapies that improve survival in SAH.
- Citation: Mishra AK, Goel A. Stratification and selection of therapies to improve survival in severe alcoholic hepatitis. World J Hepatol 2025; 17(9): 109118
- URL: https://www.wjgnet.com/1948-5182/full/v17/i9/109118.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i9.109118
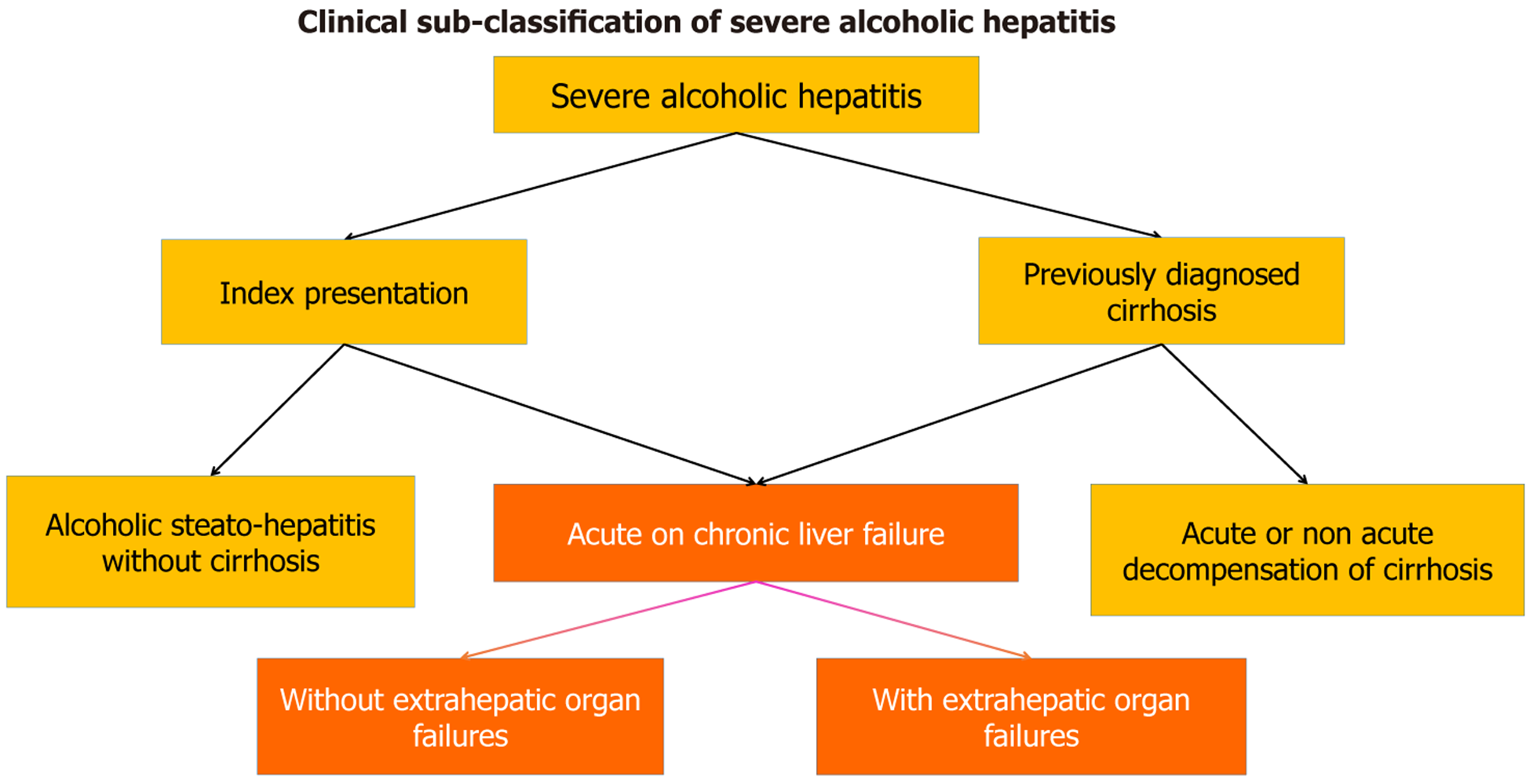
Patients with severe alcoholic hepatitis (SAH) can present with or without underlying cirrhosis. Ascites manifesting with jaundice indicate advanced disease and can develop at varied intervals after an acute insult to the liver[1]. Patients can present with alcoholic hepatitis without decompensation, acute decompensation of preexisting cirrhosis, or acute-on-chronic liver failure (ACLF). Development of jaundice with bilirubin > 5 mg% and ascites within 28 days qualifies for ACLF presentation by the Asian Pacific Association for the Study of Liver criteria indicating a severe course of the disease[2]. The development of ascites after four weeks of jaundice indicates acute decompensation, not mounting to ACLF. The prognosis differs with or without ACLF, and the rate of mortality increasing with ACLF grades. Patients with acute decompensation have a better prognosis, especially in the first episode of presentation, than those with ACLF[3]. In the absence of alcohol abstinence, long-term survival after index presentation is around 30% only, owing to the high rate of recidivism[4]. Survival is low in patients who present second or additional episodes of alcoholic hepatitis with cirrhosis and decompensation[5,6]. Hence, this heterogeneous population can be clinically classified based on prognosis, as shown in Figure 1.
Model for end stage liver disease (MELD) score is a reliable predictor of short-term mortality in patients with SAH[1]. This has been shown that patients with a MELD score > 21 have a significantly higher risk of death within 90 days than those with lower scores. This cutoff value not only aids in identifying high-risk patients but also helps clinicians make informed decisions regarding the urgency of liver transplantation. MELD > 30 should be evaluated for liver transplantation at the time of admission while considering other therapies[2]. In addition, steroid therapy is effective in SAH with MELD up to 39; however, the efficacy decreased with increasing MELD score[7]. Hence, the MELD score can be used for prognostication, identification, and selection of patients for liver transplantation or steroid therapy. Maddrey’s discriminant function (mDF) has been used to define the severity of alcoholic hepatitis. Most centers consider patients with mDF > 32 only for specific therapies, whereas those with mDF < 32 can be managed with nutrition therapy and abstinence[8]. Patients with mDF < 32 have a survival rate of > 90% at 6 months and a survival rate of > 70% at 5 years if they practice abstinence[9]. Although MELD is prognostically better, than mDF, many researchers have avoided including patients with an mDF > 90 in clinical trials. In our experience, an mDF > 90 is not a contraindication for steroids, unless sepsis and organ failures preclude its usage.
Abstinence and nutrition therapy: Abstinence is indispensable for improving survival in patients with SAH. In clinical practice, a subgroup of patients who improved rapidly with nutrition therapy alone post-abstinence were categorized as rapid fallers. Nutrition therapy should be initiated at the time of admission to achieve 35–40 kcal/kg/day in all patients. Small frequent meals can increase palatability and reduce the period of fasting. Nasogastric feeding should be considered in patients with an oral calorie intake of < 21 kcal/kg/day. The improvement in rapid fallers on nutrition and abstinence can be predicted by the trajectory of serum bilirubin, precisely by more than < 20% decrease, on day 7. Such patients can be deferred to corticosteroids due to the lack of additional survival advantage[10]. However, many such patients show partial improvement and may require subsequent therapies. Hence, the trend in liver function in the first week is crucial for therapeutic decisions. Although recommending cutoffs for improving liver function is difficult, a bilirubin reduction of more than > 20% in the first week can be significant[10]. In such patients, the absence of encephalopathy or ascites can predict near-zero mortality in the short term, thus avoiding steroid exposure[1].
Steroids: Indications, patient selection, and outcomes: Different trials have determined variable eligibility of the steroids. Patients with sepsis and organ failure were excluded from almost all clinical trials. Acute kidney injury has been considered a relative contraindication, with different investigators using variable cutoff values for steroid eligibility. Creatinine up to 5.7 mg% has been included in steroid eligibility, in the absence of the need for renal replacement therapy (Table 1)[8]. Traditionally, Gastrointestinal (GI) bleeding is considered a contraindication for steroids; however, recent evidence of steroid use in such patients could alter our future practice. Steroids increase the risk of GI bleeding mildly, which can be effectively mitigated by endo therapy and using steroids after 48 h post-bleeding. Hence, GI bleeding should not be considered an absolute contraindication for steroid use[11]. Patients with severe comorbidities, such as alcohol withdrawal, psychosis, electrolyte disturbances, or hepatic encephalopathy (HE) > grade II, should be stabilized before prescribing steroids. Thus, evidence of sepsis should be searched in this population before starting steroid therapy. Although mDF > 90 is debatable for steroid prescription, evidence suggests that the decision should not be based solely on mDF but rather on the presence of sepsis and organ failure. Also, patients were classified as steroid-eligible or -ineligible. Since the outcomes in the steroid-ineligible population are poor, alternative therapies can be considered to improve survival[6].
| Trial | Inclusion criteria | Exclusion criteria |
| STOPAH trial | Clinical diagnosis of alcoholic hepatitis with mDF > 32 | Patients with creatinine > 5.7 mg% or on renal support. Patients with gastrointestinal bleed and creatinine up to 5.7 mg% were not excluded. Also, patients with resolved sepsis were not excluded |
| Louvet et al[13] 2009 | Severe alcoholic hepatitis with mDF > 32 and onset of jaundice within 3 months. Patients with treated infections were included | Gastrointestinal bleed within 15 days, peptic ulcers, Hepatitis B and C, neoplasm |
| GpreAH study | Clinical or biopsy proven alcoholic hepatitis with mDF < 90 | Patients with sepsis, creatinine > 1.5 and recent gastrointestinal bleed were excluded |
| STASH trial | Clinical diagnosis of alcoholic hepatitis | Acute kidney injury, recent gastrointestinal bleed and active infections were excluded |
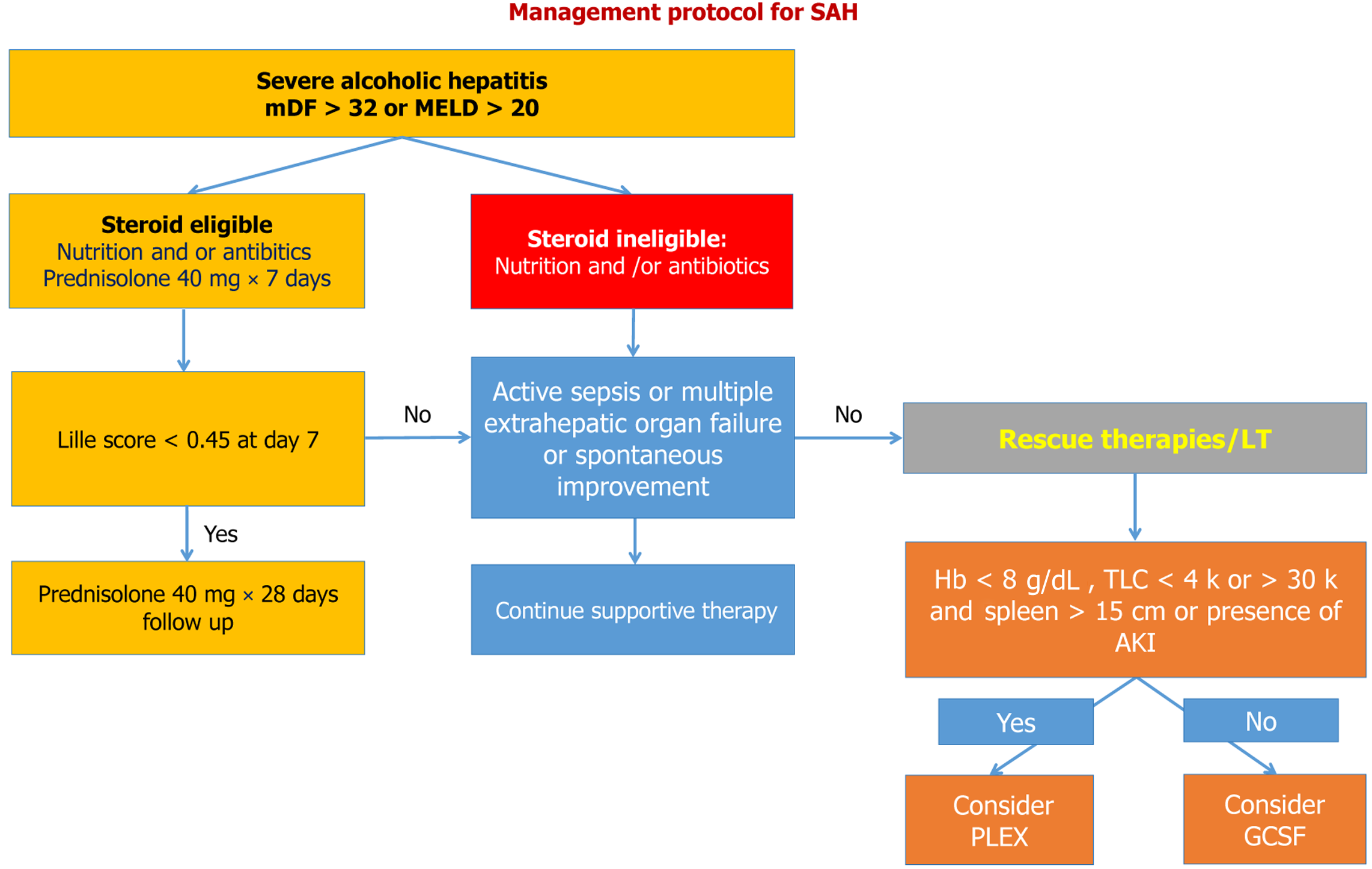
Steroid non-response: Steroid non-response was defined by a Lille score > 0.45 on day 7 (Figure 2). These patients are at increased risk of infections and high short-term mortality. Granulocyte Colony Stimulating factor (GCSF) therapy can be used as rescue therapy, as discussed subsequently.
Steroid regimens: The most common regimen used for SAH is 40 mg of prednisolone for four weeks, followed by two weeks of tapering. Since infection risk increases after the second week, several studies have attempted early tapering of steroids. A multicenter trial showed that the risk of infections is reduced with a weekly tapering regimen, i.e., by 10 mg every week starting in the second week, resulting in comparable survival rates[12,13].
Steroid-ineligible SAH: Currently, there are limited options to manage steroid-ineligible alcoholic hepatitis, although a large proportion of ineligible patients due to infections can become steroid-eligible after antibiotics treatment. Steroid therapy can be considered in > 90% of such patients, continuing antibiotic therapy, with resolved infection (13). Patients with controlled GI bleed can be considered for steroids after the bleed is controlled[11], and those with renal failure can be considered for steroid therapy if they are not on renal replacement therapy. Collectively, a large proportion of the ineligible population can be made eligible with supportive treatments. For those with more than two extrahepatic organ failures, candidature for liver transplantation should be evaluated while considering alternative therapies.
GCSF: GCSF improves liver failure and prevents infections in patients with SAH[14]. Randomized trials have shown that GCSF is safe and improves 90-day survival compared to standard medical therapy[15,16]. A recent randomized controlled trial by our group[17] suggests that GCSF improves steroid responsiveness, and combining GCSF with steroids provides better results than monotherapy[17]. However, all trials on GCSF excluded patients with active sepsis[17-19]. Additionally, GCSF can aggravate hemophagocytosis (HLH); hence, patients with cytopenia should be evaluated for HLH before considering GCSF. Also, the outcomes of GCSF alone are similar to steroids with decreased risk of infections and, hence, can be a suitable therapy as a bridge to liver transplantation in such patients[18]. Although European studies have shown opposite results, the heterogeneity of the patient population with organ failure may be one of the reasons for poor outcomes, while the other may be the variable regimens used in trials[20]. Currently, GCSF is the only medical therapy for steroid non-responders that reduces mortality. In a nutshell, GCSF therapy can be utilized alone or in combination with steroids in selected patients with SAH. The inclusion and exclusion criteria of various trials for GCSF therapy have been summarized in Table 2.
| Ref. | Intervention/control arm and inclusion criteria | Exclusion criteria | Survival at day 90 |
| Singh et al[19] (2014) | GCSF+SOC, SMT | Upper gastrointestinal bleeding during the previous 10 days, hepatocellular carcinoma or portal vein thrombosis, hepatorenal syndrome, grade 3 or 4 hepatic encephalopathy, uncontrolled bacterial infection, human immunodeficiency virus infection, and other liver disease etiologies | 18/23 (78.2%); 05/23 (21.7%) |
| Singh et al[18] (2018) | GCSF+SMT, SMT+GCSF+NAC, SMT | Same as above | 13/19 (68.42%); 16/18 (88.8%); 6/20 (30%) |
| Shasthry et al[16] (2018) | GCSF, SMT | Lille score < 0.45 at day 7. Multiorgan failure | 9/14 (64.2%); 4/14 (28.4%) |
| Tayek et al[42] (2022) | GCSF + SMT, SMT | Pregnancy, uncontrolled infection, known human immunodeficiency virus infection, recent upper gastrointestinal hemorrhage, white blood cell > 30000/mm3, creatinine > 2 mg/dL, and AH therapy for more than three days before randomization | 12/16 (75%); 15/18 (83.2%) |
| Mishra et al[17] (2024) | GCSF, GCSF+Predni-solone, Prednisolone | Serum creatinine > 1.5 mg/dL, total leukocyte count > 25000/cmm, mDF > 90, hemoglobin of less than 10 g/dL, active infection, recent upper gastrointestinal bleeding, and the existence of any other liver disease | 33/42 (78.5%); 37/42 (88.1%); 27/42 (64.2%) |
Plasma exchange: Plasma exchange (PE) lessens the onset of multiorgan failures in individuals with ACLF and improves systemic inflammation[21]. Compared to medical treatment, PE is linked to a greater resolution of systemic inflammatory response syndrome, a lower and delayed onset of multiorgan failure, and fewer deaths from liver failure in a large multinational cohort study[22]. It removes inflammatory cytokines, damage-associated molecular patterns, and endotoxins from the blood. Moreover, PE improves monocyte function and mitochondrial respiration in responders as well as removes large non-dialyzable and pathogenic macromolecules in the progression of liver failure. These findings suggest that while PE is beneficial across different etiologies of ACLF, 65% of ACLF patients treated with PE were alcoholics[22]. Notably, these studies excluded patients with multiorgan failure, sepsis, GI bleeding, and coagulopathy; these patients should be optimized before considering PE. Low-dose steroids combined with PE have been used to treat SAH in a study in India, wherein it improved one-year survival[23,24]. The authors suggested that centrifugal PE with a slow removal rate provides better results than membrane-based PE. Although patients with sepsis and GI bleeding were excluded from this study, the authors showed a positive impact of PE on renal dysfunction. Hence, patients with alcoholic hepatitis presenting as ACLF with or without renal dysfunction in the absence of sepsis or multiorgan failure can be favorable candidates for PE therapy (Table 3). The selection criteria for various therapies based on current evidence is summarized in Table 4.
| Ref. | Inclusion | Exclusion criteria | Survival at day 90 in intervention group |
| Kumar et al[24] | APASL defined alcohol ACLF | Liver illness due to other causes (such as drug- or virus-induced hepatitis, Wilson's disease, autoimmune hepatitis, or nonalcoholic fatty liver disease), sepsis, bleeding that has occurred recently (within the last three months), cancer, acute pancreatitis, or human immunodeficiency virus infection | 35.6% |
| Kumar et al[24] | EASL defined ACLF | Hepatocellular cancer, patients with PLEX contraindications, such as infection, recent gastrointestinal bleeding, and hypotension | 70% with centrifugal plasma exchange |
| Ramakrishnan et al[23] | ACLF defined by APASL with AARC score 8-10 and not responding to medical therapy | Nonalcoholic etiologies, coexisting hepatocellular carcinoma or extra-hepatic malignancy, bleeding, pregnancy, multiorgan failure (≥ 3 organ failures), severe pre-existing cardiopulmonary disease, APASL-AARC score > 10 or < 8, mechanically ventilated and requiring inotropic support at the time of enrolment were excluded from the study. | 64% |
| Therapy | Selection criteria | Remarks |
| Corticosteroids | Severe alcoholic hepatitis with mDF > 32 or MELD > 20 in absence of active sepsis and multiorgan dysfunctions. MELD > 39 should be avoided to exposed to corticosteroids | GI bleed and renal failure are relative contraindications. Corticosteroids can be considered on case to case basis in absence of other contraindications. Patients with resolved infections can be considered for steroid therapy |
| GCSF | Severe alcoholic hepatitis with mDF > 32 or MELD > 20 in absence of active sepsis and multiorgan dysfunctions. Patients with severe cytopenias, hemoglobin < 8 g/dL or leukocytosis with TLC > 30 k should be avoided for GCSF therapy. Patient with hypersplenism (spleen size > 15 cm) should be avoided | Limited literature exist regarding use of GCSF in resolved infection, GI bleed or Acute kidney injury. Hence it should be avoided till further evidence supports its use |
| Plasma exchange | Severe alcoholic hepatitis with mDF > 32 or MELD > 20 in absence of active sepsis and multiorgan dysfunctions. ACLF patients with progressive liver failure without extrahepatic failures are best candidates | Patients with acute renal injury in absence of other extrahepatic organ failure may benefit from therapy. Steroid non responders can be considered on case to case basis. Patients with GI bleed or resolved infection can be considered for plasma exchange |
| Fecal microbiota transplantation | Severe alcoholic hepatitis with mDF > 32 or MELD > 20 in absence of active sepsis and multiorgan dysfunctions | Patient with intestine paralysis should be avoided for FMT |
Fecal microbiota transplantations: Fecal microbiota transplantations (FMTs) show promising results in the treatment of SAH. Several studies have demonstrated improved survival rates and clinical outcomes in patients with SAH receiving FMT compared with those receiving standard therapies. A study comparing FMT to corticosteroids, pentoxifylline, and nutritional support showed that SAH patients receiving FMT had the highest three-month survival rate of 75% compared to 38%, 30%, and 29% in the other groups, respectively[25]. Another trial found that 90-day survival was significantly higher with FMT (75%) than prednisolone (56.6%)[26]. Interestingly, FMT modulated the gut microbiome in beneficial ways. Some studies have observed an increase in beneficial bacteria, such as Bifidobacterium, and a decrease in pathogenic species following FMT[26,27]. FMT was associated with improvements in clinical parameters, such as the resolution of HE and ascites[27,28]. Pande et al[26] and Philips et al[27] enrolled patients with mDF < 90 and without sepsis and organ failure, i.e., a steroid-eligible population. The rate of infection was lower with FMT than with steroids, although not statistically significant. Hence, FMT can be used as an alternative if steroids are contraindicated. However, the role of FMT in steroid-ineligible SAH remains unclear. The major trials evaluating FMT in SAH are summarized in Table 5.
| Ref. | Study design and inclusion criteria | Exclusion criteria | Infection rate |
| Pande et al[26] 2022 | RCT, Adults with SAH | Creatinine > 1.5, MELD > 35; mDF > 90, GI bleed within 1 months | 18.2% |
| Sharma et al[28] 2022 | Prospective cohort study. Adults with SAH | Other causes of liver disease, uncontrolled infections, uncontrolled upper gastrointestinal bleeding, grade 3 or 4 hepatic encephalopathy, more than two organ failures, malignancy, intestinal paralysis, or perforation | 30% |
| Philips et al[27] 2022 | Retrospective. Adults with Steroids ineligible SAH | Hemodialysis at admission, disseminated intravascular coagulation, multi-organ failure, uncontrolled sepsis on inotropes, and other liver disease etiologies were excluded, as was gastrointestinal bleeding within a month | NA |
Pentoxifylline: Pentoxifylline has shown mixed results in the treatment of SAH. Some studies suggested potential benefits, while others indicated limited efficacy compared to other treatments. Several studies have shown that pentoxifylline reduces the incidence of fatal hepatorenal syndrome (HRS) compared to placebo[29] and also improves short-term survival rates[30]. However, the overall effect on mortality remains controversial. The STOPAH study showed no benefit of pentoxifylline for medium- and long-term mortality[8]. Interestingly, a study by De et al[31] found pentoxifylline to be superior, with lower mortality rates and renoprotective effects compared to prednisolone[31]. Another trial compared a combination of corticosteroids and pentoxifylline with corticosteroids alone and found no additional survival advantage with combination therapy[32]. In conclusion, although pentoxifylline may offer some benefits in treating SAH, particularly in reducing the risk of HRS, its overall efficacy remains uncertain. Therefore, further investigation is essential to determine the role of pentoxifylline in SAH management.
N-acetylcysteine: N-acetylcysteine (NAC) is promising in the treatment of SAH. In a randomized controlled trial, combination therapy with prednisolone and NAC significantly improved one-month survival compared with prednisolone alone (8% vs 24% mortality) in patients with SAH. However, the primary outcome of six-month survival did not improve significantly (27% vs 38%) mortality[33,34]. Nonetheless, the improvement was attributed to the renoprotective effects, thereby reducing the incidence of kidney injury in the study group and a decline in the infection rate. NAC combined with GCSF did not provide any additional benefits for survival, suggesting that a reduction in infection rates could be the mechanism for improved survival, which is significant when combined with other steroids[18].
Combination therapies: SAH is associated with defective hepatocyte regeneration, progressive inflammation, bacterial translocation from the gut, and dysbiosis, leading to progressive liver and extrahepatic organ failures. A single therapy may not be optimal for combating all pathogenic mechanisms and rebalancing hepatocyte homeostasis. Hence, a combination of anti-inflammatory and regenerative therapies of those that modulate gut microbiota can improve survival. Combination therapies with survival advantages over monotherapy include GCSF with NAC, GCSF plus prednisolone, and PE with low-dose steroids.
Other therapies: Various anti-cytokine therapies, such as tumor necrosis factor (TNF) and interleukin-1 antagonists, have failed to show survival advantages[34,35].
Nonetheless, TNF antagonists increase the risk of infections and mortality, suggesting that some of these cytokines are essential for hepatocyte regeneration[34]. Also, S-adenosyl methionine, antibiotics, and zinc did not provide survival benefits[36-38].
Liver transplantation: Liver transplantation for SAH has been debatable due to ethical concerns and the traditional six-month abstinence rule. However, recent studies have shown promising results for early liver transplantation in carefully selected SAH patients. Mathurin et al. reported improved survival rates for patients with SAH who received early transplants compared to those who did not receive timely treatment[39]. The study observed a six-month survival rate of 77% for early transplant recipients vs 23% for non-recipients with steroid non-response defined by Lille score > 0.45. Interestingly, alcohol relapse rates in SAH patients who received early transplants were similar to or even lower than those in patients with alcoholic cirrhosis who met the six-month abstinence criterion[40]. However, there are concerns about harmful drinking patterns post-transplantation in patients with SAH, with approximately 15%–20% of the population having early relapse and poor graft function, emphasizing careful patient selection and assessment[41,42]. In conclusion, although early liver transplantation for SAH remains controversial, emerging evidence suggests that it is a lifesaving option for carefully selected patients who do not respond to medical therapy. Also, the traditional six-month abstinence rule may need to be reconsidered in light of these findings. Nevertheless, in-depth studies are required to determine the long-term outcomes and develop validated models for predicting alcohol relapse risk in this population. The expected survival on various therapies and liver transplantation is summarized in Table 6.
| Therapy | Expected 28 days survival | Expected 90 days survival | Expected 6 months survival |
| Corticosteroids[8,17] | 80% to 85% | 60% to 70% | 50% to 55% |
| GCSF[17,18] | 80% to 90% | 75% to 80% | 60% to 65% |
| Plasma exchange[23,24] | 60% to 70% | 35% to 65% | 20% to 30% |
| Fecal microbiota transplantation[26-28] | 75% to 80% | 70% to 75% | Variable |
| Liver transplantation | 80% to 85% | 80% to 85% | 69% to 85% |
Not all patients with SAH need specific therapies. Specific therapies should be given to patients not improving on supportive treatment and abstinence. The decision to start specific therapies can be based on improvement on serum bilirubin at day 7. Steroids are the mainstay of treatment for SAH and have been shown to improve survival. The GCSF and PE are potential alternatives and can be life-saving in carefully selected patients. Fecal microbiota transplantation is also emerging as disease-modifying therapy, but data are limited. The combination of therapies may improve survival compared to mono-therapies, but more research is needed before using such combinations in practice.
| 1. | Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KV, Malinchoc M, Kamath PS, Shah V. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 381] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 2. | Choudhury A, Kulkarni AV, Arora V, Soin AS, Dokmeci AK, Chowdhury A, Koshy A, Duseja A, Kumar A, Mishra AK, Patwa AK, Sood A, Roy A, Shukla A, Chan A, Krag A, Mukund A, Mandot A, Goel A, Butt AS, Sahney A, Shrestha A, Cárdenas A, Di Giorgio A, Arora A, Anand AC, Dhawan A, Jindal A, Saraya A, Srivastava A, Kumar A, Kaewdech A, Pande A, Rastogi A, Valsan A, Goel A, Kumar A, Singal AK, Tanaka A, Coilly A, Singh A, Meena BL, Jagadisan B, Sharma BC, Lal BB, Eapen CE, Yaghi C, Kedarisetty CK, Kim CW, Panackel C, Yu C, Kalal CR, Bihari C, Huang CH, Vasishtha C, Jansen C, Strassburg C, Lin CY, Karvellas CJ, Lesmana CRA, Philips CA, Shawcross D, Kapoor D, Agrawal D, Payawal DA, Praharaj DL, Jothimani D, Song DS, Kim DJ, Kim DS, Zhongping D, Karim F, Durand F, Shiha GE, D'Amico G, Lau GK, Pati GK, Narro GEC, Lee GH, Adali G, Dhakal GP, Szabo G, Lin HC, Li H, Nair HK, Devarbhavi H, Tevethia H, Ghazinian H, Ilango H, Yu HL, Hasan I, Fernandez J, George J, Behari J, Fung J, Bajaj J, Benjamin J, Lai JC, Jia J, Hu JH, Chen JJ, Hou JL, Yang JM, Chang J, Trebicka J, Kalf JC, Sollano JD, Varghese J, Arab JP, Li J, Reddy KR, Raja K, Panda K, Kajal K, Kumar K, Madan K, Kalista KF, Thanapirom K, Win KM, Suk KT, Devadas K, Lesmana LA, Kamani L, Premkumar M, Niriella MA, Al Mahtab M, Yuen MF, Sayed MH, Alla M, Wadhawan M, Sharma MK, Sahu M, Prasad M, Muthiah MD, Schulz M, Bajpai M, Reddy MS, Praktiknjo M, Yu ML, Prasad M, Sharma M, Elbasiony M, Eslam M, Azam MG, Rela M, Desai MS, Vij M, Mahmud N, Choudhary NS, Marannan NK, Ormeci N, Saraf N, Verma N, Nakayama N, Kawada N, Oidov Baatarkhuu, Goyal O, Yokosuka O, Rao PN, Angeli P, Parikh P, Kamath PS, Thuluvath PJ, Lingohr P, Ranjan P, Bhangui P, Rathi P, Sakhuja P, Puri P, Ning Q, Dhiman RK, Kumar R, Vijayaraghavan R, Khanna R, Maiwall R, Mohanka R, Moreau R, Gani RA, Loomba R, Mehtani R, Rajaram RB, Hamid SS, Palnitkar S, Lal S, Biswas S, Chirapongsathorn S, Agarwal S, Sachdeva S, Saigal S, Kumar SE, Violeta S, Singh SP, Mochida S, Mukewar S, Alam S, Lim SG, Alam S, Shalimar, Venishetty S, Sundaram SS, Shetty S, Bhatia S, Singh SA, Kottilil S, Strasser S, Shasthry SM, Maung ST, Tan SS, Treeprasertsuk S, Asthana S, Manekeller S, Gupta S, Acharya SK, K C S, Maharshi S, Asrani S, Dadhich S, Taneja S, Giri S, Singh S, Chen T, Gupta T, Kanda T, Tanwandee T, Piratvishuth T, Spengler U, Prasad VGM, Midha V, Rakhmetova V, Arroyo V, Sood V, Br VK, Wong VW, Pamecha V, Singh V, Dayal VM, Saraswat VA, Kim W, Jafri W, Gu W, Jun WY, Qi X, Chawla YK, Kim YJ, Shi Y, Abbas Z, Kumar G, Shiina S, Wei L, Omata M, Sarin SK; APASL-ACLF Research Consortium (AARC) for APASL-ACLF working party. Acute-on-chronic liver failure (ACLF): the 'Kyoto Consensus'-steps from Asia. Hepatol Int. 2025;19:1-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 29.0] [Reference Citation Analysis (1)] |
| 3. | Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, Giovo I, Uschner FE, Jansen C, Jimenez C, Mookerjee R, Gustot T, Albillos A, Bañares R, Jarcuska P, Steib C, Reiberger T, Acevedo J, Gatti P, Shawcross DL, Zeuzem S, Zipprich A, Piano S, Berg T, Bruns T, Danielsen KV, Coenraad M, Merli M, Stauber R, Zoller H, Ramos JP, Solé C, Soriano G, de Gottardi A, Gronbaek H, Saliba F, Trautwein C, Kani HT, Francque S, Ryder S, Nahon P, Romero-Gomez M, Van Vlierberghe H, Francoz C, Manns M, Garcia-Lopez E, Tufoni M, Amoros A, Pavesi M, Sanchez C, Praktiknjo M, Curto A, Pitarch C, Putignano A, Moreno E, Bernal W, Aguilar F, Clària J, Ponzo P, Vitalis Z, Zaccherini G, Balogh B, Gerbes A, Vargas V, Alessandria C, Bernardi M, Ginès P, Moreau R, Angeli P, Jalan R, Arroyo V; PREDICT STUDY group of the EASL-CLIF CONSORTIUM. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021;74:1097-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (1)] |
| 4. | Lourens S, Sunjaya DB, Singal A, Liangpunsakul S, Puri P, Sanyal A, Ren X, Gores GJ, Radaeva S, Chalasani N, Crabb DW, Katz B, Kamath PS, Shah VH; TREAT Consortium. Acute Alcoholic Hepatitis: Natural History and Predictors of Mortality Using a Multicenter Prospective Study. Mayo Clin Proc Innov Qual Outcomes. 2017;1:37-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Potts JR, Howard MR, Verma S. Recurrent severe alcoholic hepatitis: clinical characteristics and outcomes. Eur J Gastroenterol Hepatol. 2013;25:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Singeap AM, Minea H, Petrea O, Robea MA, Balmuș IM, Duta R, Ilie OD, Cimpoesu CD, Stanciu C, Trifan A. Real-World Utilization of Corticosteroids in Severe Alcoholic Hepatitis: Eligibility, Response, and Outcomes. Medicina (Kaunas). 2024;60:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Arab JP, Díaz LA, Baeza N, Idalsoaga F, Fuentes-López E, Arnold J, Ramírez CA, Morales-Arraez D, Ventura-Cots M, Alvarado-Tapias E, Zhang W, Clark V, Simonetto D, Ahn JC, Buryska S, Mehta TI, Stefanescu H, Horhat A, Bumbu A, Dunn W, Attar B, Agrawal R, Haque ZS, Majeed M, Cabezas J, García-Carrera I, Parker R, Cuyàs B, Poca M, Soriano G, Sarin SK, Maiwall R, Jalal PK, Abdulsada S, Higuera-de la Tijera MF, Kulkarni AV, Rao PN, Guerra Salazar P, Skladaný L, Bystrianska N, Prado V, Clemente-Sanchez A, Rincón D, Haider T, Chacko KR, Cairo F, de Sousa Coelho M, Romero GA, Pollarsky FD, Restrepo JC, Castro-Sanchez S, Toro LG, Yaquich P, Mendizabal M, Garrido ML, Narvaez A, Bessone F, Marcelo JS, Piombino D, Dirchwolf M, Arancibia JP, Altamirano J, Kim W, Araujo RC, Duarte-Rojo A, Vargas V, Rautou PE, Issoufaly T, Zamarripa F, Torre A, Lucey MR, Mathurin P, Louvet A, García-Tsao G, González JA, Verna E, Brown RS, Roblero JP, Abraldes JG, Arrese M, Shah VH, Kamath PS, Singal AK, Bataller R. Identification of optimal therapeutic window for steroid use in severe alcohol-associated hepatitis: A worldwide study. J Hepatol. 2021;75:1026-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 8. | Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, Downs N, Gleeson D, MacGilchrist A, Grant A, Hood S, Masson S, McCune A, Mellor J, O'Grady J, Patch D, Ratcliffe I, Roderick P, Stanton L, Vergis N, Wright M, Ryder S, Forrest EH; STOPAH Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 593] [Article Influence: 53.9] [Reference Citation Analysis (1)] |
| 9. | Degré D, Stauber RE, Englebert G, Sarocchi F, Verset L, Rainer F, Spindelboeck W, Njimi H, Trépo E, Gustot T, Lackner C, Deltenre P, Moreno C. Long-term outcomes in patients with decompensated alcohol-related liver disease, steatohepatitis and Maddrey's discriminant function <32. J Hepatol. 2020;72:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Parker R, Cabezas J, Altamirano J, Arab JP, Ventura-Cots M, Sinha A, Dhanda A, Arrese M, McCune CA, Rowe IA, Schnabl B, Mathurin P, Shawcross D, Abraldes JG, Lucey MR, Garcia-Tsao G, Verna E, Brown RS Jr, Bosques-Padilla F, Vargas V, Louvet A, Holt AP, Bataller R. Trajectory of Serum Bilirubin Predicts Spontaneous Recovery in a Real-World Cohort of Patients With Alcoholic Hepatitis. Clin Gastroenterol Hepatol. 2022;20:e289-e297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Rudler M, Mouri S, Charlotte F, Lebray P, Capocci R, Benosman H, Poynard T, Thabut D. Prognosis of treated severe alcoholic hepatitis in patients with gastrointestinal bleeding. J Hepatol. 2015;62:816-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Kulkarni AV, Kumar K, Giri S, Arab JP, Venishetty S, Premkumar M, Kadnur HB, Sharma M, Alla M, Iyengar S, Nayak G, Saraswat VA, Gupta R, Rao PN, Reddy KR, Reddy DN. Infections in Standard or Tapered Dose of Prednisolone for Alcohol-Associated Hepatitis: A Randomized Trial (STASH Trial). Am J Gastroenterol. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Louvet A, Wartel F, Castel H, Dharancy S, Hollebecque A, Canva-Delcambre V, Deltenre P, Mathurin P. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology. 2009;137:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 281] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 14. | Garg V, Garg H, Khan A, Trehanpati N, Kumar A, Sharma BC, Sakhuja P, Sarin SK. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:505-512.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 301] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 15. | Marot A, Singal AK, Moreno C, Deltenre P. Granulocyte colony-stimulating factor for alcoholic hepatitis: A systematic review and meta-analysis of randomised controlled trials. JHEP Rep. 2020;2:100139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 16. | Shasthry SM, Sharma MK, Shasthry V, Pande A, Sarin SK. Efficacy of Granulocyte Colony-stimulating Factor in the Management of Steroid-Nonresponsive Severe Alcoholic Hepatitis: A Double-Blind Randomized Controlled Trial. Hepatology. 2019;70:802-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Mishra AK, Shasthry SM, Vijayaraghavan R, Kumar G, Sarin SK. Granulocyte Colony-Stimulating Factor Improves Prednisolone Responsiveness and 90-Day Survival in Steroid-Eligible Severe Alcohol-Associated Hepatitis: The GPreAH Study a Randomized Trial. Am J Gastroenterol. 2025;120:1087-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Singh V, Keisham A, Bhalla A, Sharma N, Agarwal R, Sharma R, Singh A. Efficacy of Granulocyte Colony-Stimulating Factor and N-Acetylcysteine Therapies in Patients With Severe Alcoholic Hepatitis. Clin Gastroenterol Hepatol. 2018;16:1650-1656.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 19. | Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, Sharma R. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol. 2014;109:1417-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 20. | Engelmann C, Herber A, Franke A, Bruns T, Reuken P, Schiefke I, Zipprich A, Zeuzem S, Goeser T, Canbay A, Berg C, Trebicka J, Uschner FE, Chang J, Mueller T, Aehling N, Schmelzle M, Splith K, Lammert F, Lange CM, Sarrazin C, Trautwein C, Manns M, Häussinger D, Pfeiffenberger J, Galle PR, Schmiedeknecht A, Berg T. Granulocyte-colony stimulating factor (G-CSF) to treat acute-on-chronic liver failure: A multicenter randomized trial (GRAFT study). J Hepatol. 2021;75:1346-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 21. | Ballester MP, Sittner R, Jalan R. Alcohol and Acute-on-Chronic Liver Failure. J Clin Exp Hepatol. 2022;12:1360-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Maiwall R, Bajpai M, Choudhury AK, Kumar A, Sharma MK, Duan Z, Yu C, Hu J, Ghazinian H, Ning Q, Ma K, Lee GH, Lim SG, Shah S, Kalal C, Dokmeci A, Kumar G, Jain P, Rao Pasupuleti SS, Paulson I, Kumar V, Sarin SK; AARC working Party. Therapeutic plasma-exchange improves systemic inflammation and survival in acute-on-chronic liver failure: A propensity-score matched study from AARC. Liver Int. 2021;41:1083-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Ramakrishnan S, Hans R, Duseja A, Sharma RR. Therapeutic plasma exchange is a safe and effective bridge therapy in patients with alcohol-associated ACLF not having immediate prospects for liver transplantation-A case-control, pilot study. J Clin Apher. 2022;37:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 24. | Kumar SE, Chellaiya GK, Singh KA, Karuppusami R, Daniel D, David VG, Nair SC, Varughese S, Mammen J, Elias E, Eapen CE, Zachariah UG, Goel A. Centrifugal technique of plasma exchange and low-dose steroid to treat very severe alcoholic hepatitis patients: A retrospective analysis. Indian J Gastroenterol. 2025;44:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Philips CA, Phadke N, Ganesan K, Ranade S, Augustine P. Corticosteroids, nutrition, pentoxifylline, or fecal microbiota transplantation for severe alcoholic hepatitis. Indian J Gastroenterol. 2018;37:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 26. | Pande A, Sharma S, Khillan V, Rastogi A, Arora V, Shasthry SM, Vijayaraghavan R, Jagdish R, Kumar M, Kumar G, Mondot S, Dore J, Sarin SK. Fecal microbiota transplantation compared with prednisolone in severe alcoholic hepatitis patients: a randomized trial. Hepatol Int. 2023;17:249-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 27. | Philips CA, Ahamed R, Rajesh S, Singh S, Tharakan A, Abduljaleel JK, Augustine P. Clinical outcomes and gut microbiota analysis of severe alcohol-associated hepatitis patients undergoing healthy donor fecal transplant or pentoxifylline therapy: single-center experience from Kerala. Gastroenterol Rep (Oxf). 2022;10:goac074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Sharma A, Roy A, Premkumar M, Verma N, Duseja A, Taneja S, Grover S, Chopra M, Dhiman RK. Fecal microbiota transplantation in alcohol-associated acute-on-chronic liver failure: an open-label clinical trial. Hepatol Int. 2022;16:433-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 29. | Parker R, Armstrong MJ, Corbett C, Rowe IA, Houlihan DD. Systematic review: pentoxifylline for the treatment of severe alcoholic hepatitis. Aliment Pharmacol Ther. 2013;37:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 515] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 31. | De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol. 2009;15:1613-1619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Sidhu SS, Goyal O, Singla P, Gupta D, Sood A, Chhina RS, Soni RK. Corticosteroid plus pentoxifylline is not better than corticosteroid alone for improving survival in severe alcoholic hepatitis (COPE trial). Dig Dis Sci. 2012;57:1664-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Nguyen-Khac E, Thevenot T, Piquet MA, Benferhat S, Goria O, Chatelain D, Tramier B, Dewaele F, Ghrib S, Rudler M, Carbonell N, Tossou H, Bental A, Bernard-Chabert B, Dupas JL; AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 309] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 34. | Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, Boardman L, Gores GJ, Harmsen WS, McClain CJ, Kamath PS, Shah VH. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953-1960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 263] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 35. | Gawrieh S, Dasarathy S, Tu W, Kamath PS, Chalasani NP, McClain CJ, Bataller R, Szabo G, Tang Q, Radaeva S, Barton B, Nagy LE, Shah VH, Sanyal AJ, Mitchell MC; AlcHepNet Investigators. Randomized trial of anakinra plus zinc vs. prednisone for severe alcohol-associated hepatitis. J Hepatol. 2024;80:684-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 36. | Tkachenko P, Maevskaya M, Pavlov A, Komkova I, Pavlov C, Ivashkin V. Prednisolone plus S-adenosil-L-methionine in severe alcoholic hepatitis. Hepatol Int. 2016;10:983-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Louvet A, Labreuche J, Dao T, Thévenot T, Oberti F, Bureau C, Paupard T, Nguyen-Khac E, Minello A, Bernard-Chabert B, Anty R, Wartel F, Carbonell N, Pageaux GP, Hilleret MN, Moirand R, Nahon P, Potey C, Duhamel A, Mathurin P. Effect of Prophylactic Antibiotics on Mortality in Severe Alcohol-Related Hepatitis: A Randomized Clinical Trial. JAMA. 2023;329:1558-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 38. | Szabo G, Mitchell M, McClain CJ, Dasarathy S, Barton B, McCullough AJ, Nagy LE, Kroll-Desrosiers A, Tornai D, Min HA, Radaeva S, Holbein MEB, Casey L, Cuthbert J. IL-1 receptor antagonist plus pentoxifylline and zinc for severe alcohol-associated hepatitis. Hepatology. 2022;76:1058-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 39. | Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, Castel H, Duhamel A, Pageaux GP, Leroy V, Dharancy S, Louvet A, Boleslawski E, Lucidi V, Gustot T, Francoz C, Letoublon C, Castaing D, Belghiti J, Donckier V, Pruvot FR, Duclos-Vallée JC. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 678] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 40. | Zafar Y, Siddiqi AK, Shaikh N, Imran M, Javaid SS, Manzoor L, Iqbal AZ, Petrasek J. Alcohol Relapse After Early Liver Transplantation in Patients With Alcoholic Liver Disease: A Meta-Analysis. Gastroenterology Res. 2024;17:10-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 41. | Lee BP, Im GY, Rice JP, Lazar A, Weinberg E, Han H, Maddur H, Ghobrial RM, Therapondos G, Hsu C, Fix OK, Eswaran S, Shetty K, Chhatwal J, Dalgic OO, Jakhete N, Mobley C, Victor DW, Mehta N, Dinges L, Rinella M, Schiano TD, Lucey MR, Terrault N. Patterns of Alcohol Use After Early Liver Transplantation for Alcoholic Hepatitis. Clin Gastroenterol Hepatol. 2022;20:409-418.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 42. | Tayek JA, Stolz AA, Nguyen DV, Fleischman MW, Donovan JA, Alcorn JM, Chao DC, Asghar A, Morgan TR; Southern California Alcoholic Hepatitis (SCAH) Consortium. A phase II, multicenter, open-label, randomized trial of pegfilgrastim for patients with alcohol-associated hepatitis. EClinicalMedicine. 2022;54:101689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/