Published online Sep 27, 2025. doi: 10.4254/wjh.v17.i9.108259
Revised: May 18, 2025
Accepted: August 13, 2025
Published online: September 27, 2025
Processing time: 169 Days and 18.5 Hours
Metabolic dysfunction-associated steatotic liver disease (MASLD) poses a sub
Core Tip: Metabolic dysfunction-associated steatotic liver disease (MASLD) is a growing global health concern that progresses from simple steatosis to cirrhosis. Extracellular vesicles (EVs) have emerged as key mediators of MASLD pathogenesis, influencing lipotoxicity, inflammation, and fibrosis through bioactive cargoes such as microRNAs. This review highlights the role of EVs in MASLD progression, their diagnostic and prognostic potential, and EV-based therapeutic strategies. We also address current challenges, emerging research trends, and future perspectives, emphasizing the translational potential of EVs for improving patient outcomes.
- Citation: Boonkaew B, Charoenthanakitkul D, Suntornnont N, Ariyachet C, Tangkijvanich P. Extracellular vesicles in metabolic dysfunction-associated steatotic liver disease: From intercellular signaling to clinical translation. World J Hepatol 2025; 17(9): 108259
- URL: https://www.wjgnet.com/1948-5182/full/v17/i9/108259.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i9.108259
The prevalence of metabolic dysfunction-associated steatotic liver disease (MASLD), the updated nomenclature for steatotic liver disease associated with metabolic syndrome, has rapidly increased, becoming a leading global health challenge. It affects approximately 30% of the worldwide population and represents the most prevalent form of chronic liver disease[1-4]. Indeed, the global prevalence of MASLD is expected to rise in the coming decade, mirroring the growing worldwide epidemics of obesity and type 2 diabetes[5,6]. A 2022 systematic review reported a global MASLD prevalence of 32.4%, a substantial increase from the 25.5% reported between 1990 and 2022[7]. While cardiovascular disease (CVD) remains the primary cause of mortality in patients with MASLD, those with advanced liver fibrosis face a dramatically increased risk of liver-related death[8,9]. The incidence of MASLD closely parallels the increasing prevalence of obesity and type 2 diabetes, underscoring its strong association with metabolic syndrome. The role of environmental modifiers such as diet, lifestyle, and the gut microbiota further contributes to the increasing prevalence of metabolic syndrome components, including obesity, diabetes, and hypertension, which in turn drive MASLD incidence[10,11].
MASLD encompasses a spectrum of chronic liver disorders, beginning with simple hepatic steatosis, characterized by excessive triglyceride accumulation in hepatocytes[12,13]. This condition can progress to more severe metabolic dysfunction-associated steatohepatitis (MASH), which is characterized by inflammation and hepatocyte injury. Untreated MASH can lead to fibrosis, cirrhosis, and ultimately, hepatocellular carcinoma (HCC)[14,15]. While the disease affects a large portion of the population and poses a significant risk of liver-related mortality, especially in advanced stages, there is a lack of approved drug therapies. This critical gap underscores the urgent need for innovative therapeutic and diagnostic strategies[16].
Central to the pathogenesis of MASLD is the complex interplay of intercellular communication, where extracellular vesicles (EVs) are increasingly recognized as pivotal mediators. EVs are lipid bilayer-enclosed, nonreplicative particles released by all cells into the extracellular space and circulation. EVs are classified on the basis of size: Large EVs (100-1000 nm, primarily < 400 nm) are associated with microvesicle markers, whereas small EVs (40-200 nm) are characterized by exosome markers. The two major classes of EVs - exosomes and microvesicles - differ in their biogenesis and functional roles. Microvesicles range from 70 nm to nearly 1 μm in size and bud directly from the plasma membrane, whereas exosomes originate within the endosomal system before secretion and typically range from 50 nm to 150 nm in size[17,18]. These vesicles serve as crucial vehicles for intercellular communication, and transport of cargo of proteins, DNA, and RNA, including mRNAs, microRNAs (miRNAs), and long noncoding RNAs (lncRNAs), thereby modulating molecular pathways in recipient cells[19]. The composition of EVs reflects the physiological or pathological state of their originating cells, making them dynamic indicators of disease progression[20-22]. In MASLD, hepatocyte-derived EVs, particularly in response to lipotoxicity, contribute significantly to the inflammatory milieu by influencing macrophage activity, thus playing a critical role in disease pathogenesis[23,24].
This review aims to provide a comprehensive overview of the role of EVs in MASLD, bridging the gap between basic mechanistic insights and potential clinical applications. Specifically, we explore the fundamental biology of EVs, delineate their involvement in MASLD pathogenesis, and critically assess their potential as diagnostic biomarkers, therapeutic delivery vehicles, and targets for therapeutic intervention. We also address the inherent challenges associated with EV research and clinical translation, providing a holistic perspective on the current state of knowledge in this rapidly evolving field.
Biogenesis and secretion by liver cells: The biogenesis and secretion of EVs are complex processes, and their precise regulation remains incompletely understood. As mentioned above, research on EVs has focused widely on exosomes and microvesicles. While exosomes and microvesicles arise through distinct biogenetic pathways, they share fundamental regulatory mechanisms for their formation and release[25]. Exosomes originate from the endosomal pathway, where inward budding of endosomal membranes results in the formation of intraluminal vesicles within multivesicular bodies (MVBs)[26,27]. These MVBs either fuse with the plasma membrane to release exosomes or are directed to other cellular compartments. Exosome biogenesis can be categorized into endosomal sorting complex required for transport (ESCRT)-dependent and ESCRT-independent pathways. The ESCRT complex, comprising several protein subunits, is crucial for cargo sorting and intraluminal vesicle formation, facilitating the selection, clustering, and membrane scission of cargo proteins[28,29]. However, even in the absence of ESCRT components, exosomes can still form, with lipids such as bismonoacylglycerophosphate and phosphatidic acid playing alternative roles[30,31]. Ceramides also contribute to ESCRT-independent exosome formation, influencing cargo selection through sphingosine 1-phosphate (S1P) signaling[32]. Cargo sorting into exosomes is a selective process, with tetraspanins and various signaling pathways determining the inclusion of specific proteins and RNAs. Rab GTPases regulate MVB trafficking, docking, and fusion with the plasma membrane, impacting exosome secretion in a cell type-specific manner[33]. By contrast, microvesicles form through outward budding of the plasma membrane and are influenced by protein aggregation, lipid composition, and cy
The release of EVs is an energy-dependent process that is intricately regulated by cellular stress, nutrient availability, and signaling pathways, thereby modulating EV secretion across diverse cell types[35]. Although directly detecting EV production in vivo remains a technological hurdle, in vitro studies have demonstrated that numerous liver cell types, including hepatocytes, hepatic stellate cells (HSCs), liver sinusoidal endothelial cells (LSECs), and Kupffer cells, are capable of EV production. In particular, hepatocytes release EVs, the production of which exhibits both qualitative and quantitative variations in response to cellular stimuli and disease states. Specifically, stressors such as lipotoxic en
Mechanisms of EV uptake by recipient cells: Once released, they initiate cellular responses to induce functional changes in recipient cells by transporting bioactive molecules such as proteins and RNAs to distant targets. This delivery necessitates the internalization of EVs through two primary mechanisms: Direct membrane fusion or endocytic internalization[37,38]. Direct membrane fusion involves the integration of the EV lipid bilayer with the recipient cell plasma membrane, whereas endocytosis involves the engulfment of EVs by the target cell[39]. Several proposed endocytosis pathways include clathrin-mediated endocytosis, caveolin-dependent endocytosis, macropinocytosis, phagocytosis, and lipid raft-mediated internalization[40-42]. The precise mechanisms governing endocytosis remain a subject of ongoing investigation; however, evidence suggests that EV uptake can be cell type-specific[43]. The specific mechanisms of EV uptake can vary among recipient cell types. Studies using fluorescently labeled EVs have demonstrated that recipient cells primarily internalize EVs through controlled endocytosis. For example, neurons predominantly utilize clathrin-mediated endocytosis, epithelial cells may utilize caveolin-dependent endocytosis, and tumor cells may rely on lipid raft-dependent endocytosis[44-46]. Additionally, specific protein-protein interactions play crucial roles in EV uptake. EVs typically attach to recipient cells and are internalized through interactions involving membrane receptors, ligands, or contact proteins. Proteins such as tetraspanins, lectins, and integrins, along with glycosylation processes, facilitate these interactions and influence EV uptake[47-51]. Notably, EVs may utilize multiple routes of internalization, depending on the surface proteins present on both the vesicle and the target cell[38].
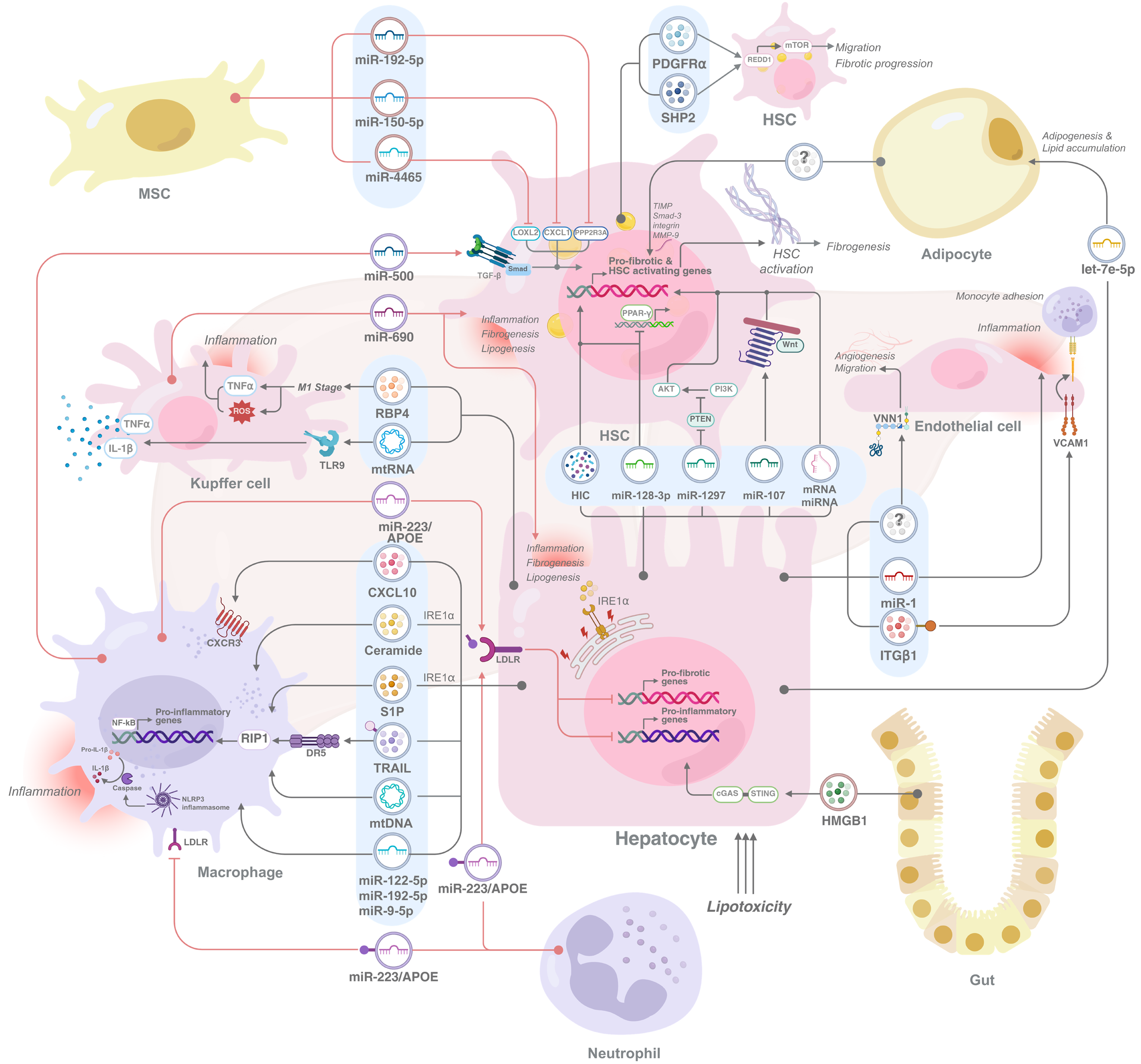
In the liver, these cells are internalized by various cell types, contributing to critical physiological processes such as macrophage modulation and HSC activation (Figure 1). Previous studies have shown that liver macrophages can take up melanoma cell line-derived EVs labeled with PKH26 fluorescent dye following intravenous administration[52]. Notably, EVs loaded with miRNA-155 (miR-155) showed rapid distribution and uptake in the liver, with the majority of miR-155 found in both hepatocytes and liver mononuclear cells within minutes of injection. This quick uptake and clearance of EVs from the circulation highlights the significant role of the liver in EV processing[53]. Moreover, EVs released from hepatocytes transfer components necessary for S1P synthesis to recipient hepatocytes, thereby promoting cell growth and liver repair following ischemia/reperfusion injury or partial hepatectomy[54]. While these studies collectively highlight the capacity of EV cargo to elicit biological responses in recipient liver cells, the precise mechanisms governing EV internalization by these cells remain incompletely understood. A recent study revealed that in MASLD, lipotoxic hepatocytes induced by palmitic acid (PA) selectively take up apolipoprotein E (ApoE)-containing EVs from neutrophils, facilitating miR-223 transfer through a low-density lipoprotein receptor (LDLR)-dependent mechanism[55]. This LDLR/ApoE pathway is also involved in the uptake of macrophage-derived miR-223-enriched EVs by lipotoxic hepatocytes. However, the impact of lipotoxicity on hepatocyte LDLR expression remains unclear, with studies reporting both increased and decreased LDLR levels[56]. Furthermore, molecules such as statins, which are known to influence LDLR expression and cholesterol uptake, may also play a role in EV uptake in the liver. While statins have been shown to increase LDLR expression and low-density lipoprotein-derived cholesterol uptake in HepG2 hepatoma cells, further research is needed to determine whether they similarly increase EV uptake in hepatocytes[57,58]. In summary, while evidence suggests that liver cells internalize EVs through various mechanisms, further investigation is needed to fully elucidate the specific pathways involved.
EVs play a crucial role in intercellular communication by transferring bioactive cargo from donor cells to recipient cells, thereby influencing various pathological processes in MASLD. Previous studies have demonstrated that elevated levels of circulating EVs in both patients and animal models correlate with liver injury, inflammation, and fibrosis, highlighting their importance in disease progression. EVs released from hepatocytes, immune cells, and HSCs carry diverse molecular cargo, including proteins, lipids, and nucleic acids, which alter the phenotype and function of recipient cells[59-62]. For example, the transfer of EVs from high-fat diet (HFD)-fed mice to healthy mice induces liver inflammation and damage by activating and directing immature myeloid cells to the liver, resulting in elevated levels of proinflammatory molecules and liver enzymes[63]. These findings underscore the role of EVs as mediators of intercellular communication in MASLD pathogenesis. The following sections discuss the involvement of EVs in different cell types, as summarized in Table 1 and Figure 1.
| Donor cells/ EV sources | Recipient cells | EV cargoes | EV effects | Ref. |
| Hepatocyte-derived EVs to other cells | ||||
| Lipotoxic hepatocytes, primary mouse hepatocytes and Huh7 cells treated with PA or LPC | Macrophages, BMDMs | CXCL10 | Recruits macrophages and exacerbates inflammation | [65,67] |
| Lipotoxic hepatocytes, mouse hepatocyte cell lines, primary mouse hepatocytes, and Huh7 cells treated with PA or OA | Macrophages, BMDMs | Ceramide, S1P | Activates IRE1α, recruits macrophages, and amplifies liver inflammation | [36,68] |
| Lipotoxic hepatocytes, primary mouse hepatocytes and Huh7 cells treated with LPC | Macrophages, BMDMs | TRAIL | Activates macrophages via RIP1-DR5 pathway, worsening inflammation | [64] |
| Primary mouse hepatocytes | Macrophages, RAW264.7 cells | mtDNA | Activates macrophages, increasing inflammatory signaling | [24] |
| Lipotoxic hepatocytes, LO2 cells treated with PA, Huh7 cells treated with ox-LDL and MβCD cholesterol | Macrophages, THP-1 cell diferentiation | miR-122-5p, miR-192-5p, miR-9-5p | Polarizes macrophages to M1, enhances inflammatory responses | [70,71,72] |
| Hypoxia in fat-laden liver cells, primary mouse hepatocytes and HepG2 treated with PA + OA | HFD-mouse Kupffer cells | mtDNA | Activates TLR9, inducing TNFα and IL-1β secretion | [73] |
| Primary rat hepatocytes | Primary rat Kupffer cells | N/A | Stimulates inflammation in Kupffer cells | [74] |
| Lipotoxic hepatocytes, LO2 cells treated with PA | Primary human Kupffer cells | RBP4 | Promotes M1 polarization, increases ROS and TNF-α production | [75] |
| Lipotoxic hepatocytes, HepG2 treated with PA + OA, chemical hypoxia induction hepatocytes, treated with CoCl2 | HSCs, LX-2 cells | Hypoxia-induced cargo | Promotes pro-fibrotic gene expression and fibrosis | [66,76] |
| Lipotoxic hepatocytes, primary mouse hepatocytes and HepG2 cells treated with PA | HSCs, LX-2 cells, primary mouse HSCs | miR-128-3p | Suppresses PPAR-γ, enhances pro-fibrogenic gene expression, proliferation, and chemotactic responses | [66] |
| Lipotoxic hepatocytes, primary hepatocytes, LO2 cells treated with PA | HSCs, LX-2 cells | miR-1297 | Targets PTEN, leading to HSC activation and proliferation via PI3K/AKT signaling | [77] |
| Lipotoxic hepatocytes, primary hepatocytes treated with PA | HSCs, LX-2 cells | miR-107 | Activates Wnt signaling, promoting HSC activation | [78] |
| Lipotoxic hepatocytes, primary rat hepatocytes treated with PA + OA | HSCs, primary rat HSCs | Various mRNAs and miRNAs | Induces pro-fibrotic and pro-senescent phenotype, reduce fibrosis, increase ROS and senescence markers, mediated via AKT-mTOR pathway | [79] |
| Lipotoxic hepatocytes, primary rat hepatocytes, HepG2 cells treated with PA | Endothelial cells, primary rat endothelial cells, HUVECs | VNN1 | Promotes angiogenesis and endothelial migration | [61] |
| Lipotoxic hepatocytes, primary mouse hepatocytes, Huh7 cells treated with PA | Endothelial cells, primary rat endothelial cells, HUVECs | miR-1 | Promotes endothelial inflammation and atherogenesis | [80] |
| Lipotoxic hepatocytes, primary mouse hepatocytes, Huh7 cells treated with LPC | Endothelial cells, primary mouse LSECs | ITGβ1 | Induces monocyte adhesion to LSECs, facilitating inflammation and fibrosis | [81] |
| Primary mouse hepatocytes | Adipocytes, 3T3-L1 cell differentiation | let-7e-5p | Promotes adipogenesis and lipid accumulation | [82] |
| Immune cell-derived EVs to other cells | ||||
| Neutrophills isolated from CCL4- and MCD-treated mice | Macrophages, mouse primary hepatic macrophages | miR-223 | Promotes restorative macrophage phenotype, reduces HSC activation and fibrosis | [84] |
| Neutrophills isolated from HFD-treated mice | Lipotoxic hepatocytes, AML12 cells treated with PA | miR-223 | Inhibits hepatic inflammatory and fibrogenic gene expression in LDLR/ApoE dependent manner | [55] |
| Macrophages and neutrophils isolated from IL-6 knockout HFD-treated mice | Hepatocytes, primary mouse hepatocytes | miR-223 | Suppresses fibrotic gene and immflmatory gene expression, reducing liver fibrosis | [87] |
| Macrophages, human PBMC dirferentiation | Lipotoxic hepatocytes, Huh7 treated with PA | miR-223 | Reduces inflammatory and fibrotic responses within the liver by suppressing FOXO3 and TAZ through LDLR/ApoE axis | [56] |
| Macrophages, RAW264.7 cells | HSCs, primary mouse HSCs isolated from CCL4-induced mice | miR-500 | Activates TGF-β/Smad pathway, accelerating fibrosis | [88] |
| Kupffer cells isolated from MASH mice | Hepatocytes, HSCs isolated from MASH mice | miR-690 | Regulates inflammation, fibrogenesis, and lipogenesis | [89] |
| Other cell-derived EVs | ||||
| Visceral adipose tissue isolated from obese and lean patients | HSCs | N/A | Alters liver matrix regulation by increasing TIMP, Smad-3, integrin, and MMP-9 expression in HSCs | [90] |
| HSCs, primary human HSCs, LX-2 cells | HSCs, primary human HSCs, LX-2 cells | PDGFRα, SHP2 | Promotes HSC migration and fibrosis progression by suppressing REDD1 and enhancing mTOR | [91,92] |
| BMSCs | HSCs | miR-192-5p | Inhibits HSC activation by targeting PPP2R3A | [93] |
| ADMSCs isolated from CCl4-induced mice | HSCs | miR-150-5p | Suppresses CXCL1, reducing fibrosis | [94] |
| MSCs isolated from the human umbilical cords | HSCs, LX-2 cells | miR-4465 | Reduces fibrosis by altering LOXL2 expression | [95] |
| Gut-derived EVs, HFD-fed mice | Hepatocytes, HSCs, HFD-fed mice, Mki67 mice | HMGB1 | Activates cGAS/STING pathway, driving inflammation and fibrosis | [96,97] |
Hepatocyte-derived EVs: Hepatocytes, the primary cells affected by lipotoxicity, are the most extensively studied source of EVs in MASLD. Given the complexity of MASLD pathology, EV-mediated crosstalk has gained significant attention, particularly with a focus on EVs released from hepatocytes. Researchers have modeled lipotoxicity by inducing lipid accumulation in hepatocytes via saturated free fatty acids (FFAs), such as PA (C16:0), oleic acid (C18:1), or lysophosphatidylcholine[64]. Notably, PA not only stimulates lipid storage but also promotes EV release from hepatocytes[65,66]. Upon lipotoxicity induction, mixed linage kinase activation triggers the mitogen-activated protein kinase signaling cascade, leading to signal transducer and activator of transcription 1 phosphorylation and enhanced C-X-C motif chemokine ligand (CXCL) 10 transcription. The subsequently released CXCL10-bearing EVs are internalized into macrophages by binding to C-X-C motif chemokine receptor 3, thereby driving inflammation[65,67]. Similarly, he
In addition to direct inflammatory signaling, lipotoxic hepatocytes release EVs carrying specific miRNAs, which influence the phenotype of recipient cells. For example, cholesterol-exposed hepatocytes release EVs containing miR-122-5p, which polarizes macrophages into the proinflammatory M1 subtype[70]. EVs carrying miR-192-5p activate macro
EVs facilitate communication between lipotoxic hepatocytes and Kupffer cells, the resident macrophages of the liver, perpetuating liver inflammation. Hepatocyte-derived EVs containing mtDNA act as danger signals, activating Toll-like receptor 9 on Kupffer cells and triggering the release of TNFα and IL-1β, thereby driving liver damage[73]. EVs released from hypoxic, lipid-laden hepatocytes stimulate inflammation in Kupffer cells, as determined by the increased levels of proinflammatory cytokines and inflammasome components, including IL-1β, NOD-like receptor pyrin domain containing 3 (NLRP3), and apoptosis-associated speck-like protein containing[74]. Hepatocytes release EVs carrying retinol-binding protein 4, which triggers M1-like polarization in Kupffer cells. This activation, which is mediated by nicotinamide adenine dinucleotide phosphate oxidase 2 and nuclear factor kappa B (NF-κB), leads to increased ROS and TNF-α production. Moreover, TNF-α stimulates fatty acid (FA) uptake and lipogenesis in hepatocytes while suppressing FA degradation. Additionally, TNF-α amplifies retinol-binding protein 4 production in hepatocytes, creating a positive feedback loop that exacerbates MASLD[75]. These findings establish a clear pathway by which hepatocyte-derived EVs directly and powerfully impact Kupffer cell behavior.
In addition to Kupffer cells, EVs from lipotoxic hepatocytes also target HSCs, which are key players in liver fibrosis. FA-treated hepatocytes exposed to chemical hypoxia with cobalt chloride (referred to as CoCl2) release elevated levels of EVs enriched with hypoxia-induced cargo. These EVs stimulate profibrotic gene expression in HSCs, promoting fibrosis. In vivo, HFD-fed mice exposed to intermittent hypoxia present increased numbers of circulating EVs, which correlate with increased portal inflammation and fibrosis[76]. The exposure of hepatocytes to PA increases EV release and alters their miRNA expression, specifically increasing the levels of miR-192 and miR-122. These modified EVs, when taken up by HSCs, stimulate fibrotic gene expression and promote HSC activation. Notably, EVs carrying miR-128-3p suppress peroxisome proliferator-activated receptor gamma (PPAR-γ) in HSCs, further enhancing profibrogenic gene expression, proliferation, and chemotactic responses. EVs are efficiently internalized by HSCs, a process that relies, at least in part, on the expression of vanin 1 (VNN1) on the EV surface[66]. A study identified miR-1297 as the most upregulated miRNA in PA-treated hepatocytes through miRNA sequencing. EVs derived from these cells induce HSC activation and proliferation. Mechanistically, phosphatase and tensin homolog is a target of miR-1297, and the silencing of phosphatase and tensin homolog leads to HSC activation and proliferation via phosphoinositide 3-kinase/AKT signaling[77]. Another key player, miR-107, which is transferred from hepatocyte-derived EVs, activates Wnt signaling in HSCs and IL-9 signaling in CD4+ T cells. In CD4+ T cells, miR-107 suppresses Foxp1, a protein that normally represses IL-9 expression, thereby increasing IL-9 production. This, in turn, activates the Raf/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathway in HSCs, further promoting their activation[78]. Moreover, lipotoxic hepatocyte-derived EVs induce a profibrotic and prosenescent phenotype in HSCs characterized by reduced collagen and alpha-smooth muscle actin (α-SMA) expression, increased ROS production, and increased the expression of senescence markers, including IL-6, IL-1β, and p21, and senescence-associated β-galactosidase activity, likely through the AKT-mTOR pathway. Cellular senescence, a state of near-permanent cell cycle arrest, is increasingly recognized as a mechanism for modulating HSC activation. Interestingly, these EVs have also been shown to inhibit HSC activation by inducing senescence. To further elucidate the molecular mechanisms underlying these observations, RNA sequencing profiling of the RNA content of lipotoxic and vehicle-derived hepatocyte EVs revealed significant differences in mRNA and miRNA expression between the two groups[79].
The communication mediated by EVs from lipotoxic hepatocytes extends beyond HSCs, encompassing other cell types involved in MASLD pathogenesis, including endothelial cells and adipocytes. Lipotoxic hepatocyte stress leads to the release of EVs that, when internalized by endothelial cells via VNN1, promote angiogenesis and endothelial migration[61]. Furthermore, miR-1-containing EVs suppress Kruppel-like transcription factor 4 a transcription factor that maintains endothelial homeostasis, leading to NF-κB activation and endothelial inflammation. Blocking miR-1 prevents these effects, offering potential therapeutic benefits[80]. Additionally, integrin subunit beta 1-enriched EVs from hepatocytes promote monocyte adhesion to LSECs, facilitating inflammation and fibrosis[81]. Hepatocyte-derived EVs also influence adipose tissue remodeling. HFD consumption upregulates geranylgeranyl diphosphate synthase expression in he
Immune cell-derived EVs: While hepatocyte-derived EVs play a significant role in the pathogenesis of this disease, the contributions of immune cells are equally substantial. A striking characteristic of MASLD is the infiltration of neutrophils, the primary effectors of innate immunity, into liver tissue. Indeed, the presence of neutrophils is correlated with the progression of the disease[83]. Subsequent investigations revealed intricate mechanisms by which neutrophils modulate the hepatic microenvironment. Specifically, neutrophils contribute by releasing miR-223-enriched EVs to inhibit NLRP3 in proinflammatory macrophages and promote a restorative macrophage phenotype. This, in turn, polarizes macrophages to facilitate the release of IL-10, ultimately reducing HSC activation and collagen deposition[84]. Fur
Further investigations revealed the contributions of other miRNAs to MASLD pathogenesis. In both in vitro ma
Other cell-derived EVs: In the context of MASLD, other cell-derived EVs play significant roles in disease progression. In obese individuals, visceral adipose tissue secretes EVs that are internalized by hepatocytes, and function as signaling molecules that alter gene expression in the TGF-β pathway. Specifically, these EVs increased tissue inhibitor matrix metalloproteinase 1 and integrin expression while reducing matrix metalloproteinase-7 and plasminogen activator inhibitor 1 Levels in liver cancer cells. In HSCs, these EVs promote the expression of tissue inhibitor matrix metalloproteinase, small mothers against decapentaplegic-3, integrin, and matrix metalloproteinase-9, leading to dysregulation of the extracellular matrix and promoting fibrosis[90]. Similarly, HSCs themselves release EVs carrying phosphorylated platelet-derived growth factor receptor alpha at tyrosine 720 and Src homology 2-containing protein tyrosine phosphatase 2, which, upon interacting with other HSCs, activate them in a paracrine manner, promoting HSC migration and driving liver fibrosis[91]. Previous studies have demonstrated that platelet-derived growth factor and Src homology 2-containing protein tyrosine phosphatase 2 suppress the regulation of development and DNA damage response 1 in HSCs, thereby increasing mTOR signaling, which facilitates the release of EVs. These EVs stimulate the migration of other HSCs, perpetuating profibrotic signals and exacerbating liver fibrosis. Moreover, CoCl2 treatment upregulated DNA damage response 1, and rapamycin administration inhibited mTOR, disrupting this signaling cascade and emphasizing its role in fibrosis progression[92]. Conversely, bone marrow mesenchymal stem cell (MSC)-derived EVs carrying miR-192-5p targeted protein phosphatase 2 regulatory subunit B alpha, effectively inhibiting HSC activation. These findings suggest a potential therapeutic avenue through bone marrow MSC-derived EVs[93]. Similarly, EVs derived from adipose-derived MSCs deliver miR-150-5p, which suppresses CXCL1 expression in HSCs, reducing HSC proliferation and activation and thereby mitigating hepatic fibrosis[94]. Furthermore, MSC-derived EVs transport miR-4465 to HSCs, modulating lysyl oxidase-like protein 2 expression and subsequently alleviating liver fibrosis[95]. These findings highlight the protective role of MSC-derived EVs in liver fibrosis.
Notably, the dysbiotic gut environment significantly influences hepatic pathology through the release of EVs. Spe
Collectively, EVs emerge as pivotal mediators in MASLD pathogenesis, facilitating intercellular communication among lipotoxic hepatocytes, macrophages, Kupffer cells, HSCs, endothelial cells, and adipocytes. These EVs carry a complex cargo of miRNAs, lipids, and proteins that orchestrate inflammatory and fibrotic responses. While lipotoxic hepatocyte-derived EVs contribute to MASLD/MASH progression, immune cell-derived EVs exert protective effects by transferring regulatory molecules to hepatocytes, ameliorating disease progression. Further exploration of EV biogenesis, cargo selection, and uptake mechanisms would provide critical insights into MASLD pathogenesis and uncover novel therapeutic targets.
MASLD progresses through different stages after liver steatosis by fibrosis development and eventually results in cirrhosis[98,99]. The activation of HSCs plays a role in liver fibrosis by increasing EV production and altering their proteomic profile. Additionally, EVs from activated HSCs are linked to extracellular matrix remodeling, proliferation, and migration[100]. Hepatocyte-derived EVs contribute to fibrosis in MASLD through multiple mechanisms. EVs released from hepatocytes stimulate HSCs through the hypoxia signaling pathway. This activation is mediated by iron accumulation in an ROS-dependent manner, arising from mitochondrial oxidative stress and ultimately leading to hepatocyte inflammation[74,101]. EVs containing VNN1 from hepatocytes contribute to the internalization process in HSCs. These EVs influence the LX-2 cell line by inducing HSC activation and decreasing the levels of regulators of quiescent HSCs, such as PPAR-γ. This process aligns with changes observed in liver endothelial cells, leading to angiogenesis and aggravating MASH, which is more severe than MASLD[61,102]. PPAR-γ participates in macrophage polarization, activating the Toll-like receptor 4/NF-κB pathway, which contributes to lipid dysregulation and inflammation[74,101]. Moreover, some EVs play roles in the dysregulation of the mitophagy pathway, which plays a vital role in clearing dysfunctional mitochondria. These EVs, such as those containing the miR-27A and LIM domain and actin binding 1, downregulate the expression of putative protein kinase 1, which is a key modulator of mitophagy[103,104]. In addition to hepatocytes, other cell types, such as adipocytes, can also incorporate their EVs into HSCs, possibly causing cellular dysregulation. EVs secreted by adipocytes in obese individuals might affect the regulation of TGF-β, a vital component of fibrosis, after incubation with hepatic cell lines[90].
MASLD has manifestations that can lead to disease progression and result in HCC[105]. Modifications in EV content during MASLD progression can influence HCC onset and progression[106]. In patients with MASLD, elevated levels of miR-504-3p-enriched EVs have been observed[107]. These EVs facilitate HCC progression by modulating frizzled class receptor 7, thereby inhibiting Wnt/β-catenin signaling and enhancing tumor-associated characteristics[108]. One of the mechanisms driving HCC progression is the polarization of tumor-associated macrophages. EVs derived from HCC cells enriched with miR-21-5p modulate macrophage polarization by influencing the specificity protein 1/X-box binding protein 1 pathway[109]. Higher levels of miR-21-5p were observed in patients with MASLD, with a progressive increase observed in more severe stages of the disease[110]. Recently, five EV-miRNAs, specifically miR-19-3p, miR-16-5p, miR-223-3p, miR-30d-5p, and miR-451a, have been identified as promising biomarkers for MASLD-related HCC. Among these, miR-19-3p has the highest diagnostic accuracy, particularly for early-stage and patients lacking alpha-fetoprotein, indicating its potential for both diagnosis and prognosis[59].
Following MASLD, patients are at high risk of developing CVDs, which stem from cardiometabolic imbalances and may result in death[111,112]. Recent findings have even highlighted a potential link between MASLD and nonischemic etiologies of sudden cardiac death (SCD). A study investigating post-mortem findings in adults experiencing SCD revealed a higher prevalence of MASLD among individuals of Hispanic ethnicity and a lower prevalence of coronary artery disease in this group. Given the increasing burden of MASLD, these ethnicity-based disparities and the specific nature of nonischemic SCD warrant immediate and in-depth investigation, underscoring the systemic ramifications of this liver disease[113]. One key mechanism through which MASLD influences the cardiovascular (CV) system involves the release of EVs carrying miRNAs from steatotic hepatocytes. For example, miRNA-containing EVs such as miR-1 from hepatocytes contribute to atherogenesis by altering Kruppel-like transcription factor 4 in the NF-κB axis, affecting smooth and cardiac muscle development and promoting inflammation in vein endothelial cells[80]. MASLD is also linked to atherosclerosis progression. EVs derived from steatotic hepatocytes carrying miRNA-30a-3p suppress ATP binding cassette subfamily A 1, restricted cholesterol removal to apolipoprotein A-I, and contributed to foam cell buildup in blood vessels[114]. Endothelial hyperpermeability in MASLD is caused by high levels of miRNA-7 EVs, which lead to lysosome-associated membrane protein 1 Lysosomal permeabilization, activate the cathepsin B/NLRP3 inflammasome pathway, and drive inflammation, which contributes to coronary microvascular dysfunction[115]. Moreover, CVD might arise from disruptions in the ceramide-related pathway caused by acid ceramidase depletion, leading to lysosomal ceramide accumulation and MASLD. EV levels subsequently increase and activate the NLRP3 inflammasome, ultimately causing endothelial injury in the carotid arteries of mice[116].
In addition to the molecular mechanisms involving circulating factors such as EVs and miRNAs, these vesicles also play a significant role in mediating histological and tissue-level changes that contribute to CV complications in MASLD. Indeed, EVs released in the context of MASLD are pivotal in driving these complications by inducing significant alterations, particularly in the heart and blood vessels. These EVs contribute to endothelial dysfunction by impairing nitric oxide production and increasing oxidative stress, affecting the proper dilation and constriction of blood vessels[113,117,118]. Furthermore, they can alter cardiomyocyte function, leading to decreased survival, reduced contractility, and increased fibrosis, thereby increasing the risk of heart failure and arrhythmias[119,120]. The pro-fibrotic signals carried by EVs promote the development of fibrosis in both the liver and the heart, exacerbating MASLD progression and stiffening the heart muscle[121]. By delivering inflammatory molecules, EVs also contribute to systemic inflammation, further damaging the endothelium. Additionally, EVs influence vascular structure and function, potentially leading to detrimental remodeling and narrowing of blood vessels[122]. These diverse effects are mediated through mechanisms such as lipid accumulation, the promotion of oxidative stress, and the alteration of cellular signaling pathways in target cells within the CV system, ultimately establishing a strong link between MASLD and an elevated risk of CV events.
The influence of EVs in MASLD extends beyond the CV system, impacting a range of extrahepatic organs, including the pancreas, colon, and nervous system[123]. This underscores the systemic nature of MASLD and the far-reaching consequences of EV-mediated signaling. In the context of type 2 diabetes, a common comorbidity of MASLD, hepatocyte-derived EVs play a significant role in modulating pancreatic beta cell function. Specifically, these EVs promote beta cell proliferation and insulin production. For example, miR-7218-5p, carried within EVs, regulates beta cell survival through the CD74 pathway, leading to the overproduction of insulin[124]. Conversely, chronic obesity, a condition often as
The limitations of existing noninvasive diagnostics for MASLD and MASH, which rely on imaging-based methods and multiple algorithm tests, have been recognized. While techniques such as magnetic resonance elastography and transient elastography (TE) offer high diagnostic accuracy, magnetic resonance elastography is costly, and TE lacks specificity for MASH-associated fibrosis[130]. Other ultrasound-based elastography methods, such as point shear wave elastography and two-dimensional shear wave elastography, exhibit similar diagnostic capabilities[131]. Multiple algorithms include direct commercial assays such as the “enhanced liver fibrosis panel” test, the Fibro Test, and procollagen III N-terminal peptide, as well as indirect indices such as the fibrosis-4 index, the aspartate aminotransferase-to-platelet ratio index, and the MASLD fibrosis score. However, these tests were originally developed for hepatitis C-related fibrosis and have been repurposed for MASLD, leading to inconsistent diagnostic performance and a need for more specific and reliable biomarkers[132,133]. The invasiveness and expense of liver biopsies, the gold standard, limit their applicability for screening. Therefore, researchers have focused on developing noninvasive biomarkers based on liquid biopsy methods involving the analysis of molecules in body fluids such as blood. Elevated serum alanine aminotransferase levels detect MASLD with high sensitivity but not MASH, whereas increased cytokeratin 18 in circulation indicates MASH in patients with MASLD[134].
EVs serve as promising biomarkers for MASLD by carrying lipids, proteins, miRNAs, and lncRNAs, reflecting pathological alterations in liver metabolism and inflammation[135]. The diverse cargo of EVs, which is influenced by their cellular origin and physiological/pathological state, prompted exploration of their potential for tracking disease progression and therapeutic response. A pivotal study, which analyzed patient-derived EVs, confirmed the diagnostic potential of EV proteins by identifying refined biomarker panels, comparing EV proteomes from patients with cancer to those from healthy individuals, and differentiating between cancer types[136]. In the context of MASLD, a study showed that patients with MASLD or MASH presented with significantly elevated levels of EVs derived from invariant natural killer T cells and macrophages/monocytes. These elevated EV levels correlated with alanine aminotransferase levels and the histological severity of MASH. In line with this, the levels of these EVs effectively distinguished between patients with MASLD/MASH and patients with chronic hepatitis C, achieving a high degree of accuracy, as indicated by an area under the receiver operating characteristic curve (AUROC) of 0.99[137].
EV-associated lipids have been particularly investigated as biomarkers, given the close link between MASLD and lipid metabolism. A study demonstrated an increase in C16: 0 ceramide-containing EVs in both murine models and human subjects with MASH[36]. Further exploration of urinary EVs from patients with MASLD and MASH revealed a lipidomic signature comprising four lipid molecules - FFA (18:0), lysophosphatidylcholine (22:6/0:0), FFA (18:1), and phospha
Proteomic analysis of EV proteins reveals their potential as biomarkers in MASLD. Liquid chromatography-mass spectrometry/mass spectrometry analysis of EVs from human serum samples revealed various liver diseases, identifying alpha-2-macroglobulin for alcoholic hepatitis and apolipoprotein C3 for MASLD as significantly upregulated proteins[140]. A study indicated that the levels of hepatocyte-specific markers, such as ASGR1, were significantly higher in patients with cirrhotic MASH[62]. A slow off-rate modified aptamer protein array identified seven circulating EV proteins - Wnt-induced secreted protein 1, aminoacyl-tRNA synthetase complex-interacting multifunctional protein 1, IL-27 receptor alpha subunit, intercellular adhesion molecule 2, IL-1β, serine/threonine kinase 16, and repulsive guidance molecule A - that were increased in precirrhotic and cirrhotic MASH samples compared with healthy controls, suggesting their potential for MASH diagnosis[62]. Proteomic analyses initially revealed eight candidate proteins, among which fibulin-3 and fibulin-1 exhibited significant alterations in advanced fibrosis. Subsequent validation confirmed that only fibulin-3 Levels were significantly elevated in patients with advanced fibrosis, distinguishing them from those with nonadvanced fibrosis. Furthermore, fibulin-3, but not fibulin-1, independently predicts liver-related events, with AUROC values of 0.727, 0.761, and 0.726 at 3 years, 5 years, and 7 years, respectively, establishing it as a promising biomarker for MASLD risk stratification[141]. Further analyses of EV protein cargo in plasma samples from healthy controls, patients with MASLD, and patients with MASH revealed that hepatogenic EVs carrying glucose transporter 1 were highly indicative of MASLD. Notably, glucose transporter 1-derived EVs exhibited an AUROC of 0.85 for MASLD diagnosis, which further improved to 0.91 when combined with the fibrosis-4 index score, demonstrating their potential as early diagnostic markers[142].
MiRNAs carried by EVs have been extensively investigated for their potential as MASLD biomarkers. The diagnostic performance of EV-derived miRNAs in MASLD has been previously reviewed[143]. Recent evidence specifically highlights the role of EV-derived miRNAs as biomarkers in patients with MASLD. For example, a study analyzing serum samples from patients with chronic hepatitis C and healthy controls identified a nine-miRNA signature (miR-1225-5p, miR-1275, miR-638, miR-762, miR-320c, miR-451, miR-1974, miR-1207-5p, and miR-1246) that effectively differentiated liver diseases, including MASH, from healthy controls, with specific miRNAs reflecting the extent of liver fibrosis and inflammation[144]. Among the most studied miRNAs, miR-122, a liver-specific miRNA, is widely established as a biomarker of liver diseases[145]. A recent study demonstrated that analyzing miRNAs within EVs, particularly miR-122 (AUROC = 0.79), provided more accurate differentiation between individuals with MASLD and healthy controls than examining total serum miRNAs. The enrichment of miR-122 and miR-192 in circulating EVs, coupled with their depletion in liver tissue, suggested active EV-mediated transfer of these molecules, potentially contributing to systemic disease propagation[145]. Furthermore, investigations analyzing miR-122, miR-192, and miR-128-3p revealed that miR-128-3p exhibited high diagnostic potential for MASLD, with the highest AUROCs for total cell-free RNA, global EVs, and liver-specific (ASGR1+) EVs (0.92, 0.89, and 0.80, respectively)[146]. Distinct miRNA patterns have been observed in both mice and humans with MASLD, with decreases in the expression of miR-135a-3p, miR-129b-5p, and miR-504-3p and increases in the expression of miR-122-5p in circulating EVs and serum samples. Notably, miR-135a-3p within circulating EVs has strong diagnostic potential for MASLD, with an AUROC of 0.85[107]. Additionally, miR-192-5p was significantly elevated in the serum of patients with MASLD and MASH, which was strongly correlated with liver damage markers and hepatic steatosis severity[71]. Finally, in a cohort with MASLD-related HCC, miR-223-3p was significantly upregulated in plasma EVs from patients compared with healthy controls, highlighting its potential as a biomarker in this specific context[59].
However, a crucial point to consider is that miRNAs, while promising for both diagnosis and treatment, encounter obstacles because of their relatively low specificity. This lack of precise targeting can impede their successful translation into routine clinical practice. The challenge lies in the fact that a single miRNA can interact with multiple mRNAs, making it difficult to pinpoint and modulate a specific biological pathway with the accuracy needed for effective diagnosis and targeted therapies[147,148]. To address this, researchers are actively pursuing strategies such as developing targeted delivery systems, designing more specific miRNA molecules, and utilizing miRNA panels to identify unique disease expression patterns. Crucially, rigorous validation of miRNA-mRNA interactions is essential to ensure the functional relevance of identified targets, ultimately aiming to balance the broad regulatory potential of miRNAs with the need for precise clinical interventions[147].
lncRNAs, a diverse class of noncoding RNAs ranging from over 200 base pairs to 100 kilobases, play crucial roles in regulating gene expression, notably by acting as miRNA sponges to control mRNA levels. Previous research has demonstrated a strong association between lncRNAs and various pathophysiological conditions, including liver diseases and MASLD. RNA sequencing of samples from patients with liver fibrosis and MASLD has revealed distinct lncRNA expression profiles[149-151], revealing differential expression patterns in MASLD samples compared with control samples. These dynamic alterations in lncRNA expression are linked to the development and progression of MASLD. Recent systematic reviews highlight the altered expression of noncoding RNAs in MASLD, identifying lncRNAs such as nuclear paraspeckle assembly transcript 1 (NEAT1), maternally expressed gene 3 (MEG3), and mucosa-associated lymphoid tissue lymphoma translocation protein 1 as promising diagnostic biomarkers in MASLD. Specifically, several lncRNAs, such as HOX transcript antisense RNA, H19, Gm15622, and NEAT1, are upregulated, whereas others, such as MEG3, are downregulated[152,153]. Functional studies have elucidated the roles of these lncRNAs in MASLD pa
In summary, EVs hold considerable promise as biomarkers in MASLD, which makes them attractive candidates for noninvasive liquid biopsies, potentially offering earlier and more accurate diagnoses than traditional methods do. However, despite this potential, the translation of EV-based biomarkers into routine clinical practice faces significant limitations. One major hurdle is the variability in EV isolation and analysis methods, leading to low sensitivity and specificity in diagnostic tests. The absence of standardized protocols for EV isolation, characterization, and cargo analysis has contributed to inconsistent results across studies[160]. Moreover, the lack of commercially available clinical test kits hinders their widespread adoption and application. Critically, many candidate biomarkers identified in smaller studies lack robust validation in large, diverse patient cohorts. This raises concerns about their generalizability and clinical utility[161]. Without large-scale verification, the true diagnostic and prognostic value of these biomarkers remains uncertain. Furthermore, the complexity of MASLD, which involves multiple cell types and pathways, necessitates a comprehensive approach that considers a panel of biomarkers rather than relying on a single marker. This adds to the challenge of developing reliable and clinically applicable EV-based diagnostic tools[162,163].
While significant progress has been made in MASLD drug therapies, with novel agents such as thyroid hormone receptor beta, farnesoid X receptor, PPAR, sodium-glucose cotransporter-2, and glucagon-like peptide-1 receptor agonists demonstrating efficacy in clinical trials, challenges remain[164]. Current pharmacological approaches often target specific disease stages, such as inflammation, fat accumulation, and fibrosis, and synthetic drugs can pose risks due to off-target effects[165]. Furthermore, surgical interventions, while effective in select cases, require careful patient selection and carry inherent risks[166]. Even promising small interfering RNA-based therapies face hurdles in terms of delivery, pharmacokinetics, and side effect profiles[167]. These limitations underscore the urgent need for innovative therapeutic strategies that offer improved targeting and reduced systemic toxicity.
In response to these limitations, EVs have emerged as a highly promising avenue for targeted MASLD treatment. These naturally occurring, membrane-bound nanoparticles offer a unique advantage by shielding their molecular cargo from degradation within biological environments. As inherent carriers, EVs can effectively transport therapeutic payloads directly to the site of action, encompassing a diverse range of molecules, such as proteins, lipids, miRNAs, and nucleic acids[168]. Notably, EVs derived from stem cells also have the potential to stimulate cellular regeneration[169]. The ability of EVs to interact with specific target organs or receptors stems from their unique surface protein barcodes and adaptable bimolecular coronas. This versatility allows for the encapsulation and delivery of various therapeutic agents, including nucleic acids, chemotherapeutic drugs, small molecules, and even viruses, to precise locations[168,169]. The biocompatibility, low immunogenicity, capacity to traverse biological barriers, and adaptable cargo loading of EVs make them highly attractive therapeutic vehicles[168]. Future EV-based therapies aim to leverage these properties by loading them with specific therapeutic cargo or drugs, ensuring targeted delivery and minimizing off-target effects. Con
Given the inherent potential of EVs as therapeutic tools, distinguishing between their two primary modes of application is crucial: As delivery vehicles and as therapeutic agents[171]. This functional distinction leads to a classification into two main categories: Naturally occurring EVs and engineered EVs[44]. Naturally occurring EVs, derived from cells such as immune cells and MSCs, carry endogenous cargo that reflects their cellular origin, endowing them with inherent therapeutic capabilities. Conversely, engineered EVs undergo modifications through biological or chemical methods, including surface alterations and physical or biological treatments, to facilitate the loading of specific therapeutic cargo[172]. While various loading techniques exist, the selection of an appropriate method is critical for efficient cargo encapsulation and depends on factors such as the physicochemical properties of the cargo, its source, and the EV subtype[172]. The diverse applications of EVs as both “delivery vehicles” and “therapeutic agents” are well supported by studies across a range of diseases, highlighting their versatility and potential in therapeutic development[168].
A crucial advantage of using EVs as therapeutic delivery vehicles lies in their inherent ability to protect their cargo from degradation within the complex biological milieu[173]. The lipid bilayer membrane of EVs acts as a robust barrier, effectively shielding encapsulated molecules such as proteins, nucleic acids, and small-molecule drugs from enzymatic breakdown, oxidation, and other forms of degradation. This protection is critical, as many therapeutic agents are susceptible to rapid degradation in circulation or within cellular compartments, limiting their bioavailability and efficacy[174]. By encapsulating these agents within EVs, we can significantly increase their stability and ensure that they reach their target sites intact.
Furthermore, the EV membrane is not merely a passive barrier. A variety of surface proteins that contribute to vesicle stability and functionality have been studied[175]. These proteins can modulate the interaction of EVs with their environment, influencing factors such as cellular uptake and intracellular trafficking. Additionally, the internal environment of EVs can be carefully controlled during loading, ensuring optimal conditions for the stability and activity of the therapeutic cargo. For example, maintaining an appropriate pH or redox potential within the EV lumen can prevent degradation or premature activation of encapsulated enzymes or drugs[176]. This sophisticated level of pro
HSCs are central to the progression of liver fibrosis, a critical aspect of MASLD. EVs offer a promising avenue for delivering therapeutic miRNAs that can modulate HSC activity and reduce collagen production. In a dietary-induced mouse model of MASH characterized by steatosis, inflammation, and fibrosis, EVs isolated from human HepG2 hepatocytes displayed notable therapeutic efficacy[177]. Administering these EVs, either as preventive measures or as treatments, led to a substantial reduction in hepatic fibrosis, as evidenced by decreased collagen deposition and α-SMA expression. This is accompanied by a reduction in hepatic inflammation. Mechanistically, these EVs transport a collection of 205 miRNAs, with seven prominent species (miR-423-5p, -483-5p, -191-5p, -148a-3p, -423-3p, -92a-3p, and -122-5p) predicted to directly target genes involved in fibrosis. These findings highlight the potential of EVs, particularly those originating from hepatocytes, as effective vehicles for delivering antifibrotic miRNAs, suggesting a novel therapeutic approach for MASLD-associated fibrosis.
In addition to the role of hepatocyte-derived EVs in MASLD, recent studies have investigated the direct impact of EVs released from steatotic hepatocytes on HSC behavior. Specifically, research employing primary rat hepatocytes, where steatosis was induced via FFAs, demonstrated that EVs originating from these lipid-laden cells (FFA-EVs) significantly suppressed the expression of collagen type 1 and α-SMA in early-stage activated HSCs[79]. This suppression coincided with an increase in ROS production and the promotion of senescence markers, including IL-6, IL-1β, and p21, and senescence-associated β-galactosidase activity within HSCs through the modulation of the AKT-mTOR signaling cascade. Analysis of EV RNA content revealed distinct profiles between those derived from normal and steatotic hepatocytes, underscoring the critical role of the FFA-EV cargo in altering HSC characteristics. These findings suggest that EVs from steatotic hepatocytes may actively regulate fibrosis progression in MASLD by triggering HSC senescence, thereby presenting potential new avenues for therapeutic intervention.
In addition to the direct impact of hepatocyte-derived EVs, the role of LSECs in modulating MASLD-related fibrosis is emerging. LSECs, which are critical for maintaining hepatic microcirculation and nutrient exchange, exert paracrine effects through the release of EVs[178]. Research indicates that LSEC-derived EVs can significantly attenuate the activation of HSCs by reducing the expression of activation markers and suppressing HSC proliferation. Interestingly, these EVs also suppress inflammatory gene expression in Kupffer cells. These findings suggest that LSEC-derived EVs play a complex regulatory role in the MASLD microenvironment, effectively dampening the fibrogenic phenotype of HSCs and modulating the inflammatory response of Kupffer cells. These findings position LSEC-derived EVs as promising therapeutic candidates for MASLD, suggesting a novel approach to simultaneously target multiple cell types involved in disease progression. Furthermore, the distinct effects of these EVs on different cell populations may also hold diagnostic potential in monitoring MASLD.
Building upon the exploration of EV-based therapies in MASLD, a significant area of interest lies in their capacity to modulate macrophage polarization, a key factor in disease progression. Given the limited availability of effective treatments for MASH, the therapeutic potential of human umbilical cord MSC derived EVs (huc-MSC-EVs) has been investigated[179]. Research employing a Western diet-induced MASH mouse model demonstrated that huc-MSC-EV administration effectively mitigated liver steatosis, inflammation, and fibrosis while also improving metabolic parameters. Notably, these EVs reduce hepatic macrophage accumulation and promote a shift toward M2 macrophage polarization, a phenotype associated with tissue repair and resolution of inflammation, over the proinflammatory M1 phenotype. Mechanistically, the observed effects are attributed, at least in part, to the high expression of miR-24-3p within huc-MSC-EVs, which target the cyclic guanosine monophosphate–adenosine monophosphate synthase/stimulator of interferon gene in macrophages, thereby regulating their polarization. The overexpression of this miRNA amplified the therapeutic benefits of the EVs, suggesting a critical role for miR-24-3p in mediating their effects. These findings highlight the potential of huc-MSC-EVs to ameliorate MASH by modulating macrophage polarization through miR-24-3p delivery, suggesting a promising avenue for clinical translation.
Continuing the exploration of the use of MSC-EVs in MASLD treatment is crucial to address the complex role of macrophage polarization. While the preceding study highlighted the beneficial shift toward M2 macrophage polarization in mitigating MASH, concerns exist regarding the potential profibrotic nature of M2 macrophages, which could theoretically exacerbate liver fibrosis. However, evidence suggests that MSC-EVs can alleviate HFD-induced MASH despite this concern[180]. To further investigate this matter, researchers confirmed the M2-polarizing capacity of their MSC-EV preparations in vitro before testing them in a MASH mouse model. Surprisingly, compared with the control treatment, treatment with MSC-EVs resulted in a reduction in MASLD activity scores and liver fibrosis. Although an increase in CD163+ M2 macrophages was observed in the liver, along with a reduction in serum IL-6 Levels, the overall outcome indicated that MSC-EV therapy was effective in reducing liver fibrosis. These findings suggest that while MSC-EVs do induce M2 macrophage polarization, their net effect in the context of MASH is antifibrotic, possibly due to other concurrent immunomodulatory mechanisms. These findings underscore the need for a nuanced understanding of macrophage polarization in EV-mediated therapies for MASLD, where the balance of pro- and antifibrotic signals ultimately determines the therapeutic outcome.
While previous research has highlighted the potential of MSC-EVs in modulating macrophage polarization to alleviate inflammation in MASLD, alternative strategies leveraging the targeted delivery of anti-inflammatory agents via EVs are also being explored. Recognizing the pivotal role of proinflammatory cytokines, such as TNF and IL-1β, in MASH pathogenesis, researchers have investigated the feasibility of inhibiting their expression in hepatic macrophages via EV-mediated delivery[181]. Given that intravenously administered EVs accumulate primarily in the liver and are readily taken up by hepatic macrophages, this approach holds promise for targeted therapeutic intervention. In this study, antisense oligodeoxynucleotides (ASOs) targeting TNF-α (ASO-TNF) and 2-deoxy-D-glucose (2DG) were used to suppress TNF-α and IL-1β expression in macrophages. EVs loaded with ASO-TNF-α or 2DG effectively reduce the expression of these inflammatory cytokines both in vitro and in vivo. Furthermore, the administration of these EV-loaded agents significantly attenuated experimental steatohepatitis in murine models of MASH. Mechanistically, RNA sequencing revealed that treatment with EV-delivered ASO-TNF-α or 2DG inhibited proinflammatory signaling pathways and upregulated the expression of superoxide dismutase 1, an antioxidant enzyme. These findings demonstrate that EV-mediated delivery of anti-inflammatory agents, such as ASO-TNF and 2DG, can effectively alleviate experimental steatohepatitis by directly targeting inflammatory pathways in hepatic macrophages, suggesting a promising therapeutic avenue for MASH treatment.
In addition to the immunomodulatory effects of EVs, particularly in terms of macrophage polarization, another promising avenue for MASLD therapy involves directly targeting lipid metabolism. Given the central role of lipid accumulation in the pathogenesis of MASLD, the potential of MSC-EVs to modulate this process has been explored. A recent study investigated whether MSC-EVs could inhibit hepatocyte lipid accumulation by regulating mitochondrial fission, a process implicated in steatosis[182]. Using both lipotoxic hepatocytes in vitro and an HFD-induced MASLD mouse model, researchers have shown that MSC-EV treatment effectively reduces the expression of dynamin-related protein 1 (DRP1), a key regulator of mitochondrial fission. This downregulation of DRP1 Led to a reduction in mi
To further explore the therapeutic potential of MSC-EVs for MASLD, researchers have investigated their role in regulating lipid homeostasis. A recent study elucidated the mechanisms by which MSC-EVs influence lipid accumulation[183]. The use of OA-treated hepatic cells and a HFD-induced MASLD mouse model demonstrated that MSC-EVs effectively promoted FA oxidation and reduced lipogenesis. Nontargeted lipidomic and transcriptome analyses revealed a positive correlation between MSC-EV treatment and the phosphorylation of AMP-activated protein kinase. Through MSC-EVs and gene manipulation experiments, calcium/calmodulin-dependent protein kinase 1, which is transferred via MSC-EVs, was shown to ameliorate lipid accumulation in an AMP-activated protein kinase-dependent manner. This mechanism involves the inhibition of SREBP-1c-mediated FA synthesis and the enhancement of PPAR-α-mediated FA oxidation. These findings highlight the therapeutic potential of MSC-EVs in preventing HFD-induced MASLD by regulating lipid homeostasis through the delivery of calmodulin-dependent protein kinase 1, suggesting a promising strategy for combating liver steatosis.
In addition to the inherent therapeutic capabilities of MSC-EVs, recent investigations have explored strategies to further augment their efficacy in treating MASLD. One such approach involves preconditioning MSCs with curcumin (Cur), a natural compound known for its anti-inflammatory and immunomodulatory properties[184]. Researchers have hypothesized that, compared with standard MSC-EVs, EVs derived from Cur-preconditioned MSCs (MSC-EVs-Cur) would exhibit superior therapeutic effects. Compared with treatment with MSC-EVs-Cur alone, treatment with MSC-EVs-Cur significantly ameliorated steatosis and inflammation in a methionine/choline-deficient diet-induced MASH mouse model, as evidenced by reduced fibrosis; decreased serum liver enzyme, triglyceride, and cholesterol levels; and increased lipid peroxidation. Notably, these beneficial effects persisted for 3 months after treatment in the MSC-EV-Cur group, whereas MASH features recurred in the MSC-EV group. In vitro studies using PA-treated HepG2 cells corroborated these findings, demonstrating that MSC-EVs-Cur effectively reversed lipotoxicity and oxidative stress. Mechanistically, MSC-EVs-Cur regulated key inflammatory and oxidative stress markers, genes involved in liver fibrogenesis, and lipid synthesis and transport. Importantly, compared with MSC-EVs, MSC-EVs-Cur significantly downregulated the ASK-c-Jun N-terminal kinase-B-cell lymphoma 2- associated X protein genes, which are implicated in mitochondrial stress and apoptosis. These results suggest that Cur preconditioning enhances the therapeutic potential of MSC-EVs in MASH, offering a promising strategy to not only ameliorate but also prevent the recurrence of this complex liver disease by modulating crucial pathways involved in inflammation, oxidative stress, and mitochondrial function.
Research on the therapeutic potential of stem cell-derived EVs in mitigating MASLD has also explored the effects of EVs from stem cells of the apical papilla (SCAPs). In one study utilizing an HFD-induced MASH mouse model and oleic acid/PA-treated HCC cells in vitro, SCAP-derived EVs significantly reduced weight gain and liver damage while alleviating hepatic fat accumulation[185]. Mechanistically, these EVs promote the expression of genes involved in FA oxidation and transport and concurrently suppress genes associated with FA synthesis. Additionally, they reduced the serum inflammatory cytokine levels and hepatic inflammatory marker expression. Similarly, in a separate study employing a methionine/choline-deficient diet-induced MASH mouse model, intravenous administration of SCAP-derived EVs at varying concentrations led to significant reductions in body weight loss and liver damage, along with a marked decrease in hepatic fat accumulation[186]. These findings collectively underscore the capacity of SCAP-derived EVs to modulate lipid metabolism and inflammatory responses in MASLD, reinforcing the potential of stem cell-derived EV therapy as a promising strategy for this complex liver disease.
The promising therapeutic potential of EVs in addressing various facets of MASLD is evident, particularly their ability to target fibrosis, modulate macrophage polarization, and regulate lipid metabolism. Compared with traditional drug delivery methods, EVs offer distinct advantages, including enhanced biocompatibility, improved bioavailability, and reduced off-target effects. However, challenges such as reduced bioavailability following systemic delivery and the need for repeated fresh EV isolation hinder their clinical translation. To overcome these limitations, innovative strategies, such as the development of “off-the-shelf” three-dimensional bioprinted hyaluronic acid-based hepatic patches, are being explored[187]. This approach enables the sustained and localized release of encapsulated EVs, potentially in combination with hepatocytes for dual therapy, promoting tissue regeneration. As demonstrated in the MASLD rat model, this bioprinted patch effectively alleviated alterations in biochemical parameters, reduced localized inflammation, and mitigated liver fibrosis. The synergistic effect between the miRNA cargo of released EVs, cell therapy, and bioprinted matrix materials highlights the potential for targeting multiple complex metabolic pathways associated with MASLD. This advancement offers a promising avenue for improving EV-based therapies, addressing current limitations and paving the way for more effective clinical interventions in this challenging liver disease. However, despite compelling preclinical evidence, the translation of EV-based therapies for MASLD into clinical practice remains in its nascent stages. To date, there are no registered clinical trials specifically investigating EV-based interventions for MASLD. This highlights the urgent need for well-designed clinical studies to evaluate the safety and efficacy of EV-based therapies in patients, ultimately paving the way for their integration into clinical management strategies for this prevalent and challenging liver disease.
Standardization of EV isolation and characterization methods: To date, there is no standard method for EV isolation and characterization. This lack of standardization arises from the inherent heterogeneity of the size, composition, and function of EVs. Common isolation techniques, such as ultracentrifugation and size-exclusion chromatography (SEC), present limitations[188]. Ultracentrifugation, while widely used, struggles to fully separate EVs from lipoproteins because of their similar densities and carries the risk of EV rupture, resulting in cargo loss. While SEC is effective for size-based separation, it coisolates EVs with certain lipoproteins, such as very low-density lipoprotein and chylomicrons, leading to sample contamination. Lipoproteins, which are far more abundant in blood plasma than EVs are, further complicate this issue[189]. To mitigate these challenges, researchers have explored optimizing density gradients and combining SEC with other purification methods. Immunoprecipitation against liver-specific EV surface proteins, such as ASGR1, is considered, although it is complex. Ultimately, different isolation methods yield distinct EV subpopulations, impacting downstream analysis, and technological advancements are necessary for efficient, large-scale, and high-purity EV isolation. A better understanding of EV biology is essential for the development of improved isolation techniques.
Similarly, characterizing isolated EVs faces challenges, as the reliability of results is affected by difficulties in isolating EVs from complex biofluids and accurately determining their contents. Techniques such as flow cytometry, resistive pulse sensing, dynamic light scattering, enzyme-linked immunosorbent assay, and nanoparticle tracking analysis are frequently employed for characterization[190]. Surface plasmon resonance approaches have the potential to become the best option for efficient biomarker panel discovery. Surface plasmon resonance holds promise for efficient biomarker panel discovery, and advanced methods such as resistive pulse sensing offer higher size resolution than dynamic light scattering and nanoparticle tracking analysis[191]. While these techniques show clinical relevance, ongoing research aims to improve their sensitivity, specificity, and standardization for broader clinical applications, with future efforts focused on the synergy between label-free EV detection and multiplexed signal analysis to facilitate the clinical translation of EVs[190,192].
Understanding the complexity of EV cargo and function: The complexity of EV cargo and function stems from their diverse molecular compositions and the intricate mechanisms governing their biogenesis. EVs carry a wide range of biomolecules, including proteins, lipids, and nucleic acids, which play essential roles in intercellular communication and disease progression. However, several challenges hinder their study and application. One major obstacle is the lack of specific marker proteins for EV subtypes, such as exosomes and microvesicles, making it difficult to isolate and purify distinct EV populations effectively[7]. Additionally, EVs exhibit significant heterogeneity in size, composition, and origin, complicating their classification and functional analysis. The mechanisms underlying EV biogenesis, including cargo sorting and release, remain incompletely understood, limiting efforts to manipulate EVs for therapeutic or diagnostic purposes. Furthermore, cargo selection and loading into EVs are influenced by cell type, physiological conditions, and external stimuli, adding another layer of complexity to their study. Addressing these challenges is crucial for advancing the understanding and application of EVs in biomedical research and clinical settings.
Challenges and issues in vivo EV tracking: In vivo tracking of EVs presents numerous challenges, particularly in the context of complex diseases such as MASLD. The use of exogenously administered EVs from cell lines fails to accurately reflect the natural interplay of endogenous EVs. Some studies have utilized cell lines engineered to express genetic reporters, such as green fluorescent protein or bioluminescence resonance energy transfer-based reporter for extracellular particle fusion proteins, to track EV release from implanted tumors in mice[193]. While fluorescent lipophilic dyes offer a convenient labeling approach, concerns regarding aggregation and signal discrepancy arise[194]. MemGlowTM, however, minimizes autofluorescence and is effective in zebrafish models[195]. Tracking EVs secreted directly by specific tissues in vivo remains crucial, but the limited applicability of genetically modified cell lines poses a challenge[196]. Researchers have used ex vivo blood models to study EV-blood cell interactions, which mimic in vivo observations. This approach allows for controlled experiments and studies across multiple species[197,198]. Furthermore, determining the functional activity of specific EV cargoes in recipient cells remains a substantial hurdle, as more robust and consistent methodologies beyond current approaches such as Cre mRNA delivery are needed[199]. Finally, the absence of standardized tracking methods across studies complicates data comparison and impedes the establishment of a cohesive knowledge base.
Addressing translational challenges in clinical applications: EV-based diagnostics for MASLD remain in early developmental stages, with a focus on identifying molecular signatures through omics approaches. Translating these findings into clinically viable tools is a significant challenge. One major limitation is the lack of comprehensive EV databases comparable to genetic repositories, making population-specific data scarce. Additionally, global variations in EV cargo across different ethnic and geographic groups require extensive validation. Another gap lies in the understanding of EV biodistribution and circulation dynamics, as factors such as patient physiology and time of sample collection remain poorly characterized. To advance EV-based diagnostics, large-scale clinical validation is necessary. Despite promising in vitro findings, studies on patient-derived samples are crucial to ensure accuracy and applicability across diverse populations before clinical implementation[196]. With respect to EV-based therapies, a more thorough exploration of their pharmacokinetics and biodistribution across various animal models, including nonrodents, is needed. For example, studies using pig-tailed macaques (Macaca nemestrina) demonstrated the value of nonhuman primate models for studying human diseases[200].
Single-EV analysis and advanced imaging techniques: Single-EV analysis and advanced imaging have become vital for addressing EV heterogeneity and exploring individual EV variations. These techniques enable the identification of specific EV subpopulations and their unique roles in cell communication[201]. Advanced imaging, such as super-resolution microscopy, allows for the visualization of EV interactions and cargo delivery at the nanoscale, which is crucial for both basic research and clinical applications[202]. By providing detailed insights into individual EVs, these techniques are essential for advancing our understanding of cell-to-cell communication and realizing the full potential of EV-based applications.
Integration of multi-omics data for comprehensive EV profiling: Integrating multiomics data is crucial for advancing EV research because it overcomes the limitations of single-omics analyses, which provide only isolated snapshots of molecular components. While valuable EV databases exist, they lack the capacity to reveal the complex interactions between DNAs, RNAs, proteins, and metabolites that drive biological functions. Combining data from different “omic” layers allows researchers to capture synergistic relationships and the intricate interplay of biological variations, offering a more comprehensive understanding of EV biology in both healthy and diseased states[203]. This integrated approach, whether guided by prior knowledge or driven by data-driven discovery, is essential for uncovering novel biomarkers, therapeutic targets, and a deeper understanding of EV-mediated processes[204].
The application of EVs derived from induced pluripotent stem cells (iPSCs) represents a significant advancement in personalized medicine. Given the inherent capacity of iPSCs to be generated from an individual’s somatic cells, they offer a platform for autologous therapies, thereby mitigating immunogenic complications[205]. The utilization of iPSC-derived EVs extends this personalized approach by leveraging their cargo for targeted therapeutic intervention[206]. Notably, studies have focused on MSC-EVs and their therapeutic potential in chronic liver diseases, including MASLD. Previous work demonstrated that MSC-EVs obtained from various sources, such as the amnion, adipose tissue, and Wharton’s jelly, effectively attenuated HSC and Kupffer cell activation, resulting in reduced inflammation and fibrogenesis[207-209]. Specifically, in murine models of hepatic injury and fibrosis, iPSC-derived EVs exhibited notable therapeutic effects on HSC modulation. Genomic analysis revealed that these iPSC-derived EVs, upon accumulation in the murine liver, were significantly enriched in miR-92a-3p[210]. In essence, the capacity to generate iPSCs from a patient’s cells and sub
EVs have emerged as critical mediators of intercellular communication, influencing key processes in MASLD, such as lipotoxicity, inflammation, and fibrogenesis. Their utility as diagnostic and prognostic biomarkers offer a pathway toward minimally invasive and targeted patient care. However, the translation of these promising findings into clinical practice necessitates addressing significant challenges. These include standardizing EV isolation and characterization, unraveling the intricate complexity of EV cargo, and developing robust in vivo tracking methods. Future research must prioritize the integration of multiomics data, the refinement of single-EV analysis, and the execution of rigorous clinical trials to overcome translational hurdles. By fostering interdisciplinary collaboration and harnessing cutting-edge technologies, we can pave the way for EV-based precision medicine, ultimately improving patient outcomes in MASLD and other liver diseases.
We would like to thank all the members of the Center of Excellence in Hepatitis and Liver Cancer, Faculty of Medicine, Chulalongkorn University, for their technical support.
| 1. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 4019] [Article Influence: 502.4] [Reference Citation Analysis (2)] |
| 2. | Chan WK, Chuah KH, Rajaram RB, Lim LL, Ratnasingam J, Vethakkan SR. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J Obes Metab Syndr. 2023;32:197-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 451] [Article Influence: 150.3] [Reference Citation Analysis (1)] |
| 3. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 3165] [Article Influence: 527.5] [Reference Citation Analysis (2)] |
| 4. | Paik JM, Henry L, Younossi Y, Ong J, Alqahtani S, Younossi ZM. The burden of nonalcoholic fatty liver disease (NAFLD) is rapidly growing in every region of the world from 1990 to 2019. Hepatol Commun. 2023;7:e0251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 115] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 5. | Karlsen TH, Sheron N, Zelber-Sagi S, Carrieri P, Dusheiko G, Bugianesi E, Pryke R, Hutchinson SJ, Sangro B, Martin NK, Cecchini M, Dirac MA, Belloni A, Serra-Burriel M, Ponsioen CY, Sheena B, Lerouge A, Devaux M, Scott N, Hellard M, Verkade HJ, Sturm E, Marchesini G, Yki-Järvinen H, Byrne CD, Targher G, Tur-Sinai A, Barrett D, Ninburg M, Reic T, Taylor A, Rhodes T, Treloar C, Petersen C, Schramm C, Flisiak R, Simonova MY, Pares A, Johnson P, Cucchetti A, Graupera I, Lionis C, Pose E, Fabrellas N, Ma AT, Mendive JM, Mazzaferro V, Rutter H, Cortez-Pinto H, Kelly D, Burton R, Lazarus JV, Ginès P, Buti M, Newsome PN, Burra P, Manns MP. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399:61-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 444] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 6. | Wong VW, Ekstedt M, Wong GL, Hagström H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023;79:842-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 456] [Article Influence: 152.0] [Reference Citation Analysis (0)] |
| 7. | Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 1460] [Article Influence: 365.0] [Reference Citation Analysis (1)] |
| 8. | Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, Stal P, Wong VW, Kechagias S, Hultcrantz R, Loomba R. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 984] [Cited by in RCA: 1506] [Article Influence: 167.3] [Reference Citation Analysis (0)] |
| 9. | Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643-54.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 1301] [Article Influence: 118.3] [Reference Citation Analysis (1)] |
| 10. | Benedé-Ubieto R, Cubero FJ, Nevzorova YA. Breaking the barriers: the role of gut homeostasis in Metabolic-Associated Steatotic Liver Disease (MASLD). Gut Microbes. 2024;16:2331460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 122] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 11. | Targher G, Byrne CD, Tilg H. MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut. 2024;73:691-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 272] [Article Influence: 136.0] [Reference Citation Analysis (1)] |
| 12. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2651] [Article Influence: 189.4] [Reference Citation Analysis (3)] |
| 13. | Burt AD, Lackner C, Tiniakos DG. Diagnosis and Assessment of NAFLD: Definitions and Histopathological Classification. Semin Liver Dis. 2015;35:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342-1359.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 586] [Cited by in RCA: 592] [Article Influence: 42.3] [Reference Citation Analysis (2)] |
| 15. | Cotter TG, Rinella M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology. 2020;158:1851-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 903] [Article Influence: 150.5] [Reference Citation Analysis (2)] |
| 16. | Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, Ye Q, Huang DQ, Zhao C, Zhang J, Liu C, Chang N, Xing F, Yan S, Wan ZH, Tang NSY, Mayumi M, Liu X, Liu C, Rui F, Yang H, Yang Y, Jin R, Le RHX, Xu Y, Le DM, Barnett S, Stave CD, Cheung R, Zhu Q, Nguyen MH. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:2809-2817.e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 455] [Article Influence: 113.8] [Reference Citation Analysis (2)] |
| 17. | Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 1125] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 18. | Meldolesi J. Exosomes and Ectosomes in Intercellular Communication. Curr Biol. 2018;28:R435-R444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 717] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 19. | Saha B, Momen-Heravi F, Kodys K, Szabo G. MicroRNA Cargo of Extracellular Vesicles from Alcohol-exposed Monocytes Signals Naive Monocytes to Differentiate into M2 Macrophages. J Biol Chem. 2016;291:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 20. | Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6453] [Cited by in RCA: 8278] [Article Influence: 1034.8] [Reference Citation Analysis (1)] |
| 21. | Kato S, Kowashi Y, Demuth DR. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb Pathog. 2002;32:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3959] [Cited by in RCA: 4410] [Article Influence: 400.9] [Reference Citation Analysis (0)] |
| 23. | Malhi H. Emerging role of extracellular vesicles in liver diseases. Am J Physiol Gastrointest Liver Physiol. 2019;317:G739-G749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, Charlton MR, Shah VH, Malhi H, Gores GJ. Lipid-Induced Signaling Causes Release of Inflammatory Extracellular Vesicles From Hepatocytes. Gastroenterology. 2016;150:956-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 418] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 25. | Record M, Silvente-Poirot S, Poirot M, Wakelam MJO. Extracellular vesicles: lipids as key components of their biogenesis and functions. J Lipid Res. 2018;59:1316-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 235] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 26. | Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4900] [Cited by in RCA: 6386] [Article Influence: 491.2] [Reference Citation Analysis (0)] |
| 27. | Traub LM. The reverse logic of multivesicular endosomes. EMBO Rep. 2010;11:79-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 994] [Cited by in RCA: 1128] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 29. | Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464:864-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 646] [Cited by in RCA: 633] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 30. | Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10:925-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 565] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 31. | Kobayashi T, Beuchat MH, Chevallier J, Makino A, Mayran N, Escola JM, Lebrand C, Cosson P, Kobayashi T, Gruenberg J. Separation and characterization of late endosomal membrane domains. J Biol Chem. 2002;277:32157-32164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 334] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 32. | Kajimoto T, Okada T, Miya S, Zhang L, Nakamura S. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat Commun. 2013;4:2712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 33. | Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2257] [Cited by in RCA: 2691] [Article Influence: 158.3] [Reference Citation Analysis (0)] |
| 34. | Haraszti RA, Didiot MC, Sapp E, Leszyk J, Shaffer SA, Rockwell HE, Gao F, Narain NR, DiFiglia M, Kiebish MA, Aronin N, Khvorova A. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles. 2016;5:32570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 375] [Cited by in RCA: 566] [Article Influence: 56.6] [Reference Citation Analysis (1)] |
| 35. | Jackson CE, Scruggs BS, Schaffer JE, Hanson PI. Effects of Inhibiting VPS4 Support a General Role for ESCRTs in Extracellular Vesicle Biogenesis. Biophys J. 2017;113:1342-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 36. | Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1α-dependent manner. J Lipid Res. 2016;57:233-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 243] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 37. | Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 1237] [Article Influence: 247.4] [Reference Citation Analysis (0)] |
| 38. | Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 2149] [Article Influence: 179.1] [Reference Citation Analysis (0)] |
| 39. | Joshi BS, de Beer MA, Giepmans BNG, Zuhorn IS. Endocytosis of Extracellular Vesicles and Release of Their Cargo from Endosomes. ACS Nano. 2020;14:4444-4455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 413] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 40. | Wadia JS, Schaller M, Williamson RA, Dowdy SF. Pathologic prion protein infects cells by lipid-raft dependent macropinocytosis. PLoS One. 2008;3:e3314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Costa Verdera H, Gitz-Francois JJ, Schiffelers RM, Vader P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J Control Release. 2017;266:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 402] [Article Influence: 44.7] [Reference Citation Analysis (3)] |
| 42. | Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q, Sui SF. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 780] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 43. | Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM, Mörgelin M, Belting M. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem. 2013;288:17713-17724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 434] [Cited by in RCA: 574] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 44. | Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, Vader P. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 534] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 45. | Nanbo A, Kawanishi E, Yoshida R, Yoshiyama H. Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J Virol. 2013;87:10334-10347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 293] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 46. | Wei F, Ma C, Zhou T, Dong X, Luo Q, Geng L, Ding L, Zhang Y, Zhang L, Li N, Li Y, Liu Y. Exosomes derived from gemcitabine-resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Mol Cancer. 2017;16:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 217] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 47. | Andreu Z, Yáñez-Mó M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 697] [Cited by in RCA: 1110] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 48. | Williams C, Pazos R, Royo F, González E, Roura-Ferrer M, Martinez A, Gamiz J, Reichardt NC, Falcón-Pérez JM. Assessing the role of surface glycans of extracellular vesicles on cellular uptake. Sci Rep. 2019;9:11920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 49. | Altei WF, Pachane BC, Dos Santos PK, Ribeiro LNM, Sung BH, Weaver AM, Selistre-de-Araújo HS. Inhibition of αvβ3 integrin impairs adhesion and uptake of tumor-derived small extracellular vesicles. Cell Commun Signal. 2020;18:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 50. | Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2697] [Cited by in RCA: 3969] [Article Influence: 360.8] [Reference Citation Analysis (0)] |
| 51. | Nishida-Aoki N, Tominaga N, Kosaka N, Ochiya T. Altered biodistribution of deglycosylated extracellular vesicles through enhanced cellular uptake. J Extracell Vesicles. 2020;9:1713527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 52. | Imai T, Takahashi Y, Nishikawa M, Kato K, Morishita M, Yamashita T, Matsumoto A, Charoenviriyakul C, Takakura Y. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J Extracell Vesicles. 2015;4:26238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 462] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 53. | Bala S, Csak T, Momen-Heravi F, Lippai D, Kodys K, Catalano D, Satishchandran A, Ambros V, Szabo G. Biodistribution and function of extracellular miRNA-155 in mice. Sci Rep. 2015;5:10721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 54. | Nojima H, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ, Gulbins E, Lentsch AB. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol. 2016;64:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 239] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 55. | He Y, Rodrigues RM, Wang X, Seo W, Ma J, Hwang S, Fu Y, Trojnár E, Mátyás C, Zhao S, Ren R, Feng D, Pacher P, Kunos G, Gao B. Neutrophil-to-hepatocyte communication via LDLR-dependent miR-223-enriched extracellular vesicle transfer ameliorates nonalcoholic steatohepatitis. J Clin Invest. 2021;131:e141513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 56. | Boonkaew B, Satthawiwat N, Pachane BC, Brett LM, Tangkijvanich P, Ariyachet C. Palmitic acid reduces LDLR-dependent uptake of macrophage-derived extracellular vesicles by hepatoma cells. Noncoding RNA Res. 2025;13:71-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 57. | Nozue T. Lipid Lowering Therapy and Circulating PCSK9 Concentration. J Atheroscler Thromb. 2017;24:895-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 58. | Choi YJ, Lee SJ, Kim HI, Lee HJ, Kang SJ, Kim TY, Cheon C, Ko SG. Platycodin D enhances LDLR expression and LDL uptake via down-regulation of IDOL mRNA in hepatic cells. Sci Rep. 2020;10:19834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Boonkaew B, Satthawiwat N, Pinjaroen N, Chuaypen N, Tangkijvanich P. Circulating Extracellular Vesicle-Derived microRNAs as Novel Diagnostic and Prognostic Biomarkers for Non-Viral-Related Hepatocellular Carcinoma. Int J Mol Sci. 2023;24:16043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 60. | Welsh JA, Scorletti E, Clough GF, Englyst NA, Byrne CD. Leukocyte extracellular vesicle concentration is inversely associated with liver fibrosis severity in NAFLD. J Leukoc Biol. 2018;104:631-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Povero D, Eguchi A, Niesman IR, Andronikou N, de Mollerat du Jeu X, Mulya A, Berk M, Lazic M, Thapaliya S, Parola M, Patel HH, Feldstein AE. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci Signal. 2013;6:ra88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 62. | Povero D, Yamashita H, Ren W, Subramanian MG, Myers RP, Eguchi A, Simonetto DA, Goodman ZD, Harrison SA, Sanyal AJ, Bosch J, Feldstein AE. Characterization and Proteome of Circulating Extracellular Vesicles as Potential Biomarkers for NASH. Hepatol Commun. 2020;4:1263-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 63. | Deng ZB, Liu Y, Liu C, Xiang X, Wang J, Cheng Z, Shah SV, Zhang S, Zhang L, Zhuang X, Michalek S, Grizzle WE, Zhang HG. Immature myeloid cells induced by a high-fat diet contribute to liver inflammation. Hepatology. 2009;50:1412-1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 64. | Hirsova P, Gores GJ. Death Receptor-Mediated Cell Death and Proinflammatory Signaling in Nonalcoholic Steatohepatitis. Cell Mol Gastroenterol Hepatol. 2015;1:17-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 65. | Ibrahim SH, Hirsova P, Tomita K, Bronk SF, Werneburg NW, Harrison SA, Goodfellow VS, Malhi H, Gores GJ. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 2016;63:731-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 66. | Lee YS, Kim SY, Ko E, Lee JH, Yi HS, Yoo YJ, Je J, Suh SJ, Jung YK, Kim JH, Seo YS, Yim HJ, Jeong WI, Yeon JE, Um SH, Byun KS. Exosomes derived from palmitic acid-treated hepatocytes induce fibrotic activation of hepatic stellate cells. Sci Rep. 2017;7:3710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 188] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 67. | Tomita K, Kabashima A, Freeman BL, Bronk SF, Hirsova P, Ibrahim SH. Mixed Lineage Kinase 3 Mediates the Induction of CXCL10 by a STAT1-Dependent Mechanism During Hepatocyte Lipotoxicity. J Cell Biochem. 2017;118:3249-3259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 68. | Liao CY, Song MJ, Gao Y, Mauer AS, Revzin A, Malhi H. Hepatocyte-Derived Lipotoxic Extracellular Vesicle Sphingosine 1-Phosphate Induces Macrophage Chemotaxis. Front Immunol. 2018;9:2980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 69. | Hwang S, He Y, Xiang X, Seo W, Kim SJ, Ma J, Ren T, Park SH, Zhou Z, Feng D, Kunos G, Gao B. Interleukin-22 Ameliorates Neutrophil-Driven Nonalcoholic Steatohepatitis Through Multiple Targets. Hepatology. 2020;72:412-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 70. | Zhao Z, Zhong L, Li P, He K, Qiu C, Zhao L, Gong J. Cholesterol impairs hepatocyte lysosomal function causing M1 polarization of macrophages via exosomal miR-122-5p. Exp Cell Res. 2020;387:111738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 71. | Liu XL, Pan Q, Cao HX, Xin FZ, Zhao ZH, Yang RX, Zeng J, Zhou H, Fan JG. Lipotoxic Hepatocyte-Derived Exosomal MicroRNA 192-5p Activates Macrophages Through Rictor/Akt/Forkhead Box Transcription Factor O1 Signaling in Nonalcoholic Fatty Liver Disease. Hepatology. 2020;72:454-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 240] [Article Influence: 40.0] [Reference Citation Analysis (1)] |
| 72. | Liu H, Niu Q, Wang T, Dong H, Bian C. Lipotoxic hepatocytes promote nonalcoholic fatty liver disease progression by delivering microRNA-9-5p and activating macrophages. Int J Biol Sci. 2021;17:3745-3759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 73. | Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, Shlomchik MJ, Coffman RL, Candia A, Mehal WZ. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest. 2016;126:859-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 414] [Cited by in RCA: 396] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 74. | Hernández A, Geng Y, Sepúlveda R, Solís N, Torres J, Arab JP, Barrera F, Cabrera D, Moshage H, Arrese M. Chemical hypoxia induces pro-inflammatory signals in fat-laden hepatocytes and contributes to cellular crosstalk with Kupffer cells through extracellular vesicles. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 75. | Yao JM, Ying HZ, Zhang HH, Qiu FS, Wu JQ, Yu CH. Exosomal RBP4 potentiated hepatic lipid accumulation and inflammation in high-fat-diet-fed mice by promoting M1 polarization of Kupffer cells. Free Radic Biol Med. 2023;195:58-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 76. | Hernández A, Reyes D, Geng Y, Arab JP, Cabrera D, Sepulveda R, Solis N, Buist-Homan M, Arrese M, Moshage H. Extracellular vesicles derived from fat-laden hepatocytes undergoing chemical hypoxia promote a pro-fibrotic phenotype in hepatic stellate cells. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 77. | Luo X, Luo SZ, Xu ZX, Zhou C, Li ZH, Zhou XY, Xu MY. Lipotoxic hepatocyte-derived exosomal miR-1297 promotes hepatic stellate cell activation through the PTEN signaling pathway in metabolic-associated fatty liver disease. World J Gastroenterol. 2021;27:1419-1434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 78. | Wang W, Li F, Lai X, Liu H, Wu S, Han Y, Shen Y. Exosomes secreted by palmitic acid-treated hepatocytes promote LX-2 cell activation by transferring miRNA-107. Cell Death Discov. 2021;7:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 79. | Wu Z, Xia M, Wang J, Aguilar MM, Buist-Homan M, Moshage H. Extracellular vesicles originating from steatotic hepatocytes promote hepatic stellate cell senescence via AKT/mTOR signaling. Cell Biochem Funct. 2024;42:e4077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 80. | Jiang F, Chen Q, Wang W, Ling Y, Yan Y, Xia P. Hepatocyte-derived extracellular vesicles promote endothelial inflammation and atherogenesis via microRNA-1. J Hepatol. 2020;72:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 205] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 81. | Guo Q, Furuta K, Lucien F, Gutierrez Sanchez LH, Hirsova P, Krishnan A, Kabashima A, Pavelko KD, Madden B, Alhuwaish H, Gao Y, Revzin A, Ibrahim SH. Integrin β(1)-enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J Hepatol. 2019;71:1193-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 82. | Zhao Y, Zhao MF, Jiang S, Wu J, Liu J, Yuan XW, Shen D, Zhang JZ, Zhou N, He J, Fang L, Sun XT, Xue B, Li CJ. Liver governs adipose remodelling via extracellular vesicles in response to lipid overload. Nat Commun. 2020;11:719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 83. | Cai J, Zhang XJ, Li H. The Role of Innate Immune Cells in Nonalcoholic Steatohepatitis. Hepatology. 2019;70:1026-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 84. | Calvente CJ, Tameda M, Johnson CD, Del Pilar H, Lin YC, Adronikou N, De Mollerat Du Jeu X, Llorente C, Boyer J, Feldstein AE. Neutrophils contribute to spontaneous resolution of liver inflammation and fibrosis via microRNA-223. J Clin Invest. 2019;129:4091-4109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 218] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 85. | Aucher A, Rudnicka D, Davis DM. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J Immunol. 2013;191:6250-6260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 86. | Ismail N, Wang Y, Dakhlallah D, Moldovan L, Agarwal K, Batte K, Shah P, Wisler J, Eubank TD, Tridandapani S, Paulaitis ME, Piper MG, Marsh CB. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013;121:984-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 428] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 87. | Hou X, Yin S, Ren R, Liu S, Yong L, Liu Y, Li Y, Zheng MH, Kunos G, Gao B, Wang H. Myeloid-Cell-Specific IL-6 Signaling Promotes MicroRNA-223-Enriched Exosome Production to Attenuate NAFLD-Associated Fibrosis. Hepatology. 2021;74:116-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 174] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 88. | Chen L, Huang Y, Duan Z, Huang P, Yao H, Zhou Y, Ji Q, Liu X. Exosomal miR-500 Derived From Lipopolysaccharide-Treated Macrophage Accelerates Liver Fibrosis by Suppressing MFN2. Front Cell Dev Biol. 2021;9:716209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 89. | Gao H, Jin Z, Bandyopadhyay G, Cunha E Rocha K, Liu X, Zhao H, Zhang D, Jouihan H, Pourshahian S, Kisseleva T, Brenner DA, Ying W, Olefsky JM. MiR-690 treatment causes decreased fibrosis and steatosis and restores specific Kupffer cell functions in NASH. Cell Metab. 2022;34:978-990.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 90. | Koeck ES, Iordanskaia T, Sevilla S, Ferrante SC, Hubal MJ, Freishtat RJ, Nadler EP. Adipocyte exosomes induce transforming growth factor beta pathway dysregulation in hepatocytes: a novel paradigm for obesity-related liver disease. J Surg Res. 2014;192:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 91. | Kostallari E, Hirsova P, Prasnicka A, Verma VK, Yaqoob U, Wongjarupong N, Roberts LR, Shah VH. Hepatic stellate cell-derived platelet-derived growth factor receptor-alpha-enriched extracellular vesicles promote liver fibrosis in mice through SHP2. Hepatology. 2018;68:333-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 92. | Gao J, Wei B, de Assuncao TM, Liu Z, Hu X, Ibrahim S, Cooper SA, Cao S, Shah VH, Kostallari E. Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. J Hepatol. 2020;73:1144-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 93. | Tan J, Chen M, Liu M, Chen A, Huang M, Chen X, Tian X, Chen W. Exosomal miR-192-5p secreted by bone marrow mesenchymal stem cells inhibits hepatic stellate cell activation and targets PPP2R3A. J Histotechnol. 2023;46:158-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 94. | Du Z, Wu T, Liu L, Luo B, Wei C. Extracellular vesicles-derived miR-150-5p secreted by adipose-derived mesenchymal stem cells inhibits CXCL1 expression to attenuate hepatic fibrosis. J Cell Mol Med. 2021;25:701-715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 95. | Wang Y, Chen Y, Yang F, Yu X, Chu Y, Zhou J, Yan Y, Xi J. MiR-4465-modified mesenchymal stem cell-derived small extracellular vesicles inhibit liver fibrosis development via targeting LOXL2 expression. J Zhejiang Univ Sci B. 2024;25:594-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 96. | Chen KY, Chang ZF. A marked increase of fucosylation of glycoproteins in IMR-90 human diploid fibroblasts during senescence in vitro. Biochem Biophys Res Commun. 1987;142:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 97. | Luo Z, Ji Y, Zhang D, Gao H, Jin Z, Yang M, Ying W. Microbial DNA enrichment promotes liver steatosis and fibrosis in the course of non-alcoholic steatohepatitis. Acta Physiol (Oxf). 2022;235:e13827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 98. | Elshaer A, Chascsa DMH, Lizaola-Mayo BC. Exploring Varied Treatment Strategies for Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Life (Basel). 2024;14:844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 99. | Svobodová G, Horní M, Velecká E, Boušová I. Metabolic dysfunction-associated steatotic liver disease-induced changes in the antioxidant system: a review. Arch Toxicol. 2025;99:1-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 100. | Li X, Chen R, Kemper S, Brigstock DR. Dynamic Changes in Function and Proteomic Composition of Extracellular Vesicles from Hepatic Stellate Cells during Cellular Activation. Cells. 2020;9:290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 101. | Wu HM, Ni XX, Xu QY, Wang Q, Li XY, Hua J. Regulation of lipid-induced macrophage polarization through modulating peroxisome proliferator-activated receptor-gamma activity affects hepatic lipid metabolism via a Toll-like receptor 4/NF-κB signaling pathway. J Gastroenterol Hepatol. 2020;35:1998-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 102. | Povero D, Panera N, Eguchi A, Johnson CD, Papouchado BG, de Araujo Horcel L, Pinatel EM, Alisi A, Nobili V, Feldstein AE. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-γ. Cell Mol Gastroenterol Hepatol. 2015;1:646-663.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 186] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 103. | Luo X, Xu ZX, Wu JC, Luo SZ, Xu MY. Hepatocyte-derived exosomal miR-27a activateshepatic stellate cells through the inhibitionof PINK1-mediated mitophagy in MAFLD. Mol Ther Nucleic Acids. 2021;26:1241-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 104. | Li S, Yang F, Cheng F, Zhu L, Yan Y. Lipotoxic hepatocyte derived LIMA1 enriched small extracellular vesicles promote hepatic stellate cells activation via inhibiting mitophagy. Cell Mol Biol Lett. 2024;29:82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 105. | Daher D, Dahan KSE, Singal AG. Non-alcoholic fatty liver disease-related hepatocellular carcinoma. J Liver Cancer. 2023;23:127-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 106. | Kim J, Seki E. Unveiling the cancer risk nexus of the steatotic liver. Trends Endocrinol Metab. 2024;35:708-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 107. | Jiang H, Qian Y, Shen Z, Liu Y, He Y, Gao R, Shen M, Chen S, Fu Q, Yang T. Circulating microRNA135a3p in serum extracellular vesicles as a potential biological marker of nonalcoholic fatty liver disease. Mol Med Rep. 2021;24:498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 108. | Quan H, Li B, Yang J. MicroRNA-504 functions as a tumor suppressor in hepatocellular carcinoma through inhibiting Frizzled-7-mediated-Wnt/β-catenin signaling. Biomed Pharmacother. 2018;107:754-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 109. | Hu Z, You L, Hu S, Yu L, Gao Y, Li L, Zhang S. Hepatocellular carcinoma cell-derived exosomal miR-21-5p promotes the polarization of tumor-related macrophages (TAMs) through SP1/XBP1 and affects the progression of hepatocellular carcinoma. Int Immunopharmacol. 2024;126:111149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 110. | Rodrigues PM, Afonso MB, Simão AL, Islam T, Gaspar MM, O'Rourke CJ, Lewinska M, Andersen JB, Arretxe E, Alonso C, Santos-Laso Á, Izquierdo-Sanchez L, Jimenez-Agüero R, Eizaguirre E, Bujanda L, Pareja MJ, Prip-Buus C, Banales JM, Rodrigues CMP, Castro RE. miR-21-5p promotes NASH-related hepatocarcinogenesis. Liver Int. 2023;43:2256-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 111. | Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69:1691-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 585] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 112. | Zisis M, Chondrogianni ME, Androutsakos T, Rantos I, Oikonomou E, Chatzigeorgiou A, Kassi E. Linking Cardiovascular Disease and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): The Role of Cardiometabolic Drugs in MASLD Treatment. Biomolecules. 2025;15:324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 113. | Vo J, Truyen TTTT, Uy-Evanado A, Sargsyan A, Chugh H, Young C, Hurst S, Miyake CY, Reinier K, Chugh SS. Sudden cardiac death associated with fatty liver disease. Int J Cardiol Heart Vasc. 2025;56:101602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 114. | Chen X, Chen S, Pang J, Huang R, You Y, Zhang H, Xiao J, Xue H, Ling W. Hepatic steatosis aggravates atherosclerosis via small extracellular vesicle-mediated inhibition of cellular cholesterol efflux. J Hepatol. 2023;79:1491-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 115. | Zuo R, Ye LF, Huang Y, Song ZQ, Wang L, Zhi H, Zhang MY, Li JY, Zhu L, Xiao WJ, Shang HC, Zhang Y, He RR, Chen Y. Hepatic small extracellular vesicles promote microvascular endothelial hyperpermeability during NAFLD via novel-miRNA-7. J Nanobiotechnology. 2021;19:396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 116. | Yuan X, Bhat OM, Zou Y, Zhang Y, Li PL. Contribution of Hepatic Steatosis-Intensified Extracellular Vesicle Release to Aggravated Inflammatory Endothelial Injury in Liver-Specific Asah1 Gene Knockout Mice. Am J Pathol. 2023;193:493-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 117. | Andrabi SM, Sharma NS, Karan A, Shahriar SMS, Cordon B, Ma B, Xie J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv Sci (Weinh). 2023;10:e2303259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 296] [Article Influence: 98.7] [Reference Citation Analysis (0)] |
| 118. | Batty M, Bennett MR, Yu E. The Role of Oxidative Stress in Atherosclerosis. Cells. 2022;11:3843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 284] [Article Influence: 71.0] [Reference Citation Analysis (1)] |
| 119. | Prakash A, Crespo-Avilan GE, Hernandez-Resendiz S, Ong SG, Hausenloy DJ. Extracellular vesicles - mediating and delivering cardioprotection in acute myocardial infarction and heart failure. Cond Med. 2020;3:227-238. [PubMed] |
| 120. | Han C, Yang J, Sun J, Qin G. Extracellular vesicles in cardiovascular disease: Biological functions and therapeutic implications. Pharmacol Ther. 2022;233:108025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 121. | Kaya E, Yilmaz Y. Metabolic-associated Fatty Liver Disease (MAFLD): A Multi-systemic Disease Beyond the Liver. J Clin Transl Hepatol. 2022;10:329-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 122. | Leroyer AS, Isobe H, Lesèche G, Castier Y, Wassef M, Mallat Z, Binder BR, Tedgui A, Boulanger CM. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J Am Coll Cardiol. 2007;49:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 303] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 123. | Li S, Cheng F, Zhang Z, Xu R, Shi H, Yan Y. The role of hepatocyte-derived extracellular vesicles in liver and extrahepatic diseases. Biomed Pharmacother. 2024;180:117502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 124. | Fu Q, Li Y, Jiang H, Shen Z, Gao R, He Y, Liu Y, Xu K, Yang T. Hepatocytes derived extracellular vesicles from high-fat diet induced obese mice modulate genes expression and proliferation of islet β cells. Biochem Biophys Res Commun. 2019;516:1159-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 125. | Ji Y, Luo Z, Gao H, Dos Reis FCG, Bandyopadhyay G, Jin Z, Manda KA, Isaac R, Yang M, Fu W, Ying W, Olefsky JM. Hepatocyte-derived exosomes from early onset obese mice promote insulin sensitivity through miR-3075. Nat Metab. 2021;3:1163-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 126. | Wu J, Dong T, Chen T, Sun J, Luo J, He J, Wei L, Zeng B, Zhang H, Li W, Liu J, Chen X, Su M, Ni Y, Jiang Q, Zhang Y, Xi Q. Hepatic exosome-derived miR-130a-3p attenuates glucose intolerance via suppressing PHLPP2 gene in adipocyte. Metabolism. 2020;103:154006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 127. | Polyzos SA, Kountouras J, Mavrouli M, Katsinelos P, Doulberis M, Gavana E, Duntas L. Selenoprotein P in Patients with Nonalcoholic Fatty Liver Disease. Exp Clin Endocrinol Diabetes. 2019;127:598-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 128. | Jin Y, Chung YW, Jung MK, Lee JH, Ko KY, Jang JK, Ham M, Kang H, Pack CG, Mihara H, Kim IY. Apolipoprotein E-mediated regulation of selenoprotein P transportation via exosomes. Cell Mol Life Sci. 2020;77:2367-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 129. | Wang Z, Kim SY, Tu W, Kim J, Xu A, Yang YM, Matsuda M, Reolizo L, Tsuchiya T, Billet S, Gangi A, Noureddin M, Falk BA, Kim S, Fan W, Tighiouart M, You S, Lewis MS, Pandol SJ, Di Vizio D, Merchant A, Posadas EM, Bhowmick NA, Lu SC, Seki E. Extracellular vesicles in fatty liver promote a metastatic tumor microenvironment. Cell Metab. 2023;35:1209-1226.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 130. | Loomba R, Wolfson T, Ang B, Hooker J, Behling C, Peterson M, Valasek M, Lin G, Brenner D, Gamst A, Ehman R, Sirlin C. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology. 2014;60:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 399] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 131. | Foncea CG, Popescu A, Lupusoru R, Fofiu R, Sirli R, Danila M, Sporea I. Comparative study between pSWE and 2D-SWE techniques integrated in the same ultrasound machine, with Transient Elastography as the reference method. Med Ultrason. 2020;22:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 132. | Stål P. Liver fibrosis in non-alcoholic fatty liver disease - diagnostic challenge with prognostic significance. World J Gastroenterol. 2015;21:11077-11087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 100] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (3)] |
| 133. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1663] [Article Influence: 87.5] [Reference Citation Analysis (1)] |
| 134. | Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 550] [Cited by in RCA: 532] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 135. | Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, Seabra MC, Round JL, Ward DM, O'Connell RM. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 2015;6:7321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 629] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 136. | Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, Zambirinis CP, Rodrigues G, Molina H, Heissel S, Mark MT, Steiner L, Benito-Martin A, Lucotti S, Di Giannatale A, Offer K, Nakajima M, Williams C, Nogués L, Pelissier Vatter FA, Hashimoto A, Davies AE, Freitas D, Kenific CM, Ararso Y, Buehring W, Lauritzen P, Ogitani Y, Sugiura K, Takahashi N, Alečković M, Bailey KA, Jolissant JS, Wang H, Harris A, Schaeffer LM, García-Santos G, Posner Z, Balachandran VP, Khakoo Y, Raju GP, Scherz A, Sagi I, Scherz-Shouval R, Yarden Y, Oren M, Malladi M, Petriccione M, De Braganca KC, Donzelli M, Fischer C, Vitolano S, Wright GP, Ganshaw L, Marrano M, Ahmed A, DeStefano J, Danzer E, Roehrl MHA, Lacayo NJ, Vincent TC, Weiser MR, Brady MS, Meyers PA, Wexler LH, Ambati SR, Chou AJ, Slotkin EK, Modak S, Roberts SS, Basu EM, Diolaiti D, Krantz BA, Cardoso F, Simpson AL, Berger M, Rudin CM, Simeone DM, Jain M, Ghajar CM, Batra SK, Stanger BZ, Bui J, Brown KA, Rajasekhar VK, Healey JH, de Sousa M, Kramer K, Sheth S, Baisch J, Pascual V, Heaton TE, La Quaglia MP, Pisapia DJ, Schwartz R, Zhang H, Liu Y, Shukla A, Blavier L, DeClerck YA, LaBarge M, Bissell MJ, Caffrey TC, Grandgenett PM, Hollingsworth MA, Bromberg J, Costa-Silva B, Peinado H, Kang Y, Garcia BA, O'Reilly EM, Kelsen D, Trippett TM, Jones DR, Matei IR, Jarnagin WR, Lyden D. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell. 2020;182:1044-1061.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 924] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 137. | Kornek M, Lynch M, Mehta SH, Lai M, Exley M, Afdhal NH, Schuppan D. Circulating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or nonalcoholic steatohepatitis. Gastroenterology. 2012;143:448-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 138. | Zhu Q, Li H, Ao Z, Xu H, Luo J, Kaurich C, Yang R, Zhu PW, Chen SD, Wang XD, Tang LJ, Li G, Huang OY, Zheng MH, Li HP, Liu F. Lipidomic identification of urinary extracellular vesicles for non-alcoholic steatohepatitis diagnosis. J Nanobiotechnology. 2022;20:349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 139. | Nakao Y, Amrollahi P, Parthasarathy G, Mauer AS, Sehrawat TS, Vanderboom P, Nair KS, Nakao K, Allen AM, Hu TY, Malhi H. Circulating extracellular vesicles are a biomarker for NAFLD resolution and response to weight loss surgery. Nanomedicine. 2021;36:102430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 140. | Nguyen HQ, Lee D, Kim Y, Bang G, Cho K, Lee YS, Yeon JE, Lubman DM, Kim J. Label-free quantitative proteomic analysis of serum extracellular vesicles differentiating patients of alcoholic and nonalcoholic fatty liver diseases. J Proteomics. 2021;245:104278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 141. | Sakane S, Hikita H, Shirai K, Sakamoto T, Narumi R, Adachi J, Kakita N, Yamada Y, Toyoda H, Takahashi H, Suda G, Kai M, Tahata Y, Sakamori R, Kumazaki S, Fukumoto K, Myojin Y, Murai K, Kodama T, Tatsumi T, Tomonaga T, Sakamoto N, Morii E, Takehara T. Proteomic analysis of serum extracellular vesicles reveals Fibulin-3 as a new marker predicting liver-related events in MASLD. Hepatol Commun. 2024;8:e0448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (1)] |
| 142. | Zhang W, Zhang J, Shi H, Liu F, Yu H, Shi H. Exosome GLUT1 derived from hepatocyte identifies the risk of non-alcoholic steatohepatitis and fibrosis. Hepatol Int. 2023;17:1170-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 143. | Newman LA, Sorich MJ, Rowland A. Role of Extracellular Vesicles in the Pathophysiology, Diagnosis and Tracking of Non-Alcoholic Fatty Liver Disease. J Clin Med. 2020;9:2032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 144. | Hu J, Xu Y, Hao J, Wang S, Li C, Meng S. MiR-122 in hepatic function and liver diseases. Protein Cell. 2012;3:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 145. | Povero D, Eguchi A, Li H, Johnson CD, Papouchado BG, Wree A, Messer K, Feldstein AE. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS One. 2014;9:e113651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 226] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 146. | Newman LA, Useckaite Z, Johnson J, Sorich MJ, Hopkins AM, Rowland A. Selective Isolation of Liver-Derived Extracellular Vesicles Redefines Performance of miRNA Biomarkers for Non-Alcoholic Fatty Liver Disease. Biomedicines. 2022;10:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 147. | What will it take to get miRNA therapies to market? Nat Biotechnol. 2024;42:1623-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 148. | Diener C, Keller A, Meese E. Emerging concepts of miRNA therapeutics: from cells to clinic. Trends Genet. 2022;38:613-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 666] [Article Influence: 166.5] [Reference Citation Analysis (0)] |
| 149. | Guo CJ, Xiao X, Sheng L, Chen L, Zhong W, Li H, Hua J, Ma X. RNA Sequencing and Bioinformatics Analysis Implicate the Regulatory Role of a Long Noncoding RNA-mRNA Network in Hepatic Stellate Cell Activation. Cell Physiol Biochem. 2017;42:2030-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 150. | Yuan X, Wang J, Tang X, Li Y, Xia P, Gao X. Berberine ameliorates nonalcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles. J Transl Med. 2015;13:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 151. | Sun C, Liu X, Yi Z, Xiao X, Yang M, Hu G, Liu H, Liao L, Huang F. Genome-wide analysis of long noncoding RNA expression profiles in patients with non-alcoholic fatty liver disease. IUBMB Life. 2015;67:847-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 152. | Zeng Q, Liu CH, Wu D, Jiang W, Zhang N, Tang H. LncRNA and circRNA in Patients with Non-Alcoholic Fatty Liver Disease: A Systematic Review. Biomolecules. 2023;13:560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 153. | Shi N, Sun K, Tang H, Mao J. The impact and role of identified long noncoding RNAs in nonalcoholic fatty liver disease: A narrative review. J Clin Lab Anal. 2023;37:e24943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 154. | Jin SS, Lin CJ, Lin XF, Zheng JZ, Guan HQ. Silencing lncRNA NEAT1 reduces nonalcoholic fatty liver fat deposition by regulating the miR-139-5p/c-Jun/SREBP-1c pathway. Ann Hepatol. 2022;27:100584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 155. | Guo B, Cheng Y, Yao L, Zhang J, Lu J, Qi H, Chen H. LncRNA HOTAIR regulates the lipid accumulation in non-alcoholic fatty liver disease via miR-130b-3p/ROCK1 axis. Cell Signal. 2022;90:110190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 156. | Liu J, Tang T, Wang GD, Liu B. LncRNA-H19 promotes hepatic lipogenesis by directly regulating miR-130a/PPARγ axis in non-alcoholic fatty liver disease. Biosci Rep. 2019;39:BSR20181722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 157. | Ma M, Duan R, Shen L, Liu M, Ji Y, Zhou H, Li C, Liang T, Li X, Guo L. The lncRNA Gm15622 stimulates SREBP-1c expression and hepatic lipid accumulation by sponging the miR-742-3p in mice. J Lipid Res. 2020;61:1052-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 158. | Yan C, Chen J, Chen N. Long noncoding RNA MALAT1 promotes hepatic steatosis and insulin resistance by increasing nuclear SREBP-1c protein stability. Sci Rep. 2016;6:22640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 159. | Zou D, Liu L, Zeng Y, Wang H, Dai D, Xu M. LncRNA MEG3 up-regulates SIRT6 by ubiquitinating EZH2 and alleviates nonalcoholic fatty liver disease. Cell Death Discov. 2022;8:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 160. | Van Dorpe S, Tummers P, Denys H, Hendrix A. Towards the Clinical Implementation of Extracellular Vesicle-Based Biomarker Assays for Cancer. Clin Chem. 2024;70:165-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 161. | González Á, López-Borrego S, Sandúa A, Vales-Gomez M, Alegre E. Extracellular vesicles in cancer: challenges and opportunities for clinical laboratories. Crit Rev Clin Lab Sci. 2024;61:435-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 162. | Cusi K, Chang Z, Harrison S, Lomonaco R, Bril F, Orsak B, Ortiz-Lopez C, Hecht J, Feldstein AE, Webb A, Louden C, Goros M, Tio F. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;60:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 163. | Gurjar S, Bhat A R, Upadhya R, Shenoy RP. Extracellular vesicle-mediated approaches for the diagnosis and therapy of MASLD: current advances and future prospective. Lipids Health Dis. 2025;24:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 164. | Ciardullo S, Muraca E, Vergani M, Invernizzi P, Perseghin G. Advancements in pharmacological treatment of NAFLD/MASLD: a focus on metabolic and liver-targeted interventions. Gastroenterol Rep (Oxf). 2024;12:goae029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 165. | van Erpecum KJ, Dalekos GN. New horizons in the diagnosis and management of patients with MASLD. Eur J Intern Med. 2024;122:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 166. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Obes Facts. 2024;17:374-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 139] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 167. | Alkhouri N, Gawrieh S. A perspective on RNA interference-based therapeutics for metabolic liver diseases. Expert Opin Investig Drugs. 2021;30:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 168. | Kumar MA, Baba SK, Sadida HQ, Marzooqi SA, Jerobin J, Altemani FH, Algehainy N, Alanazi MA, Abou-Samra AB, Kumar R, Al-Shabeeb Akil AS, Macha MA, Mir R, Bhat AA. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct Target Ther. 2024;9:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 441] [Cited by in RCA: 673] [Article Influence: 336.5] [Reference Citation Analysis (0)] |
| 169. | Kou M, Huang L, Yang J, Chiang Z, Chen S, Liu J, Guo L, Zhang X, Zhou X, Xu X, Yan X, Wang Y, Zhang J, Xu A, Tse HF, Lian Q. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: a next generation therapeutic tool? Cell Death Dis. 2022;13:580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 390] [Article Influence: 97.5] [Reference Citation Analysis (10)] |
| 170. | Kunitake K, Mizuno T, Hattori K, Oneyama C, Kamiya M, Ota S, Urano Y, Kojima R. Barcoding of small extracellular vesicles with CRISPR-gRNA enables comprehensive, subpopulation-specific analysis of their biogenesis and release regulators. Nat Commun. 2024;15:9777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 171. | Liu S, Wu X, Chandra S, Lyon C, Ning B, Jiang L, Fan J, Hu TY. Extracellular vesicles: Emerging tools as therapeutic agent carriers. Acta Pharm Sin B. 2022;12:3822-3842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 172. | Jia X, Tang J, Yao C, Yang D. Recent Progress of Extracellular Vesicle Engineering. ACS Biomater Sci Eng. 2021;7:4430-4438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 173. | Leclaire M, Gimzewski J, Sharma S. A review of the biomechanical properties of single extracellular vesicles. Nano Select. 2021;2:1-15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 174. | Jain KK. Drug delivery systems - an overview. Methods Mol Biol. 2008;437:1-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 175. | Wang J, Xing K, Zhang G, Li Z, Ding X, Leong DT. Surface Components and Biological Interactions of Extracellular Vesicles. ACS Nano. 2025;19:8433-8461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 176. | Pinheiro A, Silva AM, Teixeira JH, Gonçalves RM, Almeida MI, Barbosa MA, Santos SG. Extracellular vesicles: intelligent delivery strategies for therapeutic applications. J Control Release. 2018;289:56-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 177. | Li X, Chen R, Kemper S, Xu Z, Brigstock DR. Therapeutic Actions of Hepatocyte Extracellular Vesicles in a Murine Model of Diet-Induced Steatohepatitis with Fibrosis. Biomedicines. 2025;13:274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 178. | Wang J, Wu Z, Xia M, Salas SS, Ospina JA, Buist-Homan M, Harmsen MC, Moshage H. Extracellular vesicles derived from liver sinusoidal endothelial cells inhibit the activation of hepatic stellate cells and Kupffer cells in vitro. Biochim Biophys Acta Mol Basis Dis. 2024;1870:167020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 179. | Jiang W, Zeng Q, Liu CH, Wang Y, Wang S, Chen E, Wang M, Zhou T, Bai L, Wu D, Tang H. Huc-MSCs-derived exosomes alleviate non-alcoholic steatohepatitis by regulating macrophages polarization through miR-24-3p/STING axis. Stem Cell Res Ther. 2025;16:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 180. | Zhang B, Zhang B, Lai RC, Sim WK, Lam KP, Lim SK. MSC-sEV Treatment Polarizes Pro-Fibrotic M2 Macrophages without Exacerbating Liver Fibrosis in NASH. Int J Mol Sci. 2023;24:8092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (1)] |
| 181. | He F, Du W, Liu Y, Ling Y, Xu M, Liu J, Song P, Fang Z, Yue Z, Duan J, Wang L. Exosome-equipped TNF antisense oligodeoxynucleotide or 2-deoxy-D-glucose ameliorated nonalcoholic steatohepatitis by modulating superoxide dismutase 1 in mice. Redox Biol. 2025;80:103488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 182. | Chen Y, Yang F, Wang Y, Shi Y, Liu L, Luo W, Zhou J, Yan Y. Mesenchymal stem cell-derived small extracellular vesicles reduced hepatic lipid accumulation in MASLD by suppressing mitochondrial fission. Stem Cell Res Ther. 2025;16:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 183. | Yang F, Wu Y, Chen Y, Xi J, Chu Y, Jin J, Yan Y. Human umbilical cord mesenchymal stem cell-derived exosomes ameliorate liver steatosis by promoting fatty acid oxidation and reducing fatty acid synthesis. JHEP Rep. 2023;5:100746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 184. | Tawfeek GA, Kasem HA. Curcumin preconditioned mesenchymal stem cells derived exosomes transplantation ameliorate and protect against non- alcoholic steatohepatitis by regulation the expression of key genes of inflammation and oxidative stress. Transpl Immunol. 2023;78:101837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 185. | Nie Y, Meng W, Liu D, Yang Z, Wang W, Ren H, Mao K, Lan W, Li C, Wang Z, Lan J. Exosomes derived from apical papilla stem cells improve NASH by regulating fatty acid metabolism and reducing inflammation. Mol Med. 2024;30:186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 186. | Nie YF, Shang JM, Liu DQ, Meng WQ, Ren HP, Li CH, Wang ZF, Lan J. Apical papilla stem cell-derived exosomes regulate lipid metabolism and alleviate inflammation in the MCD-induced mouse NASH model. Biochem Pharmacol. 2024;222:116073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 187. | Saha T, Mehrotra S, Gupta P, Kumar A. Exosomal miRNA combined with anti-inflammatory hyaluronic acid-based 3D bioprinted hepatic patch promotes metabolic reprogramming in NAFLD-mediated fibrosis. Biomaterials. 2025;318:123140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 188. | Clos-Sansalvador M, Monguió-Tortajada M, Roura S, Franquesa M, Borràs FE. Commonly used methods for extracellular vesicles' enrichment: Implications in downstream analyses and use. Eur J Cell Biol. 2022;101:151227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 189. | Simonsen JB. What Are We Looking At? Circ Res. 2017;121:920-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 260] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 190. | Dilsiz N. A comprehensive review on recent advances in exosome isolation and characterization: Toward clinical applications. Transl Oncol. 2024;50:102121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 132] [Reference Citation Analysis (1)] |
| 191. | Mladenović D, Brealey J, Peacock B, Koort K, Zarovni N. Quantitative fluorescent nanoparticle tracking analysis and nano-flow cytometry enable advanced characterization of single extracellular vesicles. J Extracell Biol. 2025;4:e70031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 192. | Leggio L, Paternò G, Vivarelli S, Bonasera A, Pignataro B, Iraci N, Arrabito G. Label-free approaches for extracellular vesicle detection. iScience. 2023;26:108105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 193. | Wu AY, Sung YC, Chen YJ, Chou ST, Guo V, Chien JC, Ko JJ, Yang AL, Huang HC, Chuang JC, Wu S, Ho MR, Ericsson M, Lin WW, Cheung CHY, Juan HF, Ueda K, Chen Y, Lai CP. Multiresolution Imaging Using Bioluminescence Resonance Energy Transfer Identifies Distinct Biodistribution Profiles of Extracellular Vesicles and Exomeres with Redirected Tropism. Adv Sci (Weinh). 2020;7:2001467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 194. | Rodrigues G, Hoshino A, Kenific CM, Matei IR, Steiner L, Freitas D, Kim HS, Oxley PR, Scandariato I, Casanova-Salas I, Dai J, Badwe CR, Gril B, Tešić Mark M, Dill BD, Molina H, Zhang H, Benito-Martin A, Bojmar L, Ararso Y, Offer K, LaPlant Q, Buehring W, Wang H, Jiang X, Lu TM, Liu Y, Sabari JK, Shin SJ, Narula N, Ginter PS, Rajasekhar VK, Healey JH, Meylan E, Costa-Silva B, Wang SE, Rafii S, Altorki NK, Rudin CM, Jones DR, Steeg PS, Peinado H, Ghajar CM, Bromberg J, de Sousa M, Pisapia D, Lyden D. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat Cell Biol. 2019;21:1403-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 333] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 195. | Hyenne V, Ghoroghi S, Collot M, Bons J, Follain G, Harlepp S, Mary B, Bauer J, Mercier L, Busnelli I, Lefebvre O, Fekonja N, Garcia-Leon MJ, Machado P, Delalande F, López AA, Silva SG, Verweij FJ, van Niel G, Djouad F, Peinado H, Carapito C, Klymchenko AS, Goetz JG. Studying the Fate of Tumor Extracellular Vesicles at High Spatiotemporal Resolution Using the Zebrafish Embryo. Dev Cell. 2019;48:554-572.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 180] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 196. | Lucotti S, Kenific CM, Zhang H, Lyden D. Extracellular vesicles and particles impact the systemic landscape of cancer. EMBO J. 2022;41:e109288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 197. | Rodriguez BV, Wen Y, Shirk EN, Vazquez S, Gololobova O, Maxwell A, Plunkard J, Castell N, Carlson B, Queen SE, Izzi JM, Driedonks TAP, Witwer KW. An ex vivo model of interactions between extracellular vesicles and peripheral mononuclear blood cells in whole blood. J Extracell Vesicles. 2023;12:e12368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 198. | Erratum for Pharmacokinetics and biodistribution of extracellular vesicles administered intravenously and intranasally to Macaca nemestrina. J Extracell Biol. 2022;1:e67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 199. | Ridder K, Sevko A, Heide J, Dams M, Rupp AK, Macas J, Starmann J, Tjwa M, Plate KH, Sültmann H, Altevogt P, Umansky V, Momma S. Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. Oncoimmunology. 2015;4:e1008371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 200. | Skotland T, Iversen TG, Llorente A, Sandvig K. Biodistribution, pharmacokinetics and excretion studies of intravenously injected nanoparticles and extracellular vesicles: Possibilities and challenges. Adv Drug Deliv Rev. 2022;186:114326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 201. | Burbidge K, Zwikelmaier V, Cook B, Long MM, Balva B, Lonigro M, Ispas G, Rademacher DJ, Campbell EM. Cargo and cell-specific differences in extracellular vesicle populations identified by multiplexed immunofluorescent analysis. J Extracell Vesicles. 2020;9:1789326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 202. | Han C, Kang H, Yi J, Kang M, Lee H, Kwon Y, Jung J, Lee J, Park J. Single-vesicle imaging and co-localization analysis for tetraspanin profiling of individual extracellular vesicles. J Extracell Vesicles. 2021;10:e12047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 203. | Karczewski KJ, Snyder MP. Integrative omics for health and disease. Nat Rev Genet. 2018;19:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 720] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 204. | Shaba E, Vantaggiato L, Governini L, Haxhiu A, Sebastiani G, Fignani D, Grieco GE, Bergantini L, Bini L, Landi C. Multi-Omics Integrative Approach of Extracellular Vesicles: A Future Challenging Milestone. Proteomes. 2022;10:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 205. | Park S, Gwon Y, Khan SA, Jang KJ, Kim J. Engineering considerations of iPSC-based personalized medicine. Biomater Res. 2023;27:67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 206. | Küstermann C, Narbute K, Movčana V, Parfejevs V, Rūmnieks F, Kauķis P, Priedols M, Mikilps-Mikgelbs R, Mihailova M, Andersone S, Dzalbs A, Bajo-Santos C, Krams A, Abols A. iPSC-derived lung and lung cancer organoid model to evaluate cisplatin encapsulated autologous iPSC-derived mesenchymal stromal cell-isolated extracellular vesicles. Stem Cell Res Ther. 2024;15:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 207. | Ohara M, Ohnishi S, Hosono H, Yamamoto K, Yuyama K, Nakamura H, Fu Q, Maehara O, Suda G, Sakamoto N. Extracellular Vesicles from Amnion-Derived Mesenchymal Stem Cells Ameliorate Hepatic Inflammation and Fibrosis in Rats. Stem Cells Int. 2018;2018:3212643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 208. | Gopal A, Gangadaran P, Rajendran RL, Oh JM, Lee HW, Hong CM, Kalimuthu S, Han MH, Lee J, Ahn BC. Extracellular vesicle mimetics engineered from mesenchymal stem cells and curcumin promote fibrosis regression in a mouse model of thioacetamide-induced liver fibrosis. Regen Ther. 2024;26:911-921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 209. | Gupta S, Pinky, Vishal, Sharma H, Soni N, Rao EP, Dalela M, Yadav A, Nautiyal N, Kumar A, Nayak B, Banerjee A, Dinda AK, Mohanty S. Comparative Evaluation of Anti-Fibrotic Effect of Tissue Specific Mesenchymal Stem Cells Derived Extracellular Vesicles for the Amelioration of CCl4 Induced Chronic Liver Injury. Stem Cell Rev Rep. 2022;18:1097-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 210. | Povero D, Pinatel EM, Leszczynska A, Goyal NP, Nishio T, Kim J, Kneiber D, de Araujo Horcel L, Eguchi A, Ordonez PM, Kisseleva T, Feldstein AE. Human induced pluripotent stem cell-derived extracellular vesicles reduce hepatic stellate cell activation and liver fibrosis. JCI Insight. 2019;5:e125652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/