Published online Sep 27, 2025. doi: 10.4254/wjh.v17.i9.108144
Revised: June 13, 2025
Accepted: August 4, 2025
Published online: September 27, 2025
Processing time: 172 Days and 17.5 Hours
Metabolic endoscopy represents a promising alternative in the management of steatotic liver disease, particularly metabolic dysfunction-associated steatohepatitis (MASH), a progressive form of metabolic dysfunction-associated steatotic liver disease (MASLD). With the rising global prevalence of MASLD—affecting over one-third of the adult population—and its close association with obesity, insulin resistance, and metabolic syndrome, there is an urgent need for inno
Core Tip: This review highlights the promise of metabolic endoscopy as a minimally invasive strategy for reversing liver fibrosis in patients with metabolic dysfunction-associated steatohepatitis. It evaluates endoscopic bariatric metabolic therapies including endoscopic sleeve gastroplasty (ESG), intragastric balloon, duodenal mucosal resurfacing (DMR), and duodeno-jejunal bypass liners, along with revisional procedures such as endoscopic revisional gastroplasty (ERG) and transoral outlet reduction (TORe). Evidence indicates that ESG yields the greatest fibrosis reduction, while DMR shows minimal benefit and is not recommended. Among revisional metabolic endoscopic procedures, ERG is effective for fibrosis reduction, whereas TORe requires further evaluation.
- Citation: Sierra L, Chatterjee A, Prado R, Khurana A, Patel R, Firkins S, Simons-Linares R. Impact of metabolic endoscopy on fibrosis regression in steatotic liver disease. World J Hepatol 2025; 17(9): 108144
- URL: https://www.wjgnet.com/1948-5182/full/v17/i9/108144.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i9.108144
Steatotic liver disease per recent terminology includes metabolic dysfunction-associated steatohepatitis (MASH), previously known as non-alcoholic steatohepatitis, which is a progressive form of metabolic dysfunction-associated stea
MASLD affects approximately 38% of the global adult population and around 31.3% of adults in the United States[3] Among individuals with MASLD, 20% have type 2 diabetes mellitus, nearly 70% have hyperlipidemia (including ath
MASH can lead to liver fibrosis, cirrhosis, and an increased risk of hepatocellular carcinoma[4]. Despite the rising prevalence, there is currently only one FDA-approved pharmacological treatment for MASH advanced-stage fibrosis (i.e. resmetirom), and as such, weight loss through lifestyle interventions remains the cornerstone of management[1]. How
Bariatric surgery has demonstrated efficacy in improving MASH, but its invasive nature and limited access or insu
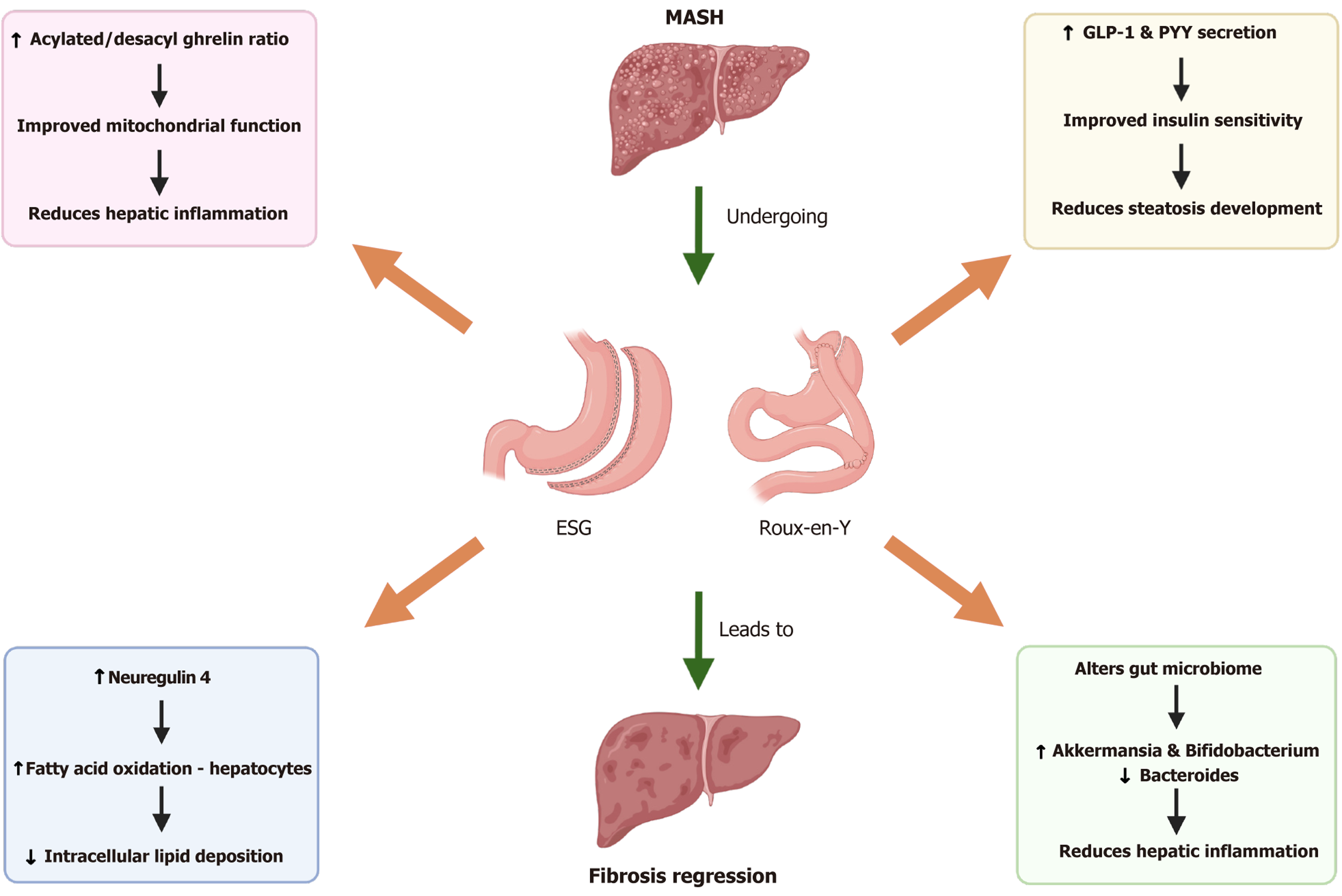
Metabolic endoscopy and surgery, including ESG and Roux-en-Y gastric bypass (RYGB) among others, plays a pivotal role in reducing hepatic inflammation, mitochondrial dysfunction, and endoplasmic reticulum (ER) stress, all of which are key drivers of MASH[5,6]. This reduction in hepatic inflammation is partially mediated by post-surgical changes in ghrelin isoforms, particularly an increase in the acylated/desacyl ghrelin ratio, which has been shown to mitigate liver inflammation and enhance mitochondrial function (Figure 1)[6,7]. The improved mitochondrial function decreases oxidative stress and prevents hepatocyte injury, contributing to the histologic regression of MASH. Alongside these benefits, a significant reduction in hepatic ER stress allows for improved protein folding and reduced accumulation of misfolded proteins, further alleviating hepatocyte dysfunction and enhancing liver health[7,8].
Beyond its effects on inflammation, bariatric surgery induces profound lipid metabolic reprogramming that contributes to MASH regression[9]. A key factor in this process is the increased circulating levels of neuregulin 4, which activates ErbB4 signaling and enhances fatty acid oxidation within hepatocytes[9]. This shift in lipid metabolism leads to decreased intracellular lipid accumulation and a marked reduction in hepatic steatosis. Additionally, bariatric surgery enhances bile acid metabolism, promoting pathways that favor lipid clearance and decrease lipotoxicity (Figure 1)[9,10].
Hormonal changes also play a crucial role in this metabolic shift, as post-surgical increases in insulin-sensitizing hor
Bariatric surgery significantly reshapes the gut microbiome, impacting systemic metabolism and liver health. Beneficial bacteria such as Akkermansia and Bifidobacterium proliferate post-surgery, promoting anti-inflammatory pathways and strengthening the gut barrier, thereby reducing the influx of gut-derived endotoxins that contribute to hepatic inflammation and insulin resistance[10,11]. Simultaneously, harmful bacteria such as Bacteroides, which are associated with increased inflammatory cytokine production, are diminished[11,12]. This shift in microbiota composition is accompanied by enhanced production of short-chain fatty acids, which improve insulin sensitivity through modulation of gut hormone secretion, particularly by increasing GLP-1 Levels[9,11]. The interplay between gut microbiota, inflammation, and metabolic health highlights the multifactorial benefits of bariatric surgery in reversing the pathophysiology of MASH.
EBMTs have emerged as promising minimally invasive interventions to indirectly manage MASLD and MASH, po
ESG is a non-surgical endoscopic procedure whereby introducing an endoscope through the mouth of the patients, the gastric lumen is invaginated luminally and suture using full-thickness endoscopic suturing to tubularize the stomach akin to a surgical sleeve gastrectomy[10]. ESG also delays gastric emptying[13], and impact gut hormones in the path
In a prospective study, Nixdorf et al[16] observed that 93 patients with obesity and MASLD/MASH achieved a sig
IGBs function as both space-occupying and anti-motility devices to induce satiety, delay gastric emptying, and promote weight loss, remaining in place for up to 6 months. IGB therapy has also shown promising results for fibrosis regression in MASH[18]. Salomone et al[18] reported that 6-month IGB placement in 26 patients with obesity and MASH (liver stiffness ≥ 9.7 kPa) led to a significant decrease in fibrosis-4 index (FIB-4) score (from 3.2 ± 0.7 to 2.7 ± 0.8) and a reduction in liver stiffness from 13.3 ± 3.2 to 11.3 ± 2.8 kPa via VCTE (P < 0.001)[18].
Interestingly, the mentioned study showed a significant improvement in degree of steatosis. As the controlled att
DMR is a novel technique whereby the metabolically-active duodenal mucosa is ablated via different endoscopic methods (i.e. thermal, electro-pulse, or radiofrequency, steam-therapy), resulting in improvement in metabolic parameters such as hemoglobin A1c, albeit with less effect on body weight compared to other EBMTs[5]. As such, DMR has exhibited only modest effects on hepatic fibrosis. Chuang et al[19] in a meta-analysis of two studies (n = 67) observed a non-significant trend toward liver fat reduction [magnetic resonance imaging proton density fat fraction (MRI-PDFF) decreased by –2.22, 95%CI: –12.79-8.34] at 12 weeks[19]. Similarly, Mingrone et al[20] in the REVITA-2 trial demonstrated that DMR in T2D patients reduced liver fat by –5.4% (P = 0.035) at 12 weeks[20]. To date, there is no study proving MASH fibrosis re
Unlike DMR, which physically alters the absorptive and metabolically-active small intestinal mucosa, DJBL are synthetic barriers that are endoscopically placed in the proximal small bowel[5]. This procedure aims to simulate the temporary effects of a gastric bypass anatomy. This prevents both absorption of nutrients as well as biochemical triggering of en
We focused on the most common, well-established endoscopic metabolic techniques and their effects on liver stiffness in MASH. We acknowledge that experimental therapies have shown potential to reverse MASH or remain untested[23-25]; however, due to their experimental nature, they were not included in this review. All the studies had a follow-up between 6 months and 2 years.
For patients experiencing weight regain or insufficient weight loss after initial EBMTs (e.g., IGB, ESG, or DJBL) and bariatric surgery, several revisional procedures can promote weight loss and potentially reduce liver fibrosis.
One popular approach is endoscopic revisional gastroplasty (ERG), which uses endoscopic suturing to reduce the size of the gastric pouch or stoma (mainly post-LSG), thereby enhancing weight loss and metabolic outcomes[26].
Another widely used, safe and effective procedure, transoral outlet reduction (TORe), decreases the size of a dilated and incompetent gastrojejunal anastomosis[21,23]. Although various revisional options exist, this review focuses on the two most used and well-established techniques: ERG and TORe.
Recent studies show that revisional procedures can also reduce hepatic fibrosis. In one retrospective study, endoscopic gastric plication significantly improved non-invasive fibrosis markers—NFS decreased from 0.48 to –1.18, FIB-4 declined from 1.4 to 1.2, and liver stiffness reduced from 13.9 to 8.9 kPa (all P < 0.001), with 63% of patients achieving ≥ 10% total weight loss[27].
Similarly, TORe in post RYGB patients with weight regain and MASLD led to significant improvements in FIB-4, with a trend toward reduced liver stiffness[26]. It also produced an average TBWL of 8.8% at 12 months, along with enhanced glycemic control[28-30]. For TORe, however, there are no dedicated studies that has focused on liver stiffness reduction after procedure (Table 1).
| Ref. | Study design | Population | Intervention | Key findings |
| Abad et al[12], 2024 | Multicenter, randomized, clinical trial | 40 patients with MASH | ESG with lifestyle modification vs sham endoscopy with lifestyle intervention | The ESG group achieved significantly greater total body weight loss (9.47% vs 3.91%) and a greater reduction in liver stiffness (5.63 vs 0.2 kPa, both P < 0.05) |
| Hajifathalian et al[15], 2021 | Prospective, single-arm interventional study | 118 patients with obesity and MASLD or MASH | ESG | ESG reduced hepatic fibrosis risk in 20% of patients, shifting VCTE elastography readings from F3–F4 to F0–F2, while only 1% worsened (P = 0.02) |
| Nunes et al[28], 2023 | Systematic review and meta-analysis | 175 patients with obesity and MASLD | Four studies: ESG | Sgnificantly reduced hepatic steatosis index (–4.85, 95%CI –6.02 to –3.67), NFS (–0.5, 95%CI –0.80 to –0.19), TWL (–17.28%, 95%CI –18.24 to –16.31) |
| Jirapinyo et al[29], 2022 | Systematic review and meta-analysis | Study sample sizes ranged from 21 to 29 patients with obesity and MASLD | One study ESG; One study IGB | EBMTs significantly reduced liver fibrosis (SMD 0.7, 95%CI: 0.1–1.3, P = 0.02), hepatic steatosis (SMD –1.0, 95%CI: –1.2 to –0.8, P < 0.0001) and NAS (–2.50, 95%CI: –3.5 to –1.5, P < 0.0001) |
| Salomone et al[18], 2021 | Retrospective study | 26 patients with obesity and MASH (liver stiffness ≥ 9.7 kPa at baseline) | IGB | FIB-4 decreased from 3.2 ± 0.7 to 2.7 ± 0.8, liver stiffness from 13.3 ± 3.2 to 11.3 ± 2.8 kPa, and CAP from 355 (298–400) to 296 (255–352) dB/m (all P < 0.01); no severe adverse events |
| Ren et al[30], 2022 | Systematic review and meta-analysis | Study sample sizes ranged from 21 to 91 patients with obesity and MASLD or MASH | Two studies: IGB; One study DJBL | NFS decreased by –0.58 (95%CI –0.97 to –0.20), APRI lowered by 0.73 (P = 0.005) and MRE stiffness by 0.3 kPa (P = 0.03); VCTE elastography (–6.39 kPa) and FIB-4 (–0.28) changes were non-significant |
| Roehlen et al[21], 2022 | Prospective interventional study | 71 patients with T2DM, obesity and MASLD | DJBL | Significantly reduced fatty liver index (from 98.22 to 93.38, P < 0.001), NFS (from 0.19 to –0.83, P < 0.001), and APRI (from 0.36 to 0.26, P < 0.0001) |
| Karlas et al[22], 2022 | Retrospective study | 32 T2D patients with obesity undergoing DJBL | DJBL | Reduced FAST score by 0.21 (95%CI: –0.28 to –0.13, P < 0.001); fibrosis markers (LSM, NFS, ELF) were unchanged, with slight FIB4 improvement; device-related complications were noted |
| Chuang et al[19], 2023 | Systematic review and meta-analysis | 67 patients with obesity and MASLD or MASH (biopsy-proven or MRI-PDFF > 5%) | Two studies: DMR | Trend toward reduced liver fat (MRI-PDFF decreased by –2.22, 95%), though results were not statistically significant (P > 0.05) |
| Jirapinyo et al[17], 2024 | Retrospective study from a prospectively collected registry | 30 patients with obesity and MASLD | 12 patients received EGR monotherapy, 18 patients received EGR+GLP-1RA | Combination therapy led to significantly greater fibrosis improvement: NFS decreased by 181% ± 182% vs 30% ± 83% (P = 0.04), and liver stiffness reduced by 54% ± 12% vs 14% ± 45% (P = 0.05) |
Our review details commonly used endoscopic bariatric metabolic procedures (ESG, IGB, DJBL, and DMR) and revisional therapies (ERG and TORe) using non-invasive tests (VCTE, FIB-4, CAP score, and MRI-PDFF) to assess liver fibrosis and fat deposition in MASLD/MASH.
Among primary interventions, ESG provides the greatest reduction in liver fibrosis, while IGB and DJBL yield improvements to a lesser extent. DMR shows no significant effect.
Among revisional therapies, ERG is the only intervention that has demonstrated fibrosis reduction, as evidenced by improvements in VCTE and FIB-4 scores. However, the potential benefits of TORe in reducing liver fibrosis still need further evaluation.
Although individual studies suggest ESG may be superior, no direct comparative studies exist; thus, causal causality cannot be drawn from this review. Further research is needed to define the roles of both primary and revisional endoscopic metabolic therapies (with particular attention to TORe) in reducing liver fibrosis and preventing cirrhosis.
Despite the promising results outlined above, several limitations and sources of heterogeneity must be acknowledged. First, sample sizes varied widely across studies, from as few as 26 participants in certain IGB trials[18] to over 118 in some ESG cohorts[15], limiting the statistical power and generalizability of individual findings. Second, follow-up durations ranged from as short as 3 months (e.g., VCTE reassessment after ESG[16]) to up to 2 years in select meta-analyses[28], making it difficult to compare the durability of fibrosis regression or steatosis improvement across interventions. Third, endpoint definitions were inconsistent: Some studies used VCTE stiffness thresholds (e.g., shifting from F3–F4 to F0–F2[15]), while others relied on noninvasive scores such as FIB-4, NFS, or MRI-PDFF to quantify steatosis and fibrosis changes[18,19,21]. Differences in cutoff values and measurement modalities introduce variability in reported effect sizes. Fourth, patient selection criteria differed: Cohorts ranged from individuals with biopsy-proven MASH to those defined by elastography or serum biomarkers alone, thereby encompassing a spectrum of disease severity and comorbidities (e.g., type 2 diabetes, hypertension, or morbid obesity). Finally, procedural heterogeneity, such as variations in ESG suture patterns, types of IGB, or duration of DJBL implantation, complicates direct comparisons.
| 1. | Ganguly S, Rosenthal SB, Ishizuka K, Troutman TD, Rohm TV, Khader N, Aleman-Muench G, Sano Y, Archilei S, Soroosh P, Olefsky JM, Feldstein AE, Kisseleva T, Loomba R, Glass CK, Brenner DA, Dhar D. Lipid-associated macrophages' promotion of fibrosis resolution during MASH regression requires TREM2. Proc Natl Acad Sci U S A. 2024;121:e2405746121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 2. | Alsaqa M, Sierra L, Marenco-Flores A, Parraga X, Barba R, Goyes D, Ozturk NB, Curry MP, Bonder A, Saberi B. Metabolic dysfunction-associated steatotic liver disease correlates with higher lower graft survival in liver transplant recipients with hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2025;37:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Manolis AA, Manolis TA, Vouliotis A, Manolis AS. Metabolic dysfunction-associated steatotic liver disease and the cardiovascular system. Trends Cardiovasc Med. 2025;35:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Polpichai N, Saowapa S, Jaroenlapnopparat A, Sierra L, Danpanichkul P, Fangsaard P, Wattanachayakul P, Kaewdech A. Statin Use in Metabolic Dysfunction-Associated Steatotic Liver Disease and Effects on Vibration-Controlled Transient Elastography-Derived Scores—A Population-Based Inverse Probability Treatment Weighting Analysis. Livers. 2024;4:677-687. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Aitharaju V, Ragheb J, Firkins S, Patel R, Simons-Linares CR. Endoscopic bariatric and metabolic therapies and its effect on metabolic dysfunction-associated steatotic liver disease: a review of the current literature. Surg Obes Relat Dis. 2025;21:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Ezquerro S, Becerril S, Tuero C, Méndez-Giménez L, Mocha F, Moncada R, Valentí V, Cienfuegos JA, Catalán V, Gómez-Ambrosi J, Piper Hanley K, Frühbeck G, Rodríguez A. Role of ghrelin isoforms in the mitigation of hepatic inflammation, mitochondrial dysfunction, and endoplasmic reticulum stress after bariatric surgery in rats. Int J Obes (Lond). 2020;44:475-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Pournaras DJ, le Roux CW. Ghrelin and metabolic surgery. Int J Pept. 2010;2010:217267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Pedersen JS, Rygg MO, Chrøis K, Sustarsic EG, Gerhart-Hines Z, Wever Albrechtsen NJ, Serizawa RR, Kristiansen VB, Basse AL, Boilesen AEB, Olsen BH, Hansen T, Gluud LL, Madsbad S, Larsen S, Bendtsen F, Dela F. Influence of NAFLD and bariatric surgery on hepatic and adipose tissue mitochondrial biogenesis and respiration. Nat Commun. 2022;13:2931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Angelini G, Castagneto Gissey L, Del Corpo G, Giordano C, Cerbelli B, Severino A, Manco M, Basso N, Birkenfeld AL, Bornstein SR, Genco A, Mingrone G, Casella G. New insight into the mechanisms of ectopic fat deposition improvement after bariatric surgery. Sci Rep. 2019;9:17315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Wang M, Li L, Chen Y, Lian G, Wang J, Zhang J, Shan K, Shang L, Tian F, Jing C. Role of Gut Microbiome and Microbial Metabolites in Alleviating Insulin Resistance After Bariatric Surgery. Obes Surg. 2021;31:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Münzker J, Haase N, Till A, Sucher R, Haange SB, Nemetschke L, Gnad T, Jäger E, Chen J, Riede SJ, Chakaroun R, Massier L, Kovacs P, Ost M, Rolle-Kampczyk U, Jehmlich N, Weiner J, Heiker JT, Klöting N, Seeger G, Morawski M, Keitel V, Pfeifer A, von Bergen M, Heeren J, Krügel U, Fenske WK. Functional changes of the gastric bypass microbiota reactivate thermogenic adipose tissue and systemic glucose control via intestinal FXR-TGR5 crosstalk in diet-induced obesity. Microbiome. 2022;10:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 12. | Abad J, Llop E, Arias-Loste MT, Burgos-Santamaría D, Martínez Porras JL, Iruzubieta P, Graus J, Ruiz-Antorán B, Sánchez Yuste MR, Romero-Gómez M, Albillos A, Crespo J, Calleja JL. Endoscopic Sleeve Gastroplasty Plus Lifestyle Intervention in Patients With Metabolic Dysfunction-associated Steatohepatitis: A Multicentre, Sham-controlled, Randomized Trial. Clin Gastroenterol Hepatol. 2024;S1542-3565(24)01080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Camilleri M, Brown ML, Malagelada JR. Relationship between impaired gastric emptying and abnormal gastrointestinal motility. Gastroenterology. 1986;91:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 180] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Lopez-Nava G, Negi A, Bautista-Castaño I, Rubio MA, Asokkumar R. Gut and Metabolic Hormones Changes After Endoscopic Sleeve Gastroplasty (ESG) Vs. Laparoscopic Sleeve Gastrectomy (LSG). Obes Surg. 2020;30:2642-2651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Hajifathalian K, Mehta A, Ang B, Skaf D, Shah SL, Saumoy M, Dawod Q, Dawod E, Shukla A, Aronne L, Brown RS, Cohen DE, Dannenberg AJ, Fortune B, Kumar S, Sharaiha RZ. Improvement in insulin resistance and estimated hepatic steatosis and fibrosis after endoscopic sleeve gastroplasty. Gastrointest Endosc. 2021;93:1110-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 16. | Nixdorf L, Hartl L, Ströhl S, Felsenreich DM, Mairinger M, Jedamzik J, Richwien P, Mozayani B, Semmler G, Balcar L, Schwarz M, Jachs M, Dominik N, Bichler C, Trauner M, Mandorfer M, Reiberger T, Langer FB, Bauer DJM, Prager G. Rapid improvement of hepatic steatosis and liver stiffness after metabolic/bariatric surgery: a prospective study. Sci Rep. 2024;14:17558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 17. | Jirapinyo P, Jaroenlapnopparat A, Zucker SD, Thompson CC. Combination Therapy of Endoscopic Gastric Remodeling with GLP-1RA for the Treatment of MASLD. Obes Surg. 2024;34:1471-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 18. | Salomone F, Currenti W, Magrì G, Boškoski I, Zelber-Sagi S, Galvano F. Effects of intragastric balloon in patients with nonalcoholic fatty liver disease and advanced fibrosis. Liver Int. 2021;41:2112-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Chuang TJ, Ko CW, Shiu SI. The metabolic influence of duodenal mucosal resurfacing for nonalcoholic fatty liver disease. Medicine (Baltimore). 2023;102:e35147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Mingrone G, van Baar AC, Devière J, Hopkins D, Moura E, Cercato C, Rajagopalan H, Lopez-Talavera JC, White K, Bhambhani V, Costamagna G, Haidry R, Grecco E, Galvao Neto M, Aithal G, Repici A, Hayee B, Haji A, Morris AJ, Bisschops R, Chouhan MD, Sakai NS, Bhatt DL, Sanyal AJ, Bergman JJGHM; Investigators of the REVITA-2 Study. Safety and efficacy of hydrothermal duodenal mucosal resurfacing in patients with type 2 diabetes: the randomised, double-blind, sham-controlled, multicentre REVITA-2 feasibility trial. Gut. 2022;71:254-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 21. | Roehlen N, Laubner K, Nicolaus L, Schwacha H, Bettinger D, Krebs A, Thimme R, Seufert J. Impact of duodenal-jejunal bypass liner (DJBL) on NAFLD in patients with obesity and type 2 diabetes mellitus. Nutrition. 2022;103-104:111806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 22. | Karlas T, Petroff D, Feisthammel J, Beer S, Blüher M, Schütz T, Lichtinghagen R, Hoffmeister A, Wiegand J. Endoscopic Bariatric Treatment with Duodenal-Jejunal Bypass Liner Improves Non-invasive Markers of Non-alcoholic Steatohepatitis. Obes Surg. 2022;32:2495-2503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 23. | Sierra L, Chatterjee A, Khurana A, Prado R, Patel R, Firkins SA, Simons-Linares R. Tissue Remodeling for a More Homogenous Ablation in Transoral Outlet Reduction Using Suturing and Noncontact Argon Plasma Coagulation. ACG Case Rep J. 2025;12:e01631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Jirapinyo P, Zucker SD, Thompson CC. Regression of Hepatic Fibrosis After Endoscopic Gastric Plication in Nonalcoholic Fatty Liver Disease. Am J Gastroenterol. 2023;118:983-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Lassailly G, Caiazzo R, Ntandja-Wandji LC, Gnemmi V, Baud G, Verkindt H, Ningarhari M, Louvet A, Leteurtre E, Raverdy V, Dharancy S, Pattou F, Mathurin P. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology. 2020;159:1290-1301.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 437] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 26. | Manos T, Nedelcu A, Noel P, Bastid C, Cazeres C, Carandina S, Nedelcu M. Endoscopic Revisional Gastroplasty After Bariatric Surgery with a Single-Channel Endoscope. Obes Surg. 2024;34:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Jaroenlapnopparat A, Thompson CC, Jirapinyo P. Effect of Transoral Outlet Reduction on Hepatic Fibrosis in Roux-en-Y Gastric Bypass Patients with Weight Regain and Non-alcoholic Fatty Liver Disease. Obes Surg. 2023;33:2303-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Nunes BCM, de Moura DTH, Kum AST, de Oliveira GHP, Hirsch BS, Ribeiro IB, Gomes ILC, de Oliveira CPM, Mahmood S, Bernardo WM, de Moura EGH. Impact of Endoscopic Sleeve Gastroplasty in Non-alcoholic Fatty Liver Disease: a Systematic Review and Meta-analysis. Obes Surg. 2023;33:2917-2926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Jirapinyo P, McCarty TR, Dolan RD, Shah R, Thompson CC. Effect of Endoscopic Bariatric and Metabolic Therapies on Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:511-524.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 30. | Ren M, Zhou X, Zhang Y, Mo F, Yang J, Yu M, Ji F. Effects of Bariatric Endoscopy on Non-Alcoholic Fatty Liver Disease: A Comprehensive Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne). 2022;13:931519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/