Published online Sep 27, 2025. doi: 10.4254/wjh.v17.i9.107806
Revised: May 22, 2025
Accepted: July 29, 2025
Published online: September 27, 2025
Processing time: 180 Days and 21.4 Hours
Hepatitis B virus (HBV) is a serious global public health concern. Although nucleoside drugs and interferons can significantly inhibit HBV replication, issues such as drug resistance and low clinical cure rates remain. Traditional Chinese medicine (TCM) is widely used in the treatment of chronic hepatitis B (CHB) in China, with anti-inflammatory, anti-fibrotic, and liver-protective effects; however, reports on its antiviral effects are still inconsistent. We retrieved multicenter clinical studies and meta-analyses of TCM treatment for CHB over the past two decades. The results revealed that TCM has a certain anti-HBV effect, and when combined with antiviral drugs, it can significantly improve antiviral efficacy. It was demonstrated that TCM most effectively promotes serum HBV e antigen conversion to negative, followed by the ability to reduce HBV DNA levels, facilitating HBV surface antigen loss, and improving the treatment of CHB.
Core Tip: Over the past two decades, multicenter clinical studies and meta-analyses have demonstrated that traditional Chinese medicines (TCM) exhibit certain antiviral properties. When administered in conjunction with nucleoside drugs, TCM can elevate the negative conversion rates or decrease hepatitis B virus e antigen, hepatitis B virus (HBV) DNA, and HBV surface antigen, ultimately enhancing therapeutic outcomes. The underlying antiviral mechanisms may involve reducing the negative immune regulation, augmenting the host immune response, and increasing the levels of HBV-specific cytotoxic T lymphocytes.
- Citation: Feng X, Li NN, Liu GJ, An C, Liu C. Anti-hepatitis B virus effects of traditional Chinese medicine: Learning from clinical trials in the past twenty years. World J Hepatol 2025; 17(9): 107806
- URL: https://www.wjgnet.com/1948-5182/full/v17/i9/107806.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i9.107806
Hepatitis B virus (HBV) is a major global public health concern. HBV infection can lead to diseases such as acute hepatitis, chronic hepatitis, liver fibrosis, cirrhosis, and liver cancer. Globally, there are approximately 316 million individuals with chronic HBV infection, with an HBV infection rate of 4.1% across all age groups[1].
Currently, there are two primary types of therapeutic drugs for chronic hepatitis B (CHB). One is nucleotide-based drugs, including first-line antiviral medications entecavir, tenofovir disoproxil fumarate, and tenofovir alafenamide fumarate. These drugs significantly inhibit HBV replication, mainly by acting on HBV polymerase. The second category is interferon (IFN)-based drugs, which inhibit viral replication by inducing the production of antiviral proteins and modulating immunity. Current therapeutic drugs have a low clinical cure rate, and there is an urgent need to enhance their therapeutic efficacy.
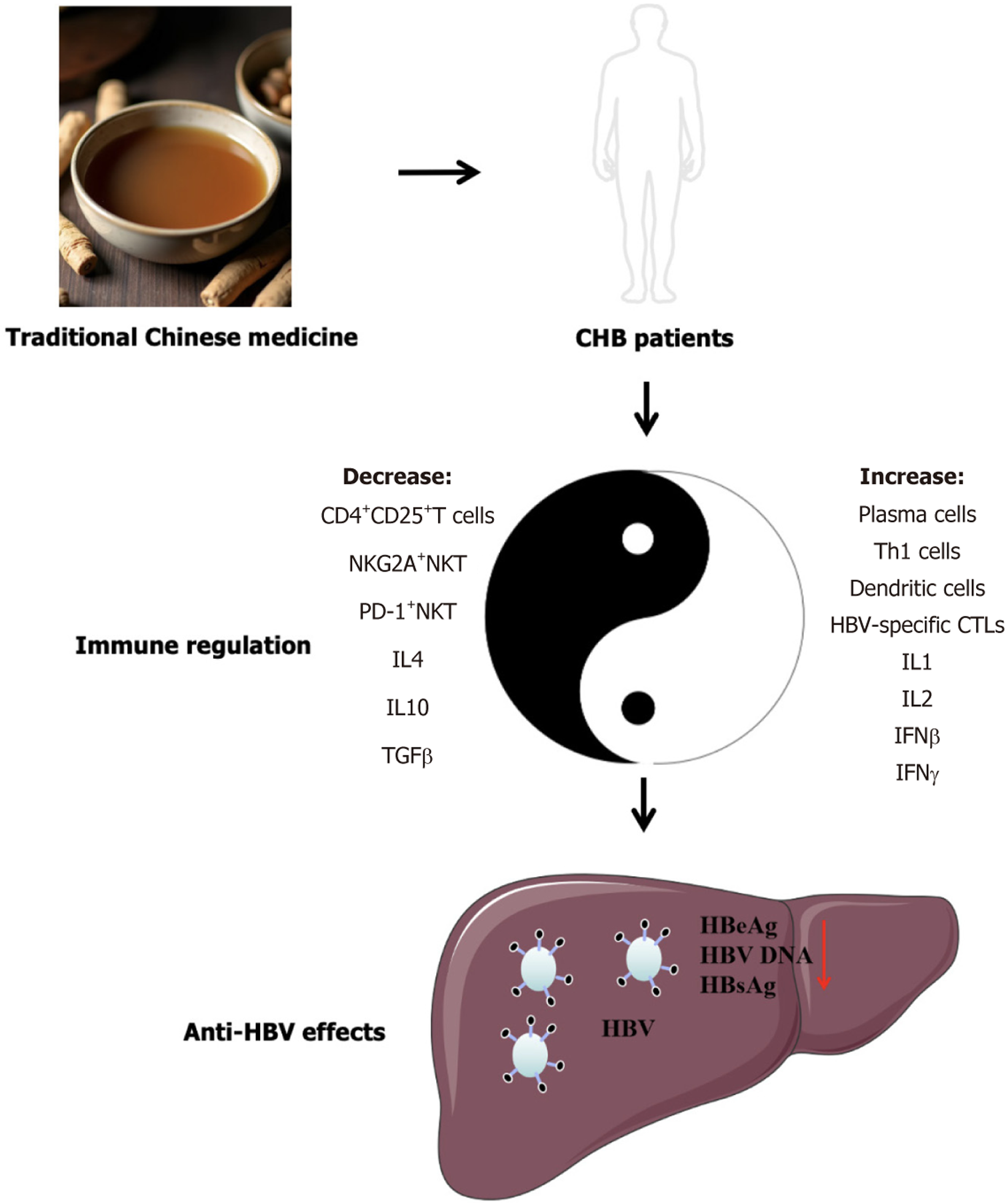
Traditional Chinese medicine (TCM) is a valuable medical resource in China that demonstrates excellent anti-inflammatory, hepatoprotective, and antifibrotic efficacy. Many Chinese patients with CHB in China receive TCM interventional therapy. Over the past two decades, numerous clinical studies have focused on the anti-HBV effects of TCM[2]. However, there are limited comprehensive reports on the anti-HBV effects, benefits, limitations, and mechanisms of action of TCM. We conducted a review and analysis of multicenter randomized controlled trials and systematic meta-analyses of clinical studies of TCM against HBV over the past two decades. This study aims to provide comprehensive evidence for the anti-HBV effects and clinical application of TCM (Figure 1).
The literature was searched in both the PubMed database and the CNKI Chinese database using the following keywords: "Traditional Chinese medicine", "HBV", "hepatitis B", "CHB", or "chronic hepatitis B". All multi-center clinical studies were incorporated into the analysis. Additionally, meta-analyses conducted by prominent TCM research institutions and those that adhered to literature evaluation systems such as Cochrane were selected and include.
The Bushen jianpi formula (BSJPF) is a TCM prescription used in The Affiliated Hospital of Shanghai University of Traditional Chinese Medicine for the treatment of CHB. This formula includes ten types of Chinese herbs: (1) Yin Yang Huo; (2) Mao Zhua Cao; (3) Huang Qi; (4) Bai Zhu; (5) Sheng Ma; (6) Ku Shen; (7) Qing Pi; (8) Dan Pi; (9) Lian Qiao; and (10) Xian He Cao. The experimental group consisted of 271 HBV e antigen (HBeAg)-negative patients with CHB treated with BSJPF combined with entecavir, whereas the control group consisted of 272 HBeAg-negative patients treated with a TCM placebo combined with entecavir. The treatment lasted for 96 weeks. This clinical study was conducted at 15 centers across China[3]. There were no significant differences in the safety between the two groups[3]. The results showed that BSJPF significantly improved the HBV surface antigen (HBsAg) seroconversion rate [5.5% (15/271) of patients converted to negative], which was significantly higher than that of the control group [1.8% (5/272) of patients converted to negative][3]. Additionally, the proportion of patients in the experimental group with a decrease in HBsAg level of more than 1 Log IU/mL was 11.1% (30 of 271 patients), which was significantly higher than 5.9% in the control group (16 of 272 patients)[3]. However, there was no significant difference in the average HBsAg levels between the two groups[3]. In terms of HBV DNA seroconversion rate, the experimental group and the control group had conversion rates of 97.8% and 98.2%, respectively, with no significant difference between the two groups[3]. This suggests that BSJPF has a weak effect on HBV DNA or is influenced by the presence of entecavir. Both groups achieved an HBV DNA seroconversion rate of approximately 98%, and the effect of BSJPF on HBV DNA was not fully demonstrated[3]. The mean decrease in cccDNA levels in the liver was not significantly different between the two groups[3]. Additional studies conducted later showed that the decrease in HBsAg levels in the long-term treatment group (192 weeks) was significantly higher than that in the short-term treatment group, indicating that the effect of BSJPF in reducing HBsAg levels increased with prolonged treatment[4]. However, this study did not report HBsAg seroconversion rates at 192 weeks.
This multicenter clinical trial demonstrated that BSJPF could enhance the rate of HBsAg seroconversion in HBeAg-negative patients with CHB; however, it did not significantly promote the rate of HBV DNA seroconversion. The article did not summarize or report the characteristics of the patients who achieved negative HBsAg seroconversion, making it difficult to identify the population most likely to benefit from BSJPF.
The Lingmao formula (LMF), also known as the Kidney-tonifying Formula, is an empirical formula for treating CHB and consists of six Chinese herbal medicines: (1) Yin Yang Huo; (2) Huang Qi; (3) Hu Huang Lian; (4) Mao Zhao Cao; (5) Nv Zhen Zi; and (6) Qing Pi. A multicenter clinical study was conducted at 12 centers to determine the anti-HBV role of LMF in patients[5]. The enrolled patients were HBeAg-positive with CHB and mildly elevated alanine aminotransferase[5]. The experimental group consisted of 136 enrolled patients who received LMF combined with entecavir treatment, whereas the control group consisted of 135 enrolled patients who received a Chinese herbal placebo combined with entecavir treatment[5]. The treatment lasted for 52 weeks[5]. At the end of 52 weeks, there was no significant difference in side effects between the two groups, and no severe side effects were observed[5]. The results showed that after 52 weeks of treatment, the HBeAg seroconversion rate in the experimental group was 22.8%, which was significantly higher than that in the control group (12.6%), indicating that LMF significantly promoted HBeAg seroconversion in patients with HBeAg-positive CHB. There was no significant difference in HBV DNA seroconversion rate between the two groups (experimental group 68.4% vs control group 67.4%). However, a slightly higher proportion of patients in the experimental group experienced a reduction of more than 2 Log copies/mL (98.5% vs 92.6%), and the average decrease in HBV DNA levels was slightly higher in the experimental group (5.5 Log copies/mL vs 5.4 Log copies/mL), suggesting that LMF had a minimal, non-significant effect on HBV DNA. There was no difference in the HBsAg seroconversion rate between the experimental and control groups (1.5% vs 0.7%, respectively), which may also be due to the short experimental period of 52 weeks, as the effect of LMF on HBsAg was not demonstrated. Additionally, the study did not report the average level or average decrease in HBsAg levels in the two groups, which would have provided a better evaluation of the effect of LMF.
A multicenter clinical study has shown that LMF can significantly promote the seroconversion rate of HBeAg in HBeAg-positive patients with CHB but has a weak effect on the seroconversion of HBV DNA and HBsAg. The study period was relatively short, which may have affected the determination of HBsAg seroconversion. Additionally, the study did not report an average decrease in HBsAg levels, which is necessary to better evaluate the effect of LMF on the decrease in HBsAg.
Another study indicated that LMF can promote the decline of HBsAg and the conversion of HBeAg to negative in HBeAg-positive CHB patients, and increase the frequency of plasma cells[6]. This suggests that LMF exerts its anti-HBV effect by regulating B cell differentiation and influencing humoral immunity. Additionally, the research shows that LMF can decrease the proportion of peripheral blood CD4+CD25+T cells in CHB patients, from 11.07% ± 4.30% before treatment to 8.70% ± 3.49% after treatment; meanwhile, it significantly increases the expression level of IFN-γ on CD4+T cells from 13.98% ± 3.25% before treatment to 15.85% ± 3.43% after treatment[7]. This suggests that LMF exerts its anti-HBV effect by regulating the number of inhibitory T cells, such as CD4+CD25+T cells. Ji et al[8] showed that LMF increased the number of peripheral blood Th1 cells and dendritic cell (DC) in patients with CHB and reduced the levels of programmed cell death ligand 1 (PD-L1) in regulatory T cells (Treg cells) and DCs. This suggests that LMF exerts its antiviral effects via negative immune regulation. The study by Le et al[9] shows that LMF can promote the conversion of HBeAg to negative by reducing the proportion of inhibitory cells such as NKG2A+NKT and PD-1+NKT in peripheral blood of HBeAg-positive CHB patients, and increasing the proportion of IFN-γ+NKT cells. Research by Zhang et al[10] indicates that LMF may exert its anti-HBV effect by regulating the signaling pathway of mitochondrial antiviral signaling protein (MAVS) to induce the expression of IFN-β. These studies suggest that LMF can exert its anti-HBV effect through mechanisms such as downregulating Treg cells and programmed cell death protein 1/PD-L1 Levels in immune cells, increasing the production of IFN-γ in CD4+T cells and natural killer T cells, and regulating the MAVS signaling pathway to induce the expression of IFN-β.
Tiao-Gan-Jian-Pi-Jie-Du granule (TGJPJD) are optimized TCM compounds for the treatment of CHB and consist of four herbs: (1) Huang Qi; (2) Chai Hu; (3) Di Ding; and (4) Yu Jin. A multicenter, randomized, controlled clinical study showed that TGJPJD significantly improved the HBeAg seroconversion rate among HBeAg-positive CHB patients[11]. Among the 285 patients in the experimental group treated with TGJPJD combined with entecavir, 107 converted to HBeAg negativity after 108 weeks of treatment (negative conversion rate: 37.54%); among the 283 patients in the control group treated with a TCM placebo combined with entecavir, 77 converted to HBeAg negativity (negative conversion rate: 27.21%)[11]. TGJPJD significantly improved the HBeAg-negativity rate among patients with HBeAg-positive CHB. However, there was no statistical difference in the reduction in HBsAg levels and HBV DNA seroconversion rates between the two groups[11]. The safety profiles of the two groups were comparable, with no statistical difference[11]. These results indicate that the combination of TGJPJD and entecavir is safe and can significantly enhance the HBeAg seroconversion in HBeAg-positive patients, although its effects on HBsAg level and HBV DNA reduction are limited.
In summary, this multicenter clinical study indicated that the combination of TGJPJD and antiviral drugs can significantly enhance the HBeAg seroconversion rate by approximately 10%. However, it had minimal effect on the HBV DNA seroconversion rate and HBV DNA levels. This reduced the average HBsAg level; however, the difference was not statistically significant. This study did not analyze the proportion of patients with varying degrees of HBsAg reduction, limiting the ability to fully assess the impact and effectiveness of TGJPJD on HBsAg levels. Additionally, the detection limits for HBsAg and HBV DNA analyses in the study were 1000 IU/mL and 500 IU/mL, respectively, which may affect the accuracy of the seroconversion rate analysis.
Shuanghu Qinggan Granules (SQG) and Yiqi Jieyu Granules are patented Chinese medicines used to treat CHB. SQG is composed of twelve TCMs: (1) Shuang Hua; (2) Hu Zhang; (3) Gua Lou; (4) Pu Gong Ying; (5) Fa Ban Xia; (6) Zi Hua Di Ding; (7) Bai Hua She Cao; (8) Ye Ju Hua; (9) Dan Shen; (10) Huang Lian; (11) Zhi Shi; and (12) Gan Cao. YYJG consists of sixteen TCMs: (1) Chai Hu (processed with vinegar); (2) Bai Shao; (3) Dan Shen; (4) Dang Shen; (5) Fu Ling; (6) Gua Lou; (7) Huang Lian; (8) Shan Zha; (9) Zhi Ke; (10) Ju Ye; (11) Huang Qi; (12) Gui Zhi; (13) Ci Wu Jia; (14) Fa Ban Xia; (15) Jue Ming Zi; and (16) Wu Wei Zi. To investigate the therapeutic effects of SQG and YYJG on CHB, a multicenter, randomized, double-blind, controlled clinical study involving ten subcenters was conducted[12]. The experimental group consisted of 134 HBeAg-positive patients with CHB who received a combination treatment of SQG, YYJG, and lamivudine for 48 weeks, whereas the control group consisted of 134 HBeAg-positive patients with CHB who received a TCM placebo combined with lamivudine for the same duration[12]. The results indicated that the two groups were similar in terms of safety[12]. The HBeAg conversion rate in the experimental group (38%) was significantly higher than that in the control group (24%)[12]. Additionally, the average HBeAg levels in the experimental group were significantly lower than those in the control group[12]. However, there was no significant difference in the HBV DNA conversion rates between the two groups[12]. The study indicated that the combination treatment with SQG, YYJG, and lamivudine could improve the HBeAg conversion rate and reduce HBeAg levels in HBeAg-positive CHB patients compared with lamivudine monotherapy, but its effect on HBV DNA was weak. This study did not report the impact of SQG and YYJG on HBsAg conversion to negative or HBsAg levels.
Mechanism research shows that SQG can significantly reduce the levels of interleukin (IL)-10 and transforming growth factor (TGF)-β in the peripheral blood of patients with CHB[13]. This suggests that SQG exerts its anti-HBV effects by reducing the levels of immunosuppressive cytokines.
This multicenter clinical study demonstrated the combined anti-HBV effect of SQG and YYJG; however, the anti-HBV effects of SQG or YYJG alone have not been reported.
The BSJPF is a traditional Chinese medicinal compound consisting of fourteen herbs: (1) Yin Yang Huo; (2) Tu Si Zi; (3) Du Zhong; (4) Huai Niu Xi; (5) Yin Du Ye Xia Zhu; (6) Huang Qi; (7) Bai Zhu; (8) Fu Ling; (9) Zhu Ling; (10) Zhi Ke; (11) Dan Shen; (12) San Qi; (13) Yu Jin; and (14) Gou Qi Zi. To investigate the antiviral effects of BSJPFb in the treatment of CHB, a multicenter, randomized, double-blind, controlled clinical study involving 19 research centers was conducted[14]. The experimental group consisted of 200 HBeAg-positive HBV carriers treated with BSJPFb, whereas the control group consisted of 100 HBeAg-positive HBV carriers treated with a TCM placebo[14]. The treatment lasted for 52 weeks[14]. The results showed no significant difference in safety between the two groups, with no serious adverse reactions, suggesting that BSJPFb is safe for CHB treatment[14].
At 24 weeks of treatment, HBV DNA levels in the experimental and control groups decreased by 0.346 Log IU/mL and 0.019 Log IU/mL, respectively, compared to baseline, and decreased by 0.996 Log IU/mL and 0.077 Log IU/mL at 52 weeks, with significant differences between the two groups[14]. At 24 weeks, the proportions of patients with HBV DNA levels decreasing by > 1 Log IU/mL in the experimental and control groups were 22.65% and 5.32%, respectively, with significant differences between the two groups, while the proportions of patients with HBV DNA levels decreasing by > 2 Log IU/mL were 7.73% and 3.19%, respectively, with no significant differences between the two groups[14]. At 52 weeks, the proportions of patients with HBV DNA levels decreasing by more than 1 Log IU/mL in the experimental group and control group were 45.98% and 11.83%, respectively, with significant differences between the two groups; the proportions of patients with HBV DNA levels decreasing by more than 2 Log IU/mL were 21.84% and 5.38%, respectively, with significant differences between the two groups[14]. There were no significant differences in the proportion of patients with HBV DNA levels decreasing by > 3 Log IU/mL or those who achieved viral clearance between the two groups[14]. This indicates that BSJPFb reduces the quantity of HBV DNA, and that the decrease in HBV DNA levels is greater with prolonged treatment. However, BSJPFb did not achieve clearance of HBV DNA within the 52-week treatment period.
The HBeAg value in the experimental group decreased from the baseline of 1115.71 S/Co to 982.63 S/Co at 24 weeks and further to 824.11 S/Co at 52 weeks, showing significant differences compared with before treatment[14]. The mean HBeAg level decreased by approximately 26%. In contrast, there was no significant change in the HBeAg levels in the control group during the 52-week treatment period. However, there was no significant difference in HBeAg seroconversion between the two groups. These findings indicate that BSJPFb can significantly reduce HBeAg levels, with its inhibitory effect becoming more pronounced over time. However, despite this reduction, BSJPFb did not significantly enhance the HBeAg seroconversion rate within the 52-week treatment period.
In the experimental group, HBsAg levels decreased by 0.178 Log IU/mL and 0.416 Log IU/mL at 24th week and 52th week, respectively, compared to the baseline, with statistically significant differences; the mean HBsAg level decreased by 9.1% at 52th week[14]. At 52th week, the proportions of patients with HBsAg levels decreasing by more than 0.5 Log IU/mL was 22.99% in the experimental group and 7.53% in the control group, with statistically significant differences[14]. However, there were no statistically significant differences between the two groups in the proportions of patients with HBsAg levels decreasing by more than 1 Log IU/mL, 2 Log IU/mL, or becoming negative[14]. This suggests that BSJPFb has an effect on reducing HBsAg levels, but the reduction was less than 1 Log IU/mL.
The study reported that BSJPFb alone had an inhibitory effect on HBV, showing its effectiveness in suppressing HBsAg, HBeAg, and HBV DNA levels with a more pronounced effect as time progressed. However, seroconversion was not observed during the study period. This suggests that the use of this TCM compound alone may not be sufficient to promote negative seroconversion of HBV antigens and DNA, and it needs to be combined with antiviral drugs.
Mechanism research indicates that the BSJPFb can increase the levels of IL-1 and IFN-γ in peripheral blood of CHB patients, reduce the levels of IL-4 and IL-10, regulate the Th1/Th2 cytokine expression, and exert its anti-HBV effect[15].
This multicenter clinical study demonstrated that BSJPFb significantly reduced the HBsAg, HBeAg, and HBV DNA levels in patients with CHB. However, this did not promote the conversion to negative results within the 52-week study period. The study period was relatively short, and it was not possible to determine whether BSJPFb could promote the conversion of HBV antigens and DNA to negative antibodies over a longer study period.
Oxymatrine is an alkaloid extracted from the roots of the traditional Chinese medicinal plant Sophora flavescens. A multicenter, randomized, double-blind, controlled clinical study showed that oxymatrine injections and capsules exhibited significant anti-HBV effects[16]. This clinical study enrolled 199 HBeAg-positive CHB patients who were divided into three groups: (1) 102 patients in the oxymatrine capsule group; (2) 30 patients in the oxymatrine injection group; and (3) 67 patients in the TCM placebo group, with a treatment duration of 24 weeks[16]. The results showed that the HBV DNA conversion rates to negative in the oxymatrine capsule and injection groups were 31.91% and 39.29%, respectively; the HBeAg conversion rates to negative were 38.61% and 43.33%, respectively, significantly higher than that in the TCM placebo control group; and the HBsAg conversion rates to negative were 3.33% and 1.98%, respectively, with no significant difference among the three groups[16]. However, this study did not report a decrease in HBsAg levels; therefore, the effect of oxymatrine on HBsAg was not reflected[16]. There were no significant differences in safety between the three groups[16]. Clinical trial results demonstrated that oxymatrine capsules and injections significantly promote HBeAg and HBV DNA conversion to negative. Another multicenter, randomized, double-blind, controlled clinical study was conducted to determine the anti-HBV effects of oxymatrine[17]. The experimental group treated 65 HBeAg-positive CHB patients with oxymatrine capsules, while the control group treated 65 patients with a TCM placebo for a treatment period of 52 weeks[17]. The results showed no significant differences in safety between the two groups[17]. The HBV DNA and HBeAg conversion rates to a negative status in the experimental group were 43.08% (28/65) and 33.33% (20/60), respectively, which were significantly higher than those in the control group[17]. The HBsAg conversion rates to a negative status in the experimental and control groups were 3.08% (2/65) and 0% (0/65), respectively, with no significant difference between the two groups. The results of the two clinical studies indicated that oxymatrine could promote negative HBeAg and HBV DNA conversion rates, improving the negative conversion of HBsAg with no statistical difference. The effect of oxymatrine on the reduction of HBsAg levels was not observed in this study.
In clinical research, the absence of a corresponding injection control for the formulation of oxymatrine may have affected the results.
Mechanism research indicate that oxymatrine can reduce the expression of Th2 cytokines IL-4 and IL-10 and increase the expression of Th1 cytokines IFN-γ and IL-2[18]. Another study demonstrated that oxymatrine exerts its anti-HBV effect by decreasing PD-L1 expression on both HBV-specific and non-specific cytotoxic T lymphocytes (CTLs) in the peripheral blood of patients with CHB, while simultaneously increasing the number of HBV-specific CTLs[19]. Current research suggests that oxymatrine primarily exerts its anti-HBV effects through immune regulation.
To systematically evaluate the anti-HBV effects of Phyllanthus niruri, a systematic meta-analysis of clinical trials using Phyllanthus niruri for CHB treatment was conducted[20]. Sixteen clinical studies were included in this analysis[20]. The meta-analysis results showed that Phyllanthus niruri significantly promoted HBV DNA clearance [relative risk (RR) = 0.69, 95%CI: 0.52-0.91, P = 0.008, I2 = 71%]. Subgroup analysis indicated that the combination of Phyllanthus niruri with IFN-α or thymosin significantly improved the HBV DNA clearance rate compared to the use of IFN-α or thymosin alone[20]. However, when combined with nucleoside antiviral drugs, such as lamivudine and adefovir dipivoxil, there was no significant difference compared with the use of antiviral drugs alone[20]. The combination of Phyllanthus niruri and antiviral drugs significantly promoted HBeAg clearance compared to the use of antiviral drugs alone (RR = 0.70, 95%CI: 0.60-0.81, I2 = 68%). The combination of Phyllanthus niruri and antiviral drugs could not significantly promote HBsAg clearance compared to the use of antiviral drugs alone (RR = 0.95, 95%CI: 0.90-1.00, P = 0.07, I2 = 0%). Systematic analysis showed that the combination of Phyllanthus niruri and antiviral drugs significantly improved HBV DNA and HBeAg clearance rates but had no significant effect on HBsAg clearance.
Although a systematic analysis based on clinical trials suggests that Phyllanthus niruri and its compound preparations can promote the conversion of HBeAg and HBV DNA levels to negative ones, the clinical trials included in the analysis were of low quality, with methodological flaws and issues. Therefore, the results of this systematic analysis require further confirmation through high-quality clinical studies.
Xiaoyao San Jiajian (XYSJJ) is a traditional Chinese medicinal compound used for the treatment of CHB that consists of eight herbs: (1) Chai Hu; (2) Bai Zhu; (3) Dang Gui; (4) Fu Ling; (5) Bai Shao; (6) Zhi Gan Cao; (7) Bo He; and (8) Wei Sheng Jiang. A meta-analysis aimed at evaluating the efficacy and safety of XYSJJ in the treatment of CHB showed that among the 17 clinical studies enrolled, XYSJJ promoted a negative conversion rate of HBV DNA (RR = 0.85, 95%CI: 0.80-0.91, P < 0.00001), and among the 14 clinical studies, Xiaoyao San significantly promoted the negative conversion of HBeAg (RR = 0.65, 95%CI: 0.56-0.76, P < 0.00001)[21]. There was no significant difference in the safety between the two groups, and the effect of XYSJJ on HBsAg was not reported in this analysis. Based on the results of this meta-analysis, XYSJJ significantly promoted the negative conversion of HBV DNA and HBeAg in patients with CHB.
The majority of clinical studies included in this systematic analysis were single-center and small-sample studies, which cannot exclude the potential impacts of selection bias, publication bias, and study heterogeneity on the results. Further high-quality clinical studies are required to validate these results.
Liuwei Wuling Tablets are a traditional Chinese patent medicine that consists of six types of TCMs: (1) Wu Wei Zi; (2) Nv Zhen Zi; (3) Lian Qiao; (4) E Zhu; (5) Qu Mai Cai; and (6) Ling Zhi Bao Zi Fen. A meta-analysis incorporating four clinical studies showed that Liuwei Wuling Tablets had a certain promoting effect on the negative conversion of HBV DNA in patients with CHB, but there was no statistical difference (RR = 1.22, 95%CI: 0.99-1.52, P = 0.07); it significantly promoted the negative conversion of HBeAg (RR = 1.26, 95%CI: 1.03-1.54, P = 0.02)[22]. However, this meta-analysis included only four clinical studies, which may have been biased. Another meta-analysis incorporating 15 clinical studies showed that Liuwei Wuling Tablets combined with drugs such as Adefovir Disoproxil and Lamivudine can significantly promote the negative conversion of HBV DNA compared to using these drugs alone; however, Liuwei Wuling Tablets combined with entecavir have a certain advantage in promoting the negative conversion of HBV DNA compared with using entecavir alone, although there was no statistical difference[23]. Thirteen clinical studies demonstrated that Liuwei Wuling Tablets combined with antiviral drugs can significantly promote negative conversion of HBeAg[23]. The above meta-analyses suggest that Liuwei Wuling Tablets combined with antiviral drugs can significantly promote the negative conversion of HBeAg and HBV DNA. However, the effects of Liuwei Wuling Tablets on HBsAg levels have not yet been reported.
The sample size in the systematic analysis was relatively small, and the results showed a degree of publication bias in the results. Therefore, further high-quality clinical studies are required to confirm these findings.
A meta-analysis involving 6514 patients revealed that the combination of Fuzheng Huayu Capsules (FZHYJN) (consisting of six Chinese herbal medicines including Dan Shen, Fa Jiao Chong Cao Jun Fen, Tao Ren, Song Hua Fen, Jiao Gu Lan, and Wu Wei Zi), Anluo Huaxian Pills (ALHXW) (consisting of fourteen Chinese herbal medicines including Di Huang, San Qi, Shui Zhi, Jiang Chan, Di Long, Bai Zhu, Yu Jin, Niu Huang, Wa Leng Zi, Mu Dan Pi, Da Huang, Sheng Mai Ya, Ji Nei Jin, and Shui Niu Jiao), and Biejia Ruangan Tablets (BJRGP) (consisting of eleven Chinese herbal medicines including Zhi Bie Jia, E Zhu, Chi Shao, Dang Gui, San Qi, Dang Shen, Huang Qi, Zi He Che, Dong Chong Xia Cao, Ban Lan Gen, and Lian Qiao) combined with entecavir exhibited a more significant advantage in improving HBV DNA negative conversion rate and HBeAg negative conversion rate compared to entecavir alone[24]. This study did not include an analysis of the effects of TCM on HBsAg levels. The conclusion drawn from the results indicated that the combination of FZHYJN, ALHXW, and Compound BJRGP with the nucleoside antiviral drug entecavir significantly promoted the negative conversion of HBV DNA and HBeAg in patients with CHB compared with entecavir alone.
The number of studies included in this analysis was relatively small, and the quality of the clinical studies was generally low, which may have affected the conclusions. The conclusions of this study require further verification by high-quality clinical studies with larger sample sizes.
TCM is safe for the treatment of CHB, with safety comparable to that of the control group and no serious adverse reactions. Analysis of the results indicated that TCM had the strongest effect on HBeAg (Table 1)[3,5,11,12,14,16,17,20-24]. Almost all clinical studies have suggested that TCM can improve HBeAg seroconversion rates. The combination of TCM and antiviral drugs increases the seroconversion rate by approximately 10%. Second, TCM affect HBV DNA. Numerous studies demonstrated that TCM can promote the seroconversion of HBV DNA to negative levels. Many studies used HBsAg seroconversion-negative status as the treatment endpoint. Currently, it is difficult for TCM to promote negative HBsAg seroconversion within the study period; however, research has shown that TCM can reduce HBsAg levels.
| Number | Traditional Chinese medicine | Experimental group | Control group | HBsAg | HBeAg | HBV DNA | Ref. |
| 1 | BSJPFa | 271 HBeAg-negative CHB patients treated with BSJPFa combined with entecavir for 96 weeks | 272 HBeAg-negative CHB patients treated with Chinese herbal placebo combined with entecavir for 96 weeks | Increase negative conversion rate (5.5% vs 1.8%, P = 0.03) | Not mentioned | Could not increase negative conversion rate (97.8% vs 98.2%, P = 0.77) | Zhang et al[3] |
| 2 | LMF | 136 HBeAg-positive CHB patients treated with LMF combined with entecavir for 52 weeks | 135 HBeAg-positive CHB patients treated with Chinese herbal placebo combined with entecavir for 52 weeks | Slightly increase negative conversion rate (1.5% vs 0.7%, P = 1.0) | Increase negative conversion rate (22.8% vs 12.6%, P = 0.038) | Increase the proportions of ≥ 2 Log copies/mL reduction compared to baseline (98.5% vs 92.6%, P = 0.019) | Zhu et al[5] |
| 3 | TGJPJD | 285 HBeAg-positive patients treated with TGJPJD combined with entecavir for 108 weeks | 283 HBeAg-positive patients treated with Chinese herbal placebo combined with entecavir for 108 weeks | Slightly increase negative conversion rate (0.7% vs 0.35%, P > 0.05) | Increase negative conversion rate (37.54% vs 27.21%, P = 0.08) | Slightly increase negative conversion rate (91.5% vs 90.11%, P > 0.05) | Li et al[11] |
| 4 | SQG and YYJG | 134 HBeAg-positive CHB patients treated with SQG and YYJG combined with Lamivudine for 48 weeks | 134 HBeAg-positive CHB patients treated with Chinese herbal placebo combined with Lamivudine for 48 weeks | Not mentioned | Increase negative conversion rate (38% vs 24%, P < 0.05) | Virological response between the two groups were similar (P > 0.05) | Ye et al[12] |
| 5 | BSJPFb | 200 HBeAg-positive HBV carriers treated with the BSJPFb for 52 weeks | 100 HBeAg-positive HBV carrier treated with Chinese herbal placebo for 52 weeks | Promote HBsAg level decline (0.416 Log IU/mL vs 0.178 Log IU/mL, P = 0.0069) | Promote HBeAg level decline (291.6 S/Co vs 14.0 S/Co, P < 0.0001) | Promote HBV DNA decline (0.996 Log IU/mL vs 0.077 Log IU/mL, P < 0.001) | Chen et al[14] |
| 6 | Oxymatrine | 102 HBeAg-positive CHB patients treated with Oxymatrine capsule for 24 weeks | 67 HBeAg-positive CHB patients treated with Chinese herbal placebo for 24 weeks | Slightly increase negative conversion rate (1.98% vs 0.0%, P = 0.269) | Increase negative conversion rate (31.91% vs 6.45%, P = 0.001) | Increase negative conversion rate (38.61% vs 7.46%, P = 0.001) | Lu et al[16], Lu et al[17] |
| 7 | Phyllanthus urinaria | CHB patients treated with Phyllanthus urinaria combined with antiviral drugs | CHB patients treated with antiviral drugs | Could not significantly promote HBsAg clearance | Increase negative conversion rate (P < 0.05) | Increase negative conversion rate (P = 0.008) | Xia et al[20] |
| 8 | XYSJJ | CHB patients treated with XYSJJ combined with antiviral drugs | CHB patients treated with antiviral drugs | Not mentioned | Increase negative conversion rate (P < 0.00001) | Increase negative conversion rate (P < 0.00001) | Yu et al[21] |
| 9 | LWWLP | CHB patients treated with LWWLP and enticavir | CHB patients treated with enticavir | Not mentioned | Increase negative conversion rate (P = 0.0002) | Slightly increase negative conversion rate (P = 0.17) | Zhou et al[22], He et al[23] |
| 10 | FZHYJN | CHB patients treated with FZHYJN and enticavir | CHB patients treated with enticavir | Not mentioned | Increase negative conversion rate (P < 0.05) | Increase negative conversion rate (P < 0.05) | Lou[24] |
| 11 | ALHXW | CHB patients treated with ALHXW and enticavir | CHB patients treated with enticavir | Not mentioned | Increase negative conversion rate (P < 0.05) | Increase negative conversion rate (P < 0.05) | Lou[24] |
| 12 | BJRGP | CHB patients treated with BJRGP with enticavir | CHB patients treated with enticavir | Not mentioned | Increase negative conversion rate (P < 0.05) | Increase negative conversion rate (P < 0.05) | Lou[24] |
Clinical studies have shown that TCM has anti- HBV effects, but its effect is not as good as that of first-line antiviral drugs. Currently, it is often used as an adjuvant anti-HBV drug, combined with first-line antiviral drugs for antiviral treatment, and combined treatment with TCM and antiviral drugs has a certain effect in improving antiviral efficacy.
Different TCM prescriptions exhibit different anti-HBV effects. This variability is partly due to differences in clinical study durations, which ranged from 24 weeks to 108 weeks, potentially affecting the observed drug efficacy. Additionally, the heterogeneity of the enrolled patients may have contributed to the varying effects of the drugs. Different clinical studies may have recruited patients with HBeAg-positive and HBeAg-negative statuses or HBV carriers. The mechanism of action of the drugs is also a significant factor affecting their anti-HBV effects. However, current research on the anti-HBV mechanisms of TCM remains insufficient. The prevailing mechanisms suggest that most TCMs enhance host immunity and increase the number of HBV-specific CTLs (Table 2)[6-10,13,15,18,19] potentially boosting HBeAg/HBcAg-specific cellular immunity and significantly facilitating the decrease and seroconversion of HBeAg. This may help further relieve the inhibition of host anti-HBV-specific responses by HBeAg, subsequently leading to a reduction in HBV DNA and HBsAg levels.
| Number | Immune cells or cytokines | Mechanism | Traditional Chinese medicine | Ref. |
| 1 | Plasma cells | The frequency increased | LMF | Li et al[6] |
| 2 | CD4+CD25+T cells | The frequency decreased | LMF | Li et al[7] |
| 3 | CD4+T cells | The expression level of IFN-γ on CD4+T cells increased | LMF | Li et al[7] |
| 4 | Th1 cells | The count increased | LMF | Ji et al[8] |
| 5 | Dendritic cells | The count increased; the levels of PD-L1 decreased | LMF | Ji et al[8] |
| 6 | Regulatory T cells | The levels of PD-L1 decreased | LMF | Ji et al[8] |
| 7 | Natural killer T cells | The proportion of NKG2A+NKT and PD-1+NKT decreased | LMF | Le et al[9] |
| 8 | IFN-β | Increased through mitochondrial antiviral signaling protein pathway | LMF | Zhang et al[10] |
| 9 | IL-10 | Decreased | SQG; BSJPFb, oxymatrine | Zhu et al[13], Chen et al[15], Dong et al[18] |
| 10 | Transforming growth factor-β | Decreased | SQG | Zhu et al[13] |
| 11 | IFN-γ | Increased | BSJPFb, oxymatrine | Chen et al[15], Dong et al[18] |
| 12 | IL-1 | Increased | BSJPFb | Chen et al[15] |
| 13 | IL-4 | Decreased | BSJPFb, oxymatrine | Chen et al[15], Dong et al[18] |
| 14 | IL-2 | Increased | Oxymatrine | Dong et al[18] |
| 15 | HBV-specific CTLs | The count increased, the PD-L1 Levels of HBV-specific CTLs decreased | Oxymatrine | Gu et al[[19] |
This review has some limitations. The multicenter clinical studies included generally had small sample sizes, and the clinical studies included in the systematic analysis were of low quality, which may have affected the final conclusions. Some meta-analyses were included, which may have introduced selection bias. This review primarily focuses on the anti-HBV effects of TCM, without delving deeply into its mechanisms of action.
In the future, large-sample, multicenter clinical studies should be conducted to further investigate the effects of TCM on serum markers such as HBsAg. Patients can be further grouped based on their HBsAg levels, and studies can be conducted on the negative conversion rate of TCM in patients with low HBsAg levels, as well as the reduction of HBsAg levels by TCM in patients with high HBsAg concentrations. Additionally, the mechanism of TCM's anti-HBV effect should be explored to expand the application scenarios of TCM and enhance its anti-HBV efficacy.
| 1. | GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:796-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 513] [Article Influence: 128.3] [Reference Citation Analysis (1)] |
| 2. | Ye YA. [An integrated traditional Chinese and Western Medicine study on the comprehensive prevention and treatment of chronic hepatitis B and its related diseases]. Linchuang Gandanbing Zazhi. 2023;6:1257-1266. [DOI] [Full Text] |
| 3. | Zhang JH, Zhang X, Zhou ZH, Zhu XJ, Zheng C, Li M, Jin SG, Mao DW, Xue JD, Shi WB, Chi XL, Wang XB, Li XD, Li Y, Wang H, Li Q, Zhou DQ, Wang CB, Shi CH, Li CZ, Wu JH, Kong XN, Sun XH, Gao YQ. Bushen Jianpi Formula Combined with Entecavir for the Treatment of HBeAg-Negative Chronic Hepatitis B: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Evid Based Complement Alternat Med. 2022;2022:6097221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Zhu XJ, Zhang JH, Sun XH, Zhang X, Gao YQ. [Clinical efficacy and mechanism of action of kidney-tonifying,spleen-strengthening,and diuresis-promoting therapy in treatment of chronic hepatitis B]. Linchuang Gandanbing Zazhi. 2023;6:1274-1279. [DOI] [Full Text] |
| 5. | Zhu XJ, Sun XH, Zhou ZH, Liu SQ, Lv H, Li M, Li L, Gao YQ. Lingmao Formula Combined with Entecavir for HBeAg-Positive Chronic Hepatitis B Patients with Mildly Elevated Alanine Aminotransferase: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Evid Based Complement Alternat Med. 2013;2013:620230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Li M, Zhou ZH, Bao T, Zhang X, Zhu XJ, Jin SG, Gao YT, Sun XH, Gao YQ. Beneficial Effects of Bushen Formula Combined with Enticavir on Chronic Hepatitis B Patients with Suboptimal Response to Enticavir by Regulating B-Cell Differentiation. Cell Physiol Biochem. 2018;48:633-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Li M, Sun XH, Zhou ZH, Yu Z, Jin SG, Zhu XJ, Gao YQ. Beneficial therapeutic effect of Chinese herbal Bushen formula on CHB patients with mildly elevated alanine aminotransferase by down-regulating CD4+CD25+T cells. J Ethnopharmacol. 2013;146:614-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Ji LS, Gao QT, Guo RW, Zhang X, Zhou ZH, Yu Z, Zhu XJ, Gao YT, Sun XH, Gao YQ, Li M. Immunomodulatory Effects of Combination Therapy with Bushen Formula plus Entecavir for Chronic Hepatitis B Patients. J Immunol Res. 2019;2019:8983903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Le F, Zhang X, Zhu XJ, Zhang JH, Li M, Zhou ZH, Gao YT, Sun XH, Gao YQ. [Effects of Bushen Jianpi Recipe on NKT cell function in positive HBeAg chronic hepatitis B patients after entecavir treatment]. Shanghai Zhongyiyao Daxue Xuebao. 2018;3:22-27. [DOI] [Full Text] |
| 10. | Zhang JH, Zheng C, Zhu XJ, Zhang X, Zhou ZH, Li M, Gao YQ, Sun XH. [Anti-inflammation antiviral effects and mechanisms of Bushen recipe based on MAVS mediated signal pathway]. Zhongxiyi Jiehe Ganbing Zazhi. 2019;155-158. |
| 11. | Li X, Zhou D, Chi X, Li Q, Wang L, Lu B, Mao D, Wu Q, Wang X, Zhang M, Xue J, Li Y, Lu W, Guo J, Jiang F, Zhang X, Li Z, Yang X, Guo H, Gan D, He L, Luo L, Zhang L, Du H, Ye Y. Entecavir combining Chinese herbal medicine for HBeAg-positive chronic hepatitis B patients: a randomized, controlled trial. Hepatol Int. 2020;14:985-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Ye YA, Tian DL, Jiang J, Li J, Chen JJ, Li ZH, Ma WG, Zhao YM, Wang RB, Yang SZ, Shao FZ, Ji G, Zhou DQ, Liu TJ, Cheng DS, Zhang W, Sun KW, Wang YF, Min LQ, Li XK. Effect of Shuanghu Qinggan Granule () and Yigan Yiqi Jieyu Granule () plus lamivudine on chronic hepatitis B patients: A randomized double-blind placebo-controlled trial. Chin J Integr Med. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Zhu LC, Bi X, Chen XY. [Influence and Efficacy of Shuanghu Qinggan Granules on IL-10 and TGF-β in Patients with Chronic Hepatitis B]. Zhongyiyao Xinxi. 2017;6:74-77. [DOI] [Full Text] |
| 14. | Chen YJ, Tong GD, He JS, Xing YF, Gao H, Zhou XZ, Qiu M, Zheng YJ, Xu WJ, Xu SM, Chen L, Tang HH, Zhang L, Zhan BL, Ma WF, Sun XF, Li Q, Zhang XH, Zhou DQ. [Antiviral therapeutic effects of Bushen Jianpi prescription on hepatitis B virus carriers with positive e antigen]. Zhongxiyi Jiehe Ganbing Zazhi. 2012;4:200-204. |
| 15. | Chen YJ, Zhou DQ, Tong GD, He JS, Xing YF. [Immunomodulatory effect of the Bushen Jianpi recipe on HBeAg-positive hepatitis B virus carriers]. Zhongyi Linchuang Yanjiu. 2013;12:1-5. [DOI] [Full Text] |
| 16. | Lu LG, Zeng MD, Mao YM, Li JQ, Wan MB, Li CZ, Chen CW, Fu QC, Wang JY, She WM, Cai X, Ye J, Zhou XQ, Wang H, Wu SM, Tang MF, Zhu JS, Chen WX, Zhang HQ. Oxymatrine therapy for chronic hepatitis B: a randomized double-blind and placebo-controlled multi-center trial. World J Gastroenterol. 2003;9:2480-2483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Lu LG, Zeng MD, Mao YM, Wan MB, Li CZ, Chen CW, Fu QC, Wang JY, She WM, Cai X, Ye J, Zhou XQ, Wang H, Wu SM, Tang MF, Zhu JS, Chen WX. [Oxymatrine in the treatment of chronic hepatitis B for one year: a multicenter random double-blind placebo-controlled trial]. Zhonghua Gan Zang Bing Za Zhi. 2004;12:597-600. [PubMed] |
| 18. | Dong Y, Xi H, Yu Y, Wang Q, Jiang K, Li L. Effects of oxymatrine on the serum levels of T helper cell 1 and 2 cytokines and the expression of the S gene in hepatitis B virus S gene transgenic mice: a study on the anti-hepatitis B virus mechanism of oxymatrine. J Gastroenterol Hepatol. 2002;17:1299-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Gu XB, Yang XJ, Hua Z, Lu ZH, Zhang B, Zhu YF, Wu HY, Jiang YM, Chen HK, Pei H. Effect of oxymatrine on specific cytotoxic T lymphocyte surface programmed death receptor-1 expression in patients with chronic hepatitis B. Chin Med J (Engl). 2012;125:1434-1438. [PubMed] |
| 20. | Xia Y. [Systematic study on the treatment of chronic hepatitis B using licorice preparations and Phyllanthus urinaria]. PhD Thesis. Beijing University of Traditional Chinese Medicine. |
| 21. | Yu LN, Wang ZY, Zi CX, Mei ZG. [Efficacy and safety of modified Xiaoyao Powder against chronic hepatitis B: A Meta-analysis]. Zhongcaoyao. 2022;24:7831-7842. |
| 22. | Zhou YF, Liu HY, Fang JX, Dong Y, Qiao Y, Zhang RR, Zhang P. [Meta-analysis of Liuwei Wuling Tablets Combined with Entecavir in the Treatment of Chronic Hepatitis B]. Zhongguo Yaofang. 2017;36:5111-5115. [DOI] [Full Text] |
| 23. | He X, Yang YX, Wen JX, Zhao YL, Zhang L, Zhou HQ, Li Y, Lu XH, Huang F. [Systematic Review on Liuweiwuling Tablets Combined with Nucleotide Analogues in Treatment of Chronic Hepatitis BΔ]. Zhongguo Yiyuan Yongyao Pingjia Yu Fenxi. 2017;1:1-6. [DOI] [Full Text] |
| 24. | Lou Y. [Mesh meta-analysis of 5 Chinese patent medicine combined with Entecavir in treatment of liver fibrosis or cirrhosis of Chronic hepatitis B]. PhD Thesis. Hubei University of Chinese Medicine. |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/