Published online May 27, 2025. doi: 10.4254/wjh.v17.i5.106916
Revised: April 7, 2025
Accepted: May 7, 2025
Published online: May 27, 2025
Processing time: 77 Days and 15.6 Hours
The Mac-2 binding protein glycosylated isomer (M2BPGi) is a serum marker for fibrosis that correlates with the fibrosis stages in various liver diseases.
To examine the M2BPGi’s threshold for staging fibrosis in patients with chronic hepatitis B (CHB), and its changes during treatment.
This was a prospective, longitudinal study. A total of 348 eligible patients were recruited from the Hepatology Department, Medic Medical Center between March 2020 and December 2023. Liver enzyme tests, platelet counts, M2BPGi levels, and FibroScan were conducted at baseline and at 3-month intervals until six months post-treatment. Correlation plots of M2BPGi, FibroScan, and the other parameters were generated. Receiver operating characteristic curves were constructed for M2BPGi and the other parameters to evaluate their performance.
M2BPGi levels correlated well with FibroScan results and increased as the fibrosis stage advanced. The median M2BPGi levels at the different stages of fibrosis showed statistically significant differences. The cut-off values of M2BPGi for diagnosing significant fibrosis (F ≥ 2), advanced fibrosis (F3), and cirrhosis (F4) were determined to be 1.08, 1.4, and 1.52, respectively. In the context of fibrosis regression in CHB patients during the first 6-month of treatment, M2BPGi levels appeared to decrease before this pattern occurred in the FibroScan results.
M2BPGi levels were strongly correlated with FibroScan. M2BPGi can be used to assess liver fibrosis, and to serve as a tool for monitoring fibrosis regression in CHB patients undergoing treatment.
Core Tip: The study highlights the innovative use of the Mac-2 binding protein glycosylated isomer (M2BPGi) as a non-invasive serum marker for assessing liver fibrosis in patients with chronic hepatitis B. It establishes specific cut-off values for M2BPGi to distinguish between significant fibrosis (F ≥ 2), advanced fibrosis (F3), and cirrhosis (F4). The research demonstrates a strong correlation between M2BPGi levels and transient elastography results, suggesting its reliability. Moreover, the study reveals M2BPGi’s potential in monitoring fibrosis regression during antiviral treatment, offering a valuable tool for clinical practice, particularly when advanced imaging options are unavailable.
- Citation: Pham TTT, Ho DT, Phan HT, Nguyen TB, Nguyen KM. Assessing the role of Mac-2 binding protein glycosylation isomer in the management of patients with chronic hepatitis B. World J Hepatol 2025; 17(5): 106916
- URL: https://www.wjgnet.com/1948-5182/full/v17/i5/106916.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i5.106916
Hepatitis B is a liver disease caused by the hepatitis B virus (HBV). Approximately 80–90% of infants infected in the first year of life and 30%–50% of children infected in the first 5 years of life develop chronic infections. Chronic HBV (CHB) can progress to liver fibrosis, cirrhosis, hepatocellular carcinoma, and death[1]. A 2020 World Health Organization (WHO) report estimated that 295.9 million (3.8%) people had chronic HBV infection, and more than 3.0 million had new infections with HBV and hepatitis C virus (HCV) in 2019[2]. The number of HBV-related chronic liver disease deaths was estimated to be 523003 in 2019, and this number is expected to increase by 2030[3]. Vietnam is a low-medium income country where HBV is endemic, and has the 9th highest burden of HBV infection globally[4]. A recent study found that the prevalence of hepatitis B was 9.0% and higher in men and older people[5]. A recent study in countries with high viral hepatitis burdens found that Vietnam has a low policy score for the management of viral hepatitis[6]. Consequently, there is an imperative need to implement treatment and prevention strategies for HBV infections in Vietnam.
As mentioned above, hepatitis B can progress to cirrhosis and hepatocellular carcinoma. This progression occurs in a series of stages of liver fibrosis from non-cirrhotic to cirrhotic. Thus, fibrosis staging is crucial for treatment initiation. According to the 2024 WHO Guidelines for the Prevention, Diagnosis, Care, and Treatment of People with Chronic Hepatitis B Infection, treatment is recommended in adults and adolescents with CHB if they are diagnosed with significant fibrosis (F ≥ 2) or cirrhosis (F4), regardless of viral load and alanine transaminase (ALT) levels[7]. CHB can be treated with nucleoside analogs (NAs), such as entecavir and tenofovir. These drugs can induce regression and reversal of fibrosis, thereby reducing hepatocellular cancer development and death[8]. Liver biopsy is the gold standard method for staging fibrosis. However, it is invasive, difficult to repeat, and impractical for large-scale screening[9]. Radiologic methods include ultrasound elastography (vibration-controlled transient elastography, point-shear wave elastography, two-dimensional shear wave elastography) and magnetic resonance elastography, which are highly accurate and reproducible[10]. However, they require expensive equipment, which may not be practical in low- to middle-income countries and resource-limited settings. Furthermore, transient elastography cannot be performed in patients with morbid obesity or ascites. Serum marker levels were also assessed. These include aspartate transaminase (AST)/alanine transaminase (ALT) ratio (AAR), Aspartate Aminotransferase to Platelet Ratio Index (APRI), Fibrosis-4 (FIB-4) (age, AST, ALT, platelet count)[10]. These parameters are easy to obtain as they are part of standard laboratory tests. However, these methods have moderate accuracy and some of these parameters can change in other liver diseases.
The Mac-2 binding protein glycosylation isomer (M2BPGi) is a novel serum biomarker that correlates with the stage of liver fibrosis[11]. Other studies have found that M2BPGi can be used to track the regression of liver fibrosis after direct-acting antiviral treatment in hepatitis C infection[12], to diagnose liver fibrosis and cirrhosis in hepatitis B patients[13,14], and shows a good correlation with APRI and FIB-4[15]. Therefore, M2BPGi may be a potential test for tracking fibrosis regression in patients with HBV infection after antiviral therapy.
The objectives of this study were to define the M2BPGi cutoffs for fibrosis stages using transient elastography (FibroScan) as a reference method, determine the correlation between different methods in liver fibrosis stages, and evaluate the change in M2BPGi levels during treatment follow-up.
An observational study screened a total of 400 patients with CHB at Hepatology Department (Medic Medical Center) from September 2020 to July 2024, of whom 54 met the exclusion criteria. At the same time, we recruited 53 healthy volunteers for this study to observe M2BPGi levels in the normal population. Finally, 346 patients with CHB signed an informed consent form and were recruited for this study. Patients with F2-F4 Liver fibrosis received tenofovir disoproxil fumarate (TDF) 300 mg, one pill per day. The treatment lasted 12 months. During treatment, M2BPGi, FibroScan, APRI, FIB-4, and Hepatitis B surface Antigen (HbsAg) were tested at 3 and 6 months after treatment initiation.
Participants were recruited from the Hepatology Department, Medic Medical Center. The inclusion criteria were as follows: (1) Age ≥ 18 years; (2) Chronic HBV (positive HBsAg last > 6 months, IgM negative); (3) Infection with HBV alone, determined by investigators’ clinical assessment, routine laboratory tests, and patient history; (4) Treatment-naïve; or history of antiviral treatment but discontinuation for 3 months or longer; (5) Mild fibrosis status (F0-1/ Fibroscan); (6) Eligibility for antiviral treatment; and (7) Eligibility for FibroScan evaluation. The exclusion criteria were as follows: (1) HCV or human immunodeficiency virus (HIV) coinfection based on investigators’ clinical assessment, routine laboratory tests, and patient history; (2) Pregnant or potentially pregnant patients without contraceptive measures; (3) Cancer; (4) Severe cardiovascular or respiratory diseases; (5) End-stage renal failure; (6) Non-alcoholic steatohepatitis; (7) Non-alcoholic fatty liver disease; (8) Moderate or frequent alcohol consumption; and (9) Blood samples that did not meet the study requirements. Healthy volunteer samples serving as controls were collected from annual health examinations. The volunteers had normal blood cell counts, negative HbsAg, non-reactive anti-HCV, non-reactive HIV, normal liver function tests, normal renal function, and no symptoms or signs of liver disease.
Hematological and biochemical tests were performed using standard assays. FIB-4 index was calculated using the formula: FIB-4 = [age (years) ×AST (U/L)/platelet count (109/L) × √ALT (U/L)][16]. APRI was calculated using the formula [(AST/upper limit of the normal AST range) × 100]/platelet count[16].
Transient elastography using FibroScan Touch 502 and Echosens was used as the reference method for liver fibrosis, and the cutoff for fibrosis staging was based on the Vietnamese National Guidelines. F0-1 (no or mild fibrosis): < 7.0 kilopascal (Kpa); F2 (significant fibrosis): 7.0 - < 9.5 Kpa; F3 (advanced fibrosis): 9.5 - < 11 Kpa; F4 (cirrhosis): ≥ 11 Kpa.
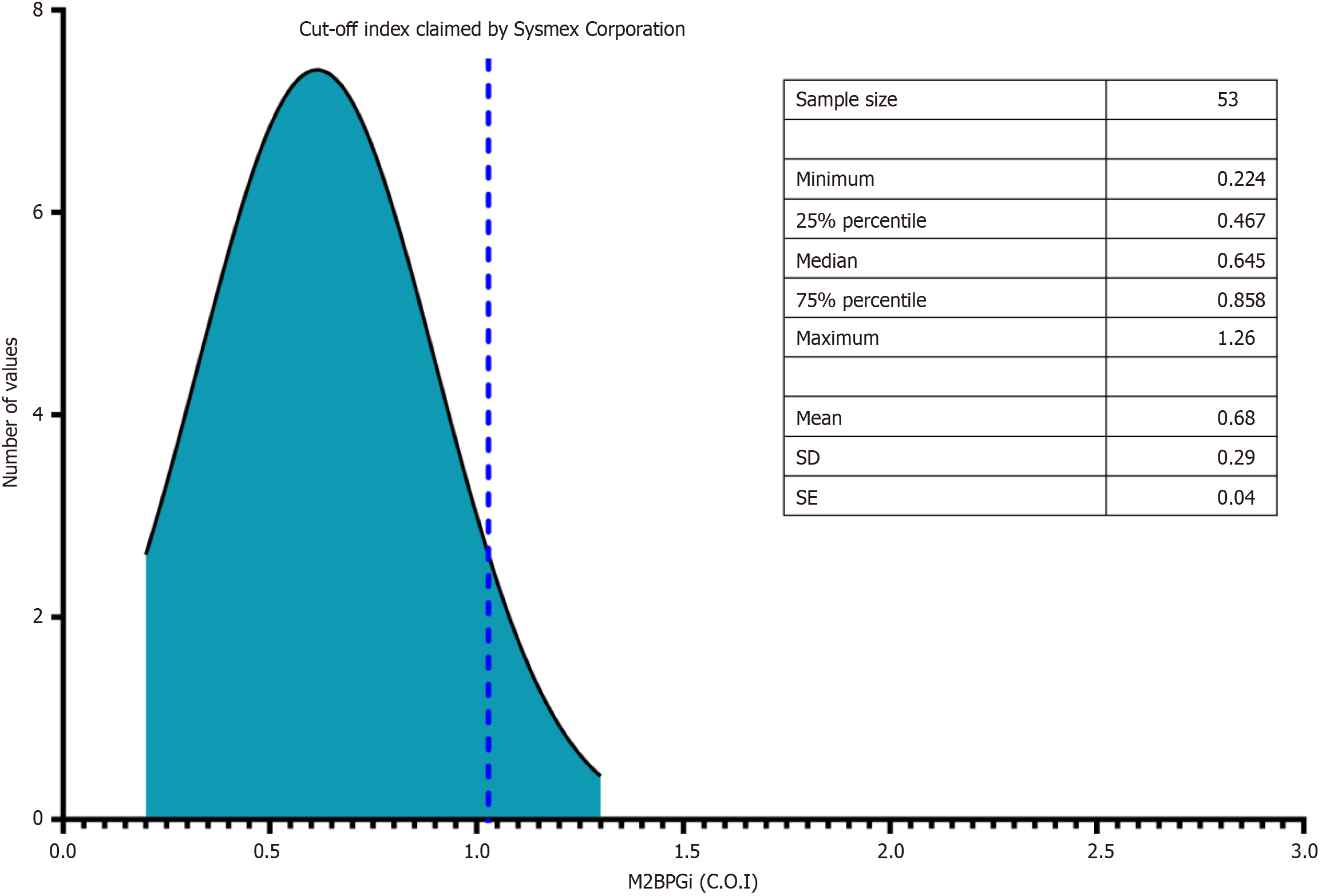
Sysmex HISCL-5000 [Sysmex Corporation, Japan] was used to measure blood M2BPGi levels and was expressed as a cutoff index (C.O.I).
C.O.I = (light intensity in sample-light intensity in negative control) (light intensity in positive control-light intensity in negative control)
The sample is deemed positive when the light intensity is above or equal to 1 C.O.I and negative when the light intensity is below 1 C.O.I.
Data were analyzed using PRISM GraphPad v10.0. The Kruskal-Wallis test was used for inter-group comparisons, and a difference was considered statistically significant at P < 0.05. The Dunn’s test was used for multiple comparisons. The Youden index was used to determine the optimal C.O.I for M2BPGi. Receiver operating characteristic (ROC) curve analysis was performed, and the area under the ROC curve (AUROC) was used to discriminate between the different fibrosis stages.
Most subjects were male (n = 206), and most subjects had F2 stage fibrosis (n = 105). The mean age was 42.6 ± 0.65 years (Table 1). We further stratified the patients according to the fibrosis stage. Table 2 shows that there were significant differences in age, platelet count, AST, ALT, FibroScan, M2BPGi, FIB-4, and APRI between the patients with different fibrosis stages.
| Variable | CHB patients (n = 346) |
| Sex | |
| Male | 206 (59.5) |
| Female | 140 (40.5) |
| Age (years) | 42.6 ± 0.65 |
| Fibrosis stage (n) | |
| F0 | 37 |
| F1 | 83 |
| F2 | 105 |
| F3 | 48 |
| F4 | 73 |
| Fibroscan (kPa) | 7.7 (0.4-46.5) |
| Platelet count (109/L) | 213 (49-394) |
| AST (µl/L) | 29 (12.9-235.6) |
| ALT (µl/L) | 31.9 (6.2-191.8) |
| Hemoglobin (g/L) | 14.5 (4.6-18.4) |
| WBC (109/L) | 6.6 (3.6-15.8) |
| M2BPGi (C.O.I) | 1.02 (0.19-12.9) |
| FIB-4 | 1.0 (0.32-11.3) |
| APRI | 0.38 (0.11-6.93) |
| Variable | F0 (n = 37) | F1 (n = 83) | F2 (n = 105) | F3 (n = 48) | F4 (n = 73) | Overall P value (< 0.05) |
| Male/Female | 12/25 | 40/43 | 69/36 | 31/17 | 54/19 | |
| Age (years) | 37 ± 8.6 | 40 ± 10.8 | 41.1 ± 11.6 | 44.2 ± 12.0 | 48.1 ± 13.2 | < 0.0001 |
| Platelet (109/L) | 232.0 (202.5-260) | 235 (210-275) | 212 (184.8-244.5) | 191 (166.5-252.8) | 167 (130.5-207.5) | < 0.0001 |
| AST (IU/L) | 21.8 (18.7-25.3) | 23.4 (19.5-26.5) | 34.4 (26.3-47.8) | 40.1 (28.6-57.3) | 45.0 (35.4-73.4) | < 0.0001 |
| ALT (IU/L) | 23.1 (14.6-26.9) | 21.7 (16.4-27.1) | 39.0 (27.6-67.4) | 49.9 (37.0-76.3) | 52.3 (33-73) | < 0.0001 |
| Hemoglobin (g/L) | 13.8 (13.1-15) | 14.3 (13.5-15.5) | 14.8 (13.9-15.9) | 14.6 (13.7-16.2) | 14.7 (13.7-16.1) | 0.006 |
| WBC (109/L) | 6.4 (5.8-7.9) | 6.5 (5.8-7.5) | 6.7 (5.5-8.5) | 6.8 (6.0-8.3) | 6.5 (5.6-8.1) | 0.621 |
| Fibroscan (kPa) | 4.6 (4-4.9) | 6.0 (5.5-6.4) | 7.8 (7.5-8.4) | 10.5 (9.8-11.5) | 22.3 (17.3-31.6) | < 0.0001 |
| M2BPGi (C.O.I) | 0.66 (0.51-1) | 0.76 (0.51-1.01) | 0.93 (0.74-1.34) | 1.54 (1.13-1.94) | 3.2 (1.56-3.9) | < 0.0001 |
| FIB-4 | 0.74 (0.58-0.94) | 0.83 (0.65-1.03) | 1.08 (0.75-1.5) | 1.28 (0.93-2.2) | 2.18 (1.5-3.4) | < 0.0001 |
| APRI | 0.25 (0.21-0.31) | 0.26 (0.21-0.33) | 0.47 (0.34-0.72) | 0.68 (0.43-1.1) | 1.37 (0.69-3.19) | < 0.0001 |
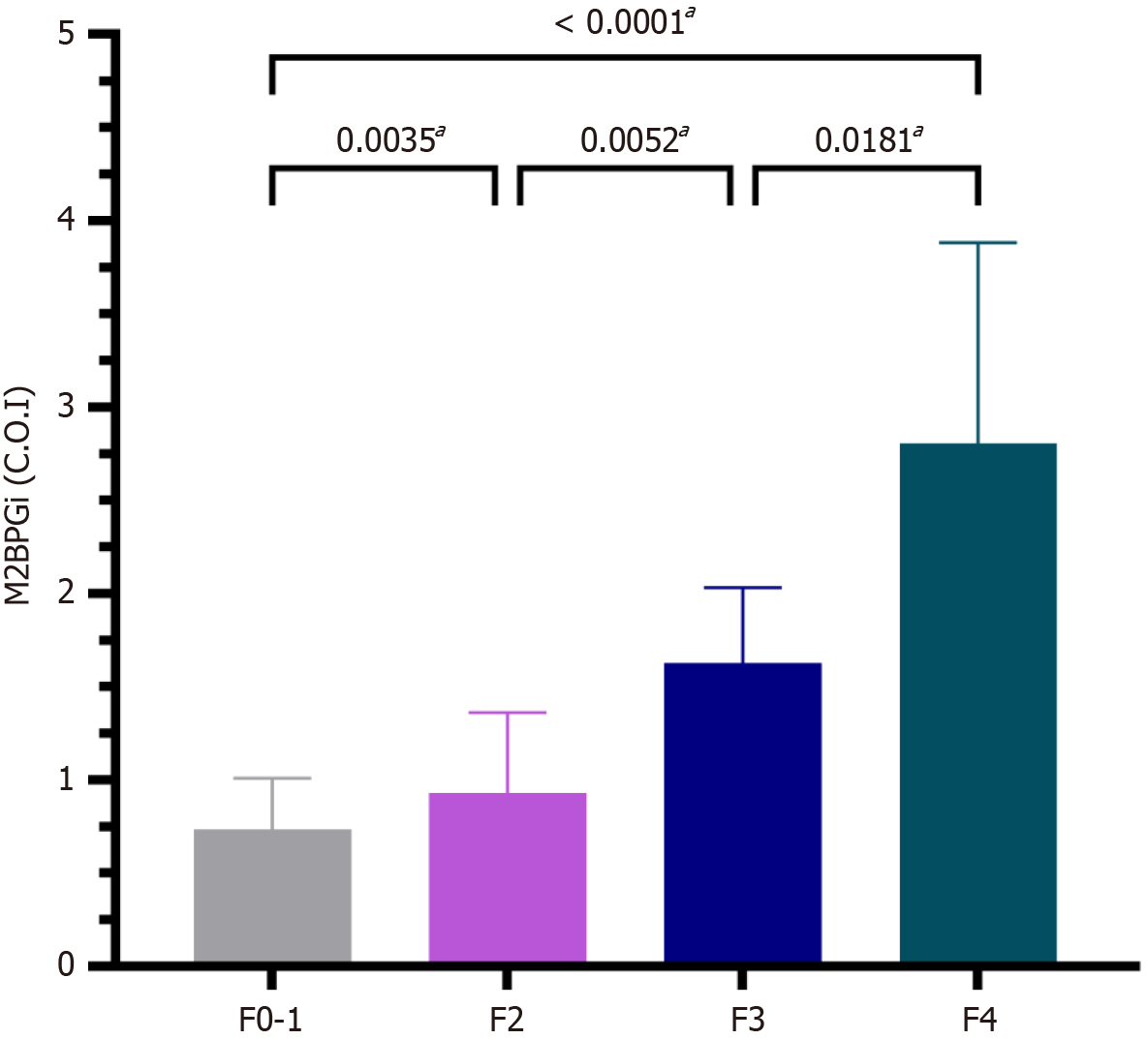
The M2BPGi level in healthy volunteers was 0.68 ± 0.29 and lower than the threshold stated by the manufacturer (Figure 1). Figure 2 shows that M2BPGi levels increased as the fibrosis stage increased, which is consistent with previous studies. The difference in M2BPGi levels between the different fibrosis stages was statistically significant (F2 vs F0-1, P = 0.0035; F3 vs F2, P = 0.0052; F4 vs F3, P = 0.0181; F4 vs F0-1, P < 0.001).
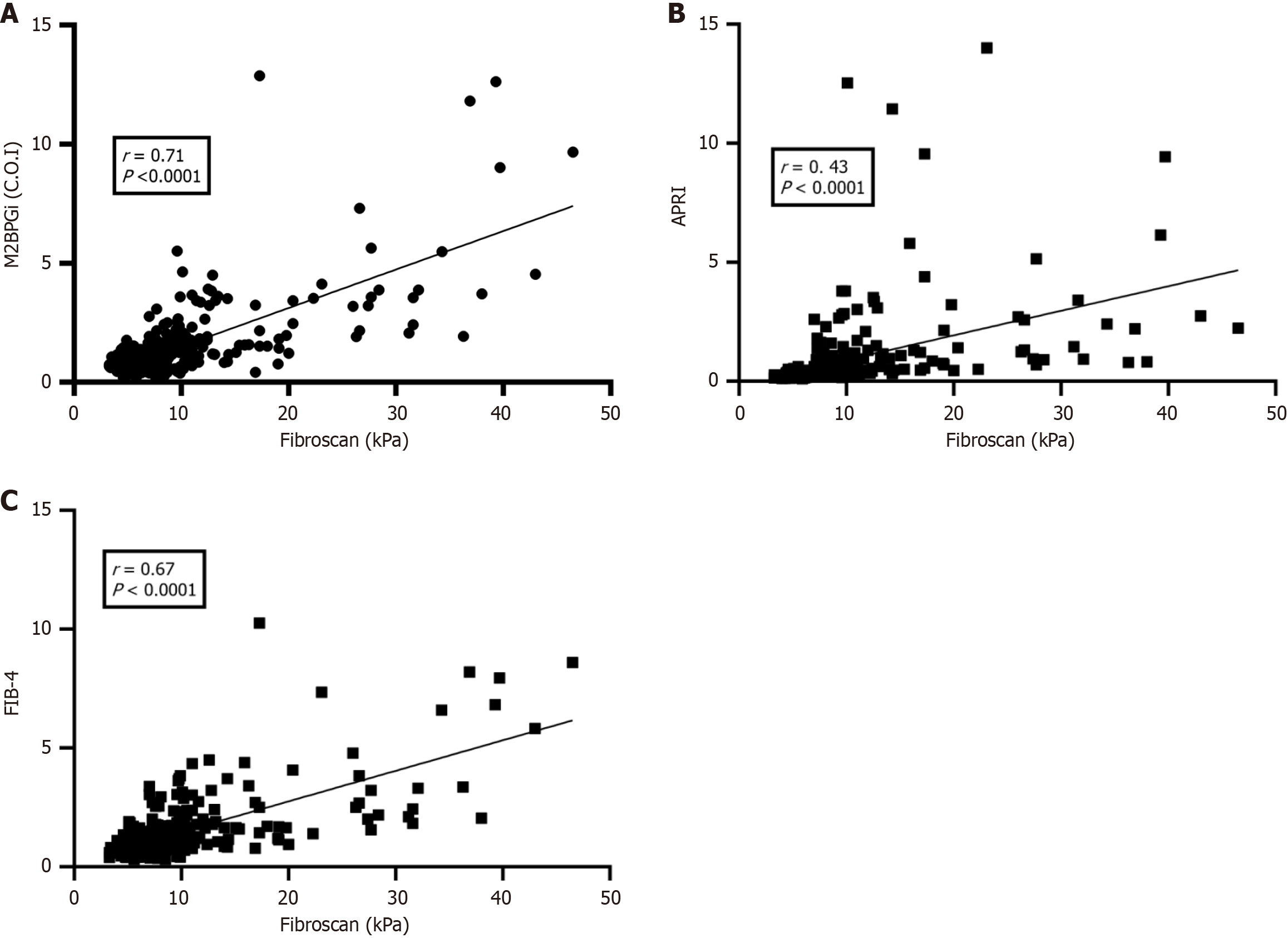
FibroScan results were used as a reference for comparison with APRI, FIB-4, and M2BPGi. The results showed that M2BPGi (Figure 3A) had a better correlation with FibroScan than with APRI (Figure 3B) and FIB-4 (Figure 3C) (all P < 0.001).
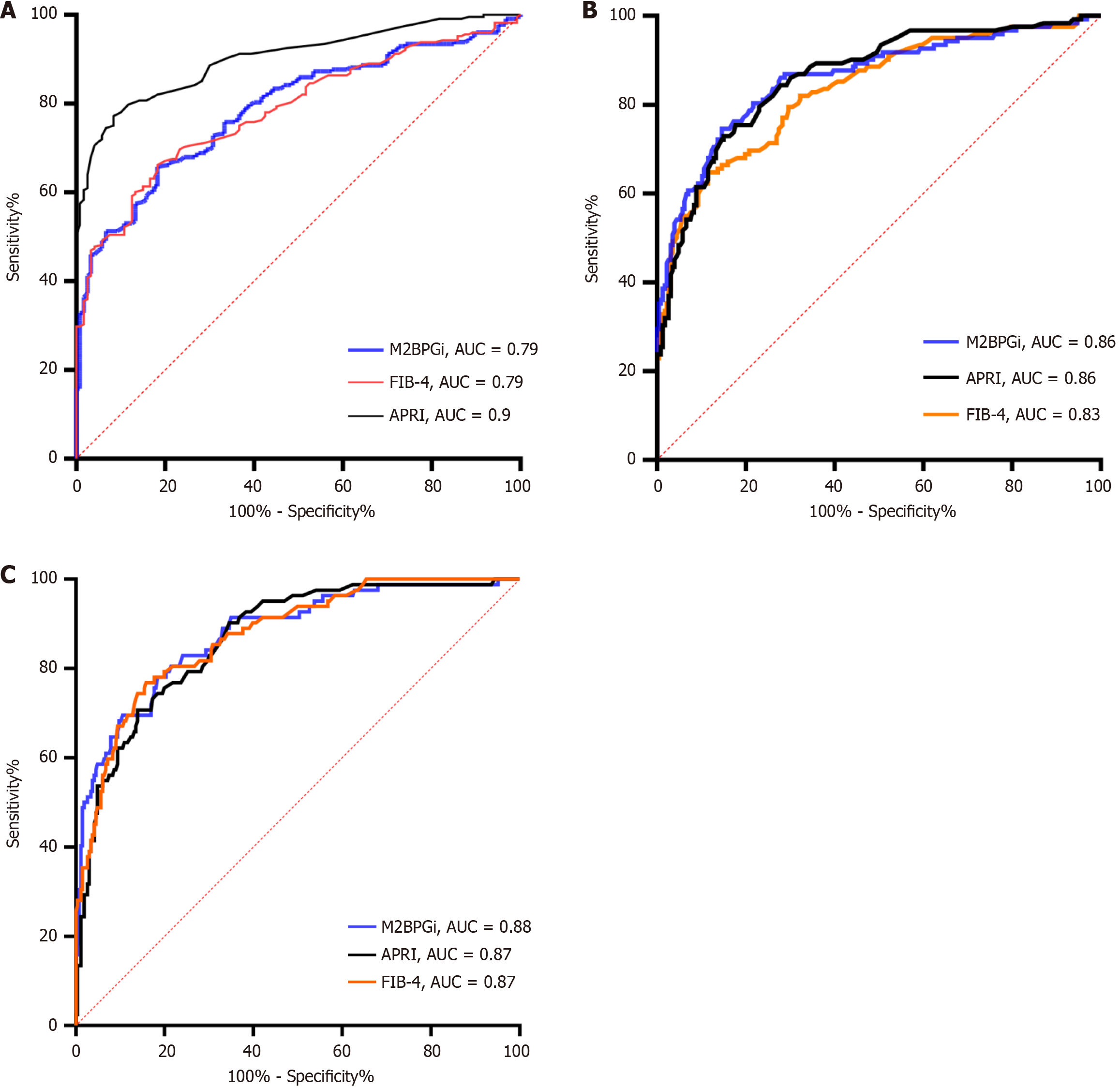
We further stratified the patients into significant fibrosis (F ≥ 2), advanced fibrosis (F3), and cirrhosis (F4) subgroups to determine the M2BPGi cutoff values for each stage. The AUROC values for M2BPGi were just above 0.80 and above in the three different subgroups, indicating its effectiveness in determining fibrosis stages. For diagnosing significant fibrosis
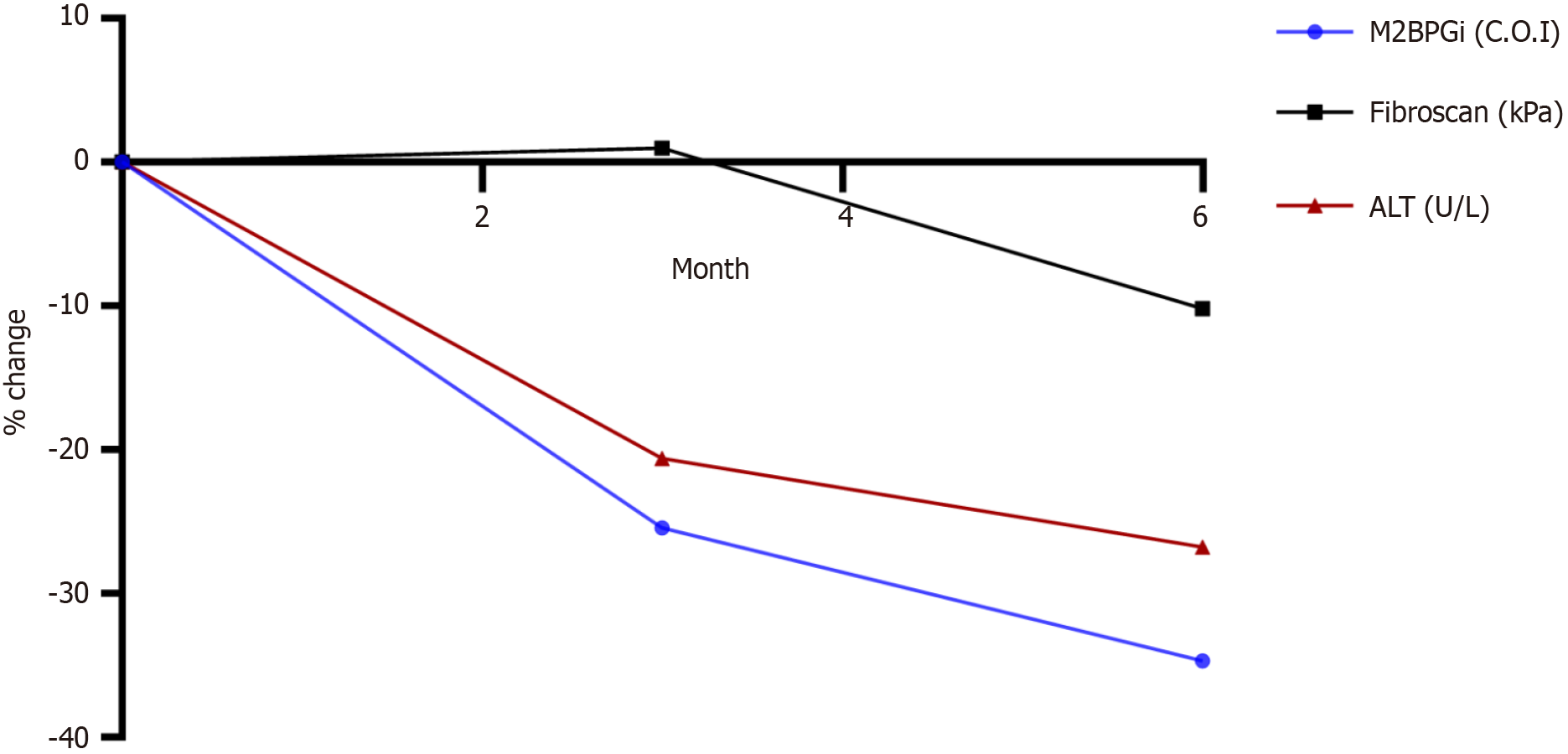
The patients were followed up for six months during treatment. The correlation between M2BPGi and Fibroscan was 0.72 (95%CI: 0.62-0.8). The results showed that ALT, M2BPGi, and FibroScan results decreased as treatment duration increased (Figure 5). From Figure 5, we can see that M2BPGi decreased from 1.73 at baseline to 1.13 at Month 6, while ALT decreased from 56.8 IU/mL at baseline to 30.05 IU/mL at Month 6 and Fibroscan decreased from 10.3 at baseline to 9.25 at Month 6.
M2BPGi is an extracellular matrix component secreted by many different cell types, such as hepatocytes, hepatic stellate cells, and fibroblasts, and induces profibrotic cytokine expression in Kupffer cells to induce fibrogenesis[17]. Therefore, its levels are correlated with the stages of fibrosis. Many studies have demonstrated that M2BPGi is associated with liver fibrosis of different etiologies, such as hepatitis B, hepatitis C, and non-alcoholic fatty liver disease[18-20]. Furthermore, M2BPGi has been shown to be a predictor of hepatocellular carcinoma in patients[21-23].
We investigated M2BPGi levels in a healthy population before measuring the M2BPGi levels in patients with CHB. A total of 53 healthy volunteers were recruited, and M2BPGi was measured in their annual health examinations. The median M2BPGi level in this population (0.68 C.O.I) appeared to be significantly lower than the cutoff of 1 C.O.I claimed by Sysmex, which indicates a high probability of liver fibrosis; however, it was remarkably higher than that reported by Tangvoraphonkchai et al[24]. who found that the mean M2BPGi level in 63 donors was 0.1 C.O.I but is similar to Sulaiman et al[25], who obtained a result of 0.59 C.O.I.
We demonstrated M2BPGi performance in staging liver fibrosis in CHB patients, allowing us to start the treatment regimen in patients with significant fibrosis or higher. The results of our study are similar to those of previous studies. In our study, the proportion of patients with significant fibrosis (≥ F2) was 66%, which was similar to that reported by Bui et al[14], who conducted liver fibrosis in CHB patients in Vietnam. We found that M2BPGi levels increased with fibrosis stage and were correlated with FibroScan liver stiffness. Specifically, our results showed a positive correlation across the fibrosis stages. The medians of M2BPGi, APRI, and FIB-4 correlated with the severity of fibrosis. M2BPGi levels differed significantly among the stages (P < 0.0001). In a meta-analysis conducted by Tamaki et al[26], M2BPGi levels were different between various studies, and the mean M2BPGi levels according to liver fibrosis stages were 0.26–0.9 (F1), 0.34–1.36 (F2), 0.57–1.65 (F3) and 1.21–3,1(F4). In our study, we found that the median M2BPGi values in F0, F1, F2, F3, and F4 were 0.66, 0.77, 0.93, 1.63, and 2.81, respectively (P < 0.05). The M2BPGi levels in the non-fibrotic group appeared to be similar to those in the healthy group. A post-hoc analysis was performed to compare the M2BPGi levels between the four stages. We found that M2BPGi levels differed significantly at all stages, particularly in significant fibrosis vs no fibrosis and mild fibrosis. M2BPGi elevation was observed in the early stages of fibrosis, consistent with Bui et al[14], but their median M2BPGI at F2-3(0.865) was much lower than our result. William Keddeas et al[27] found that M2BPGi increased with fibrosis severity, with M2BGPi levels of 0.719, 1.322, 1.65, and 1.904 at F0-1, F2, F3, and F4, respectively. In another study by Yeh et al[28] in 160 CHB patients, fibrosis was stratified by biopsy; M2BPGi levels were 0.63, 0.64, 1.36, 1.65 and 2.7 in F0, F1, F2, F3, and F4, respectively. This result is similar to that of our study, particularly for mild fibrosis, advanced fibrosis, and cirrhosis.
With regard to the correlation, our results showed a good correlation between M2BPGi and FibroScan in naïve patients, similar to that reported by Bui et al[14] (r = 0.77, P < 0.0001), and an exact correlation was found in the study by Zou et al[29] (r = 0.614, P < 0.0001).
The performance of M2BGPi was comparable to that of FIB-4 and APRI for staging significant fibrosis. The cut-off generated was 1.08 C.O.I with 66% sensitivity and 82% specificity. Our result was nearly the same as the result of the study by Zou et al[29] in 680 CHB patients with a cut-off of 1.06 C.O.I, 60% sensitivity, and 82% specificity. Compared to the study by Bui et al[14], our cut-off was higher (1.08 vs 0.79) with a slightly higher sensitivity and specificity (66%, 82% vs 62%, 80%). The sensitivity of M2BPGi in our study was lower than that of FIB-4 and APRI, which differs from the study by Bui et al[14], who found that M2BPGi had the greatest sensitivity. This may be due to the higher C.O.I for M2BPGi used in our study (1.08) compared to that used by Bui et al[14] (0.79). In the study by Yeh et al[28], the diagnostic performance was similar to our result, with an area under the curve (AUC) of 0.78, and the cut-off was 1.345, which was higher than our cut-off with similar sensitivity and specificity (66% and 81%, respectively). However, the APRI showed a much poorer performance, with an AUC of 0.573, which indicated that the APRI was unable to differentiate significant fibrosis from mild conditions. The APRI performance in our study was excellent, which was due to the high liver function levels in the significant fibrosis and higher groups compared with other studies. Furthermore, our cutoff for staging significant fibrosis was significantly different from that of Tsuji et al[30], who found a cut-off of 0.89 C.O.I with 93% sensitivity and 82% specificity when they investigated 93 CHB patients and used biopsy to stratify fibrosis stages. M2BPGi also showed better performance than platelets, hyaluronic acid, type 4 collagen 7S, type III procollagen peptide, tissue inhibitor of metalloproteinase 1, N-terminal pro-peptide of type III collagen, FIB-4, APRI, and enhanced liver fibrosis[30].
With regard to advanced fibrosis diagnosis, we demonstrated that M2BPGi performed better in triaging advanced fibrosis than significant fibrosis, as the AUC, sensitivity, and specificity in our study were higher than those reported by Yeh et al[28]. Tsuji et al[30] found that M2BPGi showed good performance in differentiating advanced fibrosis from milder stages with an AUC of 0.865, an optimal cut-off of 0.45, 70% sensitivity, and 74% specificity.
For cirrhosis diagnosis, our results were similar to those of the previous studies. Specifically, Bui et al[14] established an M2BPGi cut-off of 1.4 with an excellent AUC of 0.91, and the highest sensitivity compared to APRI and FIB-4. Similarly, M2BPGi showed the highest AUC in cirrhosis diagnosis as opposed to APRI, FIB-4, AAR, and red cell distribution width to platelet ratio in the study by Wei et al[31], but the cut-off was higher, at 1.825 C.O.I, with 55% sensitivity and 94% specificity. In addition, the best performance of M2BPGi was also observed in the study of Yeh at el[28]. In summary, M2BPGi yielded the best performance in differentiating cirrhosis from lower fibrosis stages compared with APRI and FIB-4.
A combination of M2BPGi and other markers such as APRI[14] and AGAP score[32] has been found to increase the fibrosis prediction performance. In future studies, combinations of M2BPGi and other markers could be used to determine whether this would increase the sensitivity and specificity for fibrosis and cirrhosis.
In addition, we observed that M2BPGi levels changed during antiviral treatment. Considering the correlation between FibroScan and M2BPGi, we found that M2BPGi correlated well with FibroScan, and the coefficient was similar to that of the treatment-naïve group (0.72 vs 0.71). M2BPGi levels gradually decreased during 6 months of treatment. More specifically, M2BPGi levels decreased in the first 3 months of treatment before continuously decreasing in the next 3 months of treatment. This is consistent with previous studies showing that M2BPGi levels decrease in hepatitis B patients after treatment with PEGylated interferon-α[33] and NAs[21,34]. These studies and our results show that fibrosis regression occurred after treatment in patients with hepatitis B infection. In contrast, FibroScan showed a slight increase in the first three months before it decreased subsequently. Thus, the M2BPGi reduction is likely to be more significant before FibroScan starts to decrease. In this study, we also intentionally investigated M2BPGi changes simultaneously with the observation of ALT levels, which represent liver inflammation. We found that M2BPGi and ALT showed similar patterns. This implies that M2BPGi may be affected by liver inflammation. Uojima et al[35] also demonstrated that liver inflammation leads to elevated M2BPGi levels[35]. Compared to previous study, our result of M2BPGi changes during the course of treatment was similar to study of Mak et al[13], who found that M2BPGi decreases by 0.1 C.O.I after 1-year treatment (0.32 at baseline and 0.21 at 1-year measurement). Interestingly, the authors demonstrated that Ishak score decreased after 12 months in patients with decreased M2BPGi[13]. Zou et al[29] also reported that M2BPGi reduction occurred simultaneously with FibroScan reduction during a 96-week treatment, whereas FIB-4, APRI, and AAR did not show the same pattern. By monitoring NAs treatment for an average of 6.6 years (1.1–10.9 years), Murata et al[36] found that M2BPGi levels decreased gradually at each time point, namely baseline, week 48, and the last examination. In particular, it decreased sharply at the last examination compared to that at week 48 examination (0.59 vs 0.77, P < 0.001). Hence, M2BPGi levels can be used to evaluate pathological improvement during long-term treatment.
This study has some limitations. First, it was carried out in a Vietnamese population; therefore, the results cannot be generalized to other countries. However, studies in West Africa[37], China[13], South Korea[38], and Japan[26] have found that M2BPGi levels increase with fibrosis stage in patients with hepatitis B. Second, liver biopsy, the gold standard, was not used to determine the fibrosis stage because it is invasive and impractical for screening a large population of patients. However, elastography (FibroScan) was used as a reference in this study, and studies have shown that it has high concordance with liver biopsy[39]. Third, the number of patients with different fibrosis stages was not balanced, which may have led to a selection bias and affected the validity of the C.O.I determination. Finally, the number of patients undergoing NAs treatment in the follow-up stages dropped steeply as the coronavirus disease of 2019 pandemic impeded patients from regular visits. Therefore, we only collected data from patients who completed follow-up visits in the first six months of treatment, which hindered our results from demonstrating the M2BPGi value in a one-year follow-up in terms of the course of liver regression. Hence, a further comprehensive investigation on predicting the downward trend of fibrosis needs to be considered in the context of inflammation ruled out for study subjects in the future.
M2BPGi is an effective tool in the aid of staging fibrosis stages in HBV patients, particularly significant fibrosis (F2), advanced fibrosis (F3) and cirrhosis (F4) with cut-offs of 1.08 C.O.I, 1.4 C.O.I and 1.52 C.O.I, respectively. Furthermore, M2BPGi is useful for tracking fibrosis regression in patients with CHB to evaluate treatment response. This is the first study in Vietnam to investigate the M2BPGi cut-offs in each liver fibrosis stage and the dynamic changes in treatment response in patients with CHB. As a non-invasive test, M2BPGi can be used in conjunction with other blood tests to define the fibrosis status of patients with HBV. Early diagnosis enables proper treatment, thereby preventing disease progression. Finally, we found that dynamic changes in M2BPGi can provide reliable follow-up for fibrosis progression in patients undergoing treatment, especially when FibroScan or enhanced imaging tools are unavailable in hospital settings.
| 1. | GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:796-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 515] [Article Influence: 128.8] [Reference Citation Analysis (1)] |
| 2. | Cui F, Blach S, Manzengo Mingiedi C, Gonzalez MA, Sabry Alaama A, Mozalevskis A, Séguy N, Rewari BB, Chan PL, Le LV, Doherty M, Luhmann N, Easterbrook P, Dirac M, de Martel C, Nayagam S, Hallett TB, Vickerman P, Razavi H, Lesi O, Low-Beer D. Global reporting of progress towards elimination of hepatitis B and hepatitis C. Lancet Gastroenterol Hepatol. 2023;8:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 208] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 3. | Ou TY, Huy LD, Mayne J, Shih CL, Mai Xuan H, Thi Hong Nguyen N, Nguyen Hoai L, Thi My Bui L, Chang YM, Abdi AA, Hsu SC, Lin HJ, Huang CC. Global mortality of chronic liver diseases attributable to Hepatitis B virus and Hepatitis C virus infections from 1990 to 2019 and projections to 2030. J Infect Public Health. 2024;17:102443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 4. | Kim TV, Pham TND, Le DH, Dao DVB, Phan LTB, Le A, Trang A, Tang HK, Liu JJ, Dao DY. Significant gaps in hepatitis B vaccination in adults in Viet Nam: Important targets toward hepatitis B elimination by 2030. Vaccine. 2023;41:976-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Cam Huong NT, Van Luu N, Nam NH, Ghula S, Atieh Qarawi AT, Mai Truc PT, Trung An DN, Huy NT, Le Hoa PT. Prevalence of hepatitis B virus infection in health checkup participants: a cross-sectional study at University Medical Center, Ho Chi Minh City, Vietnam. Hosp Pract (1995). 2023;51:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Palayew A, Razavi H, Hutchinson SJ, Cooke GS, Lazarus JV. Do the most heavily burdened countries have the right policies to eliminate viral hepatitis B and C? Lancet Gastroenterol Hepatol. 2020;5:948-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | World Health Organization. Guidelines for the prevention, diagnosis, care and treatment for people with chronic hepatitis B infection. 2024 Available from: https://www.who.int/publications/i/item/9789240090903. |
| 8. | Kumada T, Toyoda H, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Niinomi T, Yasuda S, Andou Y, Yamamoto K, Tanaka J. Effect of nucleos(t)ide analogue therapy on hepatocarcinogenesis in chronic hepatitis B patients: a propensity score analysis. J Hepatol. 2013;58:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Sharma S, Khalili K, Nguyen GC. Non-invasive diagnosis of advanced fibrosis and cirrhosis. World J Gastroenterol. 2014;20:16820-16830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 125] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 10. | Lai JC, Liang LY, Wong GL. Noninvasive tests for liver fibrosis in 2024: are there different scales for different diseases? Gastroenterol Rep (Oxf). 2024;12:goae024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 11. | Toshima T, Shirabe K, Ikegami T, Yoshizumi T, Kuno A, Togayachi A, Gotoh M, Narimatsu H, Korenaga M, Mizokami M, Nishie A, Aishima S, Maehara Y. A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA(+)-M2BP), for assessing liver fibrosis. J Gastroenterol. 2015;50:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 12. | Nozaki A, Chuma M, Hara K, Moriya S, Fukuda H, Numata K, Tanaka K, Morimoto M, Sakamaki K, Yamanaka T, Kondo M, Maeda S. Sofosbuvir-based therapies associated with regression of liver fibrosis in patients with hepatitis C virus infection: A prospective observational study. Medicine (Baltimore). 2021;100:e25110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Mak LY, Wong DK, Cheung KS, Seto WK, Lai CL, Yuen MF. Role of serum M2BPGi levels on diagnosing significant liver fibrosis and cirrhosis in treated patients with chronic hepatitis B virus infection. Clin Transl Gastroenterol. 2018;9:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Bui HH, Nguyen ST, Phan ST, Nguyen KM, Nguyen CD. Evaluating M2BPGi as a Marker for Liver Fibrosis in Patients with Chronic Hepatitis B. Dig Dis Sci. 2023;68:4407-4417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Pham TTT, Ho DT, Nguyen T. Usefulness of Mac-2 binding protein glycosylation isomer in non-invasive probing liver disease in the Vietnamese population. World J Hepatol. 2020;12:220-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. 2015;61:292-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 413] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 17. | Shirabe K, Bekki Y, Gantumur D, Araki K, Ishii N, Kuno A, Narimatsu H, Mizokami M. Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: more than a biomarker of liver fibrosis. J Gastroenterol. 2018;53:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 18. | Cheung KS, Seto WK, Wong DK, Mak LY, Lai CL, Yuen MF. Wisteria floribunda agglutinin-positive human Mac-2 binding protein predicts liver cancer development in chronic hepatitis B patients under antiviral treatment. Oncotarget. 2017;8:47507-47517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Saleh SA, Salama MM, Alhusseini MM, Mohamed GA. M2BPGi for assessing liver fibrosis in patients with hepatitis C treated with direct-acting antivirals. World J Gastroenterol. 2020;26:2864-2876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Kiyoaki I, Sumida Y, Nakade Y, Okumura A, Nishimura S, Ibusuki M, Kitano R, Sakamoto K, Kimoto S, Inoue T, Kobayashi Y, Fukuzawa Y, Yoneda M. Mac-2 binding protein glycosylation isomer, the FIB-4 index, and a combination of the two as predictors of non-alcoholic steatohepatitis. PLoS One. 2022;17:e0277380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 21. | Chen CH, Hu TH, Wang JH, Lai HC, Hung CH, Lu SN, Peng CY. A Mac-2 Binding Protein Glycosylation Isomer-Based Risk Model Predicts Hepatocellular Carcinoma in HBV-Related Cirrhotic Patients on Antiviral Therapy. Cancers (Basel). 2022;14:5063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Jun T, Hsu YC, Ogawa S, Huang YT, Yeh ML, Tseng CH, Huang CF, Tai CM, Dai CY, Huang JF, Chuang WL, Yu ML, Tanaka Y, Nguyen MH. Mac-2 Binding Protein Glycosylation Isomer as a Hepatocellular Carcinoma Marker in Patients With Chronic Hepatitis B or C Infection. Hepatol Commun. 2019;3:493-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Liu J, Hu HH, Lee MH, Korenaga M, Jen CL, Batrla-Utermann R, Lu SN, Wang LY, Mizokami M, Chen CJ, Yang HI. Serum Levels of M2BPGi as Short-Term Predictors of Hepatocellular Carcinoma in Untreated Chronic Hepatitis B Patients. Sci Rep. 2017;7:14352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 24. | Tangvoraphonkchai K, Suttichaimongkol T, Kularbkaew C, Sangaimwibool P, Sukeepaisarnjaroen W. Application of Mac-2 binding protein glycosylation isomer as a non-invasive biomarker for probing liver disease. Sci Rep. 2022;12:6757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 25. | Sulaiman AS, Hasan I, Hustrini NM, Lydia A, Hanifa RS, Gani RA. Diagnostic performance of Mac-2-binding protein glycosylation isomer (M2BPGi) as a liver fibrosis marker in chronic hepatitis C patients with chronic kidney disease on hemodialysis. Clin Exp Nephrol. 2023;27:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Tamaki N, Kurosaki M, Loomba R, Izumi N. Clinical Utility of Mac-2 Binding Protein Glycosylation Isomer in Chronic Liver Diseases. Ann Lab Med. 2021;41:16-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 27. | William Keddeas M, Haroun Kaisar H, Ahmed Elessawy HA, Abdel Hamid Elewa MS. Role of serum M2BPGi levels in diagnosing significant liver fibrosis and cirrhosis in patients with chronic hepatitis B. QJM: An Int J Med. 2021;114. [DOI] [Full Text] |
| 28. | Yeh ML, Huang CF, Huang CI, Dai CY, Lin IH, Liang PC, Hsieh MH, Lin ZY, Chen SC, Huang JF, Chen JJ, Yu ML, Chuang WL. Wisteria floribunda agglutinin-positive Mac-2-binding protein in the prediction of disease severity in chronic hepatitis B patients. PLoS One. 2019;14:e0220663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Zou X, Zhu MY, Yu DM, Li W, Zhang DH, Lu FJ, Gong QM, Liu F, Jiang JH, Zheng MH, Kuno A, Narimatsu H, Zhang Y, Zhang XX. Serum WFA(+) -M2BP levels for evaluation of early stages of liver fibrosis in patients with chronic hepatitis B virus infection. Liver Int. 2017;37:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 30. | Tsuji Y, Namisaki T, Kaji K, Takaya H, Nakanishi K, Sato S, Saikawa S, Sawada Y, Kitagawa K, Shimozato N, Kawaratani H, Moriya K, Noguchi R, Akahane T, Mitoro A, Yoshiji H. Comparison of serum fibrosis biomarkers for diagnosing significant liver fibrosis in patients with chronic hepatitis B. Exp Ther Med. 2020;20:985-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Wei B, Feng S, Chen E, Li D, Wang T, Gou Y, Yang T, Zhang D, Tao C, Tang H. M2BPGi as a potential diagnostic tool of cirrhosis in Chinese patients with Hepatitis B virus infection. J Clin Lab Anal. 2018;32:e22261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Pramono LK, Tjandrawati A, Turbawaty DK, Rostini T, Bestari MB, Haryono, Budiman D, Nugraha P. Macrophage-2-Binding Protein Glycosylation Isomer (M2BPGi) and AGAP Score as Markers of Noninvasive Test for Liver Fibrosis versus FibroScan in Chronic Hepatitis B Patients: A Retrospective Observational Study. Int J Hepatol. 2024;2024:6635625. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Zhu MY, Chen PZ, Li J, Yu DM, Huang D, Zhu XJ, Han Y, Chen J, Huang W, Chen YY, Gong QM, Jiang JH, Zhang DH, Zhang Y, Zhang JM, Zhang XX. Serum M2BPGi level is a novel predictive biomarker for the responses to pegylated interferon-α treatment in HBeAg-positive chronic hepatitis B patients. J Med Virol. 2018;90:721-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Hsu YC, Jun T, Huang YT, Yeh ML, Lee CL, Ogawa S, Cho SH, Lin JT, Yu ML, Nguyen MH, Tanaka Y. Serum M2BPGi level and risk of hepatocellular carcinoma after oral anti-viral therapy in patients with chronic hepatitis B. Aliment Pharmacol Ther. 2018;48:1128-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Uojima H, Yamasaki K, Sugiyama M, Kage M, Ishii N, Shirabe K, Hidaka H, Kusano C, Murakawa M, Asahina Y, Nishimura T, Iijima H, Sakamoto K, Ito K, Amano K, Kawaguchi T, Tamaki N, Kurosaki M, Suzuki T, Matsuura K, Taketomi A, Joshita S, Umemura T, Nishina S, Hino K, Toyoda H, Yatsuhashi H, Mizokami M. Quantitative measurements of M2BPGi depend on liver fibrosis and inflammation. J Gastroenterol. 2024;59:598-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 36. | Murata A, Amano N, Sato S, Tsuzura H, Tomishima K, Sato S, Matsumoto K, Shimada Y, Iijima K, Genda T. On-treatment Serum Mac-2 Binding Protein Glycosylation Isomer (M2BPGi) Level and Risk of Hepatocellular Carcinoma Development in Patients with Chronic Hepatitis B during Nucleot(s)ide Analogue Therapy. Int J Mol Sci. 2020;21:2051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Vincent JP, Ndow G, Ogawa S, Ceesay A, Njie R, Sanneh B, Baldeh I, D'Alessandro U, Mendy M, Thursz M, Chemin I, Tanaka Y, Lemoine M, Shimakawa Y. Mac-2 binding protein glycosylation isomer (M2BPGi) to evaluate liver fibrosis and cancer in HBV-infected patients in West Africa. J Glob Health. 2022;12:04076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Hur M, Park M, Moon HW, Choe WH, Lee CH. Comparison of Non-Invasive Clinical Algorithms for Liver Fibrosis in Patients With Chronic Hepatitis B to Reduce the Need for Liver Biopsy: Application of Enhanced Liver Fibrosis and Mac-2 Binding Protein Glycosylation Isomer. Ann Lab Med. 2022;42:249-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Shen QL, Chen YJ, Wang ZM, Zhang TC, Pang WB, Shu J, Peng CH. Assessment of liver fibrosis by Fibroscan as compared to liver biopsy in biliary atresia. World J Gastroenterol. 2015;21:6931-6936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/