Published online May 27, 2025. doi: 10.4254/wjh.v17.i5.105769
Revised: March 20, 2025
Accepted: March 20, 2025
Published online: May 27, 2025
Processing time: 110 Days and 5.9 Hours
In this article, we discuss the recently published article by Yang et al. This retro
Core Tip: Prognostic scoring systems, particularly the Lille model, play a crucial role in the treatment of alcoholic hepatitis by facilitating the early identification of patients who do not respond to corticosteroid therapy. The study by Yang et al highlights the predictive value of Lille model 3-7 and underlines the importance of timely treatment adjustment to minimize complications. The study also highlights the impact of nutritional deficits and renal dysfunction on mortality, suggesting a multidisciplinary approach that integrates early nutritional interventions and nephroprotective strategies to improve patient outcomes.
- Citation: Ramírez-Mejía MM, Morales-Galicia AE, Méndez-Sánchez N. Prognostic challenges in alcoholic hepatitis: From scoring systems to clinical predictors. World J Hepatol 2025; 17(5): 105769
- URL: https://www.wjgnet.com/1948-5182/full/v17/i5/105769.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i5.105769
In this article, we discuss the study by Yang et al[1], which we read with great interest in the latest issue of this journal. Alcohol consumption represents a major global health burden, representing a leading cause of preventable morbidity and mortality worldwide. Alcohol-related liver disease is the manifestation of the effect of alcohol on the liver, encompassing a clinical spectrum ranging from simple steatosis to severe forms such as steatohepatitis, fibrosis and ultimately liver failure[2]. Alcoholic hepatitis (AH) is an acute and severe inflammatory manifestation of alcohol-related liver disease that usually occurs in individuals with chronic excessive alcohol consumption. It is characterized by hepatocellular injury, jaundice and systemic inflammation[3]. The severity of AH is determined by a combination of factors, such as the extent of hepatocellular injury, the systemic inflammatory response, and the presence of complications such as infections, renal dysfunction, and malnutrition[4]. For example, severe AH (SAH) is a particularly lethal variant with a 6-month mortality rate of up to 40%[5].
To assess disease severity and guide clinical management, several prognostic scoring systems are widely used, such as the Maddrey discriminant function (MDF), the model for end-stage liver disease (MELD) score, the Lille model (LM), and the Glasgow AH score (GAHS)[6]. Despite the availability of these prognostic models, accurately predicting outcomes in AH remains a challenge owing to the heterogeneity of disease progression and the influence of comorbid conditions. The MDF has historically been one of the most widely used tools for risk stratification, with an MDF ≥ 32 indicating a high risk of short-term mortality and potential benefit from corticosteroid therapy[7]. The MELD score, which was originally developed to prioritize liver transplantation, has also been shown to be useful for predicting mortality in AH, as it considers renal function, which is a critical determinant of prognosis[8]. The LM, on the other hand, is especially valuable in assessing early response to treatment, helping clinicians determine whether corticosteroid therapy should be continued or discontinued in nonresponders[9]. However, while these scores offer valuable insights, their real-world applicability requires continuous validation, particularly in diverse patient populations. Furthermore, the dynamic nature of AH, influenced by alcohol cessation, comorbidities, and treatment responses, underscores the need for adaptive prognostic models. This editorial reflects on the findings of Yang et al[1] and their implications for the treatment of AH, emphasizing prognostic tools, clinical predictors, and individualized care.
As we previously discussed, prognostic scoring systems are essential in the clinical management of AH, as they provide valuable tools for risk stratification, guidance of therapeutic decisions, and prediction of short- and long-term outcomes. Given the significant morbidity and mortality associated with SAH, accurate early identification of high-risk patients is critical to optimize treatment strategies and prioritize resources[10]. The study by Yang et al[1] reinforced the efficacy of early LM scores (LM3-7), demonstrating that LM3 provides predictive accuracy comparable to that of later assessments (LM6 and LM7). This has significant clinical implications, as early identification of nonresponders allows timely discontinuation of corticosteroids, minimizing the risks associated with prolonged use, such as infections, hyperglycemia, and psychosis, which are complications that are particularly detrimental in this vulnerable population[11]. In addition, by optimizing early decision-making, LM3 could reduce hospital length of stay and associated healthcare costs, especially in resource-limited settings where prolonged hospitalization and high-cost interventions may not be feasible. In addition to predicting treatment response, this study validated the role of LM6 in predicting short-term mortality, with areas under the receiver operating characteristic curve values above 0.8 for both 28-day and 90-day mortality. These findings underscore the robust predictive value of the LM in AH, further supporting its integration into clinical practice to guide corticosteroid therapy and overall patient management.
While the LM provides essential guidance in corticosteroid therapy, other scoring systems contribute to broader mortality prediction and risk stratification. Yang et al[1] identified GAHS as the most effective predictor of 28-day and 90-day mortality, outperforming other models in the identification of high-risk patients. The GAHS incorporates key parameters such as bilirubin, the international normalized ratio and renal function, effectively capturing the systemic dysfunction characteristic of SAH[12]. Other studies have suggested improvements to existing models. A study by Amieva-Balmori et al[13] compared the ability of MELD-Na and MDF to predict 30-day and 90-day mortality in patients with AH. MELD-Na was found to be superior in predicting 90-day mortality, with an area under the curve of 0.871 vs 0.685 for MDF (P = 0.041). Notably, sodium levels were significantly associated with mortality, reinforcing the role of MELD-Na in prognostic assessment.
Despite the usefulness of these scoring models, limitations remain. The heterogeneity of AH, driven by systemic inflammation, renal dysfunction, and variable responses to treatment, is not fully reflected in conventional scores. The retrospective analysis by Yang et al[1] underscores this complexity, revealing that higher mortality rates are observed in patients with low nutritional intake, renal dysfunction, and underlying cirrhosis, findings that are consistent with previous research highlighting the multifactorial nature of AH[12]. The study also highlights the impact of cirrhosis, hepatorenal syndrome (HRS) and acute kidney injury (AKI) as major determinants of poor outcomes. Cirrhotic patients had a 10.9-fold increased risk of 28-day mortality, whereas AKI and HRS further exacerbated mortality risk, with odds ratios of 5.2 and 9.1, respectively[14]. These findings reinforce the critical interaction between hepatic and systemic complications, which requires a multidisciplinary approach for the treatment of AH.
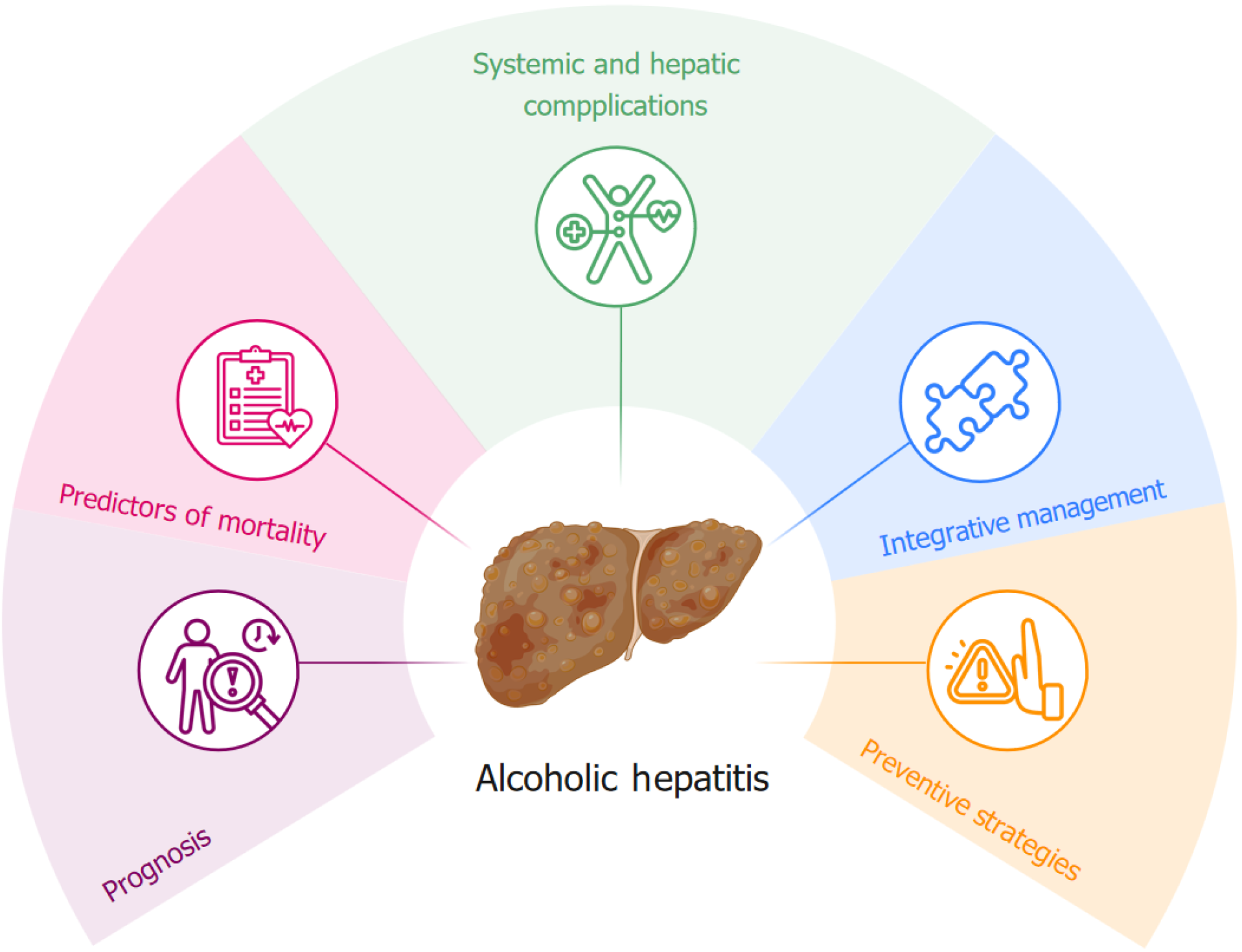
In addition, nutritional status, a critical but often overlooked factor in HA prognosis, was associated with worse outcomes. Malnutrition in patients with AH results from a combination of alcohol-induced metabolic disturbances, impaired nutrient absorption, and systemic inflammation, all of which contribute to disease progression and treatment failure[15]. A recent retrospective study analyzing 194 patients with AH evaluated the association between sarcopenia and disease severity, mortality, complications, and hospital outcomes. Patients with sarcopenia showed significantly higher MELD scores (mean 24.2 vs 21.5, P = 0.03), longer hospital stays (17.2 vs 12.4 days), and increased risk of complications, such as pneumonia, sepsis, and hepatic encephalopathy[16]. These findings highlight that sarcopenia is a critical prognostic factor in AH, underscoring the need to include it in risk stratification models to improve treatment and patient outcomes. The findings of Yang et al[1] further support the role of nutritional deficits in worsening prognosis, advocating for their integration into future scoring models. Addressing this gap could improve risk assessment, allowing for more comprehensive and targeted interventions. In addition to predicting mortality, the study highlights the prevalence of complications such as infections, gastrointestinal bleeding and hepatic encephalopathy, which significantly influence patient recovery. Early identification of these complications offers useful insights for preventive and therapeutic strategies (Figure 1).
The identification of predictive factors is crucial for individualized patient care in AH. Yang et al[1] reaffirmed the significance of renal dysfunction and nutritional deficits as independent risk factors for complications and mortality, aligning with existing evidence[17]. These factors not only influence short-term outcomes but also have long-term impli
Nutritional status, measured via albumin levels and the mean percentage of meal intake, has emerged as a critical determinant of survival. For every unit increase in the albumin level, the mortality risk decreased by 5.8-fold, whereas a higher mean percentage of meal intake was associated with reduced rates of AKI, HRS, and death[3]. These findings emphasize the importance of integrating nutritional interventions into the standard care of AH patients. Proactive measures, such as early nutritional consultations and tailored dietary plans, can significantly improve patient outcomes, highlighting the role of multidisciplinary care in AH management.
Sex and racial disparities in AH outcomes, as noted in the study, further complicate the clinical landscape. For example, female patients were more likely to develop infections, whereas non-Black patients were associated with higher rates of hepatic encephalopathy and gastrointestinal bleeding. These findings highlight the need for culturally sensitive and sex-specific interventions to address the unique needs of diverse patient populations. Additionally, addressing social determinants of health, such as access to care and socioeconomic status, is critical for mitigating these disparities and improving equity in AH management.
The clinical implications of Yang et al’s findings[1] are profound and multifaceted. Early and accurate identification of nonresponders to corticosteroid therapy is a pivotal advancement that can significantly reduce the risks associated with prolonged treatments, such as infections, hyperglycemia, and psychosis. By employing LM3 as an early decision-making tool, clinicians can quickly identify patients unlikely to benefit from corticosteroids, allowing for a timely shift to alternative therapies or supportive care strategies[19]. This approach not only enhances patient safety but also optimizes the allocation of medical resources, particularly in resource-constrained healthcare systems.
Additionally, the findings underscore the importance of addressing malnutrition as a critical component of AH management. Nutritional support, which has often been a neglected aspect of care, is increasingly recognized as a cornerstone in improving patient outcomes. The association between higher albumin levels and better survival rates highlights the need for early nutritional consultations and the integration of dietary interventions tailored to the unique needs of AH patients[20]. By incorporating proactive nutritional strategies, healthcare providers can mitigate complications such as infections and renal dysfunction, ultimately improving short-term and long-term survival.
Renal dysfunction, particularly AKI and HRS, emerged as significant predictors of mortality in Yang et al’s study[1]. These findings emphasize the necessity of vigilant renal monitoring and early intervention in patients with AH. Strategies such as the avoidance of nephrotoxic agents, optimization of intravascular volume status, and the use of pharmacological agents to improve renal perfusion are crucial in reducing the incidence and severity of renal complications. Furthermore, the integration of specialized nephrology care within multidisciplinary teams can enhance the overall management of these high-risk patients, ensuring a comprehensive approach to their complex medical needs.
From a broader perspective, the study underscores the value of multidisciplinary care models in the management of AH. The involvement of hepatologists, nutritionists, nephrologists, and infectious disease specialists is essential for addressing the diverse and interconnected challenges faced by AH patients. For example, the timely identification and treatment of infections, which are prevalent in this population, can significantly reduce morbidity and mortality. Collaborative efforts to establish standardized protocols for infection screening, prophylaxis, and treatment can further enhance clinical outcomes[21]. The findings also highlight the need for patient education and robust outpatient support systems to promote alcohol cessation, which remains the cornerstone of long-term management in AH. Structured programs that provide continuous medical follow-up, nutritional support, and psychosocial counseling can help sustain the clinical improvements achieved during hospitalization. Moreover, addressing the behavioral and social determinants of health that contribute to alcohol use disorders is critical for reducing the risk of disease recurrence and improving the overall quality of life of these patients.
While Yang et al’s study[1] provides valuable insights, several limitations warrant consideration. The retrospective design, while useful for hypothesis generation, introduces inherent biases, including selection and information bias, which may impact the generalizability of the findings. Additionally, the single-center setting of the study limits its applicability across diverse healthcare environments. Multicenter prospective studies with larger sample sizes are needed to validate these findings and establish their relevance in different patient populations and care settings[14]. Another limitation of the study is its reliance on clinical criteria for AH diagnosis without liver biopsy confirmation. Although the use of strict diagnostic criteria helps mitigate this issue, the lack of histological confirmation may lead to diagnostic inaccuracies, particularly in patients with overlapping liver conditions such as cirrhosis or nonalcoholic steatohepatitis. Future research should explore the role of noninvasive diagnostic tools, such as elastography or serum biomarkers, in improving diagnostic accuracy and differentiating AH from other liver diseases[22].
The study also highlights the need for further refinement of prognostic models. While the Lille score has proven to be a valuable tool, incorporating additional variables such as inflammatory markers, genetic predispositions, and gut microbiota profiles could increase its predictive accuracy. Advances in systems biology and bioinformatics offer promising opportunities to develop more sophisticated models that capture the complex interplay of factors influencing AH outcomes. For example, integrating biomarkers such as interleukin-6, tumor necrosis factor-alpha, and extracellular vesicles into prognostic algorithms could provide deeper insights into the pathophysiology of AH and guide personalized treatment approaches[23].
Emerging technologies such as artificial intelligence and machine learning also hold great potential in the field of hepatology. These tools can analyze large datasets to identify novel predictors of outcomes, optimize risk stratification, and support clinical decision-making in real time. By leveraging these technologies, future research can pave the way for more precise and individualized care pathways for AH patients. Additionally, there is a pressing need to investigate new therapeutic options for AH. While corticosteroids remain the mainstay of treatment, their efficacy is limited, and a significant proportion of patients are nonresponders. The exploration of novel pharmacological agents, one promising approach was the use of interleukin-1 receptor agonists (e.g., canakinumab) to reduce hepatic inflammation in AH. However, studies have yielded mixed results, with some trials indicating that it may not be as effective as initially expected[24]. This has led to the exploration of alternative therapies, such as gut microbiota modulators and immu
Finally, public health initiatives aimed at preventing AH are critical for reducing its burden on healthcare systems. Strategies to address harmful alcohol consumption, such as taxation, advertising restrictions, and public education campaigns, have been shown to be effective in decreasing alcohol-related harm. Improving access to addiction treatment services and integrating these services into primary care settings can further increase prevention efforts. Collaborative approaches involving healthcare providers, policymakers, and community organizations are essential to implementing these initiatives effectively and ensuring their sustainability. By addressing these limitations and exploring new directions in research and clinical practice, the field of hepatology can continue to advance, ultimately improving outcomes for patients with AH and reducing the societal impact of this devastating condition.
The study by Yang et al[1] highlights the critical role of LM3-7 in the treatment of AH, emphasizing the early identification of patients who do not respond to treatment, the impact of nutritional status, and the relationship between renal dysfunction and mortality. Their findings reinforce the need for early adjustment of treatment to reduce the iatrogenic risks of corticosteroids and optimize resource allocation, especially in resource-limited settings. The study highlights malnutrition as a modifiable risk factor and advocates for proactive nutritional interventions and interdisciplinary collaboration. In addition, the recognition of AKI and HRS as key predictors of mortality underscores the importance of early detection and nephroprotective strategies. In addition to clinical care, the study advocates public health initiatives targeting alcohol use disorders to reduce the incidence of AH. Ultimately, these data underscore the need for multidisciplinary, innovative, and patient-centered approaches to improve AH outcomes.
| 1. | Yang K, Nallapeta N, Hossein-Javaheri N, Carlson A, Quigley B, Mahl T. Predictors and prognosticators of outcomes in alcoholic hepatitis: A retrospective single center study. World J Hepatol. 2025;17:102152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 2. | Sehrawat TS, Liu M, Shah VH. The knowns and unknowns of treatment for alcoholic hepatitis. Lancet Gastroenterol Hepatol. 2020;5:494-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 3. | Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 698] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 4. | Dugum MF, McCullough AJ. Acute Alcoholic Hepatitis, the Clinical Aspects. Clin Liver Dis. 2016;20:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Liangpunsakul S. Clinical characteristics and mortality of hospitalized alcoholic hepatitis patients in the United States. J Clin Gastroenterol. 2011;45:714-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Jinjuvadia R, Liangpunsakul S; Translational Research and Evolving Alcoholic Hepatitis Treatment Consortium. Trends in Alcoholic Hepatitis-related Hospitalizations, Financial Burden, and Mortality in the United States. J Clin Gastroenterol. 2015;49:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 7. | De La Torre SA Jr, Morcos M, Saab S, Shetty A. Alcohol-Associated Hepatitis: Short- and Long-Term Management. Dig Dis Sci. 2025;70:74-84. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Sheth M, Riggs M, Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol. 2002;2:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Garcia-Saenz-de-Sicilia M, Duvoor C, Altamirano J, Chavez-Araujo R, Prado V, de Lourdes Candolo-Martinelli A, Holanda-Almeida P, Becerra-Martins-de-Oliveira B, Fernandez-de-Almeida S, Bataller R, Caballeria J, Duarte-Rojo A. A Day-4 Lille Model Predicts Response to Corticosteroids and Mortality in Severe Alcoholic Hepatitis. Am J Gastroenterol. 2017;112:306-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Kasper P, Lang S, Steffen HM, Demir M. Management of alcoholic hepatitis: A clinical perspective. Liver Int. 2023;43:2078-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 11. | Singal AK, Kodali S, Vucovich LA, Darley-Usmar V, Schiano TD. Diagnosis and Treatment of Alcoholic Hepatitis: A Systematic Review. Alcohol Clin Exp Res. 2016;40:1390-1402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Wakil A, Niazi M, Meybodi MA, Pyrsopoulos NT. Emerging Pharmacotherapies in Alcohol-Associated Hepatitis. J Clin Exp Hepatol. 2023;13:116-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Amieva-Balmori M, Mejia-Loza SM, Ramos-González R, Zamarripa-Dorsey F, García-Ruiz E, Pérez Y López N, Juárez-Valdés EI, López-Luria A, Remes-Troche JM. Model for end-stage liver disease-Na score or Maddrey discrimination function index, which score is best? World J Hepatol. 2015;7:2119-2126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Pan CW, Guifarro D, Poudel A, Abboud Y, Kotwal V. Racial Disparities in Alcoholic Hepatitis Hospitalizations in the United States: Trends, Outcomes, and Future Projections. Dig Dis Sci. 2024;69:2808-2816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | McClain CJ, Rios CD, Condon S, Marsano LS. Malnutrition and Alcohol-Associated Hepatitis. Clin Liver Dis. 2021;25:557-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Al-Azzawi Y, Albo B, Fasullo M, Coukos J, Watts GJ, Tai R, Radcliffe D, Kroll-Desrosiers A, Devuni D, Szabo G. Sarcopenia is associated with longer hospital stay and multiorgan dysfunction in alcoholic hepatitis. Eur J Gastroenterol Hepatol. 2020;32:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Ali H, Bolick NL, Tillmann H. Simple scoring for acute necrotizing pancreatitis: mortality in acute necrotizing pancreatitis during admission (MANP-A). Ann Gastroenterol. 2022;35:551-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Laswi H, Abusalim AR, Warraich MS, Khoshbin K, Shaka H. Trends and Outcomes of Alcoholic Liver Cirrhosis Hospitalizations in the Last Two Decades: Analysis of the Nationwide Inpatient Sample. Gastroenterology Res. 2022;15:91-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 19. | Sohail U, Satapathy SK. Diagnosis and management of alcoholic hepatitis. Clin Liver Dis. 2012;16:717-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, Morales-Ruiz M, Angeli P, Domenicali M, Alessandria C, Gerbes A, Wendon J, Nevens F, Trebicka J, Laleman W, Saliba F, Welzel TM, Albillos A, Gustot T, Benten D, Durand F, Ginès P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 585] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 21. | Hmoud BS, Patel K, Bataller R, Singal AK. Corticosteroids and occurrence of and mortality from infections in severe alcoholic hepatitis: a meta-analysis of randomized trials. Liver Int. 2016;36:721-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Rutledge SM, Im GY. Current and Future Biomarkers in Alcoholic Hepatitis. Clin Liver Dis. 2021;25:493-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Singal AK, Shah VH. Alcoholic hepatitis: prognostic models and treatment. Gastroenterol Clin North Am. 2011;40:611-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Thursz M, Mathurin P. Targeting IL-1 in severe alcohol-related hepatitis: How many frogs will we need to kiss to find an effective therapy? J Hepatol. 2024;80:678-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 25. | Singal AK, Shah VH. Current trials and novel therapeutic targets for alcoholic hepatitis. J Hepatol. 2019;70:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/