Published online May 27, 2025. doi: 10.4254/wjh.v17.i5.105706
Revised: March 31, 2025
Accepted: April 22, 2025
Published online: May 27, 2025
Processing time: 112 Days and 10 Hours
Metabolic dysfunction-associated fatty liver disease (MAFLD) and type 2 diabetes mellitus (T2DM) are independent risk factors for the development of cardio
To identify the CVD and cardiovascular event (CVE) risk in a systematic review when MAFLD and T2DM co-exist to inform better clinical practice decisions.
A systematic review was performed by compiling data by searching PubMed, EMBASE and Cochrane Library databases. Quality appraisal of retrieved studies and the meta-analysis were performed using Joanna Briggs Institute (JBI) tool and RevMan 5.4 software respectively. The effect indicators for CVE and CVD risk were expressed as odds ratios (OR) and 95%CI with P-values < 0.05 as significant.
Fourteen (5 cohort and 9 cross-sectional) studies with 370013 participants were included in this review. The meta-analysis of CVE showed that the risk of CVE in T2DM was higher in the MAFLD group when compared to the non-MAFLD group [OR 1.28 (95%CI, 1.04–1.56) P = 0.02] with follow up duration ranging between 5-6 years. The prevalence of CVD in the metanalysis of cross-sectional studies was found to be higher [OR 1.47 (95%CI, 1.21–1.78) P = 0.0001] in T2DM with MAFLD when compared to T2DM without MAFLD. Significant heterogeneity exists due to variations in study design, methodologies, and MAFLD diagnostic criteria, which may have influenced the study's findings.
The presence of MAFLD in T2DM increased the risk of CVE. The prevalence of CVD is higher in T2DM with MAFLD as compared to T2DM without MAFLD. Large well-designed multicentric long-term prospective studies are necessary to appropriately risk stratify the cardiovascular effect of the MAFLD in T2DM patients.
Core Tip: Metabolic dysfunction-associated fatty liver disease (MAFLD) and type 2 diabetes mellitus (T2DM) are independent risk factors for the development of cardiovascular disease (CVD) with an exaggerated risk of cardiovascular events (CVE) when both diseases co-exist. A systematic review of fourteen studies with 370013 participants showed that the risk of CVE in T2DM was higher in the MAFLD group when compared to the non-MAFLD group [odds ratios (OR): 1.28] with follow up duration ranging between 5-6 years. The prevalence of CVD was higher (OR: 1.47) in T2DM with MAFLD when compared to T2DM without MAFLD. In conclusion, presence of MAFLD in T2DM increased the risk of CVE and CVD as compared to T2DM without MAFLD.
- Citation: Shetty S, Suvarna R, Ambrose Fistus V, Modi S, Pappachan JM. Cardiovascular implications of metabolic dysfunction-associated fatty liver disease and type 2 diabetes mellitus: A meta-analysis. World J Hepatol 2025; 17(5): 105706
- URL: https://www.wjgnet.com/1948-5182/full/v17/i5/105706.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i5.105706
Metabolic dysfunction-associated fatty liver disease (MAFLD) has been gaining attention as the most common cause of chronic liver disease and liver-related mortality and prevalence of MAFLD has being increasing, especially in Asia[1]. A range of liver diseases in MAFLD includes from simple steatosis (storage of fat in the liver) to metabolic dysfunction-associated steatohepatitis (MASH), which can proceed to cirrhosis and hepatocellular carcinoma[2]. MAFLD is highly prevalent among individuals with type 2 diabetes mellitus (T2DM), with a high proportion of diabetics having some degree of fatty liver disease. As insulin resistance (IR) is a common risk factor for T2DM and MAFLD, they often co-exist as different manifestations of metabolic syndrome (MetS)[3]. Patients with T2DM who also have MAFLD are at a significantly increased risk of cardiovascular events (CVE)[4].
CVE, including myocardial infarction, stroke, heart failure, and cardiovascular death, are major complications associated with metabolic disorders such as T2DM and MAFLD. CVEs are a leading cause of morbidity and mortality in individuals with diabetes, particularly those with T2DM[5]. Chronic hyperglycaemia, IR, and associated metabolic abnormalities contribute to endothelial dysfunction, atherosclerosis, and increased thrombosis risk, leading to events such as myocardial infarction, stroke, heart failure, and cardiovascular death. The presence of additional risk factors, including hypertension, dyslipidemia, and obesity, further amplifies the likelihood of adverse cardiovascular outcomes in diabetic patients[6]. Individuals with T2DM and MAFLD are at an even higher risk of CVE due to the combined impact of IR, chronic inflammation, and metabolic dysfunction[7]. The presence of MAFLD exacerbates atherosclerosis, endothelial dysfunction, and arterial stiffness, leading to an increased incidence of myocardial infarction, stroke, heart failure, and cardiovascular death. Additionally, the high prevalence of comorbidities such as hypertension, dyslipidemia, and obesity in this population further amplifies cardiovascular risk[8].
Recent research suggests that the presence of MAFLD in T2DM may also be associated with increased cardiovascular disease (CVD) risk, regardless of MetS components, although larger studies are needed for further verification[9,10]. According to these findings, MAFLD in people with T2DM may increase their risk of CVD, necessitating more stringent care and screening procedures lowering the chance of adverse CVE. Finding those who have MAFLD would also help identify a subset of diabetes individuals who need to receive more intense treatment in order to lower their risk of developing CVE in the future[11]. Over 70% of patients with T2DM also have MAFLD, indicating a substantial correlation between the two conditions[12,13]. Patients with MAFLD, especially MASH, have less survival rate when compared to the general population, which is mostly due to CVD[14].
Over the past five years, the association between T2DM, MAFLD, and CVD has become increasingly evident. A large-scale study involving 3929596 individuals reported that patients with MAFLD had an age-standardized incidence rate of 2878 per 100000 person-years for CVD, compared to 1339.7 per 100000 person-years in those without liver disease, reflecting a more than twofold increased risk[15]. Additionally, MAFLD is highly prevalent among individuals with T2DM, with reported rates reaching up to 69.5%. This coexistence substantially heightens the risk of adverse CVE, emphasizing the urgent need for comprehensive management strategies that address both hepatic and cardiovascular health in this high-risk population[16].
Public health policies have increasingly acknowledged the significance of screening and preventive measures for CVD, particularly in individuals with T2DM and hepatic conditions like non-alcoholic fatty liver disease (NAFLD). The National Health Service has introduced initiatives such as the "midlife MOT" health checks, aimed at individuals aged 40 to 74, to detect and manage risks associated with heart disease, stroke, kidney disease, and diabetes[17]. These policies highlight a growing awareness of the link between metabolic disorders and cardiovascular health, reinforcing the need for comprehensive screening and prevention strategies to reduce the burden of CVD in high-risk populations[17].
With the increasing prevalence of T2DM and NAFLD, understanding their combined impact on cardiovascular health is vital for planning public healthcare policies and resource allocation. More importantly, it helps in designing appropriate screening programs and prevention measures. Studying the cardiovascular implication in T2DM with MAFLD is important to enhance the understanding of disease interactions, improve patient care through early detection and tailored interventions, inform public health strategies and clinical guidelines. Therefore, this study was conducted to assess the incidence of CVE and the risk of CVD in T2DM patients with coexisting MAFLD compared to those without MAFLD.
The PRISMA 2020 guidelines were adhered in the design and performance of this study[18] and this review has been recorded with PROSPERO (CRD42024509142) international registry.
Three common databases PubMed, EMBASE, and the Cochrane Library were searched for choosing the qualified studies from January 2005 to 25 March 2025. The specific MeSH terms used, and the full search strategy are outlined in the Supplementary Table 1.
This study included prospective studies, specifically cohort and cross-sectional studies, to ensure a robust evaluation of the relationship between MAFLD and CVE in patients with T2DM. Studies were selected based on their reporting of the incidence of CVE and risk of CVD in individuals with T2DM and MAFLD. The inclusion criteria ensured that only studies with relevant cardiovascular outcomes were analyzed. Studies that did not provide data on CVD or CVE outcomes were excluded, as they would not contribute to the primary objective of assessing cardiovascular risk. Additionally, studies that lacked a comparator group of T2DM patients without MAFLD were excluded. Case series and case reports were also excluded.
The primary outcome measure was CVE, which is a composite of cardiovascular death, nonfatal myocardial infarction, late coronary revascularization, nonfatal stroke, or hospitalization for heart failure. The secondary outcome measure evaluated was the prevalence of CVD in the study population which included coronary artery disease (CAD) and ischaemic heart disease. Each of the three authors of this study separately examined the study titles, abstracts, and data retrieved from the publications. A fourth author double-checked the information for each of the extracted items. Discrepancies were resolved by discussion with fifth author. From the qualified study, the following characteristics were extracted: The first author name, year of publication, country, study design, total number of participants, mean age, sex distribution, body mass index (BMI) and the outcome measures.
The quality appraisal of included studies was performed separately by two authors and the disagreements were resolved with the other authors. The Joanna Briggs Institute (JBI) critical appraisal tools for cross-sectional study and cohort study were used as a reliable instrument to investigate variations from the study design which would have impacted the quality of evidence in all analyzed papers including observational studies.
The meta-analysis was carried out using the RevMan 5.4 program. The odds ratio (OR) and 95%CI were used as the effect measures for the forest plot. The funnel plot was generated to detect the publication bias of eligible studies. I² statistics was used to evaluate the statistical heterogeneity between studies. A random-effect model was used to perform the meta-analysis and a P-value of less than 0.05 was considered statistically significant.
1013 articles were found in the literature search. Following the removal of 183 duplicates and the screening of titles and abstracts, the eligibility of 34 full-text articles was evaluated. Fourteen papers were eliminated because they did not provide primary outcome data, and six articles were eliminated due to the absence of comparison groups. Fourteen studies meeting the inclusion criteria involving a total of 370013 patients were included in the quantitative synthesis[19-32]. The study flow diagram is shown in Supplementary Figure 1.
The characteristics of the included studies are summarized in Table 1. Two studies were conducted in Japan[19,26], three in Italy[20,27,28], two in China[21,25], two study in India[22,32] and one study each in Turkey[24], Malaysia[23], Iran[29], Korea[30] and United Kingdom[31]. Five cohort studies reported CVE[19-21,31,32] and 9 cross-sectional studies reported the prevalence of CVD in T2DM with MAFLD as compared to T2DM without MAFLD[22-29,32].
| Ref. | Year | Country | Groups | Design | Age (years) | Male% /n | BMI | Outcome measures |
| Ichikawa et al[19] | 2021 | Japan | NAFLD-143, non- NAFLD-386 | Prospective cohort study | 65 | 61% | NAFLD: 28 ± 4, Non-NAFLD 24 ± 4 | CVE |
| Targher et al[20] | 2005 | Italy | Case: 248, control: 496 | Cohort | Case: 66 ± 4, Control: 65 ± 3 | Case: 62%, Control: 62% | Case: 29 ± 4, Control: 26 ± 3 | CVE |
| Guo et al[21] | 2022 | China | MAFLD-183, Non- MAFLD-365 | Prospective cohort | MAFLD: 42.85 ± 11.5, Non-MAFLD 35.38 ± 13.36 | NR | MAFLD 31.19 +/- 4.94, Non- MAFLD 24.85 +/- 4.08 | CVE |
| Agarwal et al[22] | 2011 | India | NAFLD-71, non-NAFLD-53 | Cross-sectional | NAFLD: 57 ± 9. Non-NAFLD: 61 ± 8 | NR | NAFLD- 27.5 ± 3.99. Non-NAFLD- 25.3 ± 3.5 | Prevalence of CAD |
| Chan et al[23] | 2014 | Malaysia | 399 T2DM | Cross-sectional | IHD: 66.4 ± 8.9. No IHD: 61.5 ± 10.7 | IHD: 50.0% Non-IHD: 40.6% | IHD: 29.3 ± 8.3. Non- IHD: 27.6 ± 6.4 | Prevalence of IHD |
| Idilman et al[24] | 2014 | Turkey | NAFLD: 59, Non-NAFLD: 214 | Cross-sectional retrospective | NAFLD: 56.6 ± 7.4. Non-NAFLD: 59.2 ± 9.8 | NAFLD: 95. Non-NAFLD: 32 | NAFLD: 32.5 ± 5.38. No-NAFLD: 31 ± 6.16 | Prevalence of CAD |
| Lu et al[25] | 2009 | China | NAFLD-421, Non-NAFLD- 139 | Cross-sectional | NAFLD: 56.42 ± 6.57 Non-NAFLD; 57.19 ± 6.61 | NAFLD: 264, Non-NAFLD: 64 | NAFL: 28.42 ± 4.33, Non- NAFL: 25.18 ± 4.19 | Prevalence of CVD |
| Takeuch et al[26] | 2012 | Japan | FLD: 77, NO-FLD: 169 | Cross-sectional | FLD: 61 ± 9, No-FLD: 65 ± 7 | NR | FLD: 26.4 ± 3.6, Non-FLD: 23.7 ± 3.7 | Prevalence of CVD |
| Targher et al[27] | 2006 | Italy | NAFLD-400, Non- NAFLD- 400 | Cross-sectional | NAFLD: 58 ± 4. Non-NAFLD: 59 ± 4 | NAFLD: 54%. Non-NAFLD: 54% | NAFLD: 28.1 ± 4. Non-NAFLD: 26.4 ± 3 | Prevalence of CVD |
| Targher et al[28] | 2007 | Italy | NAFLD-1974, Non- NAFLD- 418 | Cross-sectional | NAFLD: 65 ± 6. Non-NAFLD: 60 ± 4 | NAFLD: 57%. Non-NAFLD: 54% | NAFLD: 28.3 ± 4. Non-NAFLD: 26.5 ± 3 | Prevalence of CVD |
| Dehghani Firouzabadi et al[29] | 2024 | Iran | NAFLD- 360, Non-NAFLD-837 | Cross- sectional | NAFLD: 53.2 ± 10.4, Non-NAFLD: 57.4 ± 11.3 | NAFLD: 43%. Non-NAFLD: 43.2% | NAFLD: 30.9 ± 6.0, Non-NAFLD: 29.3 ± 5.3 | CVE and Prevalence of CVD |
| Kim et al[30] | 2024 | Korea | NAFLD: 135184, Non-NAFLD: 198322 | Cohort | MASLD: 46.1 ± 9.8, Non-MASLD: 39.6 ± 10.0 | MASLD: 86.2%, Non-MASLD: 44% | MASLD: 26.3 ± 2.4, Non-MASLD: 21.9 ± 2.3 | CVE and Prevalence of CVD |
| Riley et al[31] | 2024 | United Kingdom | MASLD: 15208, Non-MASLD: 15208 | Cohort | MASL: 56.4 ± 14.2, Non-MASLD: 56.4 ± 14.4 | MASLD: 45.5%, Non-MASLD: 44.9% | MASLD: 34.7 ± 6.7, Non-MASLD: 34.4 ± 6.7 | CVE and Prevalence of CVD |
| Lalwani and Jha[32] | 2024 | India | NAFLD-59, Non-NAFLD-41 | Cross- sectional | NAFLD: 55.08 ± 9.13, Non-NAFLD: 56.44 ± 8.55 | NR | NAFLD: 26.09 ± 2.48, Non-NAFLD: 25.72 ± 2.32 | Prevalence of CAD |
The cohort study by Ichikawa et al[19], assessed the prognostic significance of NAFLD for predicting CVE in T2DM patients who had CAD suspicions. The study had 6 years follow-up and 529 patients with no history of CVD. The coronary artery calcium score (CACS) was assessed in 172 asymptomatic patients to stratify the risk by evaluating coronary atherosclerosis. Additionally, CACS was measured in 357 patients who exhibited atypical symptoms or no symptoms but had abnormalities in their resting or stress electrocardiograms. None of the patients had a prior history of cardiovascular conditions, including CAD, heart failure, or cerebrovascular disease. CVE, which include hospitalization for heart failure, nonfatal myocardial infarction, late coronary revascularization, and cardiovascular mortality were the study's endpoint.
In the cohort study by Targher et al[20], the risk of CVE was assessed among NAFLD patients with T2DM. 248 participants developed nonfatal coronary heart disease (CHD), ischemic stroke, or cardiovascular death, over an average follow-up period of 5 years. 496 control subjects who did not have any confirmed CVE at the time of the follow-up were assigned in a 2:1 ratio, with age and sex matching to the case groups. Angina pectoris, silent CHD, or any ischemia electrocardiogram alterations were not included in the clinical end points. Medical records demonstrating new-onset neurological symptoms lasting longer than 24 hours along with diagnostic imaging studies indicated a nonfatal ischemic stroke[20].
The cohort study by Guo et al[21] studied the risk of incident CVD in patients with metabolic dysfunction-associated steatotic liver disease (MASLD). The data for this meta-analysis were taken from the analysis of the subgroup with T2DM assessing the risk of CVE in those with MAFLD (n = 183) and without MAFLD (n = 365). The primary outcome was the first diagnosed CVD event, defined as ischemic heart disease (IHD) or stroke during the follow-up period. These CVE were documented using survey-based questionnaires and review of medical files at hospitalization. The cohort studies include 11444 participants. During a median follow-up period of 4.7 years, 10.40% (1190/11444) of experienced incidence of CVD. The incidences of CVD were 9.02% and 18.38% in the MAFLD and non-MAFLD groups, respectively. In subgroup analysis for T2DM, the incident CVD was 43/183 in T2DM with MAFLD and 70/365 in T2DM without MAFLD[21].
Agarwal et al[22], in cross-sectional study estimated the prevalence of NAFLD and correlated with CAD in Indian diabetic population. Based on carotid intimal-medial thickness (CIMT) ultrasonographic measurement and an ultrasound evaluation of the liver, NAFLD was diagnosed. There was a 57.2% prevalence of NAFLD. The NAFLD subgroup had a higher prevalence of CAD (60.5%) than the non-NAFLD subgroup (45.2%)[22].
The cross-sectional study by Chan et al[23] looked at the association of NAFLD and IHD in T2DM. Asian patients with T2DM with symptoms and/or ECG changes suggestive of IHD, but without a documented history of IHD, were referred for cardiac evaluation. Participants were recruited from various Asian ethnic groups, including Indian, Chinese, and Malaysian populations. In total, 379 patients were accounted for in the analysis. Indians (34.1%) had the highest prevalence of IHD, followed by Malays (29.2%) and Chinese (20.1%). Among patients with and without NAFLD, the prevalence of IHD was 24.7 and 28.4%, correspondingly. When analyzed by ethnicity, no association was found between MAFLD and IHD[23].
The cross-sectional study by Idilman et al[24], assessed the correlation of NAFLD with CAD in T2DM patients. Patients with T2DM who underwent Computed Tomography Angiography (CTA) for chest pain but had no known liver condition were included. Plaque in the coronary arteries was indicative of CAD. Severe stenosis (≥ 50%) in one or more coronary arteries was considered significant CAD. Unenhanced CT scans acquired for calcium scoring were used to calculate the liver fat content. The purpose of the data analysis was to determine whether CAD was present in T2DM patients with or without MAFLD. Seventy-six percent of the 273 patients (n = 207) had CAD, and 48 percent of them had severe CAD (≥ 50 stenosis) according to coronary CTA[24].
In cross-sectional study by Lu et al[25] looking at prevalence of CHD in T2DM patients with MAFLD, the MAFLD was demonstrated by liver ultrasonographic scanning and CHD was identified based on patient’s symptomatology, electrocardiogram and coronary angiography.
The cross-sectional study by Takeuchi et al[26], assessed the risk of diabetic macroangiopathy in the presence of MAFLD. CHD was diagnosed based on a history of MI, angina pectoris, ECG abnormalities indicating myocardial ischemia. The study defined "diabetic macroangiopathy" as a broad term encompassing large blood vessel disorders, including CHD, CVD, or peripheral artery disease in diabetic patients[26].
In the cross-sectional study by Targher et al[28], the prevalence of CVD in T2DM with NAFLD was studied. The medical history, physical examination, ECG, and echo-Doppler scanning of the lower limb and carotid arteries were used to determine the presence of CVD. NAFLD was detected using ultrasonography, while the presence of MetS was also assessed[28].
Kim et al[30] conducted a nationwide population-based study to investigate the association between NAFLD, CVE, CVD, and all-cause mortality in patients with T2DM. Using national health records, they identified individuals with T2DM, classified NAFLD based on clinical and imaging criteria, and categorized participants into Non-NAFLD, Grade 1 NAFLD, and Grade 2 NAFLD groups. For this analysis, only the Non-NAFLD and Grade 2 NAFLD groups were considered[30].
Riley et al[31] conducted a retrospective cohort study over a 5-year period to assess the combined effects of type 2 diabetes and MASLD on cardiovascular, liver-related, diabetes-related, and cancer outcomes. They utilized clinical and population-based databases to identify individuals with MASLD and T2DM, applying diagnostic criteria, imaging techniques, and biochemical markers for classification[31].
In the included studies, the MAFLD group consistently had a higher BMI than those without MAFLD, highlighting the strong link between elevated BMI and the development of both MAFLD and CVD.
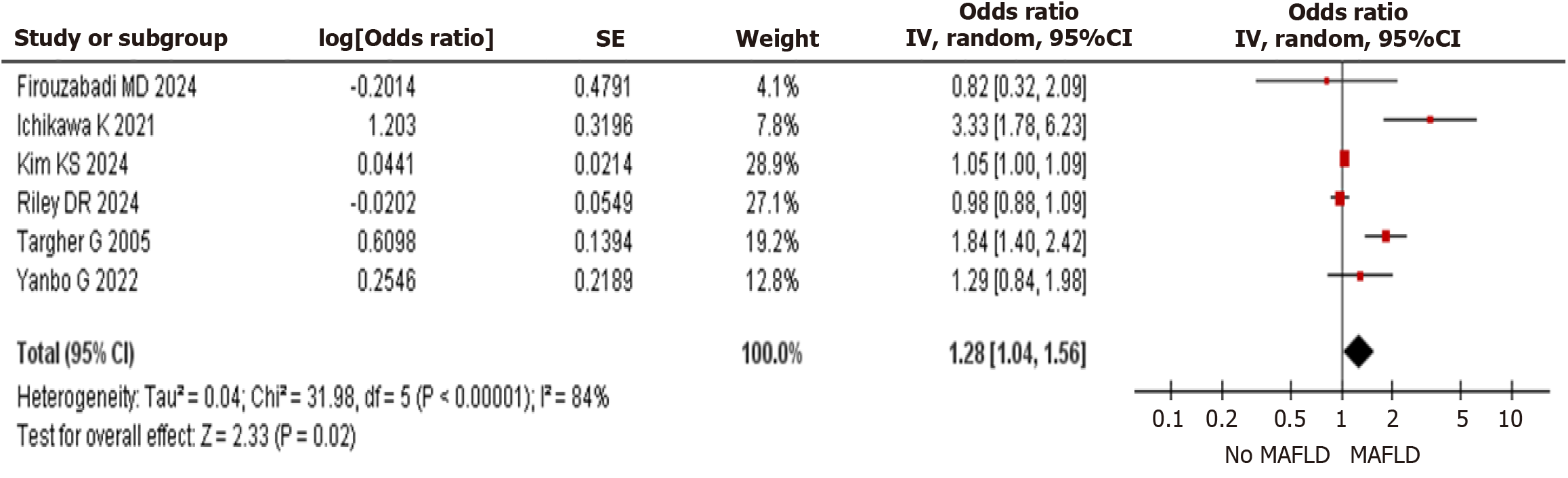
CVE: The meta-analysis comprised six trials with a total of 366940 individuals that reported CVE as the main outcome. As shown in Figure 1, Presence of MAFLD in T2DM had a statistically significant higher risk of CVE when compared to non- MAFLD group [OR: 1.28 (95%CI: 1.04–1.56) P = 0.02].
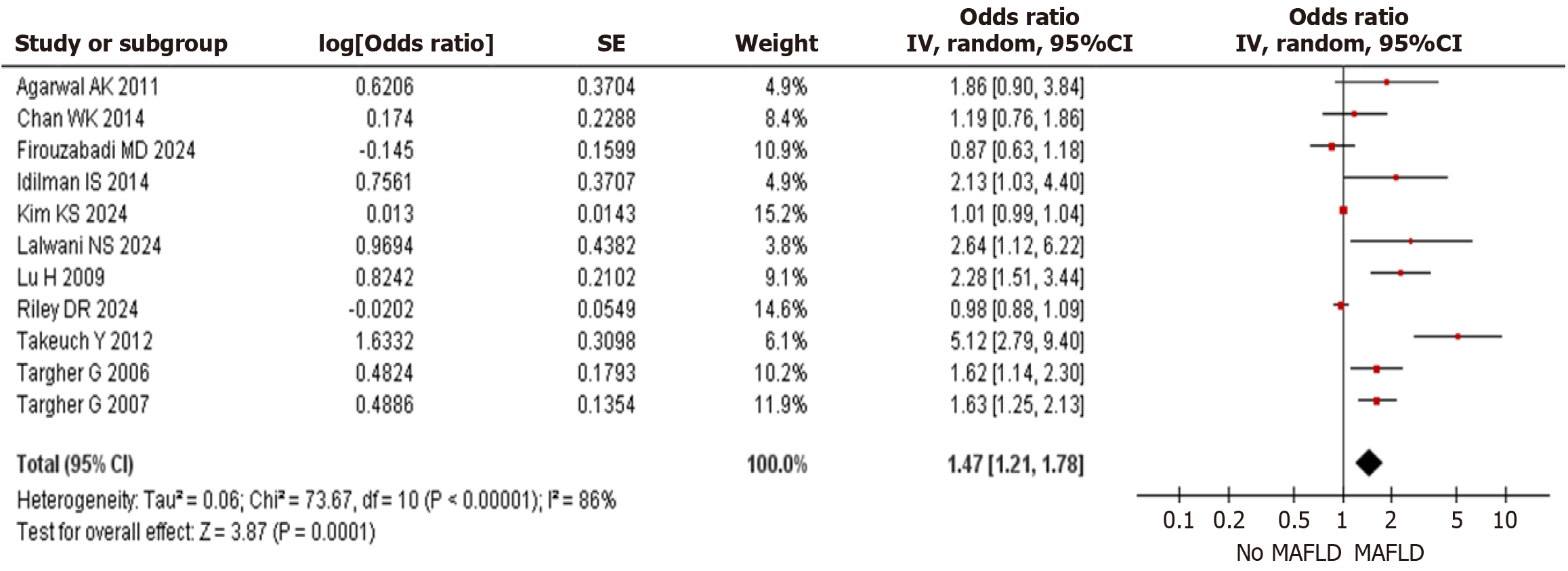
Prevalence of CVD: Eleven studies including a total of 370013 participants reporting the prevalence of CVD showed statistically significant higher prevalence of CVD in MAFLD group when compared to non- MAFLD group [OR: 1.47 (95%CI: 1.21–1.78) P = 0.0001] as shown in Figure 2.
Five cohort studies included were deemed to have a moderate to poor risk of bias, while among 9 studies with cross-sectional design, five studies were determined as having high risk of bias and others with moderate risk according to the JBI Critical Appraisal Checklist for Cross-sectional Studies (Supplementary Table 2). The funnel plot for 7 studies shows a significant publication bias (Supplementary Figure 2).
In this meta-analysis of the studies reporting the CVE and prevalence of CVD among patients with MAFLD and T2DM, we found a higher risk of CVD among patients with T2DM with MAFLD as compared to T2DM without MAFLD. Although moderate to high risk of bias was observed in view of significant heterogenicity in these studies, the analysis revealed statistically significant CVE and CVD prevalence among T2DM with MAFLD regardless of different study designs. Patients with T2DM are more prone to develop worse disease progression and advanced MAFLD, such as steatohepatitis, fibrosis, and cirrhosis, compared to those without diabetes[33]. There is also a strong bidirectional relationship between CVD and advanced MAFLD[34]. On the other hand, MAFLD can make it challenging to achieve adequate glycemic control in diabetic patients. CVDs are the leading cause of mortality in both MASLD and T2DM. Our findings highlighting the exaggerated CVD prevalence in patients with MAFLD and T2DM suggest that clinicians should consider extra-vigilance and more aggressive therapeutic interventions to prevent CVD mortality in this high-risk group[35]. The weight assigned to each study in the forest plot reflects its relative contribution to the overall pooled estimate, primarily influenced by sample size and the precision of the effect estimate[36]. Studies with larger sample sizes and narrower confidence intervals carry more weight, exerting a stronger influence on the meta-analysis results. When evaluating CVE in T2DM patients with and without MAFLD, it is essential to critically assess the methodologies, population characteristics, and potential biases of highly weighted studies, as they can significantly shape the overall findings. A substantial contribution from studies demonstrating a strong association between MAFLD and CVD may enhance the robustness of the conclusions.
MAFLD and T2DM are closely associated with common risk factors, pathophysiological abnormalities and their adverse systemic effects[37]. Excessive free fatty acids in liver cells cause disruption in the signalling pathway rising protein kinase C activity. These alterations lead to impaired insulin signalling in hepatocytes and hepatic IR[12]. Moreover, IR is exacerbated by the overactivation of proinflammatory cytokines including as interleukin (IL)-6, IL-1β, IL-18, and tumor necrosis factor-α in MAFLD[38]. A recent Mendelian randomization study examined the association between MAFLD and IR, despite the absence of direct evidence linking MAFLD to T2DM. In two distinct cohorts, the authors found a correlation between IR as evaluated by the HOMA-IR index and genetically determined hepatic fat accumulation[39]. Notably, this correlation was influenced by hepatocyte damage, indicating a progression from hepatic fat accumulation to liver cell damage and IR[40]. These results corroborate the idea of a causative relationship between MAFLD and T2DM and are consistent with clinical evidence regarding the significance of liver damage in glucose imbalance.
CVD is a major cause of mortality and morbidity in patients with T2DM. IR, proinflammatory state, oxidative stress, endothelial dysfunction, hypercoagulability, diabetic dyslipidemia contributes to atherosclerotic CVD in T2DM[41]. The complex and poorly understood pathways by which MAFLD increases the risk of CVE are ascertained. Increased arterial stiffness and endothelial dysfunction are correlated with greater histological severity of NAFLD, according to prior study[42]. Furthermore, in patients with MAFLD, inflammatory cytokines increase in direct proportion to the degree of liver disease[43]. Systemic inflammation caused by cytokines released from the liver results in endothelial dysfunction, which promotes the formation of vascular plaques[44]. Increased hepatic lipogenesis and a surplus of free fatty acid (FFA) in the liver are triggered by IR. Moreover, the build-up of FFA activates serine kinases, which alters the insulin signalling pathway and exacerbates the IR state[45]. Lowering IR levels by enhancing liver function with direct-acting antiviral medication was found to minimize the risk of CVD and the incidence of T2DM in several experimental trials[46,47].
Both T2DM and MASLD have characteristic increase in triacylglycerol content in low-density lipoprotein (LDL) and formation of small dense LDL mediated by increases in cholesteryl ester transfer protein activity by the elevated very low-density lipoprotein (VLDL)[48]. Increased levels of triglycerides and VLDL particles are caused by the interaction between MAFLD and IR. This leads to dysfunction in insulin receptor, which mobilizes liver adipose tissue to move to peripheral tissues and raises the risk of CVD[49]. In addition to IR, endothelial dysfunction and the activation of inflammatory pathways are among the complex underlying processes that link T2DM, MAFLD and CVD[50]. Patients with MAFLD may have pro-inflammatory conditions and elevated oxidative stress, which can cause endothelial dysfunction and vascular inflammation. These factors might ultimately lead to the development of atherosclerotic plaque. Changes in the anatomy of the heart and diastolic dysfunction are additional contributing factors for CVD[51].
The presence of MASLD in T2DM, further accentuates the proinflammatory state, oxidative stress and endothelial dysfunction associated with T2DM resulting in increased CVD risk[52]. The concomitant presence of MASLD and T2DM increases the risk of CVD by two-fold than in those with MASLD without T2DM. The Duo increases the risk of CAD, heart failure, cardiac arrythmias as well as cardiovascular mortality[53,54]. Thus, the increased risk of CVD in co-existence of MASLD and T2DM is mediated by several shared abnormalities predisposing to CVD, of which some of the most important proposed pathophysiological abnormalities include atherogenic dyslipidemia, prothrombotic state, IR, low-grade chronic inflammatory states and gut microbial dysbiosis[55].
The findings of this meta-analysis may have been impacted by several confounding factors present in the included studies, potentially influencing the observed association between MAFLD and CVD outcomes in individuals with T2DM. The higher prevalence of comorbid conditions such as hypertension, dyslipidemia, obesity, MetS, and smoking in the MAFLD group could have significantly contributed to the results. Hypertension and dyslipidemia are well-established risk factors for CVD, and their increased presence among MAFLD patients may have heightened the likelihood of adverse CVE[56]. Likewise, obesity and MetS, both strongly associated with MAFLD, exacerbate IR, systemic inflammation, and endothelial dysfunction, further escalating cardiovascular complications[57]. Additionally, smoking, a major independent risk factor for atherosclerosis, may have further deteriorated cardiovascular health in this population[58]. Beyond these metabolic risk factors, the use of medications among study participants may have also played a role in influencing cardiovascular outcomes[56]. Many patients were receiving statins, insulin, and oral hypoglycemic agents, all of which could have had differential effects on cardiovascular risk. Statins, widely used to manage dyslipidemia, possess anti-inflammatory and plaque-stabilizing properties that may have lessened the cardiovascular impact of MAFLD. Insulin therapy, despite being crucial for glycemic control, is known to contribute to weight gain and lipid alterations, which may affect cardiovascular outcomes. Furthermore, oral hypoglycemic agents such as metformin, sodium-glucose cotransporter-2 inhibitors, and glucagon-like peptide-1 receptor agonists are recognized for their cardioprotective effects. If these medications were more frequently used in the MAFLD group, they could have attenuated the observed cardiovascular risk, potentially leading to an underestimation of MAFLD’s true impact[59].
The strength of this study lies in its focus on assessing the risk of CVE and prevalence of CVD during the coexistence of MAFLD and T2DM both of which are increasing in epidemic proportions. The large number of participants included in the analysis and assessment of clinical outcome measures like CVE to assess the cardiovascular risk in T2DM with MAFLD are additional strengths of our study. A key limitation of our study is the considerable heterogeneity in the diagnostic evaluation of MAFLD and CVD across the included studies, along with a lack of uniformity in reported outcome measures. The criteria for diagnosing MAFLD varied, with some studies relying on imaging techniques such as ultrasound or transient elastography, while others used biochemical markers or liver biopsy, leading to inconsistencies in case identification. Similarly, the methods used to diagnose CVD differed, ranging from clinical history and medical records to imaging-based assessments, which may have affected the accuracy and comparability of cardiovascular outcomes. Additionally, variations in population characteristics, including differences in age distribution, sex ratios, ethnicity, and the prevalence of metabolic risk factors such as obesity, hypertension, and dyslipidemia, may have influenced the study findings. Patient selection also varied across studies, with data for the meta-analysis being extracted from sub-groups that met the inclusion criteria in only a few studies, potentially introducing selection bias. Furthermore, follow-up durations were inconsistent, which may have impacted the assessment of CVE incidence, with shorter follow-up periods potentially underestimating long-term cardiovascular risk. These discrepancies emphasize the need for standardized diagnostic and reporting criteria to enhance the reliability, comparability, and clinical applicability of future research in this field.
The presence of MAFLD in T2DM is associated with increased risk of CVE. T2DM patients with coexistent MAFLD have a higher prevalence of CVD compared to those without MAFLD. Thus, presence of MAFLD may be included in the CVD risk stratification of T2DM patients. This may potentiate intensifying the strategies addressing the CVD risk in the coexistence of T2DM and MAFLD. Given the strong association between MAFLD and CVD, future guidelines may need to recognize MAFLD as an independent risk factor, ensuring that T2DM patients with coexisting MAFLD receive enhanced cardiovascular monitoring and targeted preventive strategies. This approach could lead to more personalized cardiovascular risk management and improved patient outcomes. To facilitate early detection, systematic screening for MAFLD using imaging techniques such as transient elastography or controlled attenuation parameter ultrasound may be recommended. Early identification would allow timely interventions aimed at reducing both hepatic and cardiovascular complications. Combining CVD risk estimators (Atherosclerotic cardiovascular disease, QRESEARCH risk estimator version 3, Framingham Risk Score) with liver fibrosis assessment tools (Fibrosis index-4, NAFLD fibrosis score, FibroScan-AST Score) can improve risk prediction and support tailored intervention strategies. Clinicians may need to prioritize medications with proven cardiometabolic benefits alongside comprehensive lifestyle interventions, including weight loss, dietary modifications, and increased physical activity, which offer dual benefits for liver health and cardiovascular risk reduction. Further research is essential to better understand the pathophysiological links between MAFLD and CVD in T2DM and to evaluate the effectiveness of different treatment strategies in mitigating cardiovascular risk in this subgroup. Policymakers and healthcare organizations should consider incorporating MAFLD screening and management into routine diabetes care pathways to optimize long-term cardiovascular outcomes. By integrating MAFLD into CVD risk assessment for T2DM patients, healthcare providers could enable earlier detection, more individualized treatment strategies, and improved prevention of cardiovascular complications.
| 1. | Crane H, Eslick GD, Gofton C, Shaikh A, Cholankeril G, Cheah M, Zhong JH, Svegliati-Baroni G, Vitale A, Kim BK, Ahn SH, Kim MN, Strasser SI, George J. Global prevalence of metabolic dysfunction-associated fatty liver disease-related hepatocellular carcinoma: A systematic review and meta-analysis. Clin Mol Hepatol. 2024;30:436-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 2. | Mundi MS, Velapati S, Patel J, Kellogg TA, Abu Dayyeh BK, Hurt RT. Evolution of NAFLD and Its Management. Nutr Clin Pract. 2020;35:72-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 3. | Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18:599-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 588] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 4. | Pan Z, Shiha G, Esmat G, Méndez-Sánchez N, Eslam M. MAFLD predicts cardiovascular disease risk better than MASLD. Liver Int. 2024;44:1567-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 5. | Zheng H, Sechi LA, Navarese EP, Casu G, Vidili G. Metabolic dysfunction-associated steatotic liver disease and cardiovascular risk: a comprehensive review. Cardiovasc Diabetol. 2024;23:346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 86] [Reference Citation Analysis (0)] |
| 6. | Smith SC Jr. Multiple risk factors for cardiovascular disease and diabetes mellitus. Am J Med. 2007;120:S3-S11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Tanase DM, Gosav EM, Costea CF, Ciocoiu M, Lacatusu CM, Maranduca MA, Ouatu A, Floria M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J Diabetes Res. 2020;2020:3920196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 409] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 8. | Yanai H, Adachi H, Hakoshima M, Iida S, Katsuyama H. Metabolic-Dysfunction-Associated Steatotic Liver Disease-Its Pathophysiology, Association with Atherosclerosis and Cardiovascular Disease, and Treatments. Int J Mol Sci. 2023;24:15473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 76] [Reference Citation Analysis (0)] |
| 9. | Bhardwaj M, Mazumder PM. An insight on the additive impact of type 2 diabetes mellitus and nonalcoholic fatty liver disease on cardiovascular consequences. Mol Biol Rep. 2025;52:169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Barati M, Teimouri A, Feizi A, Iraj B, Karimifar M. Investigation of cardiovascular risk factors in diabetic and nondiabetic patients with nonalcoholic fatty liver disease. J Res Med Sci. 2024;29:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Zhang X, Wang L, Wang G, Li J, Mu Y, Wang S, Li X. Association Between Nonalcoholic Fatty Liver Disease and the Risk of Cardiovascular Disease in the Middle-Age and Elderly Population of Northern China: A Cross-Sectional Study. Diabetes Metab Syndr Obes. 2024;17:3079-3085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 745] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 13. | Bril F, Cusi K. Management of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Call to Action. Diabetes Care. 2017;40:419-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 252] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 14. | Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1055] [Article Influence: 105.5] [Reference Citation Analysis (0)] |
| 15. | Chang WH, Mueller SH, Chung SC, Foster GR, Lai AG. Increased burden of cardiovascular disease in people with liver disease: unequal geographical variations, risk factors and excess years of life lost. J Transl Med. 2022;20:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | He Y, Xiao F, Yi B, Lu J. Prevalence and associated factors of MAFLD in adults with type 2 diabetes. PLOS Glob Public Health. 2024;4:e0003572. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Luteijn M, Wald NJ. The NHS Health Checks programme: A better alternative. J Med Screen. 2016;23:57-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51768] [Article Influence: 10353.6] [Reference Citation Analysis (2)] |
| 19. | Ichikawa K, Miyoshi T, Osawa K, Miki T, Toda H, Ejiri K, Yoshida M, Nanba Y, Yoshida M, Nakamura K, Morita H, Ito H. Prognostic value of non-alcoholic fatty liver disease for predicting cardiovascular events in patients with diabetes mellitus with suspected coronary artery disease: a prospective cohort study. Cardiovasc Diabetol. 2021;20:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541-3546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 447] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 21. | Guo Y, Yang J, Ma R, Zhang X, Guo H, He J, Wang X, Cao B, Maimaitijiang R, Li Y, Peng X, Zhang S, Guo S. Metabolic Dysfunction-Associated Fatty Liver Disease Is Associated with the Risk of Incident Cardiovascular Disease: A Prospective Cohort Study in Xinjiang. Nutrients. 2022;14:2361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Agarwal AK, Jain V, Singla S, Baruah BP, Arya V, Yadav R, Singh VP. Prevalence of non-alcoholic fatty liver disease and its correlation with coronary risk factors in patients with type 2 diabetes. J Assoc Physicians India. 2011;59:351-354. [PubMed] |
| 23. | Chan WK, Tan AT, Vethakkan SR, Tah PC, Vijayananthan A, Goh KL. Ultrasonography-diagnosed non-alcoholic fatty liver disease is not associated with prevalent ischemic heart disease among diabetics in a multiracial Asian hospital clinic population. Clin Res Hepatol Gastroenterol. 2014;38:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Idilman IS, Akata D, Hazirolan T, Doganay Erdogan B, Aytemir K, Karcaaltincaba M. Nonalcoholic fatty liver disease is associated with significant coronary artery disease in type 2 diabetic patients: A computed tomography angiography study. J Diabetes. 2015;7:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Lu H, Zeng L, Liang B, Shu X, Xie D. High prevalence of coronary heart disease in type 2 diabetic patients with non-alcoholic fatty liver disease. Arch Med Res. 2009;40:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Takeuchi Y, Ito H, Komatsu Y, Oshikiri K, Antoku S, Abe M, Mifune M, Togane M. Non-alcoholic fatty liver disease is an independent predictor for macroangiopathy in Japanese type 2 diabetic patients: a cross-sectional study. Intern Med. 2012;51:1667-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Targher G, Bertolini L, Padovani R, Poli F, Scala L, Tessari R, Zenari L, Falezza G. Increased prevalence of cardiovascular disease in Type 2 diabetic patients with non-alcoholic fatty liver disease. Diabet Med. 2006;23:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 709] [Article Influence: 37.3] [Reference Citation Analysis (1)] |
| 29. | Dehghani Firouzabadi M, Poopak A, Sheikhy A, Dehghani Firouzabadi F, Moosaie F, Rabizadeh S, Momtazmanesh S, Nakhjavani M, Esteghamati A. Nonalcoholic Fatty Liver Disease as a Potential Risk Factor for Cardiovascular Disease in Patients with Type 2 Diabetes: A Prospective Cohort Study. Int J Endocrinol. 2024;2024:5328965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 30. | Kim KS, Hong S, Han K, Park CY. Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: nationwide population based study. BMJ. 2024;384:e076388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 81] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 31. | Riley DR, Hydes T, Hernadez G, Zhao SS, Alam U, Cuthbertson DJ. The synergistic impact of type 2 diabetes and MASLD on cardiovascular, liver, diabetes-related and cancer outcomes. Liver Int. 2024;44:2538-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 32. | Lalwani NS, Jha PR. Prevalence of non-alcoholic fatty liver disease and its correlation with coronary risk factors in patients with type 2 diabetes mellitus. Glob Med Public Health. 2024;13. |
| 33. | Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO, Shu SS, Chan AW, Yeung MW, Chan JC, Kong AP, Wong VW. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65:1359-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 371] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 34. | Eslam M, Ahmed A, Després JP, Jha V, Halford JCG, Wei Chieh JT, Harris DCH, Nangaku M, Colagiuri S, Targher G, Joshi S, Byrne CD, Khunti K, Nguyen MH, Gish RG, George J. Incorporating fatty liver disease in multidisciplinary care and novel clinical trial designs for patients with metabolic diseases. Lancet Gastroenterol Hepatol. 2021;6:743-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 35. | Matsubayashi Y, Fujihara K, Yamada-Harada M, Mitsuma Y, Sato T, Yaguchi Y, Osawa T, Yamamoto M, Kitazawa M, Yamada T, Kodama S, Sone H. Impact of metabolic syndrome and metabolic dysfunction-associated fatty liver disease on cardiovascular risk by the presence or absence of type 2 diabetes and according to sex. Cardiovasc Diabetol. 2022;21:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 36. | Schriger DL, Altman DG, Vetter JA, Heafner T, Moher D. Forest plots in reports of systematic reviews: a cross-sectional study reviewing current practice. Int J Epidemiol. 2010;39:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Kaya E, Yilmaz Y. Metabolic-associated Fatty Liver Disease (MAFLD): A Multi-systemic Disease Beyond the Liver. J Clin Transl Hepatol. 2022;10:329-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 38. | Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 693] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 39. | Dongiovanni P, Stender S, Pietrelli A, Mancina RM, Cespiati A, Petta S, Pelusi S, Pingitore P, Badiali S, Maggioni M, Mannisto V, Grimaudo S, Pipitone RM, Pihlajamaki J, Craxi A, Taube M, Carlsson LMS, Fargion S, Romeo S, Kozlitina J, Valenti L. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med. 2018;283:356-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 300] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 40. | Morrison AE, Zaccardi F, Khunti K, Davies MJ. Causality between non-alcoholic fatty liver disease and risk of cardiovascular disease and type 2 diabetes: A meta-analysis with bias analysis. Liver Int. 2019;39:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 41. | Domingueti CP, Dusse LM, Carvalho Md, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 2016;30:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 480] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 42. | Tuttolomondo A, Petta S, Casuccio A, Maida C, Corte VD, Daidone M, Di Raimondo D, Pecoraro R, Fonte R, Cirrincione A, Zafonte R, Cabibi D, Cammà C, Di Marco V, Licata A, Magliozzo F, Marchesini G, Merlino G, Craxì A, Pinto A. Reactive hyperemia index (RHI) and cognitive performance indexes are associated with histologic markers of liver disease in subjects with non-alcoholic fatty liver disease (NAFLD): a case control study. Cardiovasc Diabetol. 2018;17:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | du Plessis J, Korf H, van Pelt J, Windmolders P, Vander Elst I, Verrijken A, Hubens G, Van Gaal L, Cassiman D, Nevens F, Francque S, van der Merwe S. Pro-Inflammatory Cytokines but Not Endotoxin-Related Parameters Associate with Disease Severity in Patients with NAFLD. PLoS One. 2016;11:e0166048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 44. | Mangla N, Ajmera VH, Caussy C, Sirlin C, Brouha S, Bajwa-Dulai S, Madamba E, Bettencourt R, Richards L, Loomba R. Liver Stiffness Severity is Associated With Increased Cardiovascular Risk in Patients With Type 2 Diabetes. Clin Gastroenterol Hepatol. 2020;18:744-746.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Rinaldi L, Pafundi PC, Galiero R, Caturano A, Morone MV, Silvestri C, Giordano M, Salvatore T, Sasso FC. Mechanisms of Non-Alcoholic Fatty Liver Disease in the Metabolic Syndrome. A Narrative Review. Antioxidants (Basel). 2021;10:270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 46. | Sasso FC, Pafundi PC, Caturano A, Galiero R, Vetrano E, Nevola R, Petta S, Fracanzani AL, Coppola C, Di Marco V, Solano A, Lombardi R, Giordano M, Craxi A, Perrella A, Sardu C, Marfella R, Salvatore T, Adinolfi LE, Rinaldi L. Impact of direct acting antivirals (DAAs) on cardiovascular events in HCV cohort with pre-diabetes. Nutr Metab Cardiovasc Dis. 2021;31:2345-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 47. | Adinolfi LE, Petta S, Fracanzani AL, Nevola R, Coppola C, Narciso V, Rinaldi L, Calvaruso V, Pafundi PC, Lombardi R, Staiano L, Di Marco V, Solano A, Marrone A, Saturnino M, Rini F, Guerrera B, Troina G, Giordano M, Craxì A, Sasso FC. Reduced incidence of type 2 diabetes in patients with chronic hepatitis C virus infection cleared by direct-acting antiviral therapy: A prospective study. Diabetes Obes Metab. 2020;22:2408-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 48. | Caussy C, Aubin A, Loomba R. The Relationship Between Type 2 Diabetes, NAFLD, and Cardiovascular Risk. Curr Diab Rep. 2021;21:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 49. | Lechner K, McKenzie AL, Kränkel N, Von Schacky C, Worm N, Nixdorff U, Lechner B, Scherr J, Weingärtner O, Krauss RM. High-Risk Atherosclerosis and Metabolic Phenotype: The Roles of Ectopic Adiposity, Atherogenic Dyslipidemia, and Inflammation. Metab Syndr Relat Disord. 2020;18:176-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 50. | Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, Steffen HM. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2021;110:921-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 435] [Article Influence: 72.5] [Reference Citation Analysis (1)] |
| 51. | Galiero R, Caturano A, Vetrano E, Cesaro A, Rinaldi L, Salvatore T, Marfella R, Sardu C, Moscarella E, Gragnano F, Calabrò P, Sasso FC. Pathophysiological mechanisms and clinical evidence of relationship between Nonalcoholic fatty liver disease (NAFLD) and cardiovascular disease. Rev Cardiovasc Med. 2021;22:755-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 52. | Boutari C, Stefanakis K, Simati S, Guatibonza-García V, Valenzuela-Vallejo L, Anastasiou IA, Connelly MA, Kokkinos A, Mantzoros CS. Circulating total and H-specific GDF15 levels are elevated in subjects with MASLD but not in hyperlipidemic but otherwise metabolically healthy subjects with obesity. Cardiovasc Diabetol. 2024;23:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 53. | Yong JN, Ng CH, Lee CW, Chan YY, Tang ASP, Teng M, Tan DJH, Lim WH, Quek J, Xiao J, Chin YH, Foo R, Chan M, Lin W, Noureddin M, Siddiqui MS, Muthiah MD, Sanyal A, Chew NWS. Non-alcoholic fatty liver disease association with structural heart, systolic and diastolic dysfunction: a meta-analysis. Hepatol Int. 2022;16:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 54. | Ng CH, Chan KE, Chin YH, Zeng RW, Tsai PC, Lim WH, Tan DJH, Khoo CM, Goh LH, Ling ZJ, Kulkarni A, Mak LL, Huang DQ, Chan M, Chew NW, Siddiqui MS, Sanyal AJ, Muthiah M. The effect of diabetes and prediabetes on the prevalence, complications and mortality in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2022;28:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 55. | Chew NWS, Pan XH, Chong B, Chandramouli C, Muthiah M, Lam CSP. Type 2 diabetes mellitus and cardiometabolic outcomes in metabolic dysfunction-associated steatotic liver disease population. Diabetes Res Clin Pract. 2024;211:111652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 56. | Zhang QQ, Lu LG. Nonalcoholic Fatty Liver Disease: Dyslipidemia, Risk for Cardiovascular Complications, and Treatment Strategy. J Clin Transl Hepatol. 2015;3:78-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 57. | Shao C, Ye J, Li F, Lin Y, Wu T, Wang W, Feng S, Zhong B. Early Predictors of Cardiovascular Disease Risk in Nonalcoholic Fatty Liver Disease: Non-obese Versus Obese Patients. Dig Dis Sci. 2020;65:1850-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 58. | Luo J, Xu L, Li J, Zhao S. Nonalcoholic fatty liver disease as a potential risk factor of cardiovascular disease. Eur J Gastroenterol Hepatol. 2015;27:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Jang H, Kim Y, Lee DH, Joo SK, Koo BK, Lim S, Lee W, Kim W. Outcomes of Various Classes of Oral Antidiabetic Drugs on Nonalcoholic Fatty Liver Disease. JAMA Intern Med. 2024;184:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 72] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/