Published online May 27, 2025. doi: 10.4254/wjh.v17.i5.103852
Revised: March 24, 2025
Accepted: April 24, 2025
Published online: May 27, 2025
Processing time: 172 Days and 21.7 Hours
The global incidence of metabolic dysfunction-associated steatotic liver disease (MASLD) has increased in recent years. It has already been demonstrated that exercise and weight change are associated with the occurrence of MASLD; how
To investigate the impact of weight fluctuation and physical activity intensity on the risk of MASLD prevalence.
Data from the National Health and Nutrition Examination Survey database in
Among 3183 MASLD cases, the risk of MASLD increased with age for individuals transitioning from non-obese to obese or maintaining obesity, with odds ratio (OR) changing from 8.91 (95%CI: 7.40–10.88) and 11.87 (95%CI: 9.65–14.60) at 10 years before baseline to 9.58 (95%CI: 8.08–11.37) and 12.51 (95%CI: 9.33-16.78) at 25 years. Stable obesity correlated with age-dependent MASLD prevalence escalation, whereas increased physical activity attenuated MASLD risk in this group, with an OR changing from 13.64 (95%CI: 10.59–17.57) to 6.42 (95%CI: 4.24–9.72). Further analysis of the net weight changes revealed a paradoxical risk elevation with intensified physical activity during different time periods.
The risk of MASLD increases in individuals transitioning from non-obese to obese or maintaining obesity. High-intensity physical activity is beneficial for MASLD among individuals with stable obesity.
Core Tip: The risk of metabolic dysfunction-associated steatotic liver disease (MASLD) increases with age in individuals transitioning from non-obese to obese or maintaining obesity. High-intensity physical activity is beneficial in reducing the risk of MASLD among individuals with stable obesity. Delineation of the dose-response relationship between weight fluctuation patterns and MASLD prevalence risk will facilitate the development of personalized exercise prescriptions.
- Citation: Wang JP, Wang JY, Sun PQ, Wang XW, Yuan ZT, Cao Q, Pan SM, Jiang YY. Association between weight fluctuation and the risk of metabolic dysfunction-associated steatotic liver disease. World J Hepatol 2025; 17(5): 103852
- URL: https://www.wjgnet.com/1948-5182/full/v17/i5/103852.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i5.103852
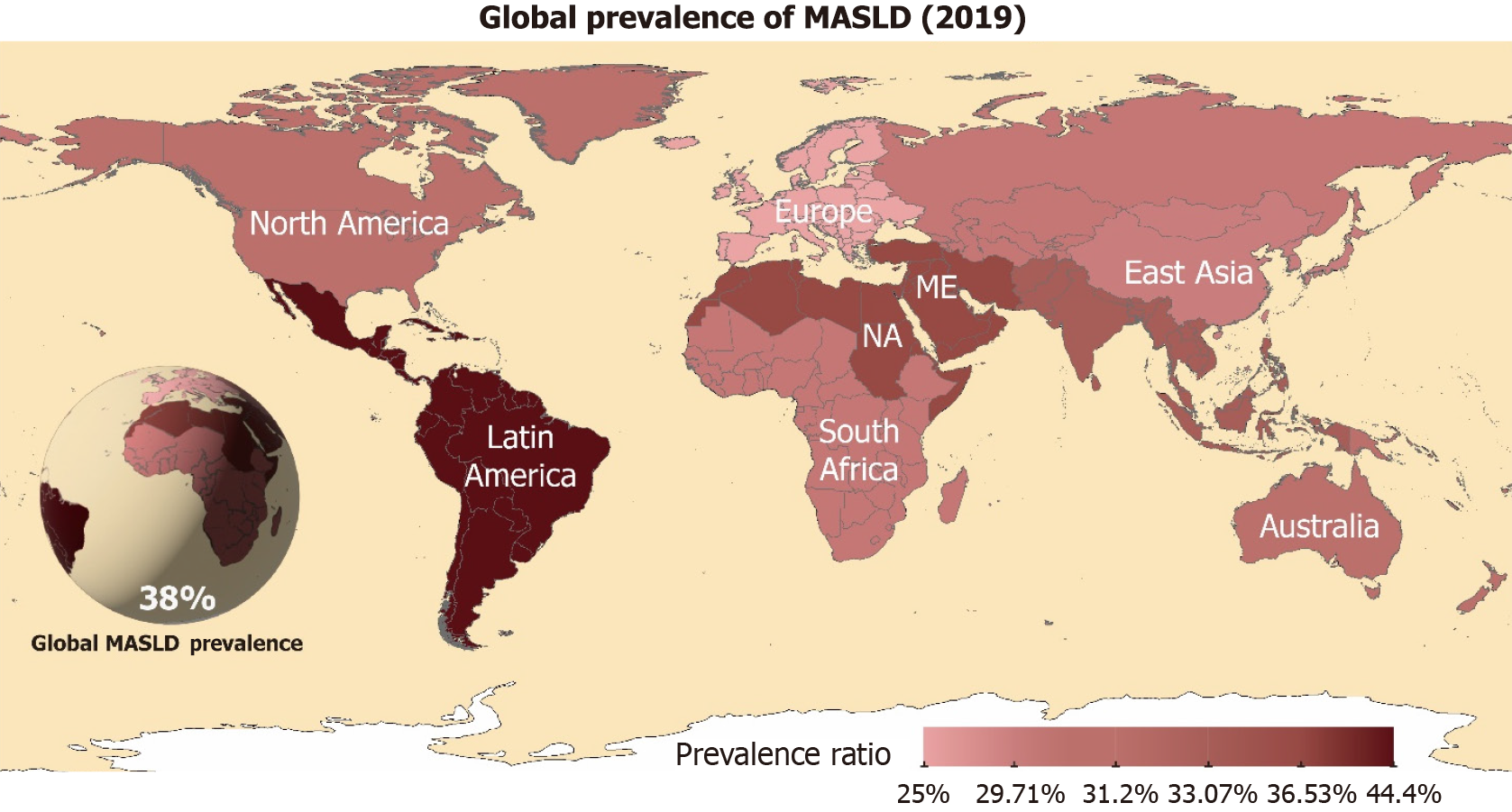
Metabolic dysfunction-associated steatotic liver disease (MASLD) is characterized by fat accumulation in the liver, ranging from simple steatotic liver disease to metabolic dysfunction-associated steatohepatitis (MASH), cirrhosis, and liver cancer. The prevalence of MASLD has been increasing worldwide. According to a global epidemiological study, MASLD was the most common cause of chronic liver disease[1]. MASLD has emerged as a pandemic-scale metabolic disorder that currently affects approximately 31.3% of the global population (Figure 1), with prevalence trajectories parallel to the obesity and type 2 diabetes epidemics[2,3]. The prevalence of MASLD has increased significantly in developed countries and it has become one of the leading causes of liver transplantation[4]. Relevant studies have shown that the prevalence of MASLD has significantly increased in Asian populations over the last few decades and is closely related to changes in lifestyle and dietary habits, including physical activity[5].
Weight change refers to changes in body weight over a relative period, during which weight can have periodic in
Exercise can be beneficial in MASLD by increasing fat oxidation and improving lipid metabolism[11]. Performing moderate to high-intensity physical activity can reduce the possibility of MASLD progression[11,12]. For example, long-term aerobic exercises such as brisk walking, running, and swimming can reduce MASLD fat deposition and are associated with improved liver function[13]. Studies have shown that a combination of aerobic exercise and resistance training is effective in reducing fat content in MASLD and improving insulin resistance[14]. In addition, 150 minutes of moderate-intensity physical activity per week has been shown to significantly reduce the risk of developing MASLD[15]. Given that patients with MASLD often present with complex metabolic comorbidities, reliance solely on BMI fails to accurately reflect specific fat distribution patterns or true metabolic health status. This study focuses on investigating age-related differentials in metabolic mechanisms of exercise intervention for MASLD. Existing research has extensively investigated lifestyle-related risk factors in MASLD pathogenesis. While given MASLD's distinct temporal cumulative effects, the interactive effects of long-term exercise patterns, body weight fluctuations, and aging on disease progression remain inadequately elucidated. Particularly, a critical knowledge gap persists regarding how weight fluctuation mediated by varying exercise intensities modulate MASLD risk across different age strata, which constitutes a pivotal yet underexplored dimension in current scientific inquiry. Our study used the National Health and Nutrition Examination Survey (NHANES) database to conduct an in-depth investigation into how weight change trajectories under varying physical activity intensities influence the prevalence risk, with the aim of providing scientifically robust and clinically actionable evidence for MASLD prevention and intervention strategies, seeking to explore the effects of weight fluctuation and physical activity intensity on the risk of MASLD by analyzing laboratory data from the NHANES database.
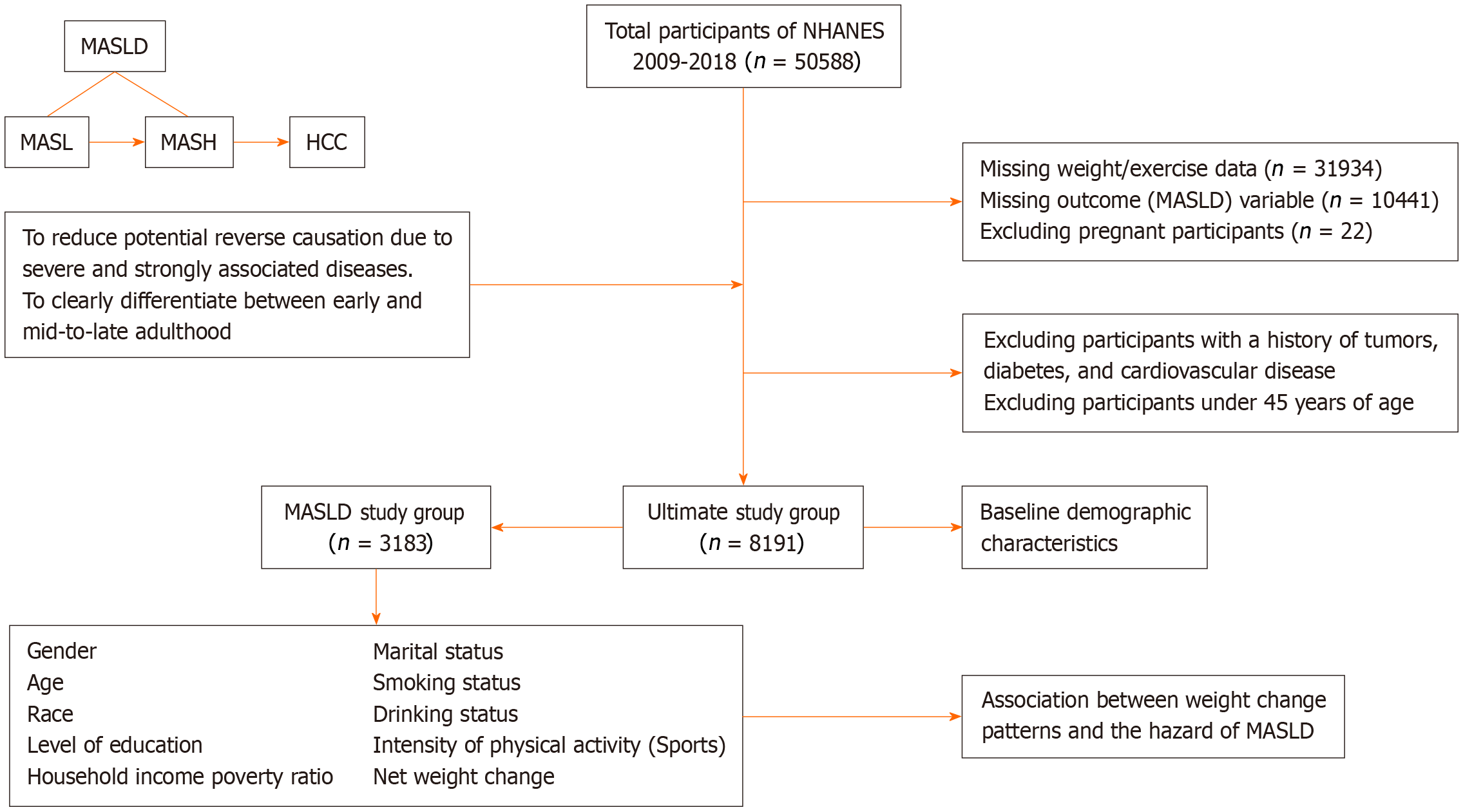
The NHANES uses a complex, multi-stage, probability sampling design to collect representative health data from the United States population. The survey has been ongoing since 1999, and survey data have been released periodically over a two-year cycle. Data were obtained via face-to-face interviews, mobile medical examinations, and laboratory tests. The current study used data from the National Health and NHANES database covering five cycles from 2009 to 2018. The exclusion criteria included (1) Missing weight data or physical activity intensity data; (2) Missing outcome (MASLD) variables; and (3) Pregnant participants (during pregnancy, female patients exhibited physiological changes, such as increased metabolic demands and heightened hepatic burden; these pregnancy-specific adaptations differ from those observed in non-pregnant individuals).
In the baseline questionnaire, participants reviewed their weight data 10 years before baseline and at 25 years of age. Height and weight were measured at baseline at a Mobile Examination Center (MEC). BMI values, calculated as weight (kg) divided by the square of height, were recorded at three time points (baseline, 10 years before baseline, and 25 years of age) and noted separately as BMI baseline, BMI 10 prior, and BMI 25. Obesity was defined as. The four patterns of weight fluctuation in early adulthood (25 years of age to 10 years before baseline) and in mid-to-late adulthood (10 years before baseline to baseline) were defined as follows: stable non-obesity (all BMI 30, the reference group), obesity to non-obesity (to), non-obesity to obesity (to), and stable obesity (all BMI 30). Considering the extensive timeframe of data collected from survey respondents, there is a dearth of research examining the patterns of long-term weight change. Furthermore, the natural trajectory of weight fluctuation is difficult to summarize and analyze because of the myriad influences of geography, cultural practices, economic status, and genetic predispositions. In light of these complexities, the present study draws upon the extant literature concerning short-term randomized controlled trials on weight re
The core diagnostic criteria for MASLD require the following three components: (1) Evidence of hepatic steatosis documented by imaging techniques, blood biomarkers, or liver histology; (2) Presence of at least one cardiometabolic risk factor, defined as: Overweight/obesity (BMI ≥ 23 kg/m² for Asian populations or ≥ 25 kg/m² for non-Asian populations, or elevated waist circumference), type 2 diabetes, hypertension (≥ 130 mmHg/85 mmHg or on antihypertensive therapy), hypertriglyceridemia (≥ 150 mg/dL or on lipid-lowering treatment), or low high-density lipoprotein-cholesterol (men < 40 mg/dL, women < 50 mg/dL); and (3) Exclusion of alternative etiologies including excessive alcohol consumption (men < 30 g/day, women < 20 g/day), viral hepatitis, drug-induced liver injury, and other competing causes of hepatic steatosis. These criteria collectively establish a positive diagnostic framework emphasizing metabolic dysfunction while maintaining compatibility with coexisting liver conditions[18,19]. It comprises two entities: (1) MASL; and (2) MASH. In this NHANES database study, MASLD was defined based on the United States Fatty Liver Index (USFLI) and NHANES data[20] using the following formula:
USFLI = e(-0.8073 × nonHispanic Black people + 0.3458 × Mexican American + 0.0093 × age + 0.6151 × ln (γ-Glutamyl transpeptidase) + 0.0249 × waist + 1.1792 × ln (insulin + 0.8242 × ln (glucose) - 14.7812))/1 + e(-0.8073 × nonHispanic Black people + 0.3458 × Mexican American + 0.0093 × age + 0.6151 × ln (γ-Glutamyl transpeptidase) + 0.0249 × waist + 1.1792 × ln (insulin + 0.8242 × ln (glucose) -14.7812)) × 100
The Fatty (Steatotic) Liver Index (FLI) (SLI) is a non-invasive assessment model for hepatic steatosis based on clinical parameters including BMI, waist circumference, gamma-glutamyl transferase, triglyceride levels etc. Its derivative version, United States FLI (SLI), incorporates optimized calibration for metabolic characteristics and disease phenotypes in multi-ethnic American populations, significantly enhancing diagnostic accuracy in heterogeneous cohorts. In epidemiological investigations (e.g., NHANES-based cohort or cross-sectional studies), threshold-based classification is commonly employed for case identification, with FLI > 30 defining high-risk individuals for fatty liver. This study utilized the NHANES database to investigate MASLD. Given the ethnic heterogeneity of the study population and region-specific metabolic profiles, the United States FLI—validated for localized applicability—was adopted as the diagnostic criterion. Participants with United States FLI values > 30 were enrolled in the MASLD case group. As the data used in this study were sourced from the NHANES database, no additional external validation of this formula could be conducted. Based on the recommended values from previous studies, MASLD was defined as a USFLI of > 30.
The physical activity levels of the participants were assessed using the Global Physical Activity Questionnaire, which converts total physical activity time into a metabolic equivalent task (MET) (minute/week). After consulting the international expert practice recommendations for MASLD[21], we categorized the physical activity intensity into the following three groups based on the conversion of metabolic equivalents: (1) Low intensity (< 600 MET minute/week); (2) Moderate-intensity (600–1200 MET minute/week); and (3) High intensity (> 1200 MET minute/week) (150 minutes of moderate-intensity exercise per week or 75 minutes of high-intensity exercise per week is approximately equivalent to engaging in 600 MET minute/week).
Based on the findings of previous studies, various covariates were used, including age, sex (male, female), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), marital status (married, other), education level (dropping out of high school or lower, high school graduate, drop out of college, college graduate, or higher), and household income poverty ratio level (0–1.0, 1.1–3.0, > 3.0). Alcohol consumption was classified as never drinking, abstaining, or drinking alcohol. Smoking status was categorized as never smoked, abstinent, or smoking. Disease history was considered separately for oncology (yes/no), diabetes mellitus (yes/no), and cardiovascular disease (yes/no), which included any of the following: (1) Congestive heart failure; (2) Coronary heart disease; (3) Angina pectoris; (4) Myocardial infarction; (5) Stroke; and (6) Hypertension.
In complex sample designs, sampling weights are used to adjust the representativeness of the sample. Each sample unit is assigned a weight to reflect its representation in the overall population. Clustering refers to dividing the population into several groups during the sampling process to improve sampling efficiency. Stratification involves dividing the population into different subpopulations (strata) based on specific variables and then drawing samples from each stratum to enhance the representativeness of the sample. The Rao-Scott χ² test is a statistical method designed to account for the complexities of survey sampling designs, such as stratification and clustering, when testing the independence of variables in a contingency table. This test is particularly useful for analyzing data from complex surveys like NHANES, where the sampling design can significantly affect the distribution of the test statistics. Weighted linear regression (WLR) is an extension of traditional linear regression that assigns different weights to each observation to address heteroscedasticity. This method is particularly useful when the reliability and importance of different observations vary, such as in survey data where certain samples may be over-sampled. By adjusting weights, WLR can reflect the true proportion of these samples in the overall population.
Appropriate sampling weights, clusters, and strata were used in this study. Data on demographic characteristics were presented according to weight fluctuation patterns in mid-to-late adulthood, with continuous variables expressed as weighted means and standard errors, and categorical variables expressed as frequencies and weighted percentages. Continuous variables were compared across weight fluctuation patterns using weight-adjusted (weighted) linear re
Weighted multivariate logistic regression was used to examine the effect of different weight fluctuation patterns on the prevalence of MASLD over two-time intervals as well as the relationship between the net weight change group and the risk of MASLD prevalence. The nonlinear relationship between net weight change and the risk of MASLD was assessed using nonparametric restricted cubic spline curves. The NHANES used a complex multistage probability sampling design comprising four hierarchical stages: (1) Counties; (2) Segments; (3) Households; and (4) Individuals. This methodology inherently generates unequal selection probabilities across participants and introduces nonindependent sampling across stages. Therefore, analytical procedures must incorporate three critical structural parameters provided in the official dataset: (1) Sample weights (accounting for differential selection probabilities); (2) Strata [reflecting primary sampling units (PSUs)]; and (3) PSUs. These parameters were pre-calculated and distributed using the NHANES datasets.
For detailed computational methodologies, refer to the official NHANES analytical guidelines at https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx#estimation-and-weighting-procedures. When pooling data across multiple NHANES survey cycles (2009–2010 through 2017–2018, comprising five biennial cycles), investigators must recalibrate the sampling weights to account for combined-cycle analyses. For the MEC-collected variables, the adjusted composite weight was calculated as adjusted weight (WTMEC_adj) = (1/5) × WTMEC2YR, where WTMEC2YR represents the original two-year MEC examination weight. Subsequent analytical procedures in Sequence Analysis and Statistics (SAS) require the systematic integration of three design elements: (1) WTMEC_adj; (2) Stratification variables; and (3) PSUs (SDMVPSU). These parameters must be explicitly declared in the survey procedures (such as PROC SURVEYMEANS/SURVEYREG) using the WEIGHT, STRATA, and CLUSTER statements. For comprehensive implementation guidance, consult https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx#analytic-guidelines. In survival analysis, the risk function generally refers to the instant-aneous risk rate at a given time point for an individual who has not yet experienced the target event (such as MASLD diagnosis) to develop the event at that specific moment.
Sensitivity analysis was performed to test the robustness of the results. First, participants with a history of tumors, diabetes, or cardiovascular disease were excluded to reduce the potential for reverse causality due to serious illness. Subsequently, participants aged < 45 years were excluded to distinguish between the time points of early and mid-to-late adulthood. Finally, underweight participants were excluded to minimize the potential effect of being underweight on the results. Following methodological adjustments, comprehensive statistical analyses were performed on the remaining study population using recalibrated analytical protocols.
All the analyses were performed using SAS (version 9.4; SAS Institute Inc., Cary, NC, United States) and the R software version 4.2.1 (R Core Team, Auckland, United States). Statistical significance was defined as a two-tailed P value less than 0.05.
A total of 8191 participants with a mean age of 55.49 years (range: 36-80 years) were included in the analysis. Most participants were female (51.28%), non-Hispanic Caucasians (73.05%), married (64.84%), educated to college graduation level or higher (31.66%), and had a household income poverty ratio of 3.0 or higher (54.57%). More than half of the participants had never smoked (51.75%), but 76.43% drank alcohol (Table 1). Notably, the observed proportion of 76.43% in this cohort was derived through the rigorous application of MASLD diagnostic criteria, which mandated the exclusion of patients with clinically significant alcohol consumption. Specifically, alcohol intake thresholds were defined according to international guidelines (male: < 30 g/day; female: < 20 g/day). Crucially, the final analytical cohort exclusively comprised MASLD-confirmed cases without a significant history of alcohol exposure, as validated using standardized alcohol use quantification tools. Notably, 62.68% of the participants had low physical activity intensity, and the overall BMI of the participants showed an increasing trend from 24.16 at age 25 years to 27.53 at 10 years before baseline and 29.16 at baseline, with a mean weight change from 10 years before baseline of 4.56 kg. A total of 3183 participants were diagnosed with MASLD, most of whom experienced a non-obese to obese weight change from 10 years before baseline to baseline, with a general weight gain of 2.5–10 kg (Table 2). A retrospective analysis of patient weight from early adulthood to baseline revealed that the number of patients with MASLD who went from a non-obese to an obese status was much greater, with most patients having a weight gain of > 20 kg. The methodological framework of this study, encompassing participant eligibility criteria, exposure variable considerations, final cohort composition, and analytical strategies, is presented schematically in Figure 2.
| Baseline characteristics | Overall population (n = 8191) | Patterns of weight change1 | P value | |||
| Stabilizing non-obesity (n = 4565) | Obesity to non-obesity (n = 491) | Non-obesity to obesity (n = 1348) | Stabilizing obesity (n = 1787) | |||
| Age (year) | 55.49 (0.22) | 55.49 (0.29) | 59.40 (0.75) | 52.75 (0.42) | 56.53 (0.31) | < 0.0001 |
| Gender | 0.0003 | |||||
| Male | 4036 (48.72) | 2346 (48.60) | 282 (57.10) | 541 (43.28) | 867 (50.97) | |
| Women | 4155 (51.28) | 2219 (51.40) | 209 (42.90) | 807 (56.72) | 920 (49.03) | |
| Race | < 0.0001 | |||||
| Non-Hispanic White | 3777 (73.05) | 2184 (74.81) | 219 (72.59) | 540 (66.61) | 834 (73.30) | |
| Non-Hispanic Black | 1568 (9.19) | 720 (7.22) | 99 (9.87) | 326 (12.80) | 423 (11.58) | |
| Mexican | 1134 (6.49) | 529 (5.10) | 94 (8.87) | 233 (9.53) | 278 (7.30) | |
| Other Hispanics and mestizos | 1712 (11.27) | 1132 (12.87) | 79 (8.66) | 249 (11.06) | 252 (7.83) | |
| Marital status | 0.0197 | |||||
| Married | 4840 (64.84) | 2783 (66.83) | 277 (63.84) | 759 (61.31) | 1021 (62.45) | |
| Else | 3348 (35.16) | 1782 (33.17) | 214 (36.16) | 588 (38.69) | 764 (37.55) | |
| Mean body mass index (kg/m2) | ||||||
| 25 years old | 24.16 (0.07) | 22.37 (0.06) | 26.24 (0.30) | 24.07 (0.14) | 28.432 (0.16) | < 0.0001 |
| 10 years before baseline | 27.53 (0.11) | 24.13 (0.06) | 32.77 (0.13) | 26.97 (0.08) | 35.64 (0.18) | < 0.0001 |
| Baseline | 29.16 (0.12) | 25.02 (0.07) | 27.56 (0.13) | 33.53 (0.12) | 37.23 (0.19) | < 0.0001 |
| Net weight change (kg) | 4.56 (0.20) | 2.50 (0.13) | -14.84 (0.49) | 18.34 (0.41) | 4.61 (0.47) | < 0.0001 |
| Level of education3 | < 0.0001 | |||||
| High school dropout or below | 2110 (16.56) | 1112 (15.45) | 153 (20.13) | 370 (18.82) | 475 (16.95) | |
| Graduate from high school | 1868 (22.34) | 1030 (21.77) | 118 (22.59) | 313 (24.08) | 407 (22.50) | |
| Drop out of college | 2203 (29.43) | 1107 (25.95) | 147 (35.14) | 413 (33.00) | 536 (34.57) | |
| University graduate or above | 2003 (31.66) | 1311 (36.83) | 73 (22.14) | 251 (24.09) | 368 (25.98) | |
| Household income poverty ratio3 | < 0.0001 | |||||
| 0-1.0 | 1434 (11.48) | 742 (10.41) | 104 (15.60) | 265 (13.44) | 323 (11.85) | |
| 1.1-3.0 | 3119 (33.95) | 1659 (31.50) | 220 (42.09) | 518 (36.14) | 722 (36.82) | |
| > 3.0 | 2982 (54.57) | 1797 (58.09) | 123 (42.32) | 444 (50.42) | 618 (51.33) | |
| Smoking status3 | < 0.0001 | |||||
| Never smoked | 4243 (51.75) | 2356 (51.87) | 222 (45.38) | 717 (51.10) | 948 (53.48) | |
| Cessation | 2373 (29.91) | 1264 (28.02) | 153 (33.58) | 379 (29.91) | 577 (34.00) | |
| Smoking | 1569 (18.34) | 941 (20.11) | 115 (21.05) | 251 (18.99) | 262 (12.52) | |
| Drinking status3 | 0.0037 | |||||
| Never | 1068 (10.63) | 571 (9.76) | 73 (14.25) | 179 (10.96) | 245 (11.73) | |
| Cessation | 1134 (12.95) | 567 (11.44) | 62 (13.69) | 189 (13.61) | 316 (16.18) | |
| Drinking | 5416 (76.43) | 3077 (78.80) | 325 (72.06) | 884 (75.44) | 1130 (72.10) | |
| Sports | < 0.0001 | |||||
| Low intensity | 5424 (62.68) | 2921 (59.41) | 337 (65.11) | 907 (63.90) | 1259 (69.78) | |
| Medium intensity | 1119 (15.71) | 680 (17.12) | 63 (16.31) | 169 (15.46) | 207 (12.01) | |
| High intensity | 1648 (21.62) | 964 (23.47) | 91 (18.57) | 272 (20.64) | 321 (18.21) | |
| History of the tumor | 0.0001 | |||||
| None | 7222 (87.32) | 4018 (87.01) | 408 (82.00) | 1236 (91.74) | 1560 (86.15) | |
| Have | 962 (12.68) | 543 (12.99) | 81 (18.00) | 112 (8.26) | 226 (13.85) | |
| History of diabetes | < 0.0001 | |||||
| None | 6667 (87.64) | 4071 (94.05) | 313 (71.79) | 1094 (87.10) | 1189 (74.57) | |
| Have | 1276 (12.36) | 401 (5.95) | 158 (28.21) | 205 (12.90) | 512 (25.43) | |
| History of cardiovascular disease | < 0.0001 | |||||
| None | 4195 (55.18) | 2771 (65.39) | 189 (40.24) | 638 (49.15) | 597 (36.40) | |
| Have | 3996 (44.82) | 1794 (34.61) | 302 (59.76) | 710 (50.85) | 1190 (63.60) | |
| Weight fluctuation assessment | Overall population | Low-intensity physical activity | Medium-intensity physical activity | High-intensity physical activity | ||||
| MASLD | OR (95%CI) | MASLD | OR (95%CI) | MASLD | OR (95%CI) | MASLD | OR (95%CI) | |
| Weight fluctuation patterns | ||||||||
| Mid-to-late adulthood | ||||||||
| Stabilizing non-obesity | 924 | 1 | 614 | 1 | 121 | 1.00 (0.71-1.40) | 189 | 0.85 (0.64-1.13) |
| Obesity to non-obesity | 159 | 1.21 (0.85-1.72) | 113 | 1.21 (0.78-1.86) | 19 | 0.90 (0.37-2.18) | 27 | 1.27 (0.60-2.66) |
| Non-obesity to obesity | 836 | 8.97 (7.40-10.88)1 | 571 | 9.46 (7.35-12.19)3 | 98 | 9.39 (5.85-15.07)3 | 167 | 6.24 (4.37-8.91)3 |
| Stabilizing obesity | 1264 | 11.87 (9.65-14.60)1 | 896 | 13.64 (10.59-17.57)3 | 145 | 11.65 (7.07-19.20)3 | 223 | 6.42 (4.24-9.72)3 |
| Early adulthood | ||||||||
| Stabilizing non-obesity | 1051 | 1 | 702 | 1 | 140 | 0.96 (0.69-1.35) | 209 | 0.84 (0.65-1.09) |
| Obesity to non-obesity | 32 | 0.56 (0.26-1.22) | 25 | 0.33 (0.15-0.71) | 0 | 0.00 (0.00-0.00) | 7 | 0.99 (0.33-2.94) |
| Non-obesity to obesity | 1685 | 9.58 (8.08-11.37)1 | 1185 | 10.29 (8.43-12.55) | 193 | 10.03 (6.44-15.61) | 307 | 6.11 (4.48-8.32) |
| Stabilizing obesity | 415 | 12.51 (9.33-16.78)1 | 282 | 16.14 (11.00-23.70) | 50 | 10.05 (5.75-17.56) | 83 | 5.74 (3.27-10.11) |
| Net weight change | ||||||||
| Mid-to-late adulthood | ||||||||
| ≤ -2.5 kg | 617 | 0.67 (0.51-0.90) | 432 | 0.65 (0.46-0.93) | 70 | 0.58 (0.33-1.02) | 115 | 0.52 (0.32-0.85) |
| -2.5 to 2.5 kg | 435 | 12 | 305 | 14 | 56 | 0.85 (0.45-1.60) | 74 | 0.74 (0.43-1.26) |
| 2.5-10 kg | 846 | 1.37 (1.09-1.73) | 570 | 1.39 (1.01-1.90) | 109 | 1.11 (0.68-1.80) | 167 | 0.98 (0.65-1.48) |
| 10-20 kg | 728 | 2.03 (1.57-2.62)2 | 485 | 1.92 (1.32-2.78) | 96 | 2.47 (1.47-4.15) | 147 | 1.20 (0.72-2.01) |
| > 20 kg | 557 | 4.78 (3.37-6.76)2 | 402 | 4.93 (3.17-7.65) | 52 | 3.10 (1.38-6.96)4 | 103 | 3.69 (2.10-6.46)4 |
| Early adulthood | ||||||||
| ≤ -2.5 kg | 163 | 0.97 (0.54-1.74) | 119 | 1.02 (0.50-2.10) | 16 | 0.62 (0.18-2.13) | 28 | 0.72 (0.32-1.62) |
| -2.5 to 2.5 kg | 111 | 1 | 73 | 1 | 12 | 0.71 (0.30-1.69) | 26 | 0.86 (0.42-1.76) |
| 2.5-10 kg | 405 | 1.36 (0.92-2.02) | 265 | 1.48 (0.87-2.51) | 53 | 1.05 (0.53-2.09) | 87 | 0.92 (0.50-1.70) |
| 10-20 kg | 885 | 2.49 (1.71-3.61) | 571 | 2.27 (1.37-3.77) | 119 | 2.56 (1.52-4.32) | 195 | 2.04 (1.25-3.36) |
| > 20 kg | 1619 | 5.73 (3.66-8.98) | 1166 | 5.89 (3.41-10.17) | 183 | 5.33 (2.88-9.89) | 270 | 3.53 (1.96-6.34) |
The association between weight fluctuation patterns and MASLD risk at each time point was assessed using stable non-obese individuals as the reference group. Participants in the non-obese to obese and stable obese groups had an increased risk of MASLD, with odds ratio (OR) of 8.97 (95%CI: 7.40–10.88) and 11.87 (95%CI: 9.65–14.60) at 10 years before baseline and 9.58 (95%CI: 8.08–11.37) and 12.51 (95%CI: 9.33–16.78) at 25 years old, respectively (Table 2). For net weight change, a 10–20 kg increase from 10 years before baseline to baseline increased the risk of MASLD by 103%, and a weight gain of > 20 kg increased the risk of MASLD by 4.78 times that of the reference group compared with the “within 2.5 kg” group, which had the smallest number of MASLD patients (Table 2). The OR for weight change from age 25 years to 10 years baseline were 2.49 (95%CI: 1.71–3.61) and 5.73 (95%CI: 3.66–8.98) for increases of 10–20 kg and > 20 kg, respectively (Table 2).
In the overall population analysis, the reference group was defined as having an OR of 1. For subgroup analyses stratified by physical activity intensity, a unified reference group was established as either "low-intensity physical activity practitioners with stable non-obese status" (for weight trajectory patterns) or "low-intensity physical activity practitioners exhibiting absolute weight changes within the range of –2.5 to +2.5 kg" (for net weight change analyses). For instance, among middle-to-late adulthood individuals demonstrating a transition pattern from non-obese to obese status, those engaged in high-intensity physical activity showed an adjusted OR of 6.24 (95%CI: 4.18–9.31), indicating a 6.24-fold increased risk of MASLD development compared to the reference group of stable non-obese individuals maintaining low-intensity physical activity. Statistical significance was determined through the simultaneous evaluation of the OR magnitude and its 95%CI, with associations deemed significant only when both values were above 1 (positive association) or below 1 (protective effect). The omission of P values in OR reporting emphasizes the precision of the effect size estimation over binary significance thresholds, aligned with contemporary epidemiological reporting standards. Further assessment of the effect of physical activity intensity on the association between weight fluctuation and MASLD risk revealed that the risk of MASLD in the non-obese to obese and stable obese groups was significantly reduced with increasing physical activity intensity in both early and mid-to-late adulthood compared to the stable non-obese low-intensity physical activity group. The MASLD risk decreased with an increase in physical activity intensity in the non-obese to obese population 10 years before baseline, with an OR trend of 9.46 (95%CI: 7.35–12.19), 9.39 (95%CI: 5.85–15.07), and 6.24 (95%CI: 4.37–8.91) for low-intensity, moderate-intensity, and high-intensity physical activity, respectively (Table 2). The ORs for MASLD risk in the stably obese population were 13.64 (95%CI: 10.59–17.57), 11.65 (95%CI: 7.07–19.20), and 6.42 (95%CI: 4.24–9.72) for low-intensity, moderate-intensity, and high-intensity physical activity, respectively (Table 2). A similar trend was observed in weight fluctuations in early adulthood (Table 2).
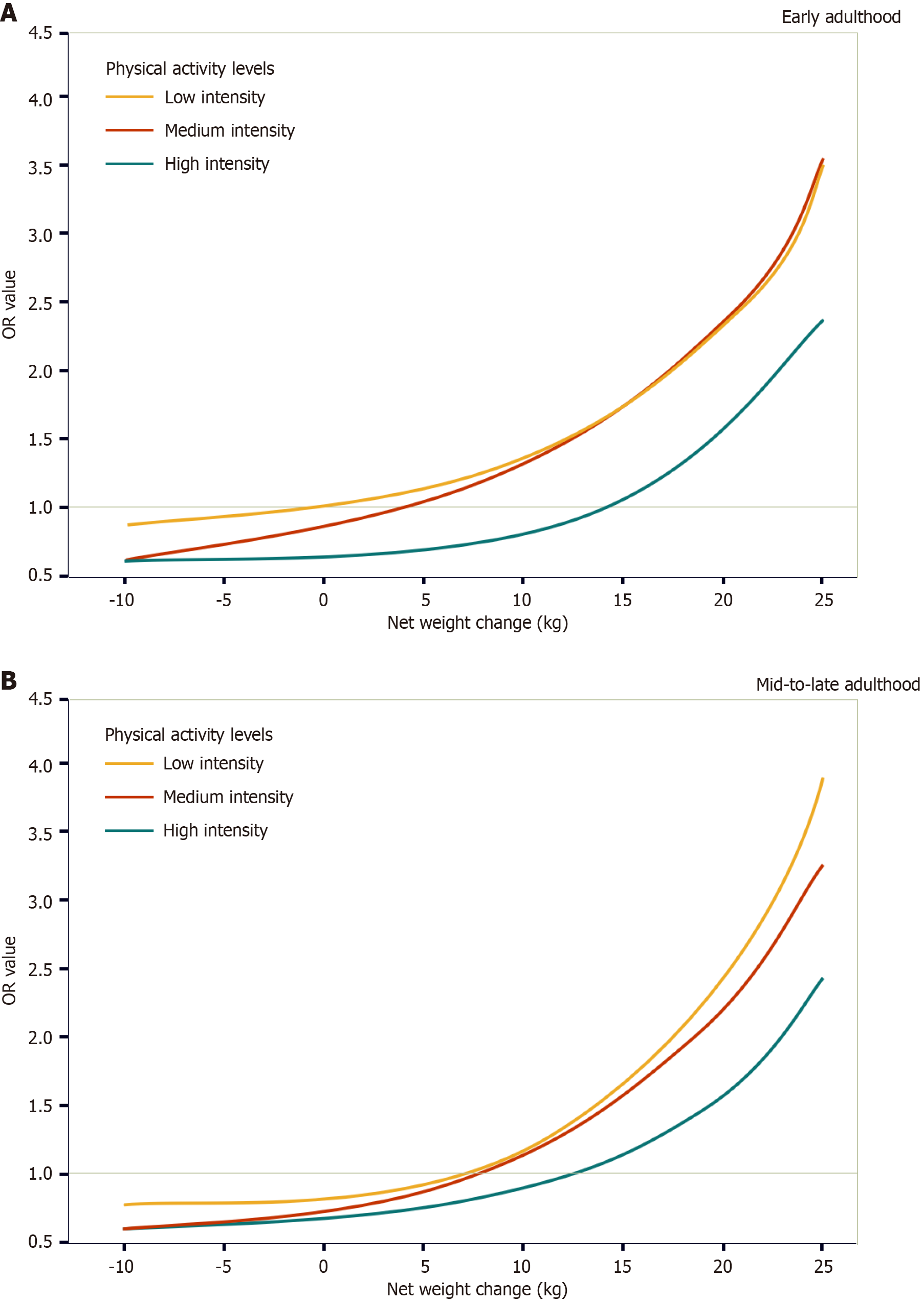
Slightly different results were obtained when exploring the effect of physical activity intensity on the association between net weight change and MASLD risk. When net weight change was considered a categorical variable, an increase in physical activity intensity increased the risk of MASLD. For example, compared with patients with a range of weight change of 2.5 kg for low-intensity physical activity, the OR for high-intensity physical activity in patients with a weight gain of > 20 kg was 3.69 (95%CI: 2.10–6.46), which was higher than that for moderate-intensity physical activity [3.10 (95%CI: 1.38–6.96)] (Table 2). However, analysis of the nonlinear association between net weight change and MASLD risk showed that for the weight gain of 5–15 kg, the risk of MASLD in mid-to-late adulthood was very close between low-intensity and medium-intensity physical activity, whereas for other values of net weight change, the risk of MASLD showed a trend of “low-intensity physical activity > medium-intensity physical activity > high-intensity physical activity” (Figure 3). In early adulthood, the risk of MASLD was lowest for high-intensity physical activity; however, after a weight gain of > 10 kg, the risk of MASLD was slightly higher for medium-intensity physical activity than for low-intensity physical activity. That’s probably because in the NHANES database, certain individuals may not have received or adhered to appropriate exercise prescriptions during the inter-survey period. Following weight gain, the decline in their physical and metabolic capacities may have exceeded expectations, rendering the initial exercise interventions not only ineffective but potentially detrimental to health. This also suggests that a weight gain of 10 kg may serve as a significant metabolic health tipping point. The lowest prevalence risk across all weight gain patterns is associated with high-intensity exercise, which may be linked to the heterogeneity of fat metabolism. Some individuals, despite being prone to weight gain, tend to accumulate fat in the hips and legs rather than in the visceral region. This heterogeneity of fat metabolism is likely related to long-term lifestyle habits and gene phenotypes. Conducting cohort studies or metabolomic analyses on individuals with high body weight but high exercise intensity may yield additional insights.
The results of the sensitivity analyses showed that high-intensity physical activity reduced the risk of MASLD in all three sensitivity analysis groups (no medical history, age ≥ 45 years, and non-underweight) in both non-obese and obese populations (Table 3). This result suggests that a certain level of physical activity intensity is beneficial for reducing the risk of developing MASLD in obese populations, which is consistent with the results of previous studies.
| Weight fluctuation patterns | Overall population | Low-intensity sports | Medium-intensity sports | High-intensity sports |
| OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| No medical history | ||||
| Stabilizing non-obesity | 1 | 1 | 0.72 (0.42-1.21) | 0.57 (0.36-0.90) |
| Obesity to non-obesity | 0.76 (0.33-1.77) | 1.10 (0.40-3.06) | 0.26 (0.04-1.68) | 0.10 (0.01-0.74) |
| Non-obesity to obesity | 9.90 (6.79-14.45) | 8.20 (5.23-12.84) | 9.13 (4.44-18.78) | 7.31 (3.84-13.92) |
| Stabilizing obesity | 11.64 (7.63-17.76) | 13.85 (8.24-23.29) | 9.77 (4.63-20.65) | 4.05 (2.08-7.90) |
| Age ≥ 45 years | ||||
| Stabilizing non-obesity | 1 | 1 | 0.96 (0.65-1.40) | 0.86 (0.63-1.19) |
| Obesity to non-obesity | 1.39 (0.94-2.07) | 1.31 (0.82-2.09) | 0.99 (0.36-2.70) | 1.78 (0.78-4.04) |
| Non-obesity to obesity | 8.39 (6.47-10.88) | 9.20 (6.70-12.63) | 9.21 (5.14-16.51) | 4.47 (2.81-7.09) |
| Stabilizing obesity | 11.96 (9.32-15.34) | 12.68 (9.56-16.83) | 16.42 (9.03-29.88) | 6.37 (3.95-10.27) |
| Not underweight | ||||
| Stabilizing non-obesity | 1 | 1 | 1.00 (0.70-1.42) | 0.90 (0.67-1.22) |
| Obesity to non-obesity | 1.23 (0.86-1.77) | 1.24 (0.79-1.93) | 0.92 (0.37-2.27) | 1.36 (0.65-2.83) |
| Non-obesity to obesity | 8.96 (7.35-10.92) | 9.55 (7.37-12.38) | 9.55 (5.86-15.55) | 6.38 (4.40-9.25) |
| Stabilizing obesity | 11.97 (9.71-14.76) | 13.99 (10.81-18.11) | 11.82 (7.17-19.48) | 6.57 (4.34-9.95) |
The present study analyzed data from a large and nationally representative sample of NHANES 2009–2018 data and showed that the risk of MASLD increased with age in non-obese to obese and stably obese participants. Previous large-scale epidemiological studies have shown that in the adult population, weight typically stabilizes after peaking in youth but may increase with midlife and old age[22]. Weight gain in the middle and old age stages may be associated with a decrease in metabolic rate, loss of muscle mass, and changes in lifestyle and dietary habits[23]. Physical activity is an important strategy for reducing the risk of MASLD in obese patients. Exercise may improve liver function and reduce fat deposition and inflammation by increasing fat oxidation, improving insulin resistance, and promoting weight loss[24], thereby reducing the risk of MASLD in obese patients. As frequent weight fluctuations may increase the risk of MASLD, long-term adherence to moderate aerobic exercise may reduce the adverse effects of weight fluctuations on MASLD[25], leading to good liver health. The current study analyzed the effects of a combination of different age stages, weight changes, and physical activity intensities on the risk of MASLD. Combined with sensitivity analyses, our findings demonstrated that high-intensity physical activity reduced the risk of MASLD in non-obese to obese and stably obese participants.
Socioeconomic status has been significantly associated with the occurrence and severity of MASLD, with a lower socioeconomic status associated with a higher risk of developing MASLD[26,27]. Generally, people with low levels of education, low income, and occupational status are more likely to be at a high risk of obesity, metabolic syndrome, and other deficient factors associated with MASLD, which increase the risk of the disease[28]. Higher socioeconomic status is basically associated with enhanced health consciousness and increased accessibility to premium fitness facilities, giving higher income population more opportunities to engage in moderate or high intensity exercise. In addition, lower socioeconomic status may lead to fewer healthcare resources and poorer disease management, which has implications for the treatment and management of patients with MASLD[29,30]. Moreover, adverse lifestyle factors, such as high-sugar and high-fat diets, lack of physical activity, and a sedentary lifestyle, are strongly associated with the occurrence and progression of MASLD[31]. The adoption of healthy dietary patterns, such as the Mediterranean diet, healthy plant-based diets, low-fat diet, and high-fiber diet can reduce the risk of developing MASLD[32,33].
Given the complex relationship between weight fluctuation and MASLD prevalence risk, it is imperative to further investigate the association between weight fluctuation and social determinants. With the rapid improvement in material living standards over the past century, socioeconomic status has always been a fundamental determinant of midlife weight fluctuation. The primary contributors to weight fluctuation/gain among middle-aged populations underlyingly stem from chronic energy surplus caused by excessive caloric intake, particularly in developed countries such as the United States where hyperpalatable, energy-dense foods demonstrate higher availability. While in developing countries such as China, lower economic level often means lower health awareness and inadequate self-health management, which can also lead to greater midlife weight fluctuations and increases. Secondly, behavioral modifications induced by social role transitions across the life course can’t be ignored. The physiological decline in the metabolic rate during midlife coincides with social role stabilization (e.g., career establishment and shifting family responsibilities), which collectively promote sedentary lifestyle tendencies. The prevalent hedonistic orientation and substantial reduction in physical activity levels around retirement age establish behavioral foundations for weight gain. Finally, the reinforcement effect of social conformity merits emphasis. When peer groups predominantly adopt dietary indiscretion and sedentary behaviors, the implicit pressure from group norms substantially weakens individual motivation for healthy behavioral modifications. This collective sedentary trend synergizes with age-related metabolic changes, exacerbating midlife weight gain.
Potential confounders, including socioeconomic status and lifestyle factors, were considered and applied to weighted multivariate logistic regression in the current study. To test the robustness of the results, comorbidities were excluded from the inclusion criteria to minimize the potential reverse causality due to serious diseases. In addition, participants under the age of 45 years were excluded to distinguish between the time points of early adulthood (25 years old, up to 10 years before baseline) and mid-to-late adulthood (10 years before and up to baseline). The effects of being underweight on the results were excluded.
Although our study conducted separate cross-sectional analyses of weight change, relative weight change, and net weight change across different periods, notably, the weight data may have been influenced by recall bias, as the NHANES database relies on participants' self-reported information regarding their weight history. We also explored many covariates, including age, sex, race/ethnicity, marital status, education level, household income poverty ratio, and physical activity intensity. As these covariates were only available at baseline, those that changed over time were not used to capture possible confounders over time. Finally, the term risk was used solely to reflect the association between the variables and the prevalence of MASLD. Therefore, this should not be interpreted directly as representing the absolute risk of MASLD development. Owing to the lack of data at different time points, we did not assess the association between other obesity-related indicators (such as waist circumference and adiposity) and MASLD, and there were no additional data on changes in body weight over these time intervals. Further studies are required to investigate the relationship between dynamic weight fluctuation and MASLD.
In summary, this study showed that, compared with stable non-obese populations, the MASLD risk increases in non-obese to obese and stably obese participants. Although elevated physical activity intensity is beneficial in reducing the risk of MASLD in a stable obese population, because of the different net weight changes and age stages, the risk of MASLD may be approximately or slightly higher for medium-intensity physical activity than for low-intensity physical activity. While enhancing physical activity intensity potentially confers benefits to MASLD risks among individuals with stable obesity, weight fluctuation patterns and heterogeneity across different life cycle stages may diminish the differential efficacy of moderate-intensity and low-intensity physical activities in MASLD risk reduction across distinct cohorts. In certain scenarios (Table 2, early adulthood, weight change assessment > 10 kg), moderate-intensity physical activity was correlated with a marginally elevated MASLD risk. Consequently, personalized weight management and exercise intervention protocols should be developed in clinical practice. For the clinical prevention and management of MASLD, a three-tiered risk-stratified management framework is recommended: (1) Stable non-obese population (risk class I); (2) Stable obese population (risk class II); and (3) Progressive obesity population (risk class III). Evidence-based exercise interventions should be designed differently, and high-intensity interval training is recommended as the primary intervention modality for risk class II and I individuals. For subjects with significant weight fluctuations (fluctuation amplitude > 10 kg), a dynamic monitoring system should be established using personalized metabolic regulation protocols that are initiated when preset thresholds are breached. In addition, young patients should focus on maintaining lean body mass, whereas middle-aged patients require prioritized quantitative control of the visceral fat area. A comprehensive analysis elucidating the mechanistic interactions between cyclical weight fluctuation and structured exercise protocols will establish a conceptual framework essential for guiding future research priorities in precision weight management. Further research is warranted to elucidate the specific effects of different exercise intensities on the risk of MASLD across various traits. A thorough examination of the disparities in risk thresholds and contributing factors between moderate-intensity and high-intensity physical activity may provide a scientific foundation for personalized health promotion strategies informed by age and genetic predispositions.
| 1. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7925] [Article Influence: 792.5] [Reference Citation Analysis (8)] |
| 2. | Younossi ZM, Kalligeros M, Henry L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin Mol Hepatol. 2025;31:S32-S50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 331] [Article Influence: 331.0] [Reference Citation Analysis (3)] |
| 3. | Miao L, Targher G, Byrne CD, Cao YY, Zheng MH. Current status and future trends of the global burden of MASLD. Trends Endocrinol Metab. 2024;35:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 422] [Article Influence: 211.0] [Reference Citation Analysis (0)] |
| 4. | Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day CP, Eguchi Y, Geier A, Kondili LA, Kroy DC, Lazarus JV, Loomba R, Manns MP, Marchesini G, Nakajima A, Negro F, Petta S, Ratziu V, Romero-Gomez M, Sanyal A, Schattenberg JM, Tacke F, Tanaka J, Trautwein C, Wei L, Zeuzem S, Razavi H. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1399] [Article Influence: 174.9] [Reference Citation Analysis (1)] |
| 5. | Wang WJ, Xiao P, Xu HQ, Niu JQ, Gao YH. Growing burden of alcoholic liver disease in China: A review. World J Gastroenterol. 2019;25:1445-1456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Lim HW, Bernstein DE. Risk Factors for the Development of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis, Including Genetics. Clin Liver Dis. 2018;22:39-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 979] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 8. | Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1009] [Article Influence: 63.1] [Reference Citation Analysis (1)] |
| 9. | Wong VW, Wong GL, Chan RS, Shu SS, Cheung BH, Li LS, Chim AM, Chan CK, Leung JK, Chu WC, Woo J, Chan HL. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J Hepatol. 2018;69:1349-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 238] [Article Influence: 29.8] [Reference Citation Analysis (34)] |
| 10. | Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 395] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 11. | Keating SE, Hackett DA, Parker HM, O'Connor HT, Gerofi JA, Sainsbury A, Baker MK, Chuter VH, Caterson ID, George J, Johnson NA. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015;63:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 12. | Perseghin G, Lattuada G, De Cobelli F, Ragogna F, Ntali G, Esposito A, Belloni E, Canu T, Terruzzi I, Scifo P, Del Maschio A, Luzi L. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care. 2007;30:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 13. | Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 448] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 14. | Sousa N, Mendes R, Silva A, Oliveira J. Combined exercise is more effective than aerobic exercise in the improvement of fall risk factors: a randomized controlled trial in community-dwelling older men. Clin Rehabil. 2017;31:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Alabdul Razzak I, Fares A, Stine JG, Trivedi HD. The Role of Exercise in Steatotic Liver Diseases: An Updated Perspective. Liver Int. 2025;45:e16220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 16. | Gardner CD, Trepanowski JF, Del Gobbo LC, Hauser ME, Rigdon J, Ioannidis JPA, Desai M, King AC. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight Loss in Overweight Adults and the Association With Genotype Pattern or Insulin Secretion: The DIETFITS Randomized Clinical Trial. JAMA. 2018;319:667-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 456] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 17. | Lingvay I, Asong M, Desouza C, Gourdy P, Kar S, Vianna A, Vilsbøll T, Vinther S, Mu Y. Once-Weekly Insulin Icodec vs Once-Daily Insulin Degludec in Adults With Insulin-Naive Type 2 Diabetes: The ONWARDS 3 Randomized Clinical Trial. JAMA. 2023;330:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 18. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1676] [Cited by in RCA: 1810] [Article Influence: 603.3] [Reference Citation Analysis (2)] |
| 19. | Israelsen M, Francque S, Tsochatzis EA, Krag A. Steatotic liver disease. Lancet. 2024;404:1761-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 169] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 20. | Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2015;41:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 350] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 21. | Younossi ZM, Corey KE, Lim JK. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2021;160:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 367] [Article Influence: 73.4] [Reference Citation Analysis (2)] |
| 22. | Sørensen TI, Rissanen A, Korkeila M, Kaprio J. Intention to lose weight, weight changes, and 18-y mortality in overweight individuals without co-morbidities. PLoS Med. 2005;2:e171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Kelley CP, Sbrocco G, Sbrocco T. Behavioral Modification for the Management of Obesity. Prim Care. 2016;43:159-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 379] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 25. | Cho EJ, Yu SJ, Jung GC, Kwak MS, Yang JI, Yim JY, Chung GE. Body weight gain rather than body weight variability associated with increased risk of nonalcoholic fatty liver disease. Sci Rep. 2021;11:14428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Mitra S, De A, Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol. 2020;5:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 355] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 27. | Cho J, Lee I, Park DH, Kwak HB, Min K. Relationships between Socioeconomic Status, Handgrip Strength, and Non-Alcoholic Fatty Liver Disease in Middle-Aged Adults. Int J Environ Res Public Health. 2021;18:1892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Pais R, Barritt AS 4th, Calmus Y, Scatton O, Runge T, Lebray P, Poynard T, Ratziu V, Conti F. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016;65:1245-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 340] [Article Influence: 34.0] [Reference Citation Analysis (1)] |
| 29. | Lazarus JV, Mark HE, Colombo M, Demaio S, Dillon JF, George J, Hagström H, Hocking S, Lee N, Nieuwenhuijsen MJ, Rinella ME, Romero-Gomez M, Soriano JB, Schattenberg JM, Tacke F, Tsochatzis EA, Valenti L, Zelber-Sagi S, Ashworth Dirac M, Huang TT. A sustainable development goal framework to guide multisectoral action on NAFLD through a societal approach. Aliment Pharmacol Ther. 2022;55:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Wong VW, Ekstedt M, Wong GL, Hagström H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023;79:842-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 456] [Article Influence: 152.0] [Reference Citation Analysis (0)] |
| 31. | Yu C, Gao J, Ge X, Wang X, Ding Y, Tian T, Xu X, Guo W, Wang Q, Ge Z, Jiang T, Zhang Q, Song C. Healthy Lifestyle Is Associated with Reduced Mortality in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients. 2022;14:3785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Thomas MS, Calle M, Fernandez ML. Healthy plant-based diets improve dyslipidemias, insulin resistance, and inflammation in metabolic syndrome. A narrative review. Adv Nutr. 2023;14:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 33. | Varkaneh HK, Poursoleiman F, Al Masri MK, Alras KA, Shayah Y, Masmoum MD, Alangari FA, Alras AA, Rinaldi G, Day AS, Hekmatdoost A, Abu-Zaid A, Kutbi E. Low fat diet versus low carbohydrate diet for management of non-alcohol fatty liver disease: A systematic review. Front Nutr. 2022;9:987921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/