Published online May 27, 2025. doi: 10.4254/wjh.v17.i5.104041
Revised: February 19, 2025
Accepted: April 17, 2025
Published online: May 27, 2025
Processing time: 170 Days and 13 Hours
Hepatocellular carcinoma (HCC), the sixth most common cancer and fourth-leading cause of cancer-related mortality globally, imposes a significant burden in Vietnam due to endemic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections. Accurate prognostication is crucial for optimizing treatment and outcomes. Numerous staging systems exist, including the Barcelona Clinic Liver Cancer (BCLC), Hong Kong Liver Cancer (HKLC), cancer of the liver Italian Program (CLIP), Italian Liver Cancer (ITA.LI.CA), Japan Integrated Staging (JIS), Tokyo Score, and model to estimate survival in ambulatory HCC patients (ME
To compare the prognostic accuracy of seven HCC staging systems in predicting survival and identify the optimal model.
This retrospective cohort study included 987 patients with HCC diagnosed at Nhan dan Gia Dinh Hospital, Vietnam, from January 2016 to December 2023. Patients were staged using BCLC, HKLC, CLIP, ITA.LI.CA, JIS, Tokyo score, and MESIAH. Overall survival was analyzed using Kaplan-Meier methods, and pro
The HKLC and BCLC systems demonstrated the highest discriminatory ability, with area under the ROC curves of 0.834 and 0.830, respectively, at 12 months and 0.859 for both systems at 36 months. CLIP and ITA.LI.CA exhibited superior calibration, particularly at 36 months. The JIS system consistently showed the poorest discriminatory performance. Subgroup analyses revealed that HKLC maintained strong performance across different viral etiologies (HBV, HCV, non-B-non-C) and treatment modalities (transarterial chemoembolization, surgery, ab
The HKLC and BCLC systems showed superior prognostic performance for Vietnamese patients with HCC, supporting HKLC adoption in clinical practice.
Core Tip: The Hong Kong Liver Cancer (HKLC) staging system demonstrated superior prognostic accuracy in Vietnamese patients with hepatocellular carcinoma (HCC), outperforming other established models such as, cancer of the liver Italian program, and Italian liver cancer. These findings highlight the HKLC system’s clinical utility, particularly in stratifying risk and guiding treatment decisions for Asian populations with diverse viral etiologies. Notably, patients with non-viral HCC exhibited poorer outcomes, emphasizing the need for improved screening strategies in this subgroup to enhance early detection and survival.
- Citation: Mai-Phan TA, Nguyen TK, Pham TN, Tran MQ, Le KL. Comparative prognostic performance of staging systems for hepatocellular carcinoma: Evidence from a Vietnamese cohort study. World J Hepatol 2025; 17(5): 104041
- URL: https://www.wjgnet.com/1948-5182/full/v17/i5/104041.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i5.104041
According to GLOBOCAN 2022, liver cancer is the sixth most commonly diagnosed cancer worldwide, with 865000 new cases annually, and the third leading cause of cancer-related mortality, accounting for 757948 deaths in 2022 alone[1,2]. Asia, particularly East and Southeast Asia, bears the highest incidence and mortality burdens of liver cancer, with Vietnam among the countries reporting the highest incidence rates globally[2-4]. The burden of hepatocellular carcinoma (HCC) in Vietnam is further exacerbated by the endemic prevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, which are the primary etiological factors contributing to HCC development in the region[3-7]. Despite advancements in diagnostic imaging and therapeutic interventions, the prognosis for HCC remains poor, largely due to late-stage diagnosis and the biological heterogeneity of the tumor[8,9].
Accurate prognostication is crucial for guiding treatment strategies and improving patient outcomes. Multiple staging systems have been developed to assess the prognosis of patients with HCC, each incorporating various clinical, labo
In response, alternative staging systems such as the Hong Kong Liver Cancer (HKLC) classification have been proposed, aiming to better reflect the clinical characteristics and treatment practices in Asian populations[11]. Other systems like the cancer of the liver Italian Program (CLIP)[12], Italian Liver Cancer (ITA.LI.CA)[13], Japan Integrated Staging (JIS)[14], Tokyo score[15], and the model to estimate survival in Ambulatory HCC patients (MESIAH)[16] have been developed, each with varying prognostic parameters and complexities.
While these staging systems have been validated in different populations, their prognostic performance can vary significantly based on geographic and ethnic factors. In Vietnam, there is a paucity of data evaluating the comparative effectiveness of these prognostic models in the local patient population. Given the unique epidemiological and clinical characteristics of Vietnamese patients with HCC, such as younger age at onset, higher prevalence of HBV infection, and differences in treatment accessibility[3,7,17], it is imperative to assess which staging system most accurately predicts survival outcomes in this context.
Which prognostic staging system among BCLC, HKLC, CLIP, ITA.LI.CA, JIS, Tokyo score, and MESIAH provides the most accurate survival prediction for Vietnamese patients with HCC?
This study was designed to primarily compare seven established staging systems in Vietnamese patients with HCC, with focus on prognostic accuracy at 12 months and 36 months. Additionally, subgroup analyses were designed to investigate the viral etiology and first-line treatment modalities.
This retrospective cohort study analyzed patients with HCC who were newly diagnosed and treated at Nhan dan Gia Dinh Hospital from January 2016 to December 2023. Nhan dan Gia Dinh Hospital is a tertiary referral center specializing in hepatobiliary diseases. The study protocol was approved by the Institutional Review Board of Nhan dan Gia Dinh Hospital (Approval No. 127/NDGD-HDDD). Due to the retrospective nature of the study, the requirement for informed consent was waived. All procedures conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Inclusion criteria: Were aged 18 years or older; had a definitive diagnosis of HCC according to the AASLD guidelines[18] and confirmed by characteristic imaging findings or histopathology; and had complete baseline clinical, laboratory, and imaging data necessary for staging according to the evaluated scoring systems.
Exclusion criteria: Prior liver transplantation; other concurrent primary malignancies; or incomplete medical records that precluded accurate staging.
To attain modest discrepancies (0.05-0.10) in discrimination metrics [e.g., area under the curve (AUC) or Harrell’s con
Patients were diagnosed with HCC according to the AASLD guidelines[18], which include diagnostic criteria based on characteristic imaging features on multiphasic CT or magnetic resonance imaging or histopathological confirmation when imaging was inconclusive.
We systematically screened the electronic medical records at Nhan dan Gia Dinh Hospital for all patients diagnosed with HCC from January 2016 to December 2023. Patients were included if they met the predefined criteria (i.e. age ≥ 18 years, a definitive HCC diagnosis based on imaging or histopathology, and complete clinical/laboratory data), while those with incomplete records or missing key variables were excluded. Relevant demographic, clinical, and laboratory information—including imaging findings, liver function tests, and alpha-fetoprotein levels—was retrieved from the hospital’s electronic database and cross-verified with paper-based charts to ensure accuracy and completeness. Tumor characteristics included tumor size, the number of nodules, the presence of portal vein thrombosis, and extrahepatic metastasis determined through ultrasound, computed tomography (CT), or magnetic resonance imaging. Treatment modalities at diagnosis were categorized as transarterial chemoembolization (TACE), surgical resection, radio frequency ablation (RFA), microwave ablation (MWA), systemic therapy, or palliative care.
All patients were followed until death or loss to follow-up. Survival status was determined through medical record reviews and when necessary direct contact with patients or next of kin. Survival time was calculated from the date of diagnosis to the date of death or last known follow-up. Due to increasing loss to follow-up over time (21.6% at 12 months, 53.3% at 36 months, and 69.3% at 60 months), prognostic evaluations focused on 12-month and 36-month outcomes to ensure data robustness.
Each patient was retrospectively staged at diagnosis using seven prognostic scoring systems. These included the BCLC system[9], the HKLC system with both the 9-tier (HKLC_9) and simplified 5-tier (HKLC_5) versions[10], the CLIP system[11], and the ITA.LI.CA system[12]. Additionally, the JIS system[13], the Tokyo score[14], and the MESIAH[15] were applied. Staging criteria and scoring were conducted according to the original publications and established guidelines for each system.
All statistical analyses were performed using R software (version 4.3.0; R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were assessed for normality using the Shapiro-Wilk test. Normally distributed data were expressed as mean ± SD, while non-normally distributed data were expressed as median with inter
The Kaplan-Meier method was employed to estimate overall survival rates at 12 months and 36 months. The log-rank test was used to compare survival distributions among different staging groups and treatment modalities. Cox proportional hazards regression models identified independent prognostic factors. Variables with a P value < 0.05 in univariate analysis were included in the multivariate model. Hazard ratios (HRs) and 95% confidence intervals (95%CIs) were calculated to assess the strength of associations. A P value < 0.05 was considered statistically significant.
The prognostic performance of each staging system was assessed. Discriminatory ability was evaluated by calculating the area under the receiver operating characteristic (ROC) curve (i.e., the AUC) and C-index using the pROC package. Higher AUC and C-index values indicate better discrimination between different prognostic groups. Calibration was assessed by comparing predicted survival probabilities with observed outcomes, calculating mean absolute errors and quantiles to evaluate the alignment between predicted and actual survival. Calibration plots were generated to visualize the agreement between predicted and observed survival. Model fit was analyzed using the Akaike information criterion (AIC) and likelihood ratio χ² tests. Lower AIC values indicate a better balance between model fit and complexity. The DeLong test was utilized to compare differences in AUCs between staging systems, determining the statistical signi
Subgroup analyses were conducted to assess the consistency of prognostic performance across different patient groups. Viral etiology was stratified into HBV, HCV, and non-B-non-C (NBNC) categories based on serological status. Initial treatment modalities were classified according to first-line treatments, including TACE, surgical resection, and RFA/MWA.
The analyses employed the following R packages: The survival package was used for Kaplan-Meier survival analysis and Cox proportional hazards modeling; the survminer package facilitated enhanced visualization of survival curves; the pROC package supported ROC curve analysis and AUC calculations; the ggplot2 package was utilized for figure creation.
Missing data were addressed through complete-case analysis. Patients with incomplete information necessary for any of the staging systems were excluded from analyses involving that particular system. The impact of missing data on the study findings was considered minimal due to the large sample size. The study included all eligible patients diagnosed during the study period, totaling 987 individuals. This sample size provided sufficient power to detect differences in prognostic performance among the staging systems.
Potential sources of bias included selection bias inherent in retrospective designs and information bias due to reliance on existing medical records. To mitigate these, we included consecutive patients meeting inclusion criteria and standardized data extraction procedures. The study acknowledged limitations related to loss to follow-up, particularly beyond 36 months.
Patient confidentiality was maintained throughout the study. Data were anonymized and securely stored in accordance with institutional policies and data protection regulations. The study was conducted in compliance with ethical standards for research involving human subjects.
A total of 987 patients diagnosed with HCC were included in this study. The mean age of the cohort was 62.28 ± 11.05 years, with a significant male predominance (male-to-female ratio of approximately 4:1). Among the cohort, 45.5% of patients had HBV infection, 32.2% had HCV infection, 21.3% were classified as NBNC, and 2.0% were co-infected with HBV and HCV. Underlying cirrhosis was present in 62.0% of patients, with varying severity across subgroups.
Key laboratory findings included a median alpha-fetoprotein level of 27.7 ng/mL (IQR: 5.9-767.3 ng/mL). Liver function tests indicated a median total bilirubin level of 16.0 μmol/L (IQR: 11.5-23.7 μmol/L) and a mean albumin level of 36.53 ± 6.52 g/L. Tumor characteristics revealed a median tumor size of 4.8 cm (IQR: 2.8-8.2 cm), and 40.8% of patients had multiple nodules. Portal vein thrombosis was observed in 15.8% of patients, with the highest occurrence in patients with NBNC (23.5%).
Treatment modalities varied among the cohort. TACE was the most common treatment, utilized in 46.4% of patients. Surgical resection was performed in 12.5% of cases, while RFA/MWA was used in 10.3%. Systemic therapy and palliative care were employed in 5.1% and 25.7% of cases, respectively. The clinical characteristics and outcomes of these patients are detailed in Table 1.
| Feature of interest | Study population, n = 987 | HBV-HCV infection status | Intergroup statistical significance | |||
| HBV, n = 446, 45.2% | HCV, n = 308, 31.2% | Non-B-non-C, n = 213, 21.6% | Co-infected HBV + HCV, n = 20, 2.0% | |||
| Demographic features | ||||||
| Male sex, n (%) | 789 (79.9) | 368 (82) | 246 (80) | 161 (76) | 15 (75) | NS (χ²) |
| Age in years, mean | 62.28 ± 11.05 | 59.10 ± 11.21 | 65.11 ± 8.77 | 64.91 ± 11.85 | 61.40 ± 11.95 | < 0.001 (A) |
| Clinical features | ||||||
| Size of tumor in cm (IQR) | 4.8 (2.8-8.2) | 5.0 (2.8-8.8) | 4.0 (2.6-6.85) | 6.0 (3.0-10.0) | 3.85 (2.5-6.65) | < 0.001 (K-W) |
| Number of tumors, n (%) | NS (χ²) | |||||
| 1 | 584 (59.2) | 261 (58.5) | 177 (57.5) | 134 (62.9) | 12 (60.0) | |
| 2 | 151 (15.3) | 69 (15.5) | 56 (18.2) | 24 (11.3) | 2 (10.0) | |
| ≥ 3 | 252 (25.5) | 116 (26.0) | 75 (24.4) | 55 (25.8) | 6 (30.0) | |
| Solidity, n (%) | NS (χ²) | |||||
| Infiltrative | 67 (6.8) | 30 (6.7) | 17 (5.5) | 20 (9.4) | 0 (0.0) | |
| Solid | 920 (93.2) | 416 (93.3) | 291 (94.5) | 193 (90.6) | 20 (100.0) | |
| Intrahepatic vascular invasion, n (%) | 17 (1.7) | 5 (1.1) | 7 (2.3) | 4 (1.9) | 1 (5.0) | NS (χ²) |
| Portal thrombosis, n (%) | 156 (15.8) | 53 (11.9) | 50 (16.2) | 50 (23.5) | 3 (15.0) | 0.002 (χ²) |
| Metastasis, n (%) | NS (χ²) | |||||
| No metastasis | 893 (90.5) | 401 (89.9) | 283 (91.9) | 190 (89.2) | 19 (95.0) | |
| Lymph nodes | 70 (7.1) | 35 (7.8) | 22 (7.1) | 12 (5.6) | 1 (5.0) | |
| Lung | 10 (1.0) | 5 (1.1) | 1 (0.3) | 4 (1.9) | 0 (0.0) | |
| Lymph nodes + Lung | 2 (0.2) | 0 (0.0) | 0 (0.0) | 2 (0.9) | 0 (0.0) | |
| Other sites | 12 (1.2) | 5 (1.1) | 2 (0.6) | 5 (2.3) | 0 (0.0) | |
| Ascites, n (%) | 216 (21.9) | 91 (20.4) | 61 (19.8) | 60 (28.2) | 4 (20.0) | NS (χ²) |
| Cirrhosis, n (%) | 612 (62.0) | 253 (56.7) | 208 (67.5) | 136 (63.8) | 15 (75.0) | 0.012 (χ²) |
| Tumor rupture, n (%) | 55 (5.6) | 26 (5.8) | 14 (4.5) | 15 (7.0) | 0 (0.0) | NS (χ²) |
| ECOG performance status, n (%) | 0.001 (χ²) | |||||
| 0: Normal activity | 655 (66.4) | 311 (69.7) | 214 (69.5) | 118 (55.4) | 12 (60.0) | |
| 1: Mildly restricted | 271 (27.5) | 114 (25.6) | 69 (22.4) | 80 (37.6) | 8 (40.0) | |
| ≥ 2: Significantly restricted | 61 (6.2) | 21 (4.7) | 25 (8.1) | 15 (7.0) | 0 (0.0) | |
| Laboratory examinations | ||||||
| AFP in ng/mL | 27.7 (5.9-767.3) | 36.55 (5.9-1000.0) | 27.45 (6.8-334.0) | 16.50 (4.3-471.8) | 65.70 (12.95-902.65) | 0.049 (K-W) |
| PT as % | 79.54 ± 16.16 | 79.98 ± 15.80 | 79.82 ± 16.18 | 78.59 ± 16.82 | 75.69 ± 17.09 | NS (A) |
| INR | 1.11 (1.00-1.23) | 1.11 (1.00-1.23) | 1.11 (1.00-1.24) | 1.09 (1.00-1.23) | 1.13 (1.01-1.27) | NS (K-W) |
| AST in U/L | 58.5 (38.0-105.0) | 56.85 (38.6-99.0) | 68.2 (36.75-107.25) | 52.0 (35.4-101.0) | 77.15 (41.2-141.0) | NS (K-W) |
| ALT in U/L | 44.7 (28.0-74.1) | 44.35 (29.2-66.9) | 46.5 (27.7-88.95) | 40.0 (25.3-70.0) | 55.65 (35.75-86.25) | NS (K-W) |
| Albumin in g/L | 36.53 ± 6.52 | 37.15 ± 6.28 | 36.23 ± 6.73 | 35.60 ± 6.68 | 37.45 ± 5.45 | 0.024 (A) |
| Total bilirubin in μmol/L | 16.0 (11.5-23.7) | 14.9 (11.3-22.2) | 17.15 (12.0-25.15) | 16.2 (11.0-24.5) | 17.3 (13.75-24.05) | NS (K-W) |
| Creatinine in μmol/L | 90.03 ± 26.91 | 87.21 ± 21.92 | 89.65 ± 26.03 | 96.73 ± 35.84 | 87.25 ± 18.41 | < 0.001 (A) |
| Platelet as 109/L1 | 186.18 ± 95.99 | 192.72 ± 96.99 | 159.71 ± 75.65 | 213.90 ± 109.61 | 152.70 ± 95.51 | < 0.001 (A) |
| Treatment | ||||||
| First choice of treatment, n (%) | < 0.001 (χ²) | |||||
| RFA/WMA | 102 (10.3) | 42 (9.4) | 39 (12.7) | 17 (8.0) | 4 (20.0) | |
| Surgery | 123 (12.5) | 70 (15.7) | 34 (11.0) | 19 (8.9) | 0 (0.0) | |
| TACE | 458 (46.4) | 218 (48.9) | 153 (49.7) | 76 (35.7) | 11 (55.0) | |
| Chemotherapy | 50 (5.1) | 22 (4.9) | 10 (3.2) | 18 (8.5) | 0 (0.0) | |
| Palliative Care | 254 (25.7) | 94 (21.1) | 72 (23.4) | 83 (39.0) | 5 (25.0) | |
| Prognosis | ||||||
| Survival status, n (%) | 0.02 (χ²) | |||||
| Alive | 731 (74.1) | 346 (77.6) | 230 (74.7) | 142 (66.7) | 13 (65.0) | |
| Deceased | 256 (25.9) | 100 (22.4) | 78 (25.3) | 71 (33.3) | 7 (35.0) | |
| Survival time, month | 15 (4-35) | 15 (4-36) | 21.5 (7-40) | 9 (1.5-22) | 18.5 (6.5-44) | < 0.001 (K-W) |
Subgroup analysis revealed notable differences in demographic and clinical features. Patients infected with HBV were younger on average (mean age: 59.10 years) compared to patients infected with HCV (mean age: 65.11 years) and patients with NBNC (mean age: 64.91 years). Cirrhosis was more common in patients infected with HCV and patients with NBNC compared to patients infected with HBV. Portal vein thrombosis was most prevalent in patients with NBNC (23.5%). Additionally, the treatment patterns varied significantly, with patients infected with HBV more likely to undergo surgical intervention, while patients with NBNC were more often managed with palliative care.
The mean survival time for the HBV group was 77.21 months (95%CI: 71.58-82.85), while the HCV group had a mean survival time of 68.12 months (95%CI: 62.77-73.46). The NBNC group exhibited the shortest mean survival time at 53.95 months (95%CI: 44.84-63.06), with a median survival time of 61.00 months. Across the overall cohort, the mean survival time was 73.36 months (95%CI: 69.50-77.22).
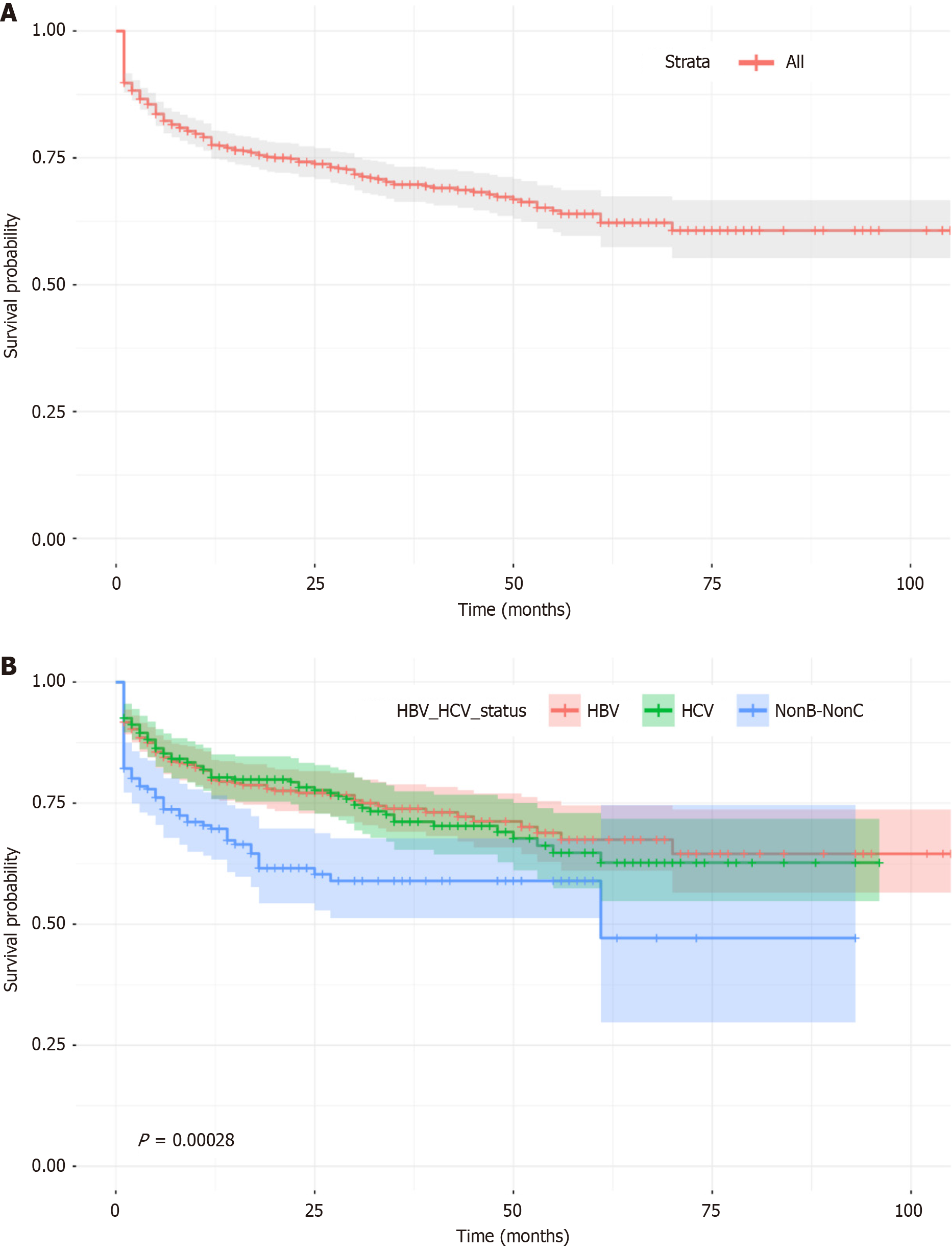
The cumulative survival rates across the cohort declined steadily over time, starting at 100% at baseline and decreasing to 78.0% at 12 months, 70.0% at 36 months, and 64.0% at 60 months (Figure 1A). Subgroup analysis revealed distinct patterns of survival and HRs among the different etiological groups (Figure 1B).
In the HBV-infected group (n = 446), survival rates were 91.0% at 1 month, 79.0% at 12 months, and 63.0% at 60 months. The highest HR (0.09) occurred at month 1 but decreased thereafter, suggesting stable mortality rates after the 1st year. The HCV-infected group (n = 308) exhibited slightly lower survival rates, declining from 92.0% at 1 month to 80.0% at 12 months and 67.0% at 60 months, with a persistently higher HR (0.08 at month 1) compared to the HBV group, indicating greater early mortality risk (Table 2). The NBNC group (n = 213) had the poorest outcomes, with survival rates of 82.0% at 1 month, 69.0% at 12 months, and 58.0% at 60 months. This group also exhibited the highest HR among all subgroups (0.20 at month 1), reflecting the greatest early mortality risk (Figure 1B).
| Variables | Univariate HR (95%CI) | Univariate P | Multivariate HR (95%CI) | Multivariate P value |
| Demographic features | ||||
| Sex | 1.208 | 0.243 | ||
| Age | 1.001 | 0.851 | ||
| Clinical features | ||||
| Tumor size | 1.130 | < 0.001 | 1.059 (1.024-1.094) | 0.001 |
| Tumor number | ||||
| 1 | < 0.001 | |||
| 2 | 0.426 | < 0.001 | ||
| ≥ 3 | 0.407 | < 0.001 | ||
| Ruptured tumor | 3.233 | < 0.001 | ||
| Solid tumor | 0.342 | < 0.001 | ||
| Intrahepatic vascular invasion | 3.295 | 0.001 | ||
| Extrahepatic vascular invasion | 9.860 | 0.023 | ||
| Portal thrombosis | 4.305 | < 0.001 | 1.905 (1.393-2.608) | < 0.001 |
| Metastasis | 1.493 | < 0.001 | 1.270 (1.069-1.509) | 0.007 |
| Ascites | 3.406 | < 0.001 | 1.560 (1.166-2.084) | 0.003 |
| Portal hypertension | 1.316 | 0.036 | ||
| Cirrhosis | 1.938 | < 0.001 | ||
| ECOG score | 3.731 | < 0.001 | 2.801 (2.308-3.401) | < 0.001 |
| HBV-HCV infection status | 0.002 | |||
| HCV | 0.571 | < 0.001 | ||
| Non-B-non-C | 0.589 | 0.001 | ||
| Co-infected | 0.818 | 0.611 | ||
| Laboratory examinations | ||||
| AFP | 1.001 | < 0.001 | 1.000 (1.000-1.000) | 0.020 |
| ALT | 1.000 | 0.538 | ||
| AST | 1.002 | < 0.001 | 1.001 (1.000-1.002) | 0.021 |
| Albumin | 0.917 | < 0.001 | 0.956 (0.936-0.977) | < 0.001 |
| PT | 0.982 | < 0.001 | ||
| INR | 0.923 | 0.632 | ||
| Platelet count | 1.003 | < 0.001 | ||
| Total bilirubin | 1.005 | < 0.001 | ||
| Creatinine | 1.003 | 0.113 | ||
| Treatment | ||||
| RFA/WMA | < 0.001 | |||
| Surgery | 0.064 | < 0.001 | ||
| TACE | 0.051 | < 0.001 | ||
| Chemotherapy | 0.123 | < 0.001 | ||
| Palliative care | 0.119 | < 0.001 |
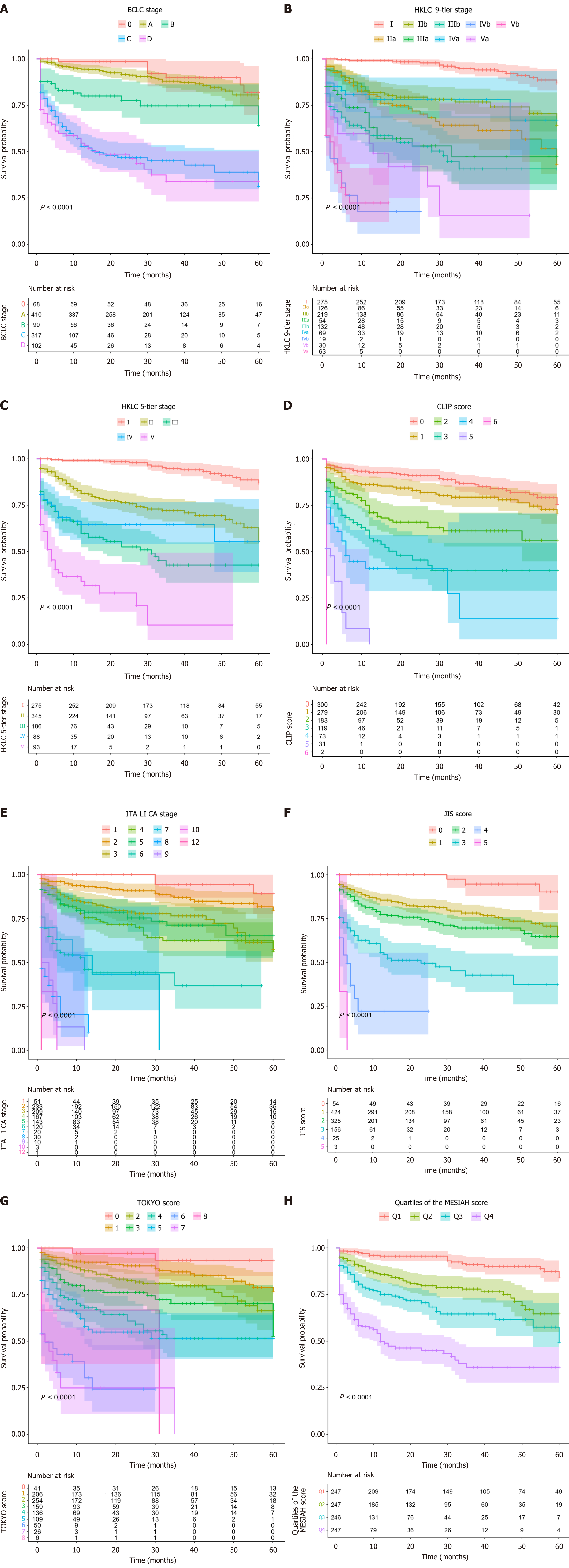
Figure 2 shows Kaplan-Meier survival curves representing 5-year overall survival probabilities stratified by the evaluated staging and scoring systems. All systems demonstrated significant survival stratification, with log-rank tests yielding P < 0.001. Each system produced distinct survival curves, reflecting clear separation of risk categories.
Table 3 summarizes the follow-up status across the entire cohort and subgroups (HBV, HCV, NBNC) at 12 months, 36 months, and 60 months. At 12 months, 57.3% of patients remained under follow-up, with moderate proportions of deceased (21.1%) and lost to follow-up (21.6%). By 36 months, patients under follow-up declined to 24.8%, while cases lost to follow-up doubled to 53.3%. At 60 months, only 7.6% of patients remained under follow-up, with 69.3% lost to follow-up. Subgroup trends were similar, with significant increases in loss to follow-up over time. For example, the NBNC group had only 5 patients (5.4%) under follow-up at 60 months.
| Group | Status | 12 months | 36 months | 60 months |
| Entire cohort | Deceased | 202 | 241 | 253 |
| Lost to follow-up | 237 | 509 | 661 | |
| Under follow-up | 548 | 237 | 73 | |
| HBV | Deceased | 80 | 93 | 99 |
| Lost to follow-up | 126 | 243 | 313 | |
| Under follow-up | 240 | 110 | 34 | |
| HCV | Deceased | 57 | 72 | 77 |
| Lost to follow-up | 47 | 148 | 199 | |
| Under follow-up | 204 | 88 | 32 | |
| Non-B-Non-C | Deceased | 59 | 70 | 70 |
| Lost to follow-up | 62 | 113 | 138 | |
| Under follow-up | 92 | 30 | 5 |
Given these patterns, prognostic evaluations were focused on the 12-month and 36-month time points. At 12 months, data quality was robust with minimal loss to follow-up (Table 3). At 36 months, although loss to follow-up increased, the remaining cohort size supported meaningful analysis. By 60 months, the sharp decline in follow-up cases rendered prognostic assessments less reliable.
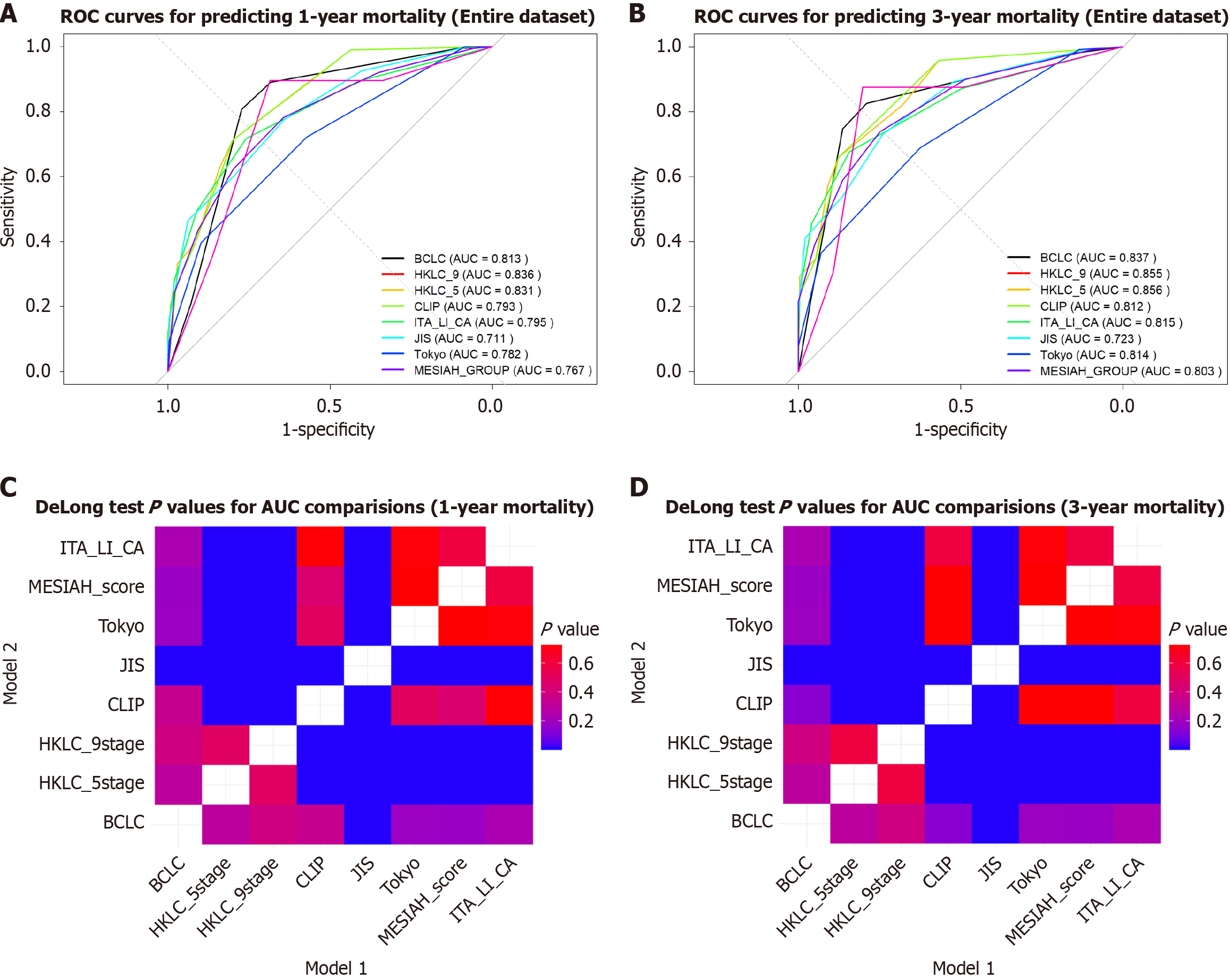
At 36 months, the AUC and C-index values improved slightly for most models compared to 12 months, indicating better discriminatory ability over time (Table 4). This trend reflects increased differentiation in survival probabilities at longer follow-ups. The HKLC_9 and HKLC_5 systems demonstrated the highest performance, with AUCs of 0.834 and 0.830, respectively at 12 months and 0.859 for both at 36 months. Conversely, the JIS system consistently exhibited the lowest AUC and C-index values at both time points (Figure 3). Pairwise comparisons of the AUCs using the DeLong test showed no significant differences between HKLC_5 and HKLC_9 (P = 0.57), while HKLC_9 significantly outperformed JIS (P < 0.001). Detailed pairwise P values are presented in Supplementary Tables 1 and 2.
| Score | Time in months | C-index | AUC | χ2 | HL P value | LRT P value | AIC | Mean error | 90% quantile |
| BCLC | 12 | 0.769 | 0.815 | 254.43 | < 0.05 | < 10-46 | 3024.30 | 0.063 | 0.280 |
| 36 | 0.765 | 0.838 | 1280.44 | < 0.05 | < 10-46 | 2774.41 | 0.072 | 0.128 | |
| HKLC_9 | 12 | 0.794 | 0.834 | 451.70 | < 0.05 | < 10-53 | 2992.47 | 0.103 | 0.183 |
| 36 | 0.798 | 0.859 | 7218.33 | < 0.05 | < 10-51 | 2750.78 | 0.104 | 0.141 | |
| HKLC_5 | 12 | 0.793 | 0.830 | 372.81 | < 0.05 | < 10-51 | 3002.21 | 0.116 | 0.175 |
| 36 | 0.795 | 0.859 | 4017.61 | < 0.05 | < 10-50 | 2758.09 | 0.102 | 0.166 | |
| CLIP | 12 | 0.756 | 0.792 | 379.93 | < 0.05 | < 10-49 | 3010.61 | 0.068 | 0.128 |
| 36 | 0.749 | 0.809 | 2490.52 | < 0.05 | < 10-44 | 2785.58 | 0.002 | 0.005 | |
| ITA.LI.CA | 12 | 0.757 | 0.795 | 300.31 | < 0.05 | < 10-45 | 3025.01 | 0.067 | 0.119 |
| 36 | 0.764 | 0.814 | 1741.14 | < 0.05 | < 10-44 | 2786.67 | 0.057 | 0.074 | |
| JIS | 12 | 0.683 | 0.712 | 221.52 | < 0.05 | < 10-25 | 3116.60 | 0.064 | 0.118 |
| 36 | 0.683 | 0.721 | 765.66 | < 0.05 | < 10-25 | 2871.86 | 0.008 | 0.018 | |
| Tokyo | 12 | 0.748 | 0.781 | 293.75 | < 0.05 | < 10-41 | 3045.51 | 0.063 | 0.118 |
| 36 | 0.755 | 0.811 | 1733.23 | < 0.05 | < 10-41 | 2797.68 | 0.010 | 0.024 | |
| MESIAH_GROUP | 12 | 0.739 | 0.765 | 242.39 | < 0.05 | < 10-34 | 3078.19 | 0.095 | 0.234 |
| 36 | 0.744 | 0.802 | 968.24 | < 0.05 | < 10-38 | 2812.91 | 0.143 | 0.214 |
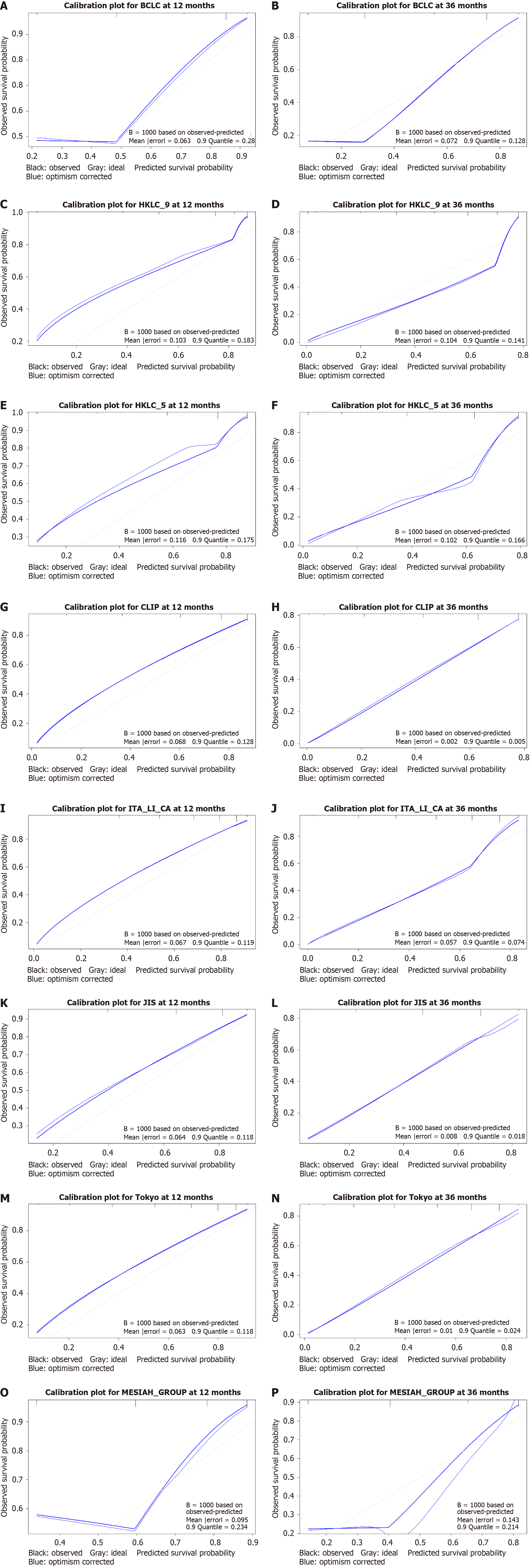
Calibration improved notably at 36 months for the CLIP, ITA.LI.CA, JIS, and Tokyo systems, with reduced mean errors and quantiles (Figure 4). The JIS system showed a significant mean error reduction from 0.064 to 0.008, reflecting improved alignment with observed survival probabilities. CLIP exhibited near-perfect calibration at 36 months, with a mean error of 0.002 and a quantile of 0.005. In contrast, MESIAH showed deteriorating calibration at 36 months, with mean error increasing from 0.095 to 0.143 and quantile decreasing from 0.234 to 0.214, indicating reduced prediction stability over time (Table 4).
All models demonstrated decreased AIC values at 36 months, suggesting improved model fit over time. The HKLC_9 and HKLC_5 systems consistently maintained the lowest AIC values, confirming their robustness in balancing model fit and complexity.
The HKLC_9 and HKLC_5 systems showed comparable discriminatory ability at both time points, with no significant differences (P > 0.5; Figure 3). At 36 months, the performance of the JIS system significantly lagged behind other models
This comparative analysis underscored the strengths and weaknesses of each prognostic system over time. For scenarios emphasizing calibration stability, CLIP and ITA.LI.CA are recommended. For superior discriminatory ability, HKLC_9 and HKLC_5 remain the preferred models.
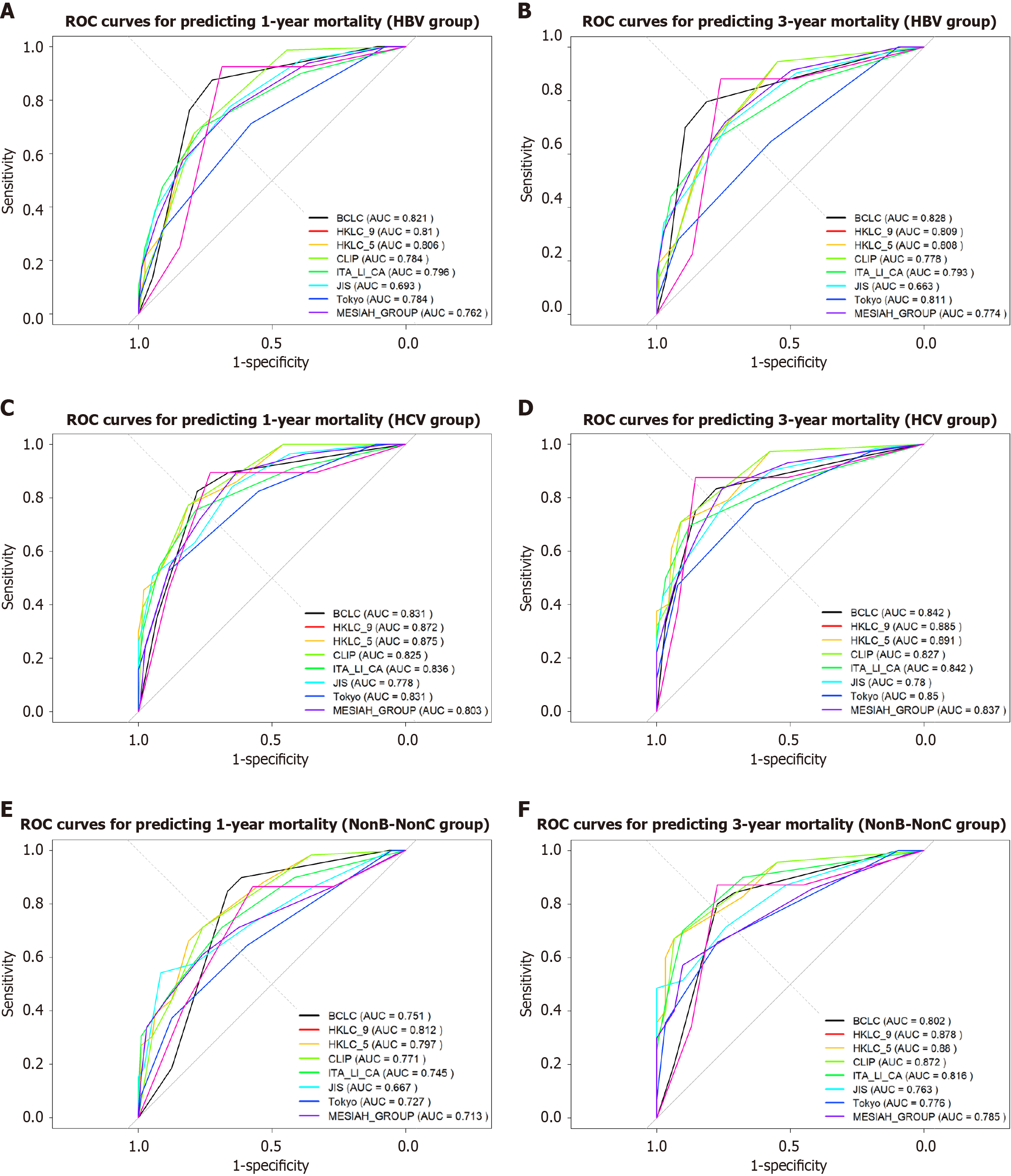
The HKLC_5 and HKLC_9 systems consistently exhibited the highest C-index and AUC values across all etiological groups and time points, with particularly strong performance in the HCV group at 36 months (AUC: 0.891). In contrast, the JIS system consistently displayed the lowest C-index and AUC values, indicating limited prognostic capability. For the NBNC group at 12 months, the BCLC and ITA.LI.CA systems also showed weaker performance compared to other groups (Figure 5).
HKLC_5 and HKLC_9 demonstrated good calibration with relatively low mean errors, especially in the HCV group at both 12 months and 36 months. In comparison, the MESIAH system exhibited higher mean errors across most groups, particularly in the HBV group at 12 months and the NBNC group at 36 months, reflecting poorer calibration.
The HKLC_5 and HKLC_9 systems consistently achieved the lowest AIC values across all viral etiology groups, indicating a good balance between model fit and complexity. Notably, the NBNC group at 36 months showed a significant reduction in AIC for HKLC_5 (80.66), reflecting strong predictive accuracy for this subgroup (Table 5).
| Infection status | Score | Time in months | AUC | χ2 | LRT P value | AIC | Mean error | 90% quantile |
| HBV | BCLC | 12 | 0.821 | 88.70 | 4.60 10-21 | 279.18 | 0.272 | 0.514 |
| 36 | 0.828 | 83.73 | 5.68 10-20 | 203.91 | 0.307 | 0.789 | ||
| HKLC_9 | 12 | 0.810 | 71.63 | 2.59 10-17 | 296.24 | 0.289 | 0.711 | |
| 36 | 0.809 | 63.58 | 1.54 10-15 | 224.05 | 0.358 | 0.723 | ||
| HKLC_5 | 12 | 0.806 | 68.18 | 1.49 10-16 | 299.69 | 0.294 | 0.641 | |
| 36 | 0.808 | 62.92 | 2.16 10-15 | 224.71 | 0.358 | 0.655 | ||
| HCV | BCLC | 12 | 0.831 | 72.13 | 2.01 10-17 | 206.34 | 0.246 | 0.614 |
| 36 | 0.842 | 70.31 | 5.06 10-17 | 156.26 | 0.298 | 0.725 | ||
| HKLC_9 | 12 | 0.872 | 93.74 | 3.60 10-22 | 184.73 | 0.211 | 0.631 | |
| 36 | 0.885 | 85.92 | 1.87 10-20 | 140.66 | 0.271 | 0.769 | ||
| HKLC_5 | 12 | 0.875 | 94.19 | 2.87 10-22 | 184.28 | 0.213 | 0.687 | |
| 36 | 0.891 | 89.75 | 2.70 10-21 | 136.83 | 0.261 | 0.666 | ||
| Non-B-Non-C | BCLC | 12 | 0.751 | 35.75 | 2.24 10-9 | 174.21 | 0.372 | 0.732 |
| 36 | 0.802 | 31.49 | 2.01 10-8 | 97.07 | 0.293 | 0.639 | ||
| HKLC_9 | 12 | 0.812 | 45.84 | 1.28 10-11 | 164.12 | 0.342 | 0.731 | |
| 36 | 0.878 | 45.90 | 1.25 10-11 | 82.66 | 0.256 | 0.669 | ||
| HKLC_5 | 12 | 0.797 | 41.21 | 1.36 10-10 | 168.74 | 0.355 | 0.750 | |
| 36 | 0.880 | 47.89 | 4.50 10-12 | 80.66 | 0.244 | 0.602 |
These findings highlight HKLC_5 and HKLC_9 as the most robust models across different viral etiologies, while the JIS system demonstrated consistent underperformance.
At 12 months, HKLC_5 and HKLC_9 consistently showed the strongest performance across treatment modalities, particularly in the TACE and RFA groups. For the surgery group, BCLC outperformed other scores. At 36 months, although performance generally declined, BCLC, HKLC_5, and HKLC_9 remained reliable choices, with BCLC showing robustness across all modalities (Table 6).
| Modality | Score | Time in months | AUC | χ2 | LRT P value | AIC | Mean error | 90% quantile |
| TACE | BCLC | 12 | 0.8000 | 3.38 | 0.066 | 26.79 | 0.063 | 0.100 |
| 36 | 0.7849 | 53.57 | < 0.001 | 214.12 | 0.321 | 0.678 | ||
| HKLC_9 | 12 | 0.9078 | 3.45 | 0.063 | 26.72 | 0.061 | 0.047 | |
| 36 | 0.7702 | 44.33 | < 0.001 | 223.36 | 0.343 | 0.702 | ||
| HKLC_5 | 12 | 0.9078 | 4.48 | 0.034 | 25.69 | 0.060 | 0.046 | |
| 36 | 0.7624 | 41.39 | < 0.001 | 226.30 | 0.349 | 0.730 | ||
| RFA | BCLC | 12 | 0.8000 | 3.38 | 0.066 | 26.79 | 0.063 | 0.100 |
| 36 | 0.7748 | 10.51 | 0.001 | 34.86 | 0.163 | 0.508 | ||
| HKLC_9 | 12 | 0.9078 | 3.45 | 0.063 | 26.72 | 0.061 | 0.047 | |
| 36 | 0.8214 | 6.15 | 0.013 | 39.22 | 0.192 | 0.632 | ||
| HKLC_5 | 12 | 0.9078 | 4.48 | 0.034 | 25.69 | 0.060 | 0.046 | |
| 36 | 0.8214 | 4.70 | 0.006 | 37.67 | 0.184 | 0.688 | ||
| Surgery | BCLC | 12 | 0.9140 | 16.44 | < 0.001 | 27.05 | 0.078 | 0.238 |
| 36 | 0.7882 | 9.69 | 0.002 | 36.04 | 0.222 | 0.565 | ||
| HKLC_9 | 12 | 0.8280 | 4.91 | 0.027 | 38.58 | 0.091 | 0.153 | |
| 36 | 0.7465 | 6.20 | 0.013 | 39.52 | 0.249 | 0.721 | ||
| HKLC_5 | 12 | 0.8120 | 4.63 | 0.031 | 38.86 | 0.091 | 0.183 | |
| 36 | 0.7292 | 4.50 | 0.034 | 41.23 | 0.264 | 0.758 |
In this retrospective cohort study of 987 Vietnamese patients with HCC, we comprehensively evaluated the prognostic performance of seven established staging systems: BCLC, HKLC (both HKLC_9 and HKLC_5), CLIP, ITA.LI.CA, JIS, Tokyo Score, and MESIAH. Our findings consistently demonstrated the superior performance of the HKLC_5 and HKLC_9 systems in terms of discriminatory ability, model fit, and prognostic accuracy, particularly at the 12-month and 36-month follow-ups. This aligns with accumulating evidence suggesting that the HKLC system, developed in an Asian context, may outperform Western-centric models like BCLC in Asian populations[11,19-22].
The incorporation of the HKLC system factors such as tumor morphology and liver functional reserve likely contributes to its enhanced prognostic accuracy. Similar to findings by Kohla et al[23], the HKLC system showed better stratification of patients within the intermediate (BCLC stage B) and advanced stages (BCLC stage C), potentially leading to a survival advantage. While the BCLC system exhibited slightly lower discriminatory ability compared to HKLC, it demonstrated strong calibration, particularly at 36 months, suggesting its reliability in estimating survival probabilities even if it does not perfectly stratify patients into distinct risk groups. This emphasizes the importance of considering both discrimination and calibration when assessing a prognostic model[24]. Therefore, the choice between HKLC and BCLC may depend on whether the clinical objective is precise risk stratification or accurate survival probability estimation.
Our subgroup analyses further highlighted the robustness of HKLC_5 and HKLC_9 across different viral etiologies (HBV, HCV, and NBNC) and treatment modalities (TACE, surgical resection, and RFA/MWA). Notably, patients with NBNC exhibited significantly poorer overall survival compared to patients infected with HBV or HCV, consistent with Kaplan-Meier survival analysis. One plausible explanation is the lack of routine surveillance programs for patients with NBNC in Vietnam and other Asian countries, where screening efforts primarily target populations infected with HBV and HCV[3,25-27]. As a result, patients with NBNC, who often also have metabolic dysfunction-associated steatotic liver disease, alcohol-associated liver disease, or cryptogenic cirrhosis, are diagnosed at more advanced stages[28,29], limiting treatment options and adversely affecting prognosis[30,31]. Our data support this hypothesis, with patients with NBNC presenting with larger tumors, higher rates of portal vein thrombosis, and a higher likelihood of receiving palliative care.
While our study observed poorer outcomes in patients with NBNC, some studies have reported more favorable prognoses in this group. Research from Japan indicated that patients with NBNC-HCC may have preserved liver function, lower rates of cirrhosis, and consequently better recurrence-free and overall survival compared to those with HBV-related or HCV-related HCC[32-34]. This discrepancy highlights the heterogeneity within populations with NBNC-HCC and underscores the potential influence of surveillance practices on prognosis.
In support of this, Shindo et al[35] demonstrated that semiannual imaging surveillance significantly improved survival outcomes in patients with NBNC-HCC. Their study revealed that patients with NBNC receiving regular surveillance had survival rates comparable to patients infected with HBV or HCV with surveillance. Conversely, patients with NBNC without regular screening presented at more advanced stages and had poorer outcomes. These findings suggest that the inferior prognosis observed in our study could be attributed primarily to the lack of routine surveillance rather than inherent differences in tumor biology.
These findings underscore the urgent need for improved screening strategies for patients with NBNC. Expanding surveillance programs to include high-risk NBNC populations, such as those with metabolic risk factors like obesity, diabetes, and alcohol abuse, could facilitate early detection and improve survival outcomes. Personalized risk assessment tools may help identify patients with NBNC who would benefit most from routine screening. Furthermore, clinicians should maintain a high index of suspicion for HCC in patients with non-viral liver diseases, ensuring timely diagnosis and intervention.
A key strength of our study was the comprehensive evaluation of multiple staging systems within a large, well-characterized Vietnamese cohort, allowing for robust subgroup analyses. The inclusion of various subgroups based on viral etiology and treatment modality enhanced the generalizability of our findings to real-world clinical settings in Vietnam and potentially other Asian countries.
However, several limitations should be acknowledged. First, the retrospective design may introduce selection bias and limit control over confounding variables; although, we attempted to mitigate this by including consecutive patients and employing standardized data collection methods. Second, the loss to follow-up increased substantially beyond 36 months, potentially affecting long-term prognostic evaluations. Despite our efforts to compare baseline characteristics between patients who remained under follow-up and those lost to follow-up, incomplete data in certain variables allowed for partial comparisons only, thereby limiting the generalizability of our findings for this extended time period. Nevertheless, focusing on the 12-month and 36-month outcomes provided robust data for meaningful analysis. Third, our study did not delve into the specific etiologies within the NBNC group, such as distinguishing between nonalcoholic fatty liver disease and alcohol-associated liver disease, which could have different prognostic implications. Future prospective studies with more robust follow-up protocols are necessary to address these limitations.
The superior performance of the HKLC staging system suggests its suitability for prognosticating Vietnamese patients with HCC. The incorporation of more granular tumor characteristics and its alignment with Asian treatment practices likely contribute to its enhanced predictive accuracy. Clinicians in Vietnam and similar settings may consider adopting the HKLC system to better stratify patients and guide therapeutic decisions.
Our findings also emphasized the importance of region-specific validation of prognostic models. Given the heterogeneity of HCC across different populations, reliance on staging systems developed predominantly in Western countries may not provide optimal prognostic information for Asian patients. Future research should focus on refining existing models or developing new ones that incorporate genetic, molecular, and socioeconomic factors pertinent to the Asian context.
Although HKLC_5 and HKLC_9 demonstrated robust prognostic performance relative to BCLC, CLIP, ITA.LI.CA, JIS, Tokyo Score, and MESIAH in a Vietnamese cohort of patients with HCC, their differences from BCLC did not always reach statistical significance. Rather than claiming outright superiority, our findings suggest that both HKLC and BCLC remain valuable tools in clinical practice, with HKLC possibly offering marginal benefits in certain subgroups. Notably, patients with NBNC etiologies had significantly poorer survival outcomes, likely due to later-stage diagnoses resulting from the absence of routine screening, underscoring the need for enhanced surveillance strategies in NBNC populations. Future prospective studies are warranted to validate the utility of these staging systems and ultimately improve early detection and patient survival.
The authors would like to extend their heartfelt gratitude to the entire liver tumor board at Nhan dan Gia Dinh Hospital for their invaluable expertise and collaboration throughout this study. In particular, we wish to thank Dr. Thu-Phuong Do-Thi, Dr. Huu-Tuan Ly, and Dr. Tien-Dung Nguyen-Thanh for their insightful clinical input and unwavering commitment to patient care. We are also grateful to the nursing and administrative staff for their dedicated support in coordinating patient services. Finally, special thanks are due to the data management team for ensuring the accuracy and completeness of the study records. Their collective efforts made this work possible.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68650] [Article Influence: 13730.0] [Reference Citation Analysis (201)] |
| 2. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12729] [Article Influence: 6364.5] [Reference Citation Analysis (8)] |
| 3. | Nguyen-Dinh SH, Do A, Pham TND, Dao DY, Nguy TN, Chen MS Jr. High burden of hepatocellular carcinoma and viral hepatitis in Southern and Central Vietnam: Experience of a large tertiary referral center, 2010 to 2016. World J Hepatol. 2018;10:116-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Rabaan AA, Bello KE, Irekeola AA, Kaabi NAA, Halwani MA, Yousuf AA, Alshengeti A, Alfaraj AH, Khamis F, Al-Subaie MF, AlShehail BM, Almuthree SA, Ibraheem NY, Khalifa MH, Alfaresi M, Fares MAA, Garout M, Alsayyah A, Alshehri AA, Alqahtani AS, Alissa M. Prevalence of Hepatocellular Carcinoma in Hepatitis B Population within Southeast Asia: A Systematic Review and Meta-Analysis of 39,050 Participants. Pathogens. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Cam Huong NT, Van Luu N, Nam NH, Ghula S, Atieh Qarawi AT, Mai Truc PT, Trung An DN, Huy NT, Le Hoa PT. Prevalence of hepatitis B virus infection in health checkup participants: a cross-sectional study at University Medical Center, Ho Chi Minh City, Vietnam. Hosp Pract (1995). 2023;51:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Nguyen-Dinh SH, Li WF, Liu YW, Wang CC, Chen YH, Wang JH, Hung CH. Comparisons of Viral Etiology and Outcomes of Hepatocellular Carcinoma Undergoing Liver Resection between Taiwan and Vietnam. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Le VQ, Nguyen VH, Nguyen VH, Nguyen TL, Sudenga SL, Trinh LH, Nguyen VT, Nguyen TTH. Epidemiological Characteristics of Advanced Hepatocellular Carcinoma in the Northern Region of Vietnam. Cancer Control. 2019;26:1073274819862793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Chen JG, Zhu J, Zhang YH, Chen YS, Ding LL, Chen HZ, Shen AG, Wang GR. Liver Cancer Survival: A Real World Observation of 45 Years with 32,556 Cases. J Hepatocell Carcinoma. 2021;8:1023-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Safri F, Nguyen R, Zerehpooshnesfchi S, George J, Qiao L. Heterogeneity of hepatocellular carcinoma: from mechanisms to clinical implications. Cancer Gene Ther. 2024;31:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 75] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 10. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 3133] [Article Influence: 783.3] [Reference Citation Analysis (61)] |
| 11. | Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691-700.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 549] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 12. | . A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 967] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 13. | Farinati F, Vitale A, Spolverato G, Pawlik TM, Huo TL, Lee YH, Frigo AC, Giacomin A, Giannini EG, Ciccarese F, Piscaglia F, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F, Cabibbo G, Felder M, Sacco R, Morisco F, Biasini E, Foschi FG, Gasbarrini A, Svegliati Baroni G, Virdone R, Masotto A, Trevisani F, Cillo U; ITA. LI.CA study group. Development and Validation of a New Prognostic System for Patients with Hepatocellular Carcinoma. PLoS Med. 2016;13:e1002006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 14. | Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 541] [Article Influence: 23.5] [Reference Citation Analysis (1)] |
| 15. | Tateishi R, Yoshida H, Shiina S, Imamura H, Hasegawa K, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Makuuchi M, Omata M. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Yang JD, Kim WR, Park KW, Chaiteerakij R, Kim B, Sanderson SO, Larson JJ, Pedersen RA, Therneau TM, Gores GJ, Roberts LR, Park JW. Model to estimate survival in ambulatory patients with hepatocellular carcinoma. Hepatology. 2012;56:614-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Le DC, Nguyen TM, Nguyen DH, Nguyen DT, Nguyen LTM. Survival Outcome and Prognostic Factors Among Patients With Hepatocellular Carcinoma: A Hospital-Based Study. Clin Med Insights Oncol. 2023;17:11795549231178171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 18. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3442] [Article Influence: 430.3] [Reference Citation Analysis (3)] |
| 19. | Chuncharunee A, Siramolpiwat S. Validation of The Hong Kong Liver Cancer Staging System in Patients with Hepatocellular Carcinoma after Curative Intent Treatment. Asian Pac J Cancer Prev. 2017;18:1697-1701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Liu PH, Hsu CY, Lee YH, Su CW, Hsia CY, Huang YH, Chiou YY, Lin HC, Huo TI. Hong Kong Liver Cancer Staging System Is Associated With Better Performance for Hepatocellular Carcinoma: Special Emphasis on Viral Etiology. Medicine (Baltimore). 2015;94:e1772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Chen C, Long PY, Chen J, Wang P, Huang QY, Zang YY, Deng GY, Lin DY, Wang ZY. Value of Barcelona Clinic Liver Cancer staging system versus Hong Kong Liver Cancer staging system in predicting the prognosis of patients with hepatocellular carcinoma. Linchuang Gandan Zazhi. 2019;35:530-534. [DOI] [Full Text] |
| 22. | Zhang YF, Shi M, Lu LH, Wang L, Guo RP. Selecting an Optimal Staging System for Intermediate-Stage Hepatocellular Carcinoma: Comparison of 9 Currently Used Prognostic Models. J Hepatocell Carcinoma. 2021;8:253-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Kohla M, Ashour R, Taha H, El-Abd O, Osman M, Abozeid M, ELKhadry SW. Prognostic performance of Hong Kong Liver Cancer with Barcelona Clinic Liver Cancer staging systems in hepatocellular carcinoma. BMC Gastroenterol. 2024;24:318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 24. | Van Calster B, McLernon DJ, van Smeden M, Wynants L, Steyerberg EW; Topic Group ‘Evaluating diagnostic tests and prediction models’ of the STRATOS initiative. Calibration: the Achilles heel of predictive analytics. BMC Med. 2019;17:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 681] [Cited by in RCA: 1124] [Article Influence: 160.6] [Reference Citation Analysis (0)] |
| 25. | Mohamed EA, Giama NH, Abdalla AO, Shaleh HM, Oseini AM, Ali HA, Ahmed F, Taha W, Ahmed Mohammed H, Cvinar J, Waaeys IA, Ali H, Allotey LK, Ali AO, Mohamed SA, Harmsen WS, Ahmmad EM, Bajwa NA, Afgarshe MD, Shire AM, Balls-Berry JE, Roberts LR. High prevalence of chronic viral hepatitis B and C in Minnesota Somalis contributes to rising hepatocellular carcinoma incidence. World J Gastroenterol. 2022;28:5217-5229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 26. | Tatemichi M, Furuya H, Nagahama S, Takaya N, Shida Y, Fukai K, Owada S, Endo H, Kinoue T, Korenaga M. A nationwide cross-sectional survey on hepatitis B and C screening among workers in Japan. Sci Rep. 2020;10:11435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Nguyen LH, Nguyen MH. Systematic review: Asian patients with chronic hepatitis C infection. Aliment Pharmacol Ther. 2013;37:921-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 628] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 29. | Hui RW, Mak LY, Cheung TT, Lee VH, Seto WK, Yuen MF. Clinical practice guidelines and real-life practice on hepatocellular carcinoma: the Hong Kong perspective. Clin Mol Hepatol. 2023;29:217-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 30. | Foog DHS, Kwok D, Yu BCY, Wong VWS. Managing HCC in NAFLD. Curr Hepatology Rep. 2017;16:374-381. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Shinkawa H, Tanaka S, Takemura S, Ito T, Aota T, Miyazaki T, Kubo S. Outcomes of Non-B Non-C Hepatocellular Carcinoma with Reference to Patients with Interferon-Induced Hepatitis C Virus Eradication. J Gastrointest Surg. 2020;24:1809-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Nishikawa H, Arimoto A, Wakasa T, Kita R, Kimura T, Osaki Y. Comparison of clinical characteristics and survival after surgery in patients with non-B and non-C hepatocellular carcinoma and hepatitis virus-related hepatocellular carcinoma. J Cancer. 2013;4:502-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Takuma Y, Nouso K, Makino Y, Gotoh T, Toshikuni N, Morimoto Y, Shimomura H, Yamamoto H. Outcomes after curative treatment for cryptogenic cirrhosis-associated hepatocellular carcinoma satisfying the Milan criteria. J Gastroenterol Hepatol. 2011;26:1417-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Utsunomiya T, Shimada M, Kudo M, Ichida T, Matsui O, Izumi N, Matsuyama Y, Sakamoto M, Nakashima O, Ku Y, Takayama T, Kokudo N; Liver Cancer Study Group of Japan. A comparison of the surgical outcomes among patients with HBV-positive, HCV-positive, and non-B non-C hepatocellular carcinoma: a nationwide study of 11,950 patients. Ann Surg. 2015;261:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 35. | Shindo K, Maekawa S, Komatsu N, Tatsumi A, Miura M, Sato M, Suzuki Y, Matsuda S, Muraoka M, Amemiya F, Fukasawa M, Yamaguchi T, Nakayama Y, Uetake T, Inoue T, Sakamoto M, Sato T, Enomoto N. Semiannual Imaging Surveillance Is Associated with Better Survival in Patients with Non-B, Non-C Hepatocellular Carcinoma. Mediators Inflamm. 2015;2015:687484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non-Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work noncommercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/