Published online Dec 27, 2025. doi: 10.4254/wjh.v17.i12.110966
Revised: August 13, 2025
Accepted: December 3, 2025
Published online: December 27, 2025
Processing time: 190 Days and 13.4 Hours
Refractory autoimmune hepatitis (AIH) is defined as intolerance of or unresponsiveness to standard immunosuppression and occurs in 10%-20% of children with AIH. Lack of response or slower than expected response to induction of remission with steroids, despite good compliance, might be the first clue to refractory AIH. Refractoriness to treatment is associated with an 11.7 times higher risk for liver transplantation or death due to liver disease. The first and foremost consideration for the management is to assess compliance with treatment. It is then important to re-evaluate the diagnosis, assess alternative aetiologies which can mimic the cli
Core Tip: Treatment of autoimmune hepatitis seeks to induce and maintain remission, prevent progression, and potentially reverse fibrosis. In the 10%-20% of children unresponsive to steroids and azathioprine, adherence to medication and diagnosis should be reassessed and mimicking diseases excluded. Mycophenolate mofetil is the preferred second-line agent. Further therapy should be individualized, with adjustments made only after 6-12 months.
- Citation: Valamparampil J, Brown RM, McKiernan P. Refractory autoimmune hepatitis in children: Considerations for assessment and management. World J Hepatol 2025; 17(12): 110966
- URL: https://www.wjgnet.com/1948-5182/full/v17/i12/110966.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i12.110966
Autoimmune hepatitis (AIH) is a chronic immune-mediated inflammatory disease caused by loss of tolerance to hepatocyte autoantigens, leading to an altered immunological response and resulting in progressive liver injury and fibrosis[1,2]. All age groups can be affected. Clinical presentation in children is highly variable, ranging from asy
The aim of treatment is to induce biochemical and histological remission, improve symptoms, prevent disease pro
| Ref. | Outcomes | Definition |
| Mack et al[2], 2020; Pape et al[8], 2022 | Biochemical remission; complete biochemical response | Normal aspartate aminotransferase, alanine amino transferase and immunoglobulin G levels |
| Mack et al[2], 2020 | Histological remission | Absence of inflammation in liver tissue after treatment |
| Mieli-Vergani et al[3], 2018 | Complete remission | Normal aspartate aminotransferase, alanine amino transferase and immunoglobulin G levels; anti-nuclear antibody/anti-smooth muscle antibody- negative or low titer (< 1:20); anti-liver-kidney microsomal antibody type 1 and anti-liver cytosol type 1 antibody - < 1:10 or negative |
| Mack et al[2], 2020 | Treatment intolerance | Inability to continue maintenance therapy due to drug-related side effects |
| Mack et al[2], 2020 | Incomplete response | Improvement of laboratory and histological findings that are insufficient to satisfy criteria for remission |
| Pape et al[8], 2022 | Non-response | Failure to achieve a more than 50% reduction of alanine amino transferase within 4 weeks of treatment |
| Mack et al[2], 2020 | Treatment failure | Worsening laboratory or histological findings despite compliance with standard therapy |
| Mack et al[2], 2020 | Relapse | Exacerbation of disease activity after induction of remission and drug withdrawal (or nonadherence) |
| Mieli-Vergani et al[3], 2018 | Refractory | Intolerant of or unresponsive to standard immunosuppression |
The European Society for Paediatric Gastroenterology, Hepatology, and Nutrition position statement[3] defines refractory AIH as patients’ intolerance of, or unresponsive to, standard immunosuppression. The term difficult-to-treat AIH includes refractory AIH as well as other clinical situations, such as acute severe hepatitis with or without liver failure, AIH in pregnancy, and AIH in patients with viral hepatitis[9,10]. The 2019 practice guidelines by the American Ass
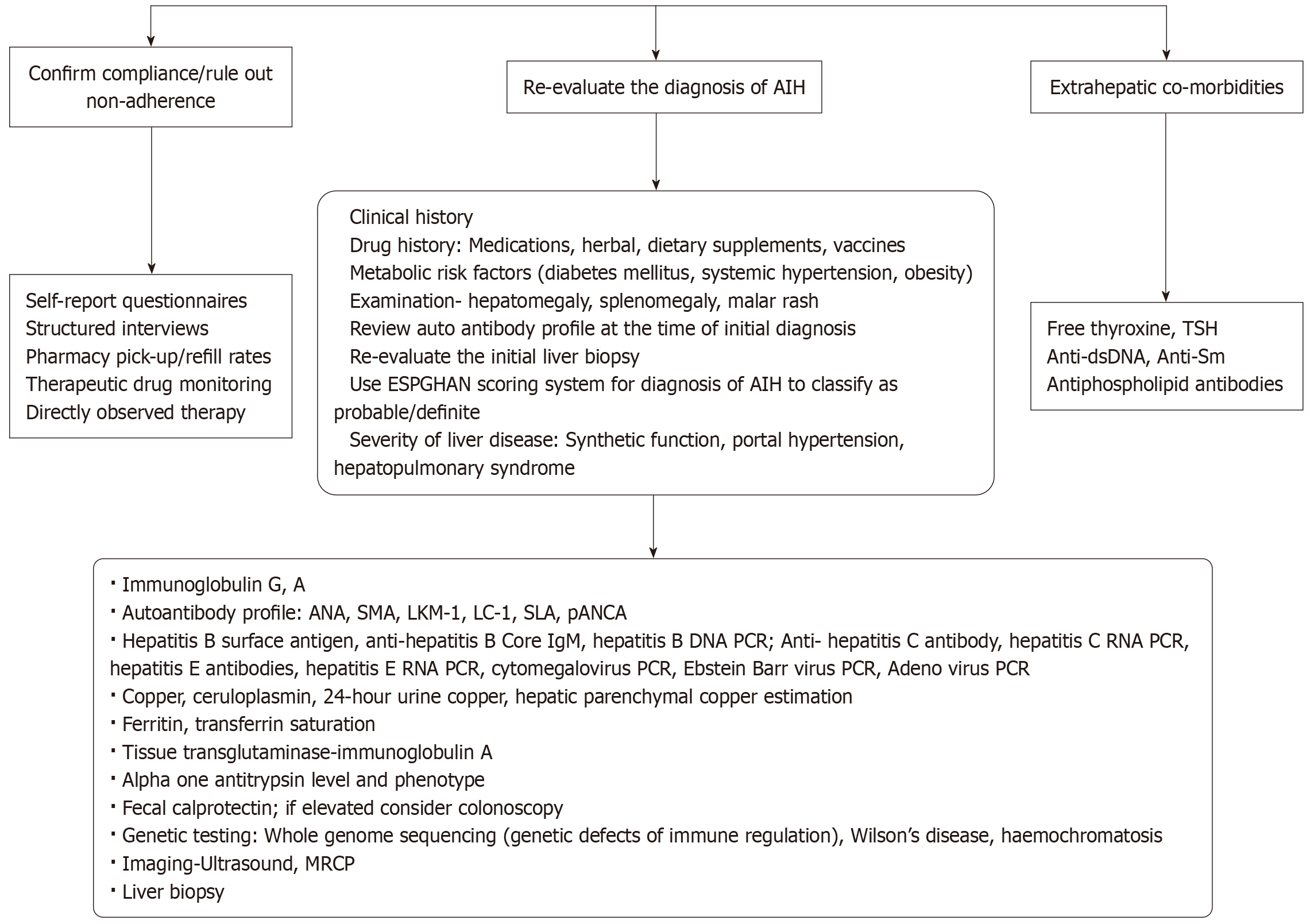
Prompt biochemical response, with a steady and consistent decline in aminotransferases on standard immunosuppression, is the norm and has been suggested as a diagnostic criterion for AIH. Therefore, the first and most important step in suspected refractory AIH is to assess compliance with treatment (Table 2). Next, it is essential to re-evaluate the diagnosis and to consider alternative or overlapping diagnoses before escalating immunosuppressive therapy. The presence of hepatic or extrahepatic comorbidities that may cause derangement of aminotransferases should also be assessed. Only after these possibilities have been excluded should a diagnosis of refractory AIH be made. Figure 1 details an algorithm for the evaluation of children not responding to or intolerant to first-line treatment.
| Considerations for assessing patients with refractory autoimmune hepatitis | |
| 1 | Confirm compliance/rule out non-adherence |
| 2 | Re-evaluate the diagnosis of AIH and evaluate for liver diseases which can mimic biochemical and/or histological features of AIH |
| 3 | Assess for extra-hepatic co-morbidities |
The most important consideration in refractory AIH is confirming adherence to therapy. Non-adherence to treatment must be ruled out in all who do not respond to first-line treatment before diagnosing and evaluating for resistant AIH[2]. The risk of non-adherence while on chronic immunosuppressive medications is higher in children and adolescents than in adults[14,15]. Assessment of adherence can be challenging, as there is no gold-standard tool. A combination of direct and indirect methods is recommended[16]. Inaccurate estimation of medication adherence can result in falsely diagnosing resistant AIH, leading to unnecessary diagnostic testing, physical and emotional distress, increased risk from invasive procedures, including liver biopsy (LB), and inappropriate intensification of immunosuppressive therapy.
Adherence assessment should include a combination of self-report questionnaires, structured interviews, pharmacy pick-up/refill rates, and therapeutic drug monitoring[16,17]. All patients on AZA should have consistent therapeutic 6-thioguanine nucleotide levels. Occasionally, directly observed therapy may be required to confirm adherence and assess treatment response. It is also important to evaluate factors contributing to non-adherence, including high medication costs, false beliefs about treatment (e.g., “medications are harmful to the liver”) held by the child or parent, cultural influences, psychiatric disorders, social adjustment difficulties, psychological distress, behavioural problems, poor family functioning, and intolerance due to side effects of immunosuppressive therapy (e.g., weight gain, abdominal pain)[14,15].
Intolerance to AZA: AZA is an antagonist of purine metabolism, inhibiting RNA and DNA synthesis and thereby affecting more rapidly dividing cells, including lymphocytes[1]. Intolerance is defined as the inability to continue therapy due to side effects. Mild nausea is the most common side effect. Significant side effects - most commonly, gastrointestinal - requiring discontinuation of therapy occur in 5%-15% of children[1,2]. AZA hypersensitivity syndrome, characterised by systemic symptoms such as fever, myalgia, rash, arthralgia, and nausea, may develop within the first few days to weeks after starting treatment and occurs in a minority of patients[1]. Other rare but important side effects requiring discontinuation of AZA include bone marrow suppression, hepatotoxicity, acute cholestatic hepatitis, pancreatitis, and opportunistic infections[1,2]. The most important dose-limiting adverse effect is bone marrow suppression, which is idiosyncratic and may be exacerbated by concomitant cytopenia due to hypersplenism.
Alternative first-line medications for AIH: Steroids, most commonly prednisolone, are used as first-line therapy either alone or in combination with AZA[1]. Steroids are the backbone of AIH treatment, as they are highly effective in inducing biochemical remission. Strict monitoring of serum transaminases, with rapid weekly tapering of the steroid to a minimum dose (most commonly 5 mg once daily) along with staged introduction of AZA, is the most common practice in paediatrics. It is important to taper the steroid to the lowest effective dose as quickly as possible, as systemic side effects can lead to reduced health-related quality of life (HRQoL) and poor adherence. These side effects include weight gain, cushingoid features, insomnia, osteoporosis, hyperglycaemia, hypertension, psychosis, hypertrichosis, and acne. My
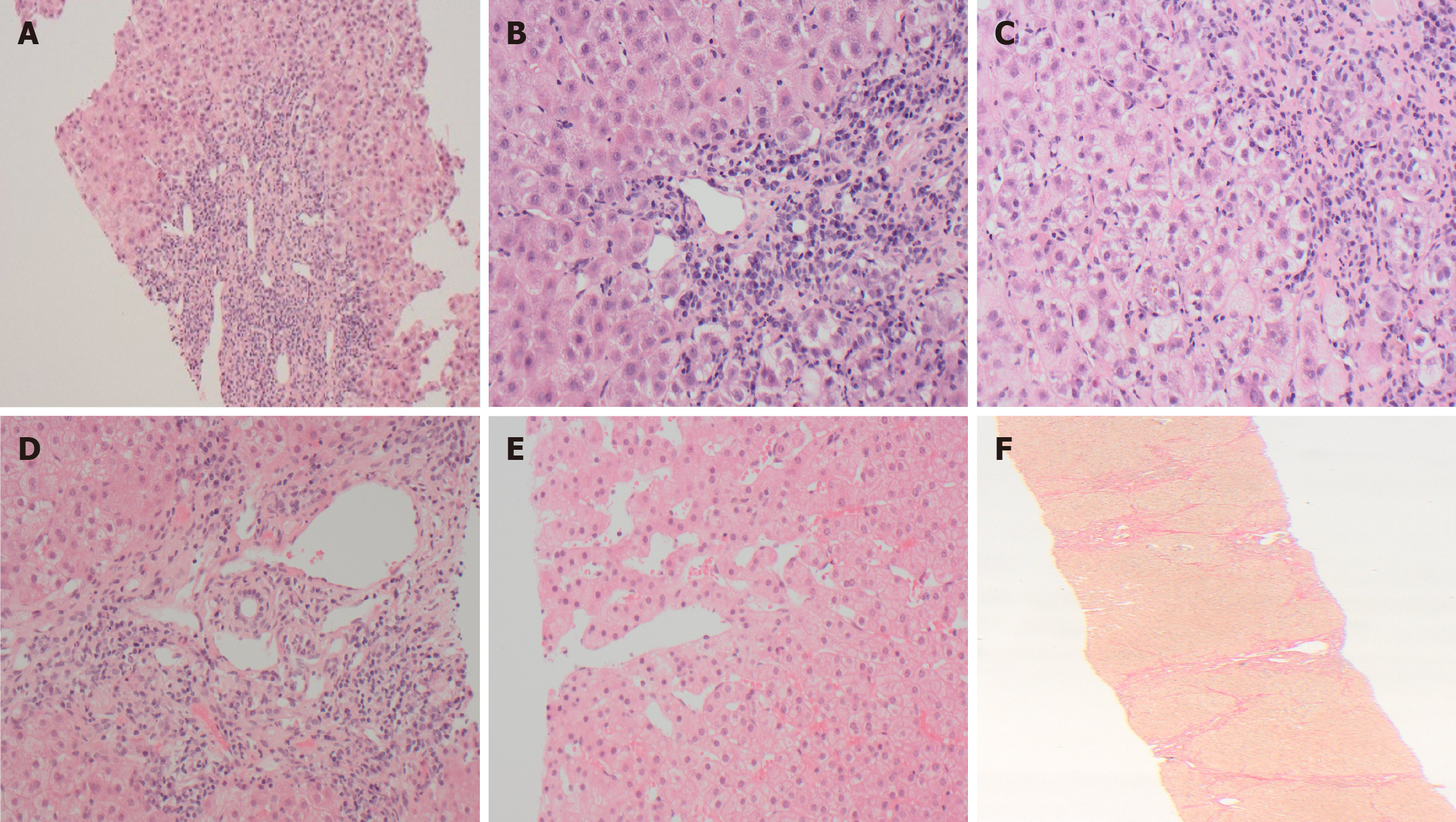
All children with refractory AIH should undergo a thorough re-evaluation to confirm the initial diagnosis and reconfirm the diagnosis of AIH. This should include a detailed history, complete clinical examination, review and reassessment of the autoantibody profile, histopathology, and evaluation for concomitant hepatic or extra-hepatic co-morbidities. The European Society of Paediatric Gastroenterology, Hepatology and Nutrition scoring system to classify AIH as probable or definite (Table 3) can be useful. A score of ≥ 7 is consistent with probable AIH, while ≥ 8 would be classed as definite AIH. The histopathology of the initial LB should be re-evaluated by an experienced liver pathologist. It is important to repeat the LB before changing the immunosuppressive regimen or escalating immunosuppression (Figure 2). This is to assess for an alternate aetiology, confirm histological features consistent with AIH, quantify the degree of inflammation, assess fibrosis stage, and plan the treatment regimen. The clinical, laboratory, radiological, and histological assessments should also evaluate for liver diseases which can mimic biochemical and/or histological features of AIH as detailed below (Table 4).
| Criteria | Titre or level | Points |
| Positive autoantibodies1,2 | ||
| Antinuclear antibodies (ANA) or anti-smooth muscle | ≥ 1:20 | + 1 |
| antibodies (SMA) | ≥ 1:80 | + 2 |
| Anti-liver kidney microsomal type 1 (anti-LKM1) | ≥ 1:10 | + 1 |
| ≥ 1:80 | + 2 | |
| Anti-liver cytosol type 1 (anti-LC1) antibody | + 2 | |
| Anti-soluble liver antigen (anti-SLA) | + 2 | |
| Anti-perinuclear neutrophil cytoplasmic antibody (pANCA) | + 1 | |
| Immunoglobulin G (IgG) | ||
| > ULN | + 1 | |
| > 1.2 × the ULN | + 2 | |
| Histological features3 | ||
| Compatible with AIH | + 1 | |
| Typical of AIH | + 2 | |
| Absence of viral hepatitis, Wilson disease, metabolic associated steatotic liver disease, drug exposure | + 2 | |
| Presence of extrahepatic autoimmunity | + 1 | |
| Family history of autoimmune disease | + 1 | |
| Cholangiography | ||
| Normal | + 2 | |
| Abnormal | - 2 | |
| Differential diagnosis in refractory autoimmune hepatitis |
| Primary sclerosing cholangitis |
| Viral infections |
| Wilsons disease |
| Hereditary hemochromatosis |
| Coeliac disease |
| Alpha one antitrypsin deficiency |
| Inflammatory bowel disease |
| Metabolic dysfunction-associated steatotic liver disease |
| Drug-induced autoimmune-like hepatitis |
| Genetic defects of immune regulation |
| Extrahepatic immune disorders (thyroid disorders, systemic lupus erythematosus) |
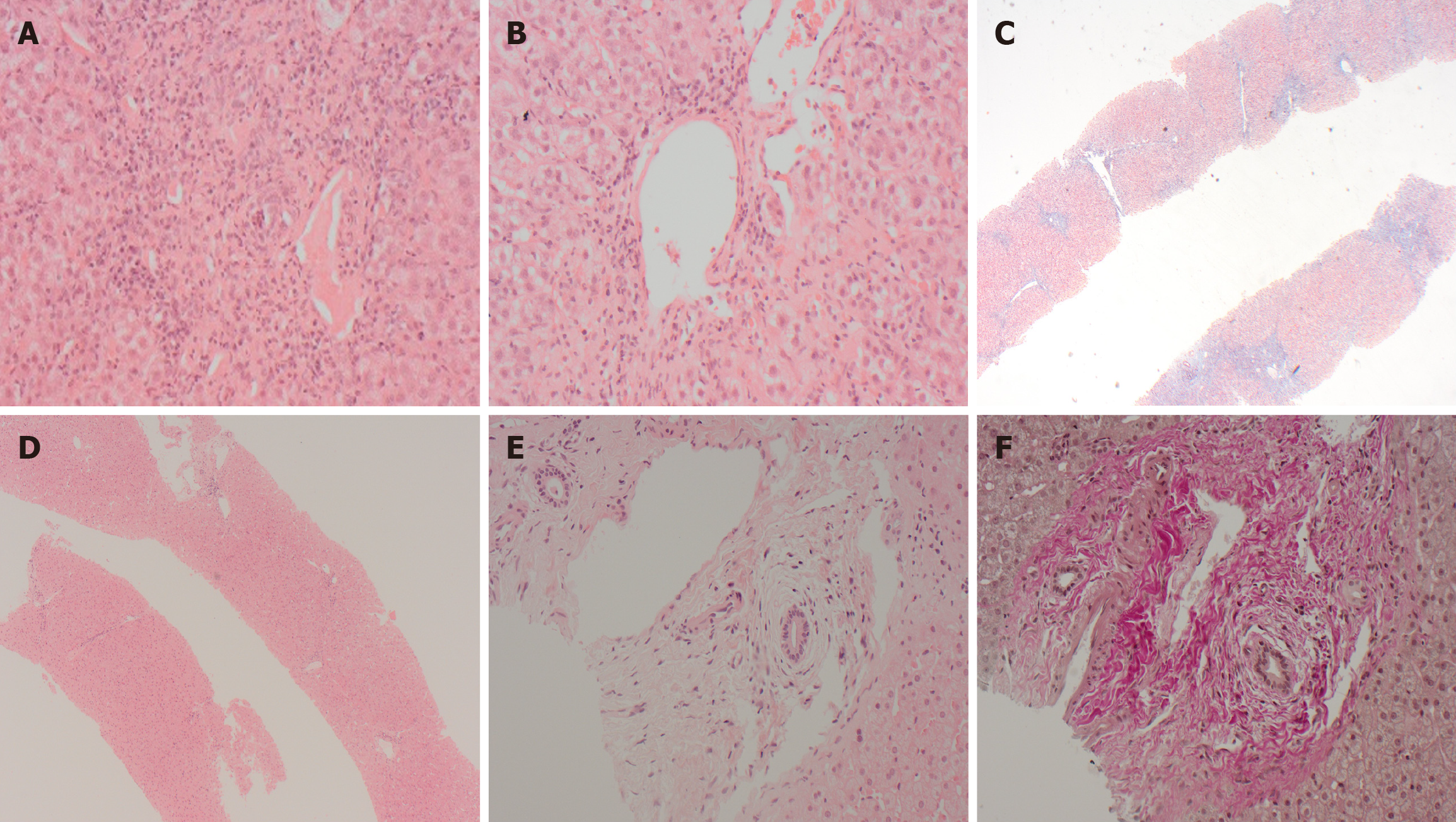
PSC/overlap syndrome: Lack of response to immunosuppressive therapy in AIH might be due to the development or progression of biliary features. This is most frequently observed in AIH-1 but can also rarely occur in AIH-2[18,19]. Long-term follow-up data, as reported by Warner et al[20], showed that approximately 20% of children with childhood onset AIH-1 developed biliary features by adulthood; 50% of these showed a phenotypical transition to primary sclerosing cholangitis (PSC), while the remainder developed an overlap syndrome.
These patients with AIH initially respond to immunosuppressive therapy with excellent biochemical response, but the progression of biliary disease is not halted and may become unresponsive to immunosuppressive medications with the change of phenotype (Figure 3)[18,19]. It has been postulated that even biliary ductular reaction considered insignificant in the context of the overall histological features in the initial LB might be an indicator of future biliary disease pro
The 2019 AASLD practice guidelines[2] recommend that AIH–PSC overlap syndrome should be considered in all patients with AIH and inflammatory bowel disease (IBD), and those non-responsive to conventional steroid therapy. Persistently raised gamma-glutamyl transferase values can be an initial clue to a change of phenotype. Gamma-glutamyl transferase levels are likely to be significantly higher in children with a phenotype change to PSC[18]. Assessment of the biliary system with ultrasonography or preferably magnetic resonance cholangiopancreatography might provide further diagnostic clues. LB is essential in this group to decide whether patients are likely to benefit from continuation or es
DI-ALH: Is defined as liver injury with laboratory and/or histological features that may be indistinguishable from those of AIH[21]. Drug-induced autoimmune-like hepatitis (DI-ALH) has been reported in 9% of the total cases of drug-induced liver injury[22]. The clinical manifestations most commonly occur within 3 months of drug exposure, but can appear after prolonged intervals, even > 500 days[21,23]. DI-ALH can have high IgG levels, positive ANA, anti-SMA, anti-liver–kidney microsomal antibody type 1 antibody, and histological features mimicking the morphological pattern of AIH[21,22]. The medications most associated with DI-ALH are nitrofurantoin, hydralazine, interferon, imatinib, adalimumab, infliximab, and statins[21,22]. Herbal, dietary supplements, and vaccines, especially the severe acute re
Wilson’s disease: Children with Wilson’s disease (WD) can have clinical, immunological, and histological features indistinguishable from AIH[24]. Very rarely, WD and AIH can co-exist, necessitating treatment for both conditions[25]. IgG, ANA, and anti-SMA can show significantly elevated titres in WD[26]. The histological features can also mimic AIH with par
Metabolic dysfunction-associated steatotic liver disease: The incidence of metabolic dysfunction-associated steatotic liver disease (MASLD) is increasing worldwide, and the incidence in AIH is likely to be similar to the general population[27]. MASLD can be present at the diagnosis of AIH or develop later. MASLD or metabolic dysfunction-associated steatohepatitis (MASH) can be associated with elevated liver enzymes. Autoantibodies and immunoglobulin [mostly immunoglobulin A (IgA)] can be elevated in some patients with MASLD. Histological findings of MASLD/MASH can be present in 17%-30% of patients with AIH[27,28]. Most studies have reported that there are no significant differences in treatment outcomes of AIH with or without MASLD[27,28]. However, the presence of MASH in patients with AIH has been reported to be associated with non-response, though this was a small cohort, and studies in larger groups are needed[27]. Children with refractory AIH should be assessed for hepatic steatosis (imaging and LB) and the presence of metabolic risk factors (diabetes, hypertension, obesity).
Viral infections: Viral hepatitis B and C should be excluded in all children prior to starting treatment for AIH. But children on treatment for AIH can contract hepatitis B or C. Reactivation of the hepatitis B virus is a known complication of immunosuppressive medications and can lead to a spectrum of manifestations, including acute hepatitis. Though chronic hepatitis E infection has mostly been described in solid organ transplant recipients, patients receiving im
Genetic defects of immune regulation: Genetic defects of immune regulation have been rarely reported as a cause of refractory AIH. The classic example of a genetic defect of immune regulation is autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, caused by biallelic mutations in the autoimmune regulator (AIRE) gene. The classic triad of manifestations is chronic mucocutaneous candidiasis, hypoparathyroidism, and adrenal insufficiency[30]. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy-associated hepatitis can present with mild asymptomatic laboratory derangements through to life-threatening fulminant hepatic failure requiring LT. Hepatitis can be the initial manifestation[30]. The laboratory and histological features are identical to classical AIH. Most patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy-associated hepatitis respond well to the classical immunosuppressive regimen of AIH, but a small fraction (10%) can be refractory to treatment[30]. AIH-2 has been reported in immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome, a rare disorder caused by mutations of the FOXP3 gene and characterized by autoimmune enteropathy, early-onset type 1 diabetes mellitus, thyroiditis, and eczema[31]. An autosomal dominant gain-of-function mutation in the signal transducer and activator of transcription 1 (STAT1) gene has also been reported as a cause of refractory AIH[32]. The Janus kinase/signal transducer and activator of transcription apparatus constitutes essential intracellular signalling pathways that induce phos
IBD: IBD can be present at the time of diagnosis of AIH or can develop over time[18]. IBD occurs in 20% of children with AIH, and the incidence is much higher (45%) in children with AIH-PSC overlap syndrome[2]. Children with histological progression of biliary features are four times more likely to have IBD compared to those without[18]. Ulcerative colitis is the most common type of IBD. Untreated IBD can be a cause of persistently deranged liver enzymes. Children with co-existent IBD can be asymptomatic or clinically silent and remain undetected for months or years unless specifically screened for[33]. Treatment with immunosuppressive therapy can also mask symptoms and signs of IBD, leading to a delay in diagnosis[34]. All children with refractory AIH should be screened for concomitant IBD with faecal calprotectin. If faecal calprotectin is elevated, colonoscopy and mucosal biopsies, with special focus on the cecum/ascending colon, should be performed[34].
Coeliac disease: Coeliac disease (CD) is more frequently seen in children with AIH than in the general population[2]. Gluten exposure, either due to non-compliance with a gluten-free diet or undiagnosed CD, can be associated with elevated transaminases in 50% of cases[35]. Transaminases will normalise only after 6-12 months of a strict gluten-free diet. Since laboratory and serological features can mimic AIH, all children with refractory AIH should be screened for CD, and compliance with a gluten-free diet should be assessed in those already diagnosed[2]. The recommended screening tests are tissue transglutaminase-IgA antibody and total IgA level. If both transglutaminase-IgA and total IgA are normal, CD is unlikely.
Other extrahepatic autoimmune diseases: Concurrent extrahepatic autoimmune disease occurs in 15%-50% of children with AIH[1,2]. Autoimmune thyroid disorders (Hashimoto’s disease, autoimmune thyroiditis, Graves’ disease) are the most common[2,36]. Autoimmune skin disorders, type 1 diabetes mellitus, and systemic lupus erythematosus are also associated. In a large adult cohort, the presence of extrahepatic autoimmune disease had no impact on the clinical course or prognosis in AIH[36]. All children with refractory AIH should be screened for thyroid disorders and systemic lupus erythematosus. Assessment for other systemic autoimmune disorders should be guided by clinical judgement and patient symptomatology.
The options for treatment in children are MMF, calcineurin inhibitors, rituximab, infliximab, and sirolimus[37]. Table 5 details the immunosuppressive therapies used in children with refractory AIH.
| Medication | Route of administration | Dose in children | Trough level | Adjuvant therapy indicated | |
| Mycophenolate mofetil | Second line | Oral | 10 mg/kg/dose twice daily; maximum dose 20 mg/kg/dose twice daily | Not applicable | Yes- steroid |
| Tacrolimus | Second or third line | Oral | 0.05 mg/kg/day- adjusted as per trough level | Initial serum trough levels 6-8 ng/mL, tapering to 3-5 ng/mL | Yes- steroid |
| Cyclosporine A | Third or fourth line | Oral | 4 mg/kg twice daily- adjusted as per trough level | Initial trough levels at 200-250 ng/mL, tapering to trough levels < 120 ng/mL after full biochemical remission | Yes- steroid |
| Sirolimus | Third/fourth line | Oral | Initial dose 1-2 mg/m2 body surface area | Trough level of 4 ng/mL to 8 ng/mL | Yes- steroid |
| Rituximab | Third/fourth line | Intravenous infusion | 375 mg/m2 2-4 doses every alternate week or fortnightly; further doses of rituximab might be needed 4-6 monthly | Not measurable. Surveillance of CD20+ B-cells is recommended; immunoglobulin supplementation may be necessary | Yes- steroid |
| Infliximab | Third/fourth line | Intravenous infusion | 5 mg/kg infliximab is infusions at 0, after two weeks, and after six weeks of initial infusion, and thereafter every four to eight weeks depending on laboratory and clinical course | Data not available | Yes- steroid |
MMF inhibits purine synthesis in B and T lymphocytes and is the most common second-line agent used in the management of refractory AIH[38]. MMF should be the first therapeutic option for patients’ intolerant or not responding to AZA[2]. Santiago et al[39], in a meta-analysis of adult patients treated with MMF, reported response rates of 82% and 32% for AZA intolerance and for treatment failure, respectively. There was a low incidence of adverse events (14%) and discontinuation of therapy (8%)[39]. In children, MMF induced a complete biochemical response in 89% of those with AZA intolerance, while, similar to adult studies, the response rate was lower (22%) in non-responders to AZA[40]. Adverse events were reported in 44%, but cessation of therapy was only needed in 11%[40].
The major disadvantages of MMF are uncertainties in how to monitor drug levels and its teratogenic potential. Across Europe, only 22% of paediatric centres used drug level monitoring, with a preference for predefined or weight-adjusted doses[38]. The trough level of mycophenolic acid depends on multiple factors, including polymorphisms, renal function, albumin levels, and concomitant medications, and there is no recommended range in paediatric AIH. MMF is teratogenic, with risk of congenital malformations, spontaneous abortions, and is hence contraindicated in pregnancy. Due to the risk of genotoxicity, contraception is also needed in men[29]. The risk of teratogenicity should be discussed with all adolescents and families, contraceptive advice should be offered, and the discussion documented. The recommended dose in children is initially 10 mg/kg body weight twice daily, increased to 20 mg/kg body weight twice daily (1-2 g/day) based on treatment response, with close monitoring for side effects, especially cytopenia and gastrointestinal symptoms (nausea, vomiting, diarrhoea).
Tacrolimus and, rarely, cyclosporine A (CsA) are commonly used as immunosuppressive agents post-transplantation; both are structurally distinct but have a similar mechanism of action. Tacrolimus causes impairment of gene expression in target cells by binding to FK506-binding protein (CsA binds to cyclophilin), inhibiting calcineurin phosphatase, and suppressing interleukin-2 synthesis and T-cell proliferation. Tacrolimus also potentiates the actions of glucocorticoids by binding to FK-binding proteins contained within the hormone–receptor complex, preventing degradation. Across Europe, tacrolimus is most commonly used as a third-line and less commonly as a second-line agent in refractory AIH[38]. As a second-line agent, tacrolimus can be used in specific situations - for example, when there are concerns about potential non-compliance, when frequent monitoring of drug levels is beneficial, or in patients concerned about the teratogenic potential of MMF. With tacrolimus, it is possible to titrate the trough level within a narrow range. Tacrolimus induced biochemical remission in approximately 90% and 50% of children when used after intolerance to previous medication and non-responsiveness to previous treatment, respectively[11,40]. The recommended target serum trough level before attaining remission is 6-8 ng/mL; the target level can be tapered to 3-5 ng/mL after full biochemical remission has been achieved[29]. Frequent monitoring of trough levels is essential until the levels are consistently within range.
CsA is very rarely used as a third-line agent in AIH. In a systematic review and meta-analysis of second-line agents in AIH, CsA induced remission in 83% at 6 months, but studies evaluating the use of CsA are all historical[11]. The long-term use of CsA is complicated by the high frequency of side effects, including cosmetic (gingival hyperplasia, hirsutism), nephrotoxicity, and neurotoxicity. Due to the undesirable side-effect profile - especially cosmetic - there is a high risk of non-adherence. Frequent drug level monitoring is needed to keep the drug level in the therapeutic range to decrease the risk of long-term complications. The target therapeutic level in AIH is not well defined, but initial trough levels of 100-300 ng/mL, tapering to trough levels < 100 ng/mL or 100-200 ng/mL after full biochemical remission, have been used[38]. The only indication for CsA use in children with AIH as a second or third-line immunosuppressant, as per the 2019 AASLD practice guidelines[2], is in the presence of concurrent diabetes mellitus.
Though AIH is characterised by elevated immunoglobulins, autoantibodies, and a plasma cell-rich histological infiltrate, it has traditionally been considered a T-cell-mediated disease, with onset and chronicity driven by T-helper cells and impaired T-regulatory cells, respectively[1]. The use of anti-B-cell therapy in AIH is supported by evidence from murine models showing B-cell facilitation of the autoimmune process through antigen presentation, stimulation of T cells, and inflammatory cytokine production, causing hepatocyte injury and cell death[1]. Rituximab, an anti-CD20 monoclonal antibody, is the only anti-B-cell therapy used in children[41]. Rituximab depletes B cells and prevents CD4 T-cell activation, thereby preventing hepatocyte damage. B-cell activating factor, a cytokine required for B-cell development and differentiation, has also been proposed as a therapeutic target in AIH[6]. Belimumab, an anti-B-cell activating factor monoclonal antibody, has been reported to induce remission in adults with AIH, but its use has not been reported in children[42].
Rituximab is recommended only as a third or fourth line-line drug in refractory AIH[38]. Evidence for the use of rituximab in refractory AIH is limited to case reports and case series[41,43]. In a large series of adult patients, Than et al[43] reported that rituximab caused a significant decline in aspartate aminotransferase, alanine aminotransferase, and IgG within a month of starting treatment, sustained for 2 years, with the absence of clinical disease flares and a reduction in prednisolone dose, without significant side effects. Saul et al[41] reported the successful use of rituximab in two children, achieving complete biochemical remission. Rituximab should always be used in combination with other immunosuppressive agents. The initial course used in children is a dose of 375 mg/m2, 2-4 doses given every other week. Further doses may be required every 4-6 months. Patients and families should be counselled regarding the increased risk of infections, and immunoglobulin infusion (1 g/kg) can be given after rituximab infusion to reduce infection risk.
Mammalian target of rapamycin inhibitors acts late in the cell cycle, preventing interleukin-2–mediated proliferation of activated CD4 and CD8 T cells. Sirolimus is the only drug in this group that has been used in refractory paediatric AIH, with its use extrapolated from experience in liver transplant recipients, particularly in recurrent and de novo AIH. Complete biochemical remission has been reported in 40%-50% of patients treated with sirolimus, without significant side effects[44,45]. Sirolimus should only be used as a third- or fourth-line medication in refractory AIH. A starting dose of 1-2 mg/m2 and a target trough level of 4-8 ng/mL have been suggested.
The only tumor necrosis factor alpha (TNF-α) inhibitor used in refractory AIH in children is infliximab, a recombinant humanised chimeric antibody that directly neutralises soluble TNF-α. Evidence for its use in AIH is limited to case reports and small series[46,47]. Infliximab induced complete remission in approximately 55% of patients unresponsive to second- or third-line treatment[47]. Another study reported complete remission in 75% of non-responders to second-line therapy and 46% of non-responders to third-line therapy[46]. Infliximab should only be used as a third- or fourth-line medication in refractory AIH. Patients and families should be counselled regarding the increased risk of infectious complications, the possibility of developing autoimmune-like hepatitis while on treatment, and the need for multiple infusions over prolonged periods. A suggested regimen is infliximab 5 mg/kg administered at week 0, 2, and 6, followed by main
There is a lack of universal consensus on the management of paediatric patients with refractory AIH. Most experts and protocols recommend MMF as the first choice for second-line treatment[2,3,37]. The use of other medications is based on case reports, case series, and treatment centre preferences rather than clinical trials[48]. The choice of treatment options in paediatric patients has been guided by the experience of using these medications in adults. Hence, it is difficult to provide a generalised recommendation for refractory AIH in children if refractory to MMF or if treatment with MMF is not an option.
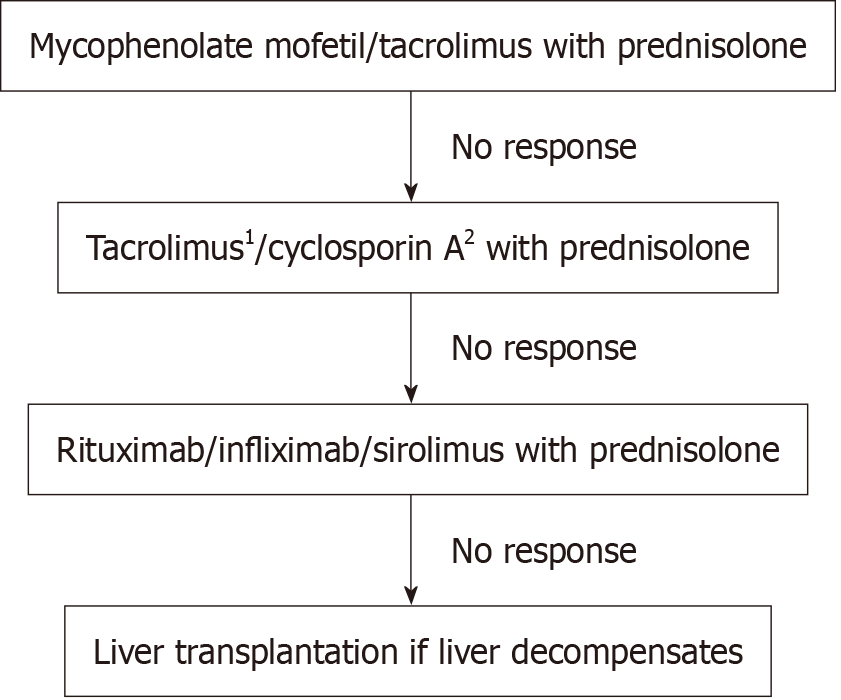
Figure 4 illustrates a treatment algorithm for children with refractory AIH. Choice of therapy has traditionally been based on local practices and experience of using the drugs in other settings, for example, post-solid organ transplantation. It is important to consider the specific clinical situations, previous therapy for AIH, compliance, options for therapeutic drug monitoring, and adverse effects profile before deciding on the most appropriate treatment regimen. Patients and families should receive extensive counselling on the benefits, risks, and long-term complications of medication(s) prior to initiation of second-line or higher therapy, and the discussion should be documented. Consideration also should be given to adherence, the need for drug level monitoring, growth potential, and availability of liquid formulations of medication while deciding on possible treatment options. Children’s and families’ preferences need to be taken into consideration before deciding on the therapy.
It is also vital that after initiating any of the regimens, a sufficient period of time is allowed before considering changing or intensifying therapy. Patients with advanced fibrosis or severe interface hepatitis may require a longer duration to achieve a complete biochemical response, and the use of more potent immunosuppressive drugs could increase the risk of unnecessary short- and long-term complications[49]. Hence, the risks vs benefits of escalating or augmenting therapy should be carefully considered prior to any changes. Overall, children who are intolerant to AZA are more likely to respond to a change of treatment than non-responders. Patients with insufficient response to first- and/or second-line treatments are expected to exhibit lower rates of response to third-line therapies compared to intolerant patients and often require combinations of two or three drugs to achieve complete biochemical response[49]. The recommended time between changes is 6 months, but it might be prudent to wait at least 12 months before changing treatment[11]. It is recommended that second-line or higher treatment options be decided and monitored only in specialised hepatology centres[29].
Non-pharmacological interventions are a crucial part of managing any chronic liver disease and become even more important in children with refractory AIH, whose HRQoL is likely lower than those responding to treatment[37]. Im
Fatigue is a common but difficult-to-quantify symptom in children with autoimmune liver diseases. Counselling and physiotherapy support should be made available for such patients. Appropriate nutrition is a critical aspect of managing refractory AIH, and regular nutritional assessment and support should be part of routine follow-up. Patient and family involvement in daily care and research should be encouraged. The benefits of participation in patient support groups and connecting with peers of similar age should be communicated to all patients and families[49]. Support from these groups can significantly enhance HRQoL and should be offered as part of comprehensive care[49].
There is a lack of multi-centre prospective registries or studies addressing the efficacy of alternative agents in AIH. Zizzo et al[11], in a systematic review and meta-analysis, reported data from multiple studies on the use of alternative agents: MMF (5 studies), calcineurin inhibitors (6 studies), one study each of MMF and calcineurin inhibitors, rituximab, sirolimus, and budesonide. The response rate for MMF (36%) was lower than cyclosporine and tacrolimus (84% and 50% respectively), while adverse events were the highest with cyclosporine (82%), followed by tacrolimus (42%) and MMF (38%)[11]. These results need to be interpreted with caution due to the small size of the studies.
Patients who do not respond to medical treatment and have progression of liver disease will require assessment for LT. Approximately 10% of children and adolescents with AIH progress to end-stage liver disease requiring transplantation within 15 years of diagnosis[37]. The indications for LT are decompensated chronic liver disease, uncontrolled portal hypertension, especially intractable/recurrent variceal bleeding which is not controlled by medical or endoscopic man
A wide range of potential therapies is currently being evaluated based on our present understanding of the immunopathogenic mechanisms in AIH. These include pharmacological and biological agents that can restore homeostatic me
| Ref. | Treatment | Mechanism of action | Stage of development | |
| Mack et al[2], 2020; Whitehead and Kriegermeier[42], 2020; Reau et al[48], 2024 | 1 | Zetomipzomib | Immunoproteasome inhibitor | Phase 2 |
| 2 | Ianalumab, Belimumab | Antibody-dependent cellular cytotoxicity mediated B-cell depletion through B-cell activating factor inhibition | Phase 2/3 | |
| 3 | JKB-122 | Toll-like receptor-4 inhibition | Phase 2 | |
| 4 | Mesenchymal stromal cells | Induction of regulatory T-cell differentiation and suppression of lymphocyte activation | Phase 1/2 | |
| 5 | Synthetic preimplantation factor (s-PIF) | Creation of immunosuppressive and immunomodulatory environment of pregnancy | Phase 1 |
At present, therapy for AIH is not specific or targeted and relies on the broad effects of immunosuppressive medi
Precision medicine holds substantial promise for transforming the management of AIH by tailoring diagnosis, prognostication, and therapy to the individual patient’s unique clinical, genetic, and biomarker profile[52]. In AIH, the integration of demographic information, clinical parameters, biomarker data, and genetic insights into predictive, self-learning systems represents a likely path forward. Such a precision medicine framework would incorporate genomics, proteomics, metabolomics, and microbiome profiling - to stratify risk of disease progression, guide the timing and selection of therapeutic interventions, predict treatment response, and identify high-risk subgroups[52]. This paradigm shift from treatment toward prevention aims to enable earlier detection and targeted intervention, supported by the application of artificial intelligence and machine learning to uncover hidden patterns and refine prognostic models[52].
In summary, for the 10%-20% of children who do not respond to first-line treatment with AZA and steroids, non-compliance should be ruled out, the diagnosis of AIH revisited, and evaluation performed for liver diseases that can mimic the biochemical and/or histological features of AIH, as well as for the presence of extrahepatic complications. MMF remains the most commonly used agent in refractory AIH. Further treatment strategies should be individualized, ideally based on the experience of specialized centers, and decided in close consultation with the patient and family.
| 1. | Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Autoimmmune hepatitis. Cell Mol Immunol. 2022;19:158-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 2. | Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, Vierling JM, Alsawas M, Murad MH, Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 648] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 3. | Mieli-Vergani G, Vergani D, Baumann U, Czubkowski P, Debray D, Dezsofi A, Fischler B, Gupte G, Hierro L, Indolfi G, Jahnel J, Smets F, Verkade HJ, Hadžić N. Diagnosis and Management of Pediatric Autoimmune Liver Disease: ESPGHAN Hepatology Committee Position Statement. J Pediatr Gastroenterol Nutr. 2018;66:345-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 4. | Muratori L, Lohse AW, Lenzi M. Diagnosis and management of autoimmune hepatitis. BMJ. 2023;380:e070201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 127] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 5. | Lohse AW, Mieli-Vergani G. Autoimmune hepatitis. J Hepatol. 2011;55:171-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 6. | Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM; American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1039] [Cited by in RCA: 1027] [Article Influence: 64.2] [Reference Citation Analysis (1)] |
| 7. | Plagiannakos CG, Hirschfield GM, Lytvyak E, Roberts SB, Ismail M, Gulamhusein AF, Selzner N, Qumosani KM, Worobetz L, Hercun J, Vincent C, Flemming JA, Swain MG, Cheung A, Chen T, Grbic D, Peltekain K, Mason AL, Montano-Loza AJ, Hansen BE; Canadian Network for Autoimmune Liver Disease (CaNAL). Treatment response and clinical event-free survival in autoimmune hepatitis: A Canadian multicentre cohort study. J Hepatol. 2024;81:227-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Pape S, Snijders RJALM, Gevers TJG, Chazouilleres O, Dalekos GN, Hirschfield GM, Lenzi M, Trauner M, Manns MP, Vierling JM, Montano-Loza AJ, Lohse AW, Schramm C, Drenth JPH, Heneghan MA; International Autoimmune Hepatitis Group (IAIHG) collaborators(‡). Systematic review of response criteria and endpoints in autoimmune hepatitis by the International Autoimmune Hepatitis Group. J Hepatol. 2022;76:841-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 9. | Parker R, Oo YH, Adams DH. Management of patients with difficult autoimmune hepatitis. Therap Adv Gastroenterol. 2012;5:421-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Lammert C, Loy VM, Oshima K, Gawrieh S. Management of Difficult Cases of Autoimmune Hepatitis. Curr Gastroenterol Rep. 2016;18:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Zizzo AN, Valentino PL, Shah PS, Kamath BM. Second-line Agents in Pediatric Patients With Autoimmune Hepatitis: A Systematic Review and Meta-analysis. J Pediatr Gastroenterol Nutr. 2017;65:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 12. | Porta G, de Carvalho E, Santos JL, Gama J, Bezerra JA; Brazilian Group for the Study of Pediatric Liver Diseases. Autoimmune Hepatitis: Predictors of Native Liver Survival in Children and Adolescents. J Pediatr. 2021;229:95-101.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Montano-Loza AJ, Mason AL, Ma M, Bastiampillai RJ, Bain VG, Tandon P. Risk factors for recurrence of autoimmune hepatitis after liver transplantation. Liver Transpl. 2009;15:1254-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Burra P, Germani G, Gnoato F, Lazzaro S, Russo FP, Cillo U, Senzolo M. Adherence in liver transplant recipients. Liver Transpl. 2011;17:760-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Kerkar N, Annunziato RA, Foley L, Schmeidler J, Rumbo C, Emre S, Shneider B, Shemesh E. Prospective analysis of nonadherence in autoimmune hepatitis: a common problem. J Pediatr Gastroenterol Nutr. 2006;43:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Lam WY, Fresco P. Medication Adherence Measures: An Overview. Biomed Res Int. 2015;2015:217047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 768] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 17. | Al-Hassany L, Kloosterboer SM, Dierckx B, Koch BC. Assessing methods of measuring medication adherence in chronically ill children-a narrative review. Patient Prefer Adherence. 2019;13:1175-1189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Deneau MR, El-Matary W, Valentino PL, Abdou R, Alqoaer K, Amin M, Amir AZ, Auth M, Bazerbachi F, Broderick A, Chan A, Cotter J, Doan S, El-Youssef M, Ferrari F, Furuya KN, Gottrand M, Gottrand F, Gupta N, Homan M, Kamath BM, Kim KM, Kolho KL, Konidari A, Koot B, Iorio R, Ledder O, Mack C, Martinez M, Miloh T, Mohan P, O'Cathain N, Papadopoulou A, Ricciuto A, Saubermann L, Sathya P, Shteyer E, Smolka V, Tanaka A, Varier R, Venkat V, Vitola B, Vos MB, Woynarowski M, Yap J, Jensen MK. The natural history of primary sclerosing cholangitis in 781 children: A multicenter, international collaboration. Hepatology. 2017;66:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 19. | Mieli-Vergani G, Vergani D. Sclerosing Cholangitis in Children and Adolescents. Clin Liver Dis. 2016;20:99-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Warner S, Rajanayagam J, Russell E, Lloyd C, Ferguson J, Kelly DA, Hirschfield GM. Biliary disease progression in childhood onset autoimmune liver disease: A 30-year follow-up into adulthood. JHEP Rep. 2024;6:100901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 21. | Andrade RJ, Aithal GP, de Boer YS, Liberal R, Gerbes A, Regev A, Terziroli Beretta-Piccoli B, Schramm C, Kleiner DE, De Martin E, Kullak-Ublick GA, Stirnimann G, Devarbhavi H, Vierling JM, Manns MP, Sebode M, Londoño MC, Avigan M, Robles-Diaz M, García-Cortes M, Atallah E, Heneghan M, Chalasani N, Trivedi PJ, Hayashi PH, Taubert R, Fontana RJ, Weber S, Oo YH, Zen Y, Licata A, Lucena MI, Mieli-Vergani G, Vergani D, Björnsson ES; IAIHG and EASL DHILI Consortium. Nomenclature, diagnosis and management of drug-induced autoimmune-like hepatitis (DI-ALH): An expert opinion meeting report. J Hepatol. 2023;79:853-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 101] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 22. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol. 2019;70:1222-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 769] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 23. | Molleston JP, Fontana RJ, Lopez MJ, Kleiner DE, Gu J, Chalasani N; Drug-Induced Liver Injury Network. Characteristics of idiosyncratic drug-induced liver injury in children: results from the DILIN prospective study. J Pediatr Gastroenterol Nutr. 2011;53:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Schilsky ML, Roberts EA, Bronstein JM, Dhawan A, Hamilton JP, Rivard AM, Washington MK, Weiss KH, Zimbrean PC. A multidisciplinary approach to the diagnosis and management of Wilson disease: 2022 Practice Guidance on Wilson disease from the American Association for the Study of Liver Diseases. Hepatology. 2025;82:E41-E90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 55.0] [Reference Citation Analysis (1)] |
| 25. | Milkiewicz P, Saksena S, Hubscher SG, Elias E. Wilson's disease with superimposed autoimmune features: report of two cases and review. J Gastroenterol Hepatol. 2000;15:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Santos BC, Guedes LR, Faria LC, Couto CA. Wilson's disease presentation resembling autoimmune hepatitis. BMJ Case Rep. 2019;12:e230721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Zachou K, Azariadis K, Lytvyak E, Snijders RJALM, Takahashi A, Gatselis NK, Robles M, Andrade RJ, Schramm C, Lohse AW, Tanaka A, Drenth JPH, Montano-Loza AJ, Dalekos GN; International Autoimmune Hepatitis Group (IAIHG). Treatment responses and outcomes in patients with autoimmune hepatitis and concomitant features of non-alcoholic fatty liver disease. JHEP Rep. 2023;5:100778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 28. | De Luca-Johnson J, Wangensteen KJ, Hanson J, Krawitt E, Wilcox R. Natural History of Patients Presenting with Autoimmune Hepatitis and Coincident Nonalcoholic Fatty Liver Disease. Dig Dis Sci. 2016;61:2710-2720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Lohse AW, Sebode M, Jørgensen MH, Ytting H, Karlsen TH, Kelly D, Manns MP, Vesterhus M; European Reference Network on Hepatological Diseases (ERN RARE-LIVER); International Autoimmune Hepatitis Group (IAIHG). Second-line and third-line therapy for autoimmune hepatitis: A position statement from the European Reference Network on Hepatological Diseases and the International Autoimmune Hepatitis Group. J Hepatol. 2020;73:1496-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 30. | Chascsa DM, Ferré EMN, Hadjiyannis Y, Alao H, Natarajan M, Quinones M, Kleiner DE, Simcox TL, Chitsaz E, Rose SR, Hallgren A, Kampe O, Marko J, Ali RO, Auh S, Koh C, Belkaid Y, Lionakis MS, Heller T. APECED-Associated Hepatitis: Clinical, Biochemical, Histological and Treatment Data From a Large, Predominantly American Cohort. Hepatology. 2021;73:1088-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | López SI, Ciocca M, Oleastro M, Cuarterolo ML, Rocca A, de Dávila MT, Roy A, Fernández MC, Nievas E, Bosaleh A, Torgerson TR, Ruiz JA. Autoimmune hepatitis type 2 in a child with IPEX syndrome. J Pediatr Gastroenterol Nutr. 2011;53:690-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Hadžić N, Deheragoda M, Worth A, Bansal S, Samyn M, Kusters M. JAK Inhibition in STAT1 Gain-of-Function-Mediated Treatment-Resistant Autoimmune Hepatitis. N Engl J Med. 2024;390:284-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Misiou M, Hope B, Lee HM, Samyn M, Vadamalayan B. P46 Review the diagnosis of IBD in children with AILD (Auto immune liver disease) -8 years’ experience in a tertiary Centre. Frontline Gastroenterol. 2021;12:A40-A41. [DOI] [Full Text] |
| 34. | Kellermayer R. Should all pediatric patients with type 1 autoimmune hepatitis be screened for inflammatory bowel disease? JHEP Rep. 2025;7:101291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 35. | Nastasio S, Sciveres M, Riva S, Filippeschi IP, Vajro P, Maggiore G. Celiac disease-associated autoimmune hepatitis in childhood: long-term response to treatment. J Pediatr Gastroenterol Nutr. 2013;56:671-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Muratori P, Fabbri A, Lalanne C, Lenzi M, Muratori L. Autoimmune liver disease and concomitant extrahepatic autoimmune disease. Eur J Gastroenterol Hepatol. 2015;27:1175-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Mieli-Vergani G, Vergani D, Czaja AJ, Manns MP, Krawitt EL, Vierling JM, Lohse AW, Montano-Loza AJ. Autoimmune hepatitis. Nat Rev Dis Primers. 2018;4:18017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 309] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 38. | Cananzi M, Jørgensen MH, Buescher G, De Bruyne R, Samyn M; ESPGHAN Hepatology Interest Group and the ERN RARE‐LIVER Autoimmune Hepatitis Working Group. Current practice in the management of paediatric autoimmune liver disease in Europe. J Pediatr Gastroenterol Nutr. 2025;80:260-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 39. | Santiago P, Schwartz I, Tamariz L, Levy C. Systematic review with meta-analysis: mycophenolate mofetil as a second-line therapy for autoimmune hepatitis. Aliment Pharmacol Ther. 2019;49:830-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 40. | Efe C, Taii HA, Ytting H, Aehling N, Bhanji RA, Hagström H, Purnak T, Muratori L, Werner M, Muratori P, Klintman D, Schiano TD, Montano-Loza AJ, Berg T, Larsen FS, Alkhouri N, Ozaslan E, Heneghan MA, Yoshida EM, Wahlin S. Tacrolimus and Mycophenolate Mofetil as Second-Line Therapies for Pediatric Patients with Autoimmune Hepatitis. Dig Dis Sci. 2018;63:1348-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Saul SA, Taylor SA, Mohammad S. Treatment of Refractory Pediatric Autoimmune Hepatitis With Rituximab. JPGN Rep. 2021;2:e069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Whitehead B, Kriegermeier A. Natural history and management of refractory autoimmune hepatitis. Clin Liver Dis (Hoboken). 2022;20:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Than NN, Hodson J, Schmidt-Martin D, Taubert R, Wawman RE, Botter M, Gautam N, Bock K, Jones R, Appanna GD, Godkin A, Montano-Loza AJ, Lammert F, Schramm C, Manns MP, Swain M, Burak KW, Adams DH, Hirschfield GM, Oo YH. Efficacy of rituximab in difficult-to-manage autoimmune hepatitis: Results from the International Autoimmune Hepatitis Group. JHEP Rep. 2019;1:437-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 44. | Chatrath H, Allen L, Boyer TD. Use of sirolimus in the treatment of refractory autoimmune hepatitis. Am J Med. 2014;127:1128-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 45. | Kurowski J, Melin-Aldana H, Bass L, Alonso EM, Ekong UD. Sirolimus as rescue therapy in pediatric autoimmune hepatitis. J Pediatr Gastroenterol Nutr. 2014;58:e4-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 46. | Efe C, Lytvyak E, Eşkazan T, Liberal R, Androutsakos T, Turan Gökçe D, Terziroli Beretta-Piccoli B, Janik M, Bernsmeier C, Arvaniti P, Milkiewicz P, Batibay E, Yüksekyayla O, Ergenç I, Arikan Ç, Stättermayer AF, Barutçu S, Cengiz M, Gül Ö, Heurgue A, Heneghan MA, Verma S, Purnak T, Törüner M, Akdogan Kayhan M, Hatemi I, Zachou K, Macedo G, Drenth JPH, Björnsson E, Montano-Loza AJ, Wahlin S, Higuera-de la Tijera F. Efficacy and safety of infliximab in patients with autoimmune hepatitis. Hepatology. 2025;81:1660-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 47. | Weiler-Normann C, Schramm C, Quaas A, Wiegard C, Glaubke C, Pannicke N, Möller S, Lohse AW. Infliximab as a rescue treatment in difficult-to-treat autoimmune hepatitis. J Hepatol. 2013;58:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 48. | Reau NS, Lammert CS, Weinberg EM. Autoimmune hepatitis: Current and future therapies. Hepatol Commun. 2024;8:e0458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 49. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of autoimmune hepatitis. J Hepatol. 2025;83:453-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 43] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 50. | Chai PF, Lee WS, Brown RM, McPartland JL, Foster K, McKiernan PJ, Kelly DA. Childhood autoimmune liver disease: indications and outcome of liver transplantation. J Pediatr Gastroenterol Nutr. 2010;50:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis: Current Status and Future Directions. Gut Liver. 2016;10:177-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/