Published online Nov 27, 2025. doi: 10.4254/wjh.v17.i11.110614
Revised: July 14, 2025
Accepted: September 28, 2025
Published online: November 27, 2025
Processing time: 169 Days and 3.4 Hours
Hepatocellular carcinoma (HCC) remains one of the leading causes of cancer-related mortality worldwide, with approximately 35%-50% of patients presenting concurrent portal vein tumor thrombus (PVTT). Untreated HCC patients with PVTT have a median survival of only 2.5-4 months, posing significant challenges to liver transplantation outcomes. Downstaging therapies play a pivotal role in improving transplant eligibility rates and optimizing post-transplant outcomes. This systematic review summarizes current downstaging therapies, including transarterial chemoembolization, transarterial radioembolization, proton beam therapy, intraportal radiofrequency ablation, and other novel systemic modalities. In-depth analysis of their clinical applications, efficacy, and safety profiles were performed. Furthermore, the review critically evaluates future challenges, inc
Core Tip: Hepatocellular carcinoma with portal vein tumor thrombus has long been considered a contraindication for liver transplantation due to poor prognosis. However, evolving downstaging strategies, including transarterial chemoembolization, transarterial radioembolization, proton beam therapy, and combination immunotherapies, have shown potential to convert select patients into transplant candidates. This review summarizes current clinical evidence and highlights future directions involving personalized medicine, novel biomaterials, and standardized criteria.
- Citation: Li ZY, Xie C, Cai HQ. Pre-transplant downstaging strategies for hepatocellular carcinoma with portal vein tumor thrombus: Current therapies and future challenges. World J Hepatol 2025; 17(11): 110614
- URL: https://www.wjgnet.com/1948-5182/full/v17/i11/110614.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i11.110614
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer-related mortality globally[1]. Its incidence continues to rise, particularly in regions with high prevalence of chronic hepatitis B virus and hepatitis C virus infections, as well as in populations increasingly affected by non-alcoholic fatty liver disease and cirrhosis[2]. One of the most formidable challenges in HCC is the presence of portal vein tumor thrombus (PVTT), which occurs in approximately 35%-50% of HCC patients at initial diagnosis[3]. PVTT represents a hallmark of aggressive tumor behavior and is classified as macrovascular invasion under the Barcelona Clinic Liver Cancer staging system with advanced stage category[4]. The formation of PVTT not only impairs portal hemodynamics and liver function, but also significantly reduces the efficacy and safety of conventional treatments[5]. Importantly, median overall survival (OS) in untreated HCC patients with PVTT remains dismal, ranging from 2.5 to 4 months, highlighting the urgent need for effective interventions[6].
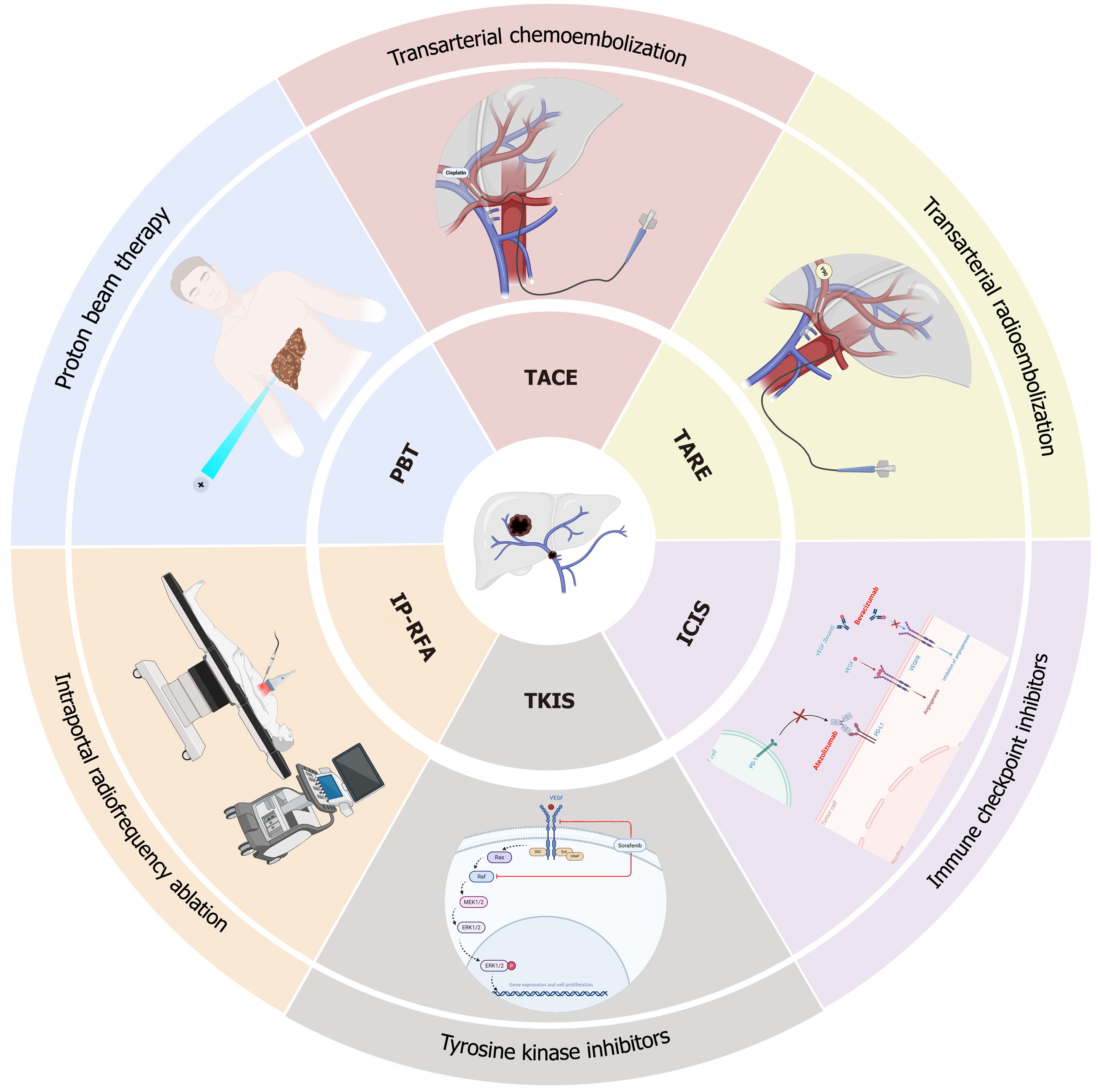
Liver transplantation (LT) is considered a potentially curative option for selected HCC patients, particularly those within the Milan or UCSF criteria[7]. However, patients with PVTT are typically excluded from transplantation due to high recurrence rates and poor post-transplant outcomes[8]. To address this unmet need, pre-transplant downstaging therapies have been developed with the aim of reducing tumor burden and vascular invasion, thereby enabling patients to meet transplant eligibility criteria and improving long-term survival[9]. Downstaging strategies encompass a broad range of locoregional therapies (Figure 1) such as transarterial chemoembolization (TACE), transarterial radioembolization (TARE), external beam radiotherapy, and more recently, proton beam therapy (PBT) and intraportal radiofrequency ablation (IP-RFA)[10-12]. In addition, systemic therapies, including tyrosine kinase inhibitors (TKIs) (e.g., sorafenib, lenvatinib) and immune checkpoint inhibitors (ICIs) (e.g., atezolizumab plus bevacizumab), have shown potential in reducing tumor size and vascular invasion, either alone or in combination with locoregional approaches[12,13]. Despite promising results from retrospective and early-phase prospective studies, several challenges remain in integrating downstaging therapies into routine clinical practice[14]. This review aims to summarize the current landscape of pre-transplant downstaging therapies for HCC patients with PVTT, critically evaluate their efficacy and safety, and highlight key challenges and opportunities for future clinical and translational research.
PVTT is a critical prognostic factor in HCC, reflecting an advanced disease stage with aggressive biological behavior[15]. Its presence alters liver hemodynamics, accelerates hepatic decompensation, and complicates treatment planning[16]. Accurate classification of PVTT is essential for risk stratification, treatment selection, and evaluating the feasibility of downstaging therapies prior to LT[17]. The Liver Cancer Study Group of Japan (LCSGJ) classification is the most widely used and clinically validated system for categorizing PVTT based on the anatomical extent of portal vein involvement[18]. Patients classified as Vp1-Vp2 often retain better liver function and are more likely to benefit from locoregional therapies such as TACE, PBT, or radioembolization. In contrast, Vp3-Vp4 denotes more advanced disease, often acc
In addition to the LCSGJ system, an alternative and widely adopted classification method, Cheng’s classification system, was developed by Chinese investigators to more precisely reflect the extent of PVTT and guide clinical decision-making in HCC treatment[21,22]. This system categorizes PVTT into four distinct types based on the anatomical location of the tumor thrombus and its extension within the portal venous system[23]. Cheng’s classification is particularly valuable in clinical practice across Asia, where surgical resection remains a central component of HCC treatment[24]. The system not only provides a basis for prognostic stratification but also aids in selecting candidates for aggressive therapies, including downstaging protocols[25]. Generally, patients with Type I and II PVTT are considered suitable for locoregional therapy or surgery, while those with Type III and IV require a multidisciplinary approach, often involving sys
| System | Grade/type | Anatomical extent | Typical characteristics | Downstaging feasibility |
| LCSGJ | Vp0 | No portal vein invasion | Normal portal flow | Not applicable |
| Vp1 | Thrombus distal to second-order branches | Peripheral PVTT; limited hepatic impact | High: TACE, TARE, PBT often effective | |
| Vp2 | Thrombus in second-order (segmental) branches | Segmental invasion; localized disease | Moderate to high | |
| Vp3 | Thrombus in first-order (right/Left) portal vein | Major branch occlusion; moderate liver function compromise | Moderate: Requires combination approaches | |
| Vp4 | Thrombus in main trunk or contralateral portal vein | Central occlusion; severe portal hypertension | Low: Systemic + radiation or experimental | |
| Cheng | Type I | Segmental branches of the portal vein | Equivalent to Vp1-Vp2; good prognosis if treated early | High |
| Type II | Right or left portal vein | Similar to Vp3; higher recurrence risk post resection | Moderate | |
| Type III | Main portal vein trunk | Equivalent to Vp4; marked hemodynamic impairment | Low | |
| Type IV | Extending into superior mesenteric vein | Extensive systemic vascular involvement | Very low: Limited to palliative/systemic care |
Downstaging therapies aim to reduce tumor burden and vascular invasion to render HCC patients with PVTT eligible for potentially curative interventions such as LT[29]. These modalities vary in their mechanisms of action, patient selection criteria, and integration with systemic therapy[30].
TACE is the most frequently applied locoregional therapy for patients with intermediate-stage HCC[31]. Its use in advanced-stage disease, particularly in patients with PVTT, has been controversial due to the potential for hepatic ischemia and subsequent liver decompensation[32]. However, a growing body of evidence suggests that carefully selected patients with segmental or lobar PVTT (e.g., Vp1-Vp2 or Cheng Type I-II) may derive meaningful survival benefit from TACE, either as a standalone modality or in combination with systemic therapy[33]. TACE combines intra-arterial delivery of chemotherapeutic agents (commonly doxorubicin, cisplatin, or epirubicin) with embolic materials such as lipiodol or gelatin sponge particles, leading to both cytotoxic and ischemic necrosis of tumor tissue[34]. In patients with PVTT, tumor regions often receive their primary blood supply via the hepatic artery, allowing for selective embolization despite compromised portal flow, thereby sparing healthy liver parenchyma[35]. The pivotal randomized controlled trial by Lo et al[36] first demonstrated the efficacy of TACE in unresectable HCC. Although patients with main trunk PVTT were excluded, those with segmental PVTT were included. The study reported a significant improvement in median OS in the TACE group (19.4 months vs 7.1 months, P < 0.001), supporting the rationale for TACE in appropriately selected PVTT patients. Further supporting evidence comes from a multi-treatment meta-analysis (2012-2019) involving 4265 PVTT patients across 12 randomized controlled trials[37]. The network analysis compared various treatments including sorafenib, hepatectomy, and TACE, as well as their combinations. Among all strategies, sorafenib combined with TACE was ranked as the most effective, with the highest probability of improving 1-year survival outcomes. Despite its efficacy, TACE is not without risks. The incidence of hepatic decompensation increases substantially in patients with Vp3-Vp4 PVTT or poor liver reserve (Child-Pugh B/C), often limiting its use in advanced cases. TACE, although widely used, may pose significant risks in patients with advanced PVTT (Vp3 and Vp4) due to the potential for hepatic ischemia, especially when portal vein patency is severely compromised. Additionally, TARE, while effective in controlling tumor growth, may not provide adequate results in patients with extensive vascular invasion or those who cannot tolerate the embolic load due to impaired hepatic reserve. Recently reported TACE-based combination therapies for HCC with PVTT were listed in Table 2[5,38-44]. In summary, TACE remains a viable and effective option for downstaging in PVTT patients with preserved liver function and limited PVTT extent[45]. The integration of TACE with systemic agents, particularly sorafenib or ICIs, represents a promising direction for future protocols aiming to improve transplant eligibility and long-term survival[46,47].
| Ref. | Population | Treatment arms | ORR (%) | Median OS (months) | Median PFS (months) | Key findings |
| Yuan et al[5] | 743 HCC with PVTT | TACE + HAIC + TKIs + PD-1 vs TACE | 53.7 vs 7.8 | Not reached vs 10.4 | 14.8 vs 2.3 | Best surgical conversion and pCR outcomes |
| You et al[38] | 265 HCC with PVTT | IT + TACE vs IT | – | 19.0 vs 13.0 | 12.0 vs 7.3 | TACE enhances immune-targeted response |
| Lu et al[39] | 227 Vp2–Vp3 PVTT | TACE + PEI + Lenvatinib vs TACE + Lenvatinib | 50.5 vs 25.8 | 17.1 vs 13.9 | 8.1 vs 6.5 | PEI boosts PVTT regression |
| Lu et al[40] | 105 Vp4 PVTT | 125I stent + TACE vs Sorafenib + TACE | – | 9.9 vs 6.3 | 6.6 vs 4.2 | 125I stent prolongs survival and patency |
| Zou et al[41] | 165 HCC with PVTT | TACE + Lenvatinib + PD-1 vs TACE + Sorafenib + PD-1 | 41.3 vs 30.6 | 21.7 vs 15.6 | 6.3 vs 3.2 | Lenvatinib triple better than Sorafenib triple |
| Lin et al[42] | 95 PVTT + APFs | HAIC + Lenvatinib + PD-1 vs TACE + Lenvatinib + PD-1 | 52.9 vs 27.9 | 25.0 vs 19.3 | 21.7 vs 8.7 | HAIC favored in APF setting |
| Zhao et al[43] | 58 PVTT + APFs | TACE + 125I vs TACE + Sorafenib | – | 12.8 vs 8.0 | – | 125I improves APF control and OS |
| Yang et al[44] | 116 PVTT | TACE + Lenvatinib vs TACE + Sorafenib | 66.8 vs 33.3 | 18.97 vs 10.77 | 10.6 vs 5.4 | TACE-L significantly outperforms TACE-S in ORR, OS, and PFS |
TARE, also known as selective internal radiation therapy (SIRT), is an increasingly utilized locoregional therapy in HCC, particularly in patients with PVTT or other forms of macrovascular invasion[48]. Unlike TACE, TARE delivers radioactive microspheres, most commonly yttrium-90 (Y-90), directly into the hepatic artery branches feeding the tumor[49]. These microspheres emit localized beta radiation over approximately two weeks, allowing high-dose radiation to the tumor with minimal embolic effect, making it particularly suitable for PVTT patients who may not tolerate the ischemia induced by TACE[50]. The minimal embolic effect of TARE enables it to preserve portal venous flow, a critical advantage in PVTT patients who often already have impaired portal circulation[51]. Radiation induces DNA damage and apoptosis in tumor cells, including the thrombus within the portal vein, offering both tumor control and potential thrombus regression[52]. Moreover, TARE allows segmental, lobar, or whole-liver treatments, which is advantageous for patients with bilobar disease or PVTT involving major branches[53]. The DOSISPHERE-01 trial demonstrated that personalized SIRT (Y-90) dosing significantly improves downstaging success compared to standard dosing, highlighting the need for tailored treatment protocols. Some clinical studies have validated the role of TARE in HCC (Table 3).
| Trial | Phase/design | Comparison | Primary endpoint | Results | Clinical insights |
| SARAH | Phase III, open-label, randomized controlled trial | SIRT (Y-90) vs Sorafenib | Overall survival (OS) | OS: 8.0 months (SIRT) vs 9.9 months (Sorafenib)- no significant difference. Fewer AEs and better QoL in SIRT group | Comparable efficacy with better safety; viable alternative in select patients |
| SIRveNIB | Phase III, open-label, randomized controlled trial | SIRT (Y-90) vs Sorafenib | OS | OS: 8.8 months (SIRT) vs 10.0 months (Sorafenib) no significant difference. Grade ≥ 3 AEs: 27.7% (SIRT) vs 50.6% (Sorafenib) | Reinforces safety advantage of SIRT; supports use in selected Asian patient populations |
| DOSISPHERE-01 | Phase II, open-label, randomized controlled trial | Personalized SIRT vs Standard-dose SIRT | OS | OS: 26.6 months (personalized) vs 10.7 months (standard) P = 0.009. Higher response rates; some downstaged to surgery | Personalized dosimetry improves outcomes; highlights need for tailored treatment planning |
A large multicenter retrospective study involving 216 HCC patients with PVTT compared TARE and (TKIs, including sorafenib and Lenvatinib)[54]. The TARE group achieved a significantly superior median OS of 28.2 months vs 7.2 months in the TKI group (P < 0.001). In the matched cohort, TARE demonstrated an objective response rate (ORR) of 53%-56.7%, far exceeding that of TKIs (12.3%-15.0%). A comprehensive network meta-analysis compared 12 treatment strategies in 1623 HCC patients with PVTT[55]. It found that TARE, either alone or combined with other modalities, ranked among the most effective in improving OS, outperforming sorafenib monotherapy in multiple endpoints. TARE is both safe and effective for HCC patients with PVTT with a pooled ORR of 50%-75% and a median OS of approximately 10 months, and a favorable toxicity profile, particularly in patients with Child-Pugh A liver function[56]. A comparison between TACE and TARE has been summarized in Table 4.
| Therapy | Vp2 PVTT | Vp3 PVTT | ORR (%) | Median OS (months) | Safety profile |
| TACE | Moderate | Moderate | 50-70 | 18-24 | Moderate |
| TARE | High | Moderate | 60-80 | 22-30 | Low |
PBT is an advanced form of external beam radiation therapy that utilizes the unique physical properties of protons, most notably the Bragg peak, to deliver highly conformal doses of radiation to tumors while sparing surrounding healthy tissues[57]. The Bragg peak in PBT allows for precise tumor irradiation with minimal damage to surrounding healthy tissue, which is particularly crucial in the treatment of PVTT where liver function and vascular integrity are com
IP-RFA is an emerging interventional strategy that applies thermal energy directly to PVTT through the portal venous system. Unlike traditional percutaneous or transarterial RFA, which targets intrahepatic tumors, IP-RFA specifically aims to debulk or ablate intravascular tumor components, particularly thrombi within the main or branch portal vein using a catheter-based approach[63]. The primary rationale for IP-RFA lies in its ability to induce localized tumor necrosis within the portal vein without impairing hepatic arterial flow or inducing significant systemic toxicity. By applying high-frequency alternating current via a radiofrequency electrode catheter inserted into the portal vein, the technique generates heat-induced coagulative necrosis of the thrombus tissue. A compelling clinical case report described a 60-year-old male with HCC-induced PVTT who underwent intraportal RFA combined with stent-assisted recanalization (VesOpen procedure)[64]. No additional systemic or locoregional therapy was administered. At 5-month follow-up, computed tomography imaging showed a significant reduction in tumor size, restored patency of the main and right portal vein, and partial recanalization of the left portal vein. Liver function, appetite, and overall clinical condition had markedly improved. This real-world case illustrates the potential for IP-RFA, particularly when combined with mechanical interventions, to reverse portal hypertension and improve quality of life in end-stage PVTT patients.
Systemic therapy has long served as the mainstay treatment for advanced HCC, particularly in patients with PVTT who are ineligible for resection or locoregional therapy[65]. Over the past decade, the landscape of systemic treatment has rapidly evolved, from TKIs such as sorafenib and lenvatinib, to ICIs targeting PD-1/PD-L1 and CTLA-4 pathways[66]. More recently, combination regimens integrating systemic therapy with locoregional modalities (e.g., TACE, TARE, radiotherapy) have shown promise in downstaging advanced HCC to resectability or even liver transplant eligibility[67]. Sorafenib was the first systemic agent approved for advanced HCC following the landmark SHARP trial, which demonstrated modest survival benefit[68]. In PVTT patients, sorafenib has been shown to prolong median OS to 5-6 months; however, response rates remain low (< 10%). Lenvatinib, a multitargeted TKI, showed non-inferiority to sorafenib in the REFLECT trial[69]. Subgroup analysis indicated that lenvatinib may be more effective in patients with macrovascular invasion (including PVTT), yielding higher ORRs approximately 24% and better progression-free survival.
The combination of atezolizumab (anti-PD-L1) and bevacizumab (anti-VEGF) has emerged as a transformative systemic therapy for advanced HCC, establishing a new standard of care after the landmark IMbrave150 trial[70]. In this global phase III randomized controlled study, the atezolizumab–bevacizumab combination demonstrated significantly superior clinical outcomes compared to sorafenib, the prior first-line standard[71]. Specifically, the ORR was 27% with atezolizumab–bevacizumab vs 12% with sorafenib, and median OS improved to 19.2 months compared to 13.4 months for sorafenib. Importantly, the IMbrave150 trial included patients with macrovascular invasion, including PVTT, a cohort historically underrepresented in pivotal studies. Subgroup analysis revealed that patients with PVTT still benefited substantially from atezolizumab–bevacizumab in terms of tumor shrinkage, disease stabilization, and vascular invasion control, indicating that immune-anti-angiogenic synergy is not abrogated by the presence of PVTT. Mechanistically, VEGF blockade may enhance T cell infiltration into tumors and normalize abnormal vasculature, thereby improving the efficacy of PD-L1 inhibition even in poorly perfused thrombotic regions[72]. VEGF inhibition reduces tumor vascularization, promoting better immune cell infiltration and enhancing the effects of PD-L1 inhibitors in thrombotic regions, leading to better downstaging outcomes. Building on this success, other ICI-based regimens are under active investigation in the PVTT population. The HIMALAYA trial, a phase III study, evaluated durvalumab (anti-PD-L1) combined with a single priming dose of tremelimumab (anti-CTLA-4) vs sorafenib[73]. Results demonstrated that the dual immune checkpoint blockade significantly improved OS while maintaining a favorable safety profile. Though PVTT-specific data were not fully stratified, subgroup analyses suggest consistent efficacy across macrovascular invasion strata. Similarly, nivolumab, alone or in combination with ipilimumab, has shown durable responses in patients with advanced HCC, including those with vascular invasion, in phase I/II trials (e.g., CheckMate040)[74,75]. In these studies, patients with PVTT achieved partial responses and stable disease, with manageable immune-related adverse events[76].
While downstaging strategies have demonstrated potential to expand the liver transplant candidate pool, a major limitation remains the absence of a globally accepted set of criteria for evaluating downstaging success. Current protocols reference varied benchmarks such as the Milan, UCSF, and Kyoto criteria, each differing in tumor size, number, and biomarker thresholds[77]. This heterogeneity complicates comparative study designs, transplant decision-making, and registry documentation. Moreover, traditional imaging-based criteria (e.g., RECIST, mRECIST) may not fully capture biologic tumor behavior, necessitating the integration of dynamic biomarkers and functional imaging parameters (e.g., PET, gadoxetate-enhanced magnetic resonance imaging) into downstaging assessment frameworks[78].
As molecular oncology advances, there is growing emphasis on tailoring downstaging regimens to individual tumor biology and host factors. Genomic profiling, including TP53, CTNNB1, and TERT mutations, may predict therapeutic resistance or recurrence risk[79]. Recent studies have demonstrated that TP53 mutations are associated with poor pro
Emerging biomaterials offer new frontiers for localized, sustained drug delivery in downstaging. Bioresponsive hydrogels and nanoscaffolds capable of releasing epigenetic drugs (e.g., decitabine), immune modulators (e.g., PD-1/PD-L1 inhibitors), or chemotherapeutics directly into the tumor microenvironment show promise in preclinical models[82]. For instance, pH- and glutathione-responsive hydrogel systems have demonstrated selective release profiles in the acidic and reductive conditions characteristic of PVTT. Additionally, biomimetic drug carriers (e.g., cell membrane-coated particles) may enable tumor-homing capabilities, thereby enhancing safety and efficacy in patients with compromised liver function[83]. The translation of such systems into clinical trials represents a critical next step. AI and machine learning (ML) can be employed to analyze complex patient data, including clinical, genomic, and imaging information, to predict which downstaging therapies will yield the best outcomes for individual patients. ML algorithms have shown promise in predicting tumor progression, therapeutic response, and even patient survival rates based on pre-treatment characteristics. Besides, real-world data (RWD) from large cohort studies can provide valuable insights into the long-term effectiveness and safety of downstaging therapies across diverse patient populations. Unlike randomized control trials, which often have strict inclusion criteria, RWD includes patients with comorbidities or advanced disease, reflecting the real-world clinical challenges faced by healthcare providers.
Downstaging introduces complex ethical dilemmas regarding organ allocation. Given the organ scarcity and the higher recurrence risk associated with downstaged patients, transparent and objective criteria must be developed to ensure equitable access. Metrics such as duration of radiologic response, biomarker normalization, and absence of vascular invasion on imaging should be validated and integrated into allocation algorithms. Moreover, global transplant networks must collaborate to balance urgency, utility, and equity, ensuring that patients who achieve successful downstaging are not disadvantaged due to procedural variability across institutions. Understanding the differential impacts of these strategies on patient prognosis is crucial for optimizing treatment planning and transplant outcomes. The combination of immunotherapy with locoregional therapies such as TACE or radiation has shown promising results in preclinical studies and early-phase trials. This multimodal approach may enhance the immune response, overcome tumor resistance mechanisms, and significantly improve downstaging efficacy for patients with advanced PVTT.
HCC with PVTT represents one of the most challenging clinical scenarios in liver oncology, historically considered a contraindication to LT. However, recent advances in locoregional, systemic, and combination therapies have significantly expanded the potential for effective downstaging, enabling selected patients with PVTT to become eligible for curative LT. Evidence from clinical trials and retrospective studies supports the efficacy of TACE, TARE, PBT, and novel techniques such as IP-RFA, especially when combined with targeted agents and ICIs. Despite these promising deve
| 1. | Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1584] [Article Influence: 396.0] [Reference Citation Analysis (42)] |
| 2. | Brown ZJ, Tsilimigras DI, Ruff SM, Mohseni A, Kamel IR, Cloyd JM, Pawlik TM. Management of Hepatocellular Carcinoma: A Review. JAMA Surg. 2023;158:410-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 495] [Reference Citation Analysis (1)] |
| 3. | Tan ZB, Zhang J. Recent advances in treatment strategies for hepatocellular carcinoma with portal vein cancer thrombus. Eur Rev Med Pharmacol Sci. 2023;27:8119-8134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Zhu M, Liu Z, Chen S, Luo Z, Tu J, Qiao L, Wu J, Fan W, Peng Z. Sintilimab plus bevacizumab combined with radiotherapy as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A multicenter, single-arm, phase 2 study. Hepatology. 2024;80:807-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 5. | Yuan Y, He W, Yang Z, Qiu J, Huang Z, Shi Y, Lin Z, Zheng Y, Chen M, Lau WY, Li B, Yuan Y. TACE-HAIC combined with targeted therapy and immunotherapy versus TACE alone for hepatocellular carcinoma with portal vein tumour thrombus: a propensity score matching study. Int J Surg. 2023;109:1222-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 6. | Zhou XH, Li JR, Zheng TH, Chen H, Cai C, Ye SL, Gao B, Xue TC. Portal vein tumor thrombosis in hepatocellular carcinoma: molecular mechanism and therapy. Clin Exp Metastasis. 2023;40:5-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 7. | Feng S, Roll GR, Rouhani FJ, Sanchez Fueyo A. The future of liver transplantation. Hepatology. 2024;80:674-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Bhangui P. Liver transplantation and portal vein tumour thrombus: futile enterprise? Curr Opin Organ Transplant. 2022;27:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, Colledan M, Salizzoni M, Romagnoli R, Antonelli B, Vivarelli M, Tisone G, Rossi M, Gruttadauria S, Di Sandro S, De Carlis R, Lucà MG, De Giorgio M, Mirabella S, Belli L, Fagiuoli S, Martini S, Iavarone M, Svegliati Baroni G, Angelico M, Ginanni Corradini S, Volpes R, Mariani L, Regalia E, Flores M, Droz Dit Busset M, Sposito C. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020;21:947-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 237] [Article Influence: 39.5] [Reference Citation Analysis (1)] |
| 10. | Frankul L, Frenette C. Hepatocellular Carcinoma: Downstaging to Liver Transplantation as Curative Therapy. J Clin Transl Hepatol. 2021;9:220-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Kutlu R, Karatoprak S. Radioembolization for Hepatocellular Carcinoma in Downstaging and Bridging for Liver Transplantation. J Gastrointest Cancer. 2020;51:1157-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Mehta N, Yao FY. Liver Transplantation After Downstaging of Hepatocellular Carcinoma With Portal Vein Tumor Thrombus Using Yttrium-90 Radioembolization: Pipe Dream or Reality? Liver Transpl. 2021;27:1706-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Tran NH, Muñoz S, Thompson S, Hallemeier CL, Bruix J. Hepatocellular carcinoma downstaging for liver transplantation in the era of systemic combined therapy with anti-VEGF/TKI and immunotherapy. Hepatology. 2022;76:1203-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 14. | Ersan V, Barut B, Yilmaz S. The Timing of Liver Transplantation Following Downstaging: Wait of Not to Wait? J Gastrointest Cancer. 2020;51:1152-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Hu Y, Qin T, Li S, Zhang T, Xue J. Efficacy and Safety of SBRT Combined With Camrelizumab and Apatinib in HCC Patients With PVTT: Study Protocol of a Randomized Controlled Trial. Front Oncol. 2020;10:1589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Yao Z, Ren Y, Cao M, Li Y, Su X, Hu Z, Han P, Yuen HK, Cheung TT. Comparative analysis of hepatectomy for HCC with PVTT: Insights from a 30-year single-center experience: Hepatectomy for HCC with PVTT. Surg Oncol. 2025;60:102211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Tao ZW, Cheng BQ, Zhou T, Gao YJ. Management of hepatocellular carcinoma patients with portal vein tumor thrombosis: A narrative review. Hepatobiliary Pancreat Dis Int. 2022;21:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Kawaoka T, Aikata H, Takaki S, Hashimoto Y, Katamura Y, Hiramatsu A, Waki K, Takahashi S, Kamada K, Kitamoto M, Nakanishi T, Ishikawa M, Hieda M, Kakizawa H, Tanaka J, Chayama K. Transcatheter chemoembolization for unresectable hepatocellular carcinoma and comparison of five staging systems. Hepatol Res. 2010;40:1082-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Jiao T, Tang H, Zhang W, Hu B, Wan T, Cao Y, Zhang Z, Wang Y, Cao J, Cui M, Lu S. Long-term survival and portal vein patency with novel PVTT surgery approach in advanced HCC patients with Vp3/4 PVTT following combination therapy of TKIs and PD-1 inhibitors. BMC Surg. 2023;23:384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 20. | Wang L, Feng JK, Lu CD, Wu JY, Zhou B, Wang K, Wei XB, Liang C, Zhou HK, Shi J, Guo WX, Lau WY, Yan ML, Cheng SQ. Salvage Surgery for Initially Unresectable HCC With PVTT Converted by Locoregional Treatment Plus Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody. Oncologist. 2024;29:e1041-e1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 21. | Peng SY, Wang XA, Huang CY, Li JT, Hong DF, Wang YF, Xu B. Better surgical treatment method for hepatocellular carcinoma with portal vein tumor thrombus. World J Gastroenterol. 2018;24:4527-4535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (6)] |
| 22. | Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci. 2011;18:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 23. | Lu CD, Wang K, Zhang CZ, Zhou FG, Guo WX, Wu MC, Cheng SQ. Outcomes of intrahepatic cholangiocarcinoma with portal vein tumor thrombus following hepatic resection. J Gastroenterol Hepatol. 2016;31:1330-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Yu J, Zhuang L, Liu P, Liu Z, Ling S, Deng Y, Li J, Yang B, Chen Z, Wang Z, Zang Y, Yang Y, Zheng S, Xu X. Long-term outcomes of deceased donor liver transplantation in hepatocellular carcinoma patients with portal vein tumor thrombus: A multicenter study. Eur J Surg Oncol. 2022;48:121-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Su K, Gu T, Xu K, Wang J, Liao H, Li X, Wen L, Song Y, Zhong J, He B, Liu X, He J, Liu Y, Li Q, Feng X, Chen S, Yang B, Huang W, Jin H, Luo X, Hu T, Chen J, Wu Z, Lu S, Zhang J, Rao M, Xie Y, Wang J, Zhu X, Chen L, Li B, Su S, Yang X, Wang J, Zeng H, Wang P, Yan M, Chen X, He K, Han Y. Gamma knife radiosurgery versus transcatheter arterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a propensity score matching study. Hepatol Int. 2022;16:858-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Zhang L, Zheng T, Wu Y, Wei H, Yang T, Zhu X, Yang J, Chen Y, Wang Y, Qu Y, Chen J, Zhang Y, Jiang H, Song B. Preoperative MRI-based multiparametric model for survival prediction in hepatocellular carcinoma patients with portal vein tumor thrombus following hepatectomy. Eur J Radiol. 2023;165:110895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Pan C, Dai F, Sheng L, Li K, Qiao W, Kang Z, Zhang X. Clinical application of spectral CT perfusion scanning in evaluating the blood supply source of portal vein tumor thrombus in hepatocellular carcinoma. Front Oncol. 2023;13:1348679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 28. | Xiang X, Lau WY, Wu ZY, Zhao C, Ma YL, Xiang BD, Zhu JY, Zhong JH, Li LQ. Transarterial chemoembolization versus best supportive care for patients with hepatocellular carcinoma with portal vein tumor thrombus: a multicenter study. Eur J Surg Oncol. 2019;45:1460-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Zhao XH, Li HL, Guo CY, Yao QJ, Xia WL, Hu HT. Downstaging and Conversation Strategy for Advanced Hepatocellular Carcinoma with Portal Vein Branch Tumor Thrombus: TACE, (125)I Seed Implantation, and RFA for Hepatocellular Carcinoma with Portal Vein Branch Tumor Thrombus. J Hepatocell Carcinoma. 2023;10:231-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 30. | Lin H, Luo B, Peng F, Fang C, Gan Y, Yang X, Li B, Li Y, Su S. The efficacy of transarterial chemoembolization in downstaging unresectable hepatocellular carcinoma to curative therapy: a predicted regression model. Invest New Drugs. 2022;40:1146-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 470] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 32. | Tsurusaki M, Murakami T. Surgical and Locoregional Therapy of HCC: TACE. Liver Cancer. 2015;4:165-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 33. | Fernández-Palanca P, Mauriz JL. Therapeutic approaches in intermediate-stage hepatocellular carcinoma (HCC): a novel insight of adjuvant transarterial chemoembolization (TACE). Chin Clin Oncol. 2023;12:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Huang D, Chen Y, Chen S, Zeng Q, Zhao J, Wu R, Li Y. TACE plus percutaneous chemotherapy-lipiodol treatment of unresectable pedunculated hepatocellular carcinoma. Medicine (Baltimore). 2017;96:e7650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Mo A, Lin B, Chen D. Efficacy of sequential TACE on primary hepatocellular carcinoma with microvascular invasion after radical resection: a systematic review and meta-analysis. World J Surg Oncol. 2023;21:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2009] [Article Influence: 83.7] [Reference Citation Analysis (2)] |
| 37. | Luo J, Xu L, Li L, Zhang J, Zhang M, Xu M. Comparison of treatments for hepatocellular carcinoma patients with portal vein thrombosis: a systematic review and network meta-analysis. Ann Transl Med. 2021;9:1450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | You R, Cheng Y, Diao L, Wang C, Leng B, Yu Z, Xu Q, Yin G. Immune-Targeted Therapy with or without Transarterial Chemoembolization (TACE) for Advanced Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis (PVTT): A Multicenter Retrospective Study. Biomedicines. 2024;12:2124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Lu H, Zheng C, Liang B, Xia X, Fan H. Efficacy and safety analysis of TACE + PEI + lenvatinib compared with TACE + lenvatinib for the treatment of hepatocellular carcinoma with PVTT: a retrospective study. Front Oncol. 2024;14:1280837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 40. | Lu J, Guo JH, Ji JS, Li YL, Lv WF, Zhu HD, Sun JH, Ren WX, Zhang FJ, Wang WD, Shao HB, Cao GS, Li HL, Gao K, Yang P, Yin GW, Zhu GY, Wu FZ, Wang WJ, Lu D, Chen SQ, Min J, Zhao Y, Li R, Lu LG, Lau WY, Teng GJ. Irradiation stent with 125 I plus TACE versus sorafenib plus TACE for hepatocellular carcinoma with major portal vein tumor thrombosis: a multicenter randomized trial. Int J Surg. 2023;109:1188-1198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Zou X, Xu Q, You R, Yin G. Evaluating the Benefits of TACE Combined with Lenvatinib Plus PD-1 Inhibitor for Hepatocellular Carcinoma with Portal Vein Tumor Thrombus. Adv Ther. 2023;40:1686-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Lin Z, Chen D, Hu X, Huang D, Chen Y, Zhang J, Li X, Zou X. Clinical efficacy of HAIC (FOLFOX) combined with lenvatinib plus PD-1 inhibitors vs. TACE combined with lenvatinib plus PD-1 inhibitors in the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombus and arterioportal fistulas. Am J Cancer Res. 2023;13:5455-5465. [PubMed] |
| 43. | Zhao XH, Yuan H, Xia WL, Zhang LL, Li Z, Cao GS, Li HL, Fan WJ, Li HL, Guo CY, Yao QJ, Zhu WB, Hu HT. Prospective study of TACE combined with sorafenib vs TACE combined with (125)I seed implantation in the treatment of hepatocellular carcinoma with portal vein tumor thrombus and arterioportal fistulas. Front Oncol. 2022;12:977462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 44. | Yang B, Jie L, Yang T, Chen M, Gao Y, Zhang T, Zhang Y, Wu H, Liao Z. TACE Plus Lenvatinib Versus TACE Plus Sorafenib for Unresectable Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Prospective Cohort Study. Front Oncol. 2021;11:821599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 45. | Yang SB, Zhang JH, Fu YF, Wang R. TACE with portal vein radioactive seeds for HCC with portal vein tumor thrombus: a meta-analysis. Minim Invasive Ther Allied Technol. 2022;31:856-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Fan W, Zhu B, Chen S, Wu Y, Zhao X, Qiao L, Huang Z, Tang R, Chen J, Lau WY, Chen M, Li J, Kuang M, Peng Z. Survival in Patients With Recurrent Intermediate-Stage Hepatocellular Carcinoma: Sorafenib Plus TACE vs TACE Alone Randomized Clinical Trial. JAMA Oncol. 2024;10:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 47. | Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 543] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 48. | Iñarrairaegui M, Sangro B. Selective Internal Radiation Therapy Approval for Early HCC: What Comes Next? Hepatology. 2021;74:2333-2335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Regnault H, Chalaye J, Galetto-Pregliasco A, Perrin C, Derbel H, Amaddeo G, Mulé S, Lequoy M, Kobeiter H, Reizine E, Itti E, Duvoux C, Laurent A, Leroy V, Sommacale D, Rasolonirina D, Luciani A, Calderaro J, Tacher V, Brustia R. Selective internal radiation therapy for unresectable HCC: The SIRT downstaging study. Hepatol Commun. 2024;8:e0475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 50. | Salem R, Johnson GE, Kim E, Riaz A, Bishay V, Boucher E, Fowers K, Lewandowski R, Padia SA. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology. 2021;74:2342-2352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 383] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 51. | Prince DS, Schlaphoff G, Davison SA, Huo YR, Xiang H, Chan MV, Lee AU, Thailakanathan C, Jebeili H, Rogan C, Al-Omary A, Gupta S, Lockart I, Tiwari N, Clark-Dickson M, Hillhouse JW, Laube R, Chang J, Nguyen V, Danta M, Cheng R, Strasser SI, Zekry A, Levy MT, Chan C, Liu K. Selective internal radiation therapy for hepatocellular carcinoma: A 15-year multicenter Australian cohort study. J Gastroenterol Hepatol. 2022;37:2173-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 52. | de la Torre-Aláez M, Matilla A, Varela M, Iñarrairaegui M, Reig M, Lledó JL, Arenas JI, Lorente S, Testillano M, Márquez L, Da Fonseca L, Argemí J, Gómez-Martin C, Rodriguez-Fraile M, Bilbao JI, Sangro B. Nivolumab after selective internal radiation therapy for the treatment of hepatocellular carcinoma: a phase 2, single-arm study. J Immunother Cancer. 2022;10:e005457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 53. | Benguerfi S, Estrade F, Lescure C, Rolland Y, Palard X, Le Sourd S, Pracht M, Bourien H, Muzellec L, Le Du F, Garin E, Edeline J. Selective internal radiation therapy in older patients with hepatocellular carcinoma: a retrospective analysis. Eur J Gastroenterol Hepatol. 2022;34:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Hur MH, Cho Y, Kim DY, Lee JS, Kim GM, Kim HC, Sinn DH, Hyun D, Lee HA, Seo YS, Lee IJ, Park JW, Kim YJ. Transarterial radioembolization versus tyrosine kinase inhibitor in hepatocellular carcinoma with portal vein thrombosis. Clin Mol Hepatol. 2023;29:763-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Lee S, Song SK, Bae B, Park Y. Comparing efficacies of different treatment regimens in patients with hepatocellular carcinoma accompanied by portal vein tumor thrombus using network meta-analysis. Ann Surg Treat Res. 2022;103:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 56. | Luo F, Li M, Ding J, Zheng S. The Progress in the Treatment of Hepatocellular Carcinoma With Portal Vein Tumor Thrombus. Front Oncol. 2021;11:635731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 57. | Lee CH, Chen AH, Hung SP, Hsieh CE, Tseng JH, Chen PJ, Cheng JY, Chang JT, Chan KM, Lin SM, Lin CC, Chen WT, Chen WY, Huang BS. Proton Beam Therapy in Managing Unresectable Hepatocellular Carcinoma with Bile Duct Invasion. Cancers (Basel). 2022;14:1616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Li Y, Shimizu S, Mizumoto M, Iizumi T, Numajiri H, Makishima H, Li G, Sakurai H. Proton Beam Therapy for Multifocal Hepatocellular Carcinoma (HCC) Showing Complete Response in Pathological Anatomy After Liver Transplantation. Cureus. 2022;14:e25744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 59. | Dionisi F, Scartoni D, Fracchiolla F, Giacomelli I, Siniscalchi B, Goanta L, Cianchetti M, Sanguineti G, Brolese A. Proton therapy in the treatment of hepatocellular carcinoma. Front Oncol. 2022;12:959552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 60. | Iizumi T, Okumura T, Hasegawa N, Ishige K, Fukuda K, Seo E, Makishima H, Niitsu H, Takahashi M, Sekino Y, Takahashi H, Takizawa D, Oshiro Y, Baba K, Murakami M, Saito T, Numajiri H, Mizumoto M, Nakai K, Sakurai H. Proton beam therapy for hepatocellular carcinoma with bile duct invasion. BMC Gastroenterol. 2023;23:267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 61. | Chen CL, Dungca LBP, Yong CC, Chen IC, Cheng YF, Cheng JY, Chen YY. Proton beam therapy for downstaging hepatocellular carcinoma with lobar portal vein tumor thrombosis to living donor liver transplantation. Hepatobiliary Surg Nutr. 2023;12:966-974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 62. | Kobeissi JM, Hilal L, Simone CB 2nd, Lin H, Crane CH, Hajj C. Proton Therapy in the Management of Hepatocellular Carcinoma. Cancers (Basel). 2022;14:2900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Kong YL, Sun JJ, Zhang HY, Xing Y, Wang C, Liu Y, He XJ, Kong LH, Liu CL. Clinical evaluation of percutaneous endovascular radiofrequency ablation for portal vein tumor thrombus: experience in 120 patients. Surg Endosc. 2023;37:1173-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 64. | Mizandari M, Azrumelashvili T, Paksashvili N, Kikodze N, Pantsulaia Ia, Janikashvili N, Chikovani T. Tumor Regression in HCC Patient with Portal Vein Tumor Thrombosis after Intraportal Radiofrequency Thermal Ablation. Case Reports Hepatol. 2016;2016:6843121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 65. | Fujiwara K, Kondo T, Fujimoto K, Yumita S, Ogawa K, Ishino T, Nakagawa M, Iwanaga T, Tsuchiya S, Koroki K, Kanzaki H, Inoue M, Kobayashi K, Kiyono S, Nakamura M, Kanogawa N, Ogasawara S, Nakamoto S, Chiba T, Koizumi J, Kato J, Kato N. Clinical risk factors for portal hypertension-related complications in systemic therapy for hepatocellular carcinoma. J Gastroenterol. 2024;59:515-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 66. | Sun L, Xu X, Meng F, Liu Q, Wang H, Li X, Li G, Chen F. Lenvatinib plus transarterial chemoembolization with or without immune checkpoint inhibitors for unresectable hepatocellular carcinoma: A review. Front Oncol. 2022;12:980214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 67. | Qiu Z, Wang G, Yan H, Qi H, Zuo M, Wang G, Jiang W, Chen Z, Xue J, Lu L, Zhang F, Gao F. TIPS plus sequential systemic therapy of advanced HCC patients with tumour thrombus-related symptomatic portal hypertension. Eur Radiol. 2022;32:6777-6787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 68. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10533] [Article Influence: 585.2] [Reference Citation Analysis (9)] |
| 69. | Yamashita T, Kudo M, Ikeda K, Izumi N, Tateishi R, Ikeda M, Aikata H, Kawaguchi Y, Wada Y, Numata K, Inaba Y, Kuromatsu R, Kobayashi M, Okusaka T, Tamai T, Kitamura C, Saito K, Haruna K, Okita K, Kumada H. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol. 2020;55:113-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (1)] |
| 70. | Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 1180] [Article Influence: 295.0] [Reference Citation Analysis (0)] |
| 71. | Kudo M, Finn RS, Galle PR, Zhu AX, Ducreux M, Cheng AL, Ikeda M, Tsuchiya K, Aoki KI, Jia J, Lencioni R. IMbrave150: Efficacy and Safety of Atezolizumab plus Bevacizumab versus Sorafenib in Patients with Barcelona Clinic Liver Cancer Stage B Unresectable Hepatocellular Carcinoma: An Exploratory Analysis of the Phase III Study. Liver Cancer. 2023;12:238-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 72. | Li S, Li K, Wang K, Yu H, Wang X, Shi M, Liang Z, Yang Z, Hu Y, Li Y, Liu W, Li H, Cheng S, Ye L, Yang Y. Low-dose radiotherapy combined with dual PD-L1 and VEGFA blockade elicits antitumor response in hepatocellular carcinoma mediated by activated intratumoral CD8(+) exhausted-like T cells. Nat Commun. 2023;14:7709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 73. | Sangro B, Chan SL, Kelley RK, Lau G, Kudo M, Sukeepaisarnjaroen W, Yarchoan M, De Toni EN, Furuse J, Kang YK, Galle PR, Rimassa L, Heurgué A, Tam VC, Van Dao T, Thungappa SC, Breder V, Ostapenko Y, Reig M, Makowsky M, Paskow MJ, Gupta C, Kurland JF, Negro A, Abou-Alfa GK; HIMALAYA investigators. Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann Oncol. 2024;35:448-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 195] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 74. | Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 908] [Cited by in RCA: 1051] [Article Influence: 175.2] [Reference Citation Analysis (0)] |
| 75. | Melero I, Yau T, Kang YK, Kim TY, Santoro A, Sangro B, Kudo M, Hou MM, Matilla A, Tovoli F, Knox J, He AR, El-Rayes B, Acosta-Rivera M, Lim HY, Soleymani S, Yao J, Neely J, Tschaika M, Hsu C, El-Khoueiry AB. Nivolumab plus ipilimumab combination therapy in patients with advanced hepatocellular carcinoma previously treated with sorafenib: 5-year results from CheckMate 040. Ann Oncol. 2024;35:537-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 76. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3454] [Article Influence: 383.8] [Reference Citation Analysis (2)] |
| 77. | Shirabe K, Taketomi A, Morita K, Soejima Y, Uchiyama H, Kayashima H, Ninomiya M, Toshima T, Maehara Y. Comparative evaluation of expanded criteria for patients with hepatocellular carcinoma beyond the Milan criteria undergoing living-related donor liver transplantation. Clin Transplant. 2011;25:E491-E498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 78. | Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol. 2020;72:288-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 503] [Cited by in RCA: 498] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 79. | Tümen D, Heumann P, Gülow K, Demirci CN, Cosma LS, Müller M, Kandulski A. Pathogenesis and Current Treatment Strategies of Hepatocellular Carcinoma. Biomedicines. 2022;10:3202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (1)] |
| 80. | Ye Q, Ling S, Zheng S, Xu X. Liquid biopsy in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA. Mol Cancer. 2019;18:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 291] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 81. | Kawka M, Dawidziuk A, Jiao LR, Gall TMH. Artificial intelligence in the detection, characterisation and prediction of hepatocellular carcinoma: a narrative review. Transl Gastroenterol Hepatol. 2022;7:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 82. | Xu X, Liu Y, Liu Y, Yu Y, Yang M, Lu L, Chan L, Liu B. Functional hydrogels for hepatocellular carcinoma: therapy, imaging, and in vitro model. J Nanobiotechnology. 2024;22:381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 83. | Shen P, Jia Y, Zhou W, Zheng W, Wu Y, Qu S, Du S, Wang S, Shi H, Sun J, Han X. A biomimetic liver cancer on-a-chip reveals a critical role of LIPOCALIN-2 in promoting hepatocellular carcinoma progression. Acta Pharm Sin B. 2023;13:4621-4637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/