Published online Nov 27, 2025. doi: 10.4254/wjh.v17.i11.110331
Revised: August 11, 2025
Accepted: October 24, 2025
Published online: November 27, 2025
Processing time: 176 Days and 8 Hours
Liver cirrhosis often leads to significant impairments in functional capacity, which are associated with disease severity and prognosis. Simple, reliable, and low-cost tests are essential to monitor these patients in clinical practice. The 6-min walk test (6MWT) is widely used in other chronic conditions, but its measurement pro
To assess the reliability of the 6MWT in patients with liver cirrhosis (LC).
This cross-sectional study was conducted at a teaching hospital in Juiz de Fora-Minas Gerais. Patients diagnosed with LC at any stage of the disease and under clinical follow-up were included. Patients with grade 2 or higher encephalopathy, respiratory, and/or musculoskeletal diseases or who did not understand the test were excluded. Initially, anamnesis and anthropometric evaluation were per
The mean difference between 6MWT-2 and 6MWT-1 was -18.9 m; the lower limit of the Bland-Altman agreement was -83.5 m, and the upper limit was 45.7 m. One participant was excluded from further analyses for being outside these limits. The typical error of measurement was 18.9 m. The ICC showed excellent reliability between the two tests (ICC = 0.97, 95% confidence internal: 0.90-0.99, P < 0.001). The Student’s one-sample t-value was -2.35 (P = 0.03). The paired t-value was 2.35 (P = 0.03). Pearson’s correlation coefficient between the 6MWT-1 and 6MWT-2 was r = 0.98 (P = 0.0001).
The 6MWT is a test with excellent reliability. It is safe, easy to administer, inexpensive, and can be introduced into routine practice without loss of diagnostic precision in estimating the functional capacity of patients with LC.
Core Tip: This study highlighted the clinical applicability of the 6-min walk test (6MWT) in individuals with liver cirrhosis. By evaluating its intra-rater reliability, the research demonstrated that the 6MWT is a safe, simple, and low-cost tool suitable for routine hepatology assessment. Its excellent reproducibility supports the use of the 6MWT to estimate functional capacity in patients with cirrhosis, facilitating objective monitoring and improving the understanding of disease-related physical limitations.
- Citation: Corrêa FCCR, Nader ISTP, Riolino MRS, Silva E. Intra-rater reliability of the 6-min walk test in people with liver cirrhosis. World J Hepatol 2025; 17(11): 110331
- URL: https://www.wjgnet.com/1948-5182/full/v17/i11/110331.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i11.110331
Metabolic alterations associated with malnutrition are a complication caused by advanced liver disease[1]. The reduction in muscle mass resulting from the pathophysiology of liver cirrhosis (LC) and malnutrition leads to functional changes in these patients, and the combination of these factors negatively affects daily activities and the quality of life of this population[2]. Patients with LC have markedly impaired functional capacity related to worsening physical, cardiorespiratory, muscular, and nutritional performance, affecting their individual and social well-being[3,4]. Among the various complications described above, functional capacity is a factor correlated with mortality in this population[5]. Functional capacity is the maximum cardiac output value to meet the demands of physical activity and daily routines and is associated with oxygen consumption[6].
Functional capacity can be measured through cardiopulmonary exercise testing with oxygen consumption and peak oxygen consumption, which is considered the gold standard[7]. However, functional capacity can also be assessed through some functional tests, such as the 6-min walk test (6MWT)[7]. The 6MWT is a simple, practical test that only requires a 30 m corridor. It does not require exercise equipment, advanced training, or specialized personnel. During the test, the volunteer performs a daily activity, i.e. walking. This test measures the distance a volunteer can quickly walk on a flat, hard surface in 6 min. Through the 6MWT integrated evaluations of the systems involved during exercise (respiratory and cardiovascular systems) can be performed[7].
The 6MWT is a submaximal exercise test that is easy and inexpensive and has good reliability when used to assess functional capacity. It is widely used in patients with chronic heart, pulmonary, neuromuscular, or kidney diseases[8-10]. Additionally, the 6MWT is better tolerated by patients and more representative of daily life activities compared with other walking tests[5]. The reliability and validity of the 6MWT have been demonstrated in various patient populations, such as those with asthma[11], chronic heart failure[12], and post-coronavirus disease 2019 syndrome[13]. Although the 6MWT can provide information about functional limitations in chronic respiratory disease, current knowledge about its measurement properties (e.g., reliability and construct validity) in patients with LC is limited. Therefore, this study aimed to investigate the reliability of the 6MWT in people with LC.
This was a cross-sectional quantitative study of the reliability of the 6MWT conducted between October and November 2023. It was approved by the Research Ethics Committee of the Faculdade de Ciências Médicas e da Saúde de Juiz de Fora (Approval No. 6.183.626).
The non-probabilistic convenience sample consisted of 20 participants diagnosed with LC at any stage of the disease who were under clinical follow-up at the hepatology clinic of the Hospital e Maternidade Therezinha de Jesus, located in Juiz de Fora, Minas Gerais, Brazil. Participants with grade 2 encephalopathy or higher (clinically diagnosed), associated respiratory and/or musculoskeletal pathologies (medically diagnosed prior to the research), participants who did not understand the proposed test, and those who did not complete the evaluations were excluded. Participants were infor
Participants underwent interviews to investigate lifestyle habits, the presence of risk factors or diseases in the cardiorespiratory system, and the medications used. Clinical and laboratory evaluation variables were obtained by reviewing patient records, considering values from the participant’s last consultation before the study procedures. Variables included the etiology of LC, Model for End-stage Liver Disease score, Child-Turcotte-Pugh classification, previous episodes of hepatic decompensation (esophageal varices, fatigue, palmar erythema, jaundice, ascites, and hepatic encephalopathy), and laboratory tests (albumin, total bilirubin, indirect bilirubin, international normalized ratio, gamma-glutamyl transferase, aspartate aminotransferase/serum transaminase oxaloacetic, alanine aminotransferase/serum glutamic-pyruvic transaminase, and creatinine).
Weight and height were measured using a scale with 0.01 kg precision and a stadiometer with 0.5 cm precision (Líder®), respectively. Body mass index was calculated by dividing weight in kilograms by height in meters squared (kg/m2). Arm and waist circumferences were measured using a Sanny® measuring tape with 1 cm precision[14].
Before and immediately after the 6MWT, the following vital signs were measured: Blood pressure; heart rate; and peripheral oxygen saturation using a portable G-Tech Led pulse oximeter[14].
The 6MWT required participants to walk the maximum distance possible in 6 min, walking as fast as possible in a straight, flat, unobstructed 30 m corridor. Participants reported their sensation of dyspnea and leg fatigue, measured using the modified Borg scale (0-10 scale) before and after the test. Blood pressure, heart rate, and peripheral oxygen saturation were recorded before and after the test using the G-Tech Led portable pulse oximeter[14]. All tests were supervised by a trained and qualified technician who walked behind the participant, providing standardized motivational phrases each minute (“That’s right, you’re doing great”, “If you can, try to pick up the pace”, “Don’t slow down, speed up”, and “Almost there”), and informing them of the remaining time in the 6MWT. Participants were allowed to stop (if necessary) during the 6MWT but were instructed to resume walking as soon as possible. The 6MWT was conducted on two consecutive days, with a 24-h interval between tests, and the same evaluator conducted both tests.
Descriptive statistics were used to present the data as appropriate. Continuous variables were tested for normality using the Shapiro-Wilk test. The reliability of the 6MWT was tested using the Bland-Altman analysis, typical error of mea
The demographic, anthropometric, and hemodynamic characteristics of participants with LC are shown in Table 1. The clinical and laboratory data of participants with LC are shown in Table 2. The associated diseases and medications of participants with LC are shown in Table 3.
| Variable | Before 6MWT-1 | After 6MWT-1 | Before 6MWT-2 | After 6MWT-2 |
| Gender (female) | 55% | |||
| Age (years) | 59 ± 11.1 | |||
| Weight (kg) | 81.3 ± 19.7 | |||
| Height (cm) | 143.7 ± 6.21 | |||
| BMI (kg/m2) | 28.5 ± 5.9 | |||
| Right arm circumference (cm) | 30 ± 5.7 | |||
| Left arm circumference (cm) | 29.8 ± 5.7 | |||
| Waist circumference (cm) | 100.4 ± 17.9 | |||
| SBP (mmHg) | 129.0 ± 14.0 | 140.0 ± 16.9 | 123.8 ± 9.2 | 138.8 ± 12.5 |
| DBP (mmHg) | 74.0 ± 7.0 | 86.6 ± 17.0 | 75.3 ± 4.3 | 84.1 ± 12.1 |
| MAP (mmHg) | 92.0 ± 9.0 | 104.4 ± 14.6 | 91.4 ± 5.1 | 102.3 ± 9.2 |
| HR (bpm) | 70 ± 12 | 98 ± 17 | 72 ± 14 | 96 ± 18 |
| SpO2 (%) | 98 ± 2 | 97 ± 2 | 97 ± 3 | 97 ± 2 |
| Borg scale | 0.6 ± 1.5 | 7.6 ± 0.5 | 0.6 ± 1.5 | 7.5 ± 0.5 |
| Variable | Data |
| Etiology | |
| Alcohol | 8 |
| Autoimmune hepatitis | 1 |
| Hepatitis C | 5 |
| Drug-induced | 4 |
| NASH | 2 |
| MELD | 11 ± 3 |
| CTP | |
| A | 19 |
| B | 1 |
| Signs1 | |
| Ascites | 2 |
| Esophageal varices | 4 |
| Fatigue | 6 |
| Palmar erythema | 1 |
| Jaundice | 2 |
| Laboratory data | |
| Albumin (g/dL) | 3.62 ± 0.55 |
| Total bilirubin (mg/dL) | 1.01 ± 0.50 |
| Indirect bilirubin (mg/dL) | 0.44 ± 0.16 |
| INR | 1.23 ± 0.25 |
| GGT (U/L) | 186.67 ± 274.75 |
| AST/SGOT (U/L) | 44.79 ± 32.75 |
| ALT/SGPT (U/L) | 31.32 ± 19.01 |
| Creatinine (mg/dL) | 1.28 ± 1.48 |
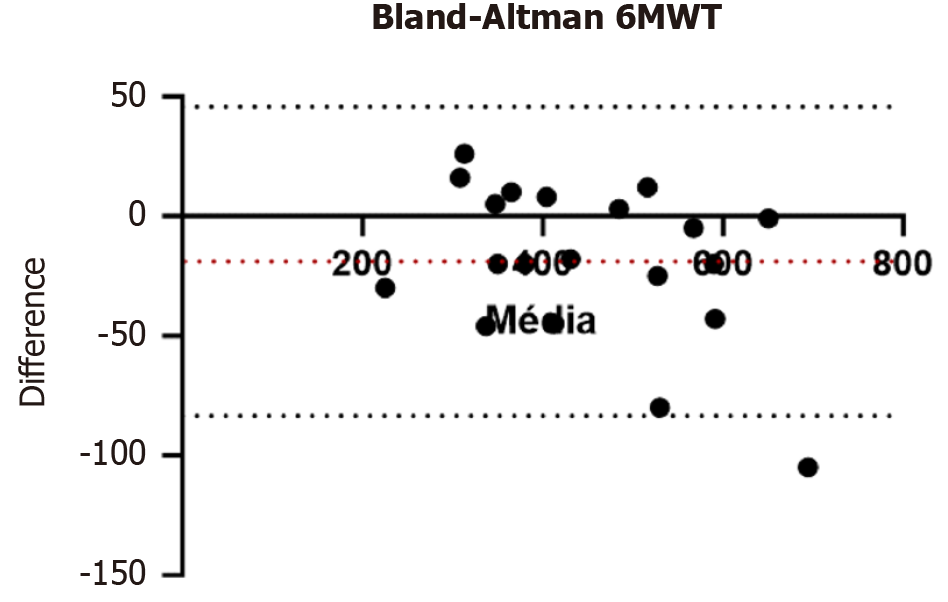
The results of the 6MWT at the first and second time points showed a normal distribution (W = 0.96, P = 0.50 and W = 0.97, P = 0.67, respectively). The differences in the 6MWT between the two timepoints also showed a normal distribution (W = 0.95, P = 0.39). The mean difference between the distances walked in the two 6MWTs was -18.9 m (central dotted line), and the Bland-Altman limits of agreement were -83.5 m to 45.7 m (lower and upper dotted lines, respectively). The Bland-Altman plot identified an outlier (694.5 m, -105 m), which was excluded from the study for being outside the Bland-Altman limits of agreement (Figure 1). The typical measurement error (n = 19), calculated by dividing the standard deviation of the differences between the distances walked in the two 6MWTs (26.7 m) by the square root of 2, was 18.9 m.
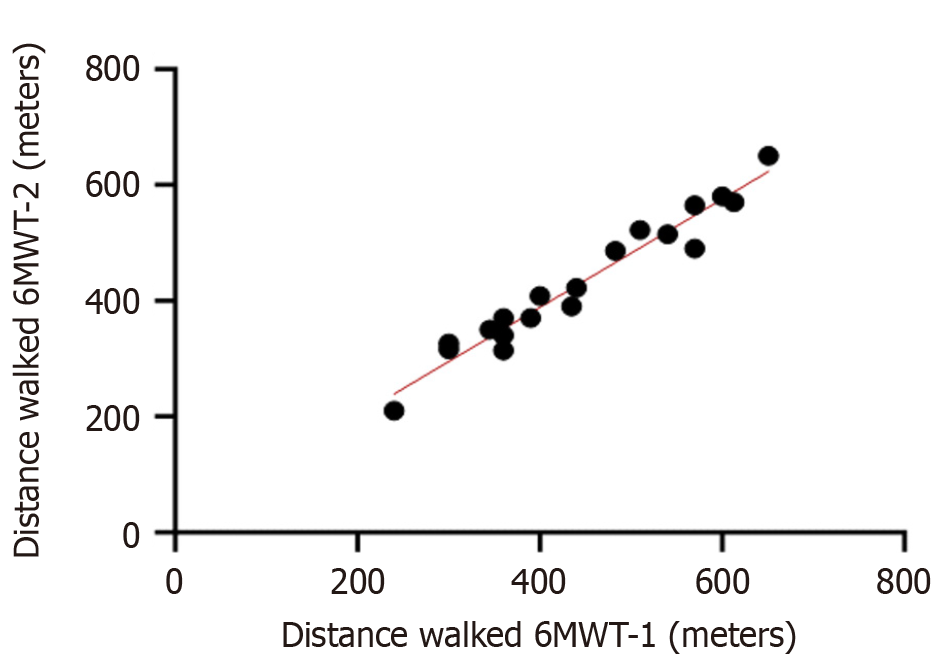
To verify whether the mean difference in the distance walked between the two 6MWTs deviated significantly from zero, a one-sample t-test was applied (Table 4). This analysis aimed to determine if participants maintained consistent performance between test sessions or if there was evidence of variation across trials. Additionally, a paired Student’s t-test was performed to compare the distances walked in the first and second 6MWTs (Table 5). This procedure was used to assess whether any statistically significant difference existed between the two measurements, indicating possible learning or familiarization effects with the test. The Pearson correlation coefficient between the first 6MWT and the second 6MWT was r = 0.98, P < 0.0001 (Figure 2).
| Variable | One-sample t-test compared with the zero constant | |||||||
| Mean (m) | SD (m) | n | SE (m) | Constant | t-value | df | P value | |
| 6MWT-2 - 6MWT-1 (m) | -14.4 | 26.7 | 19 | 6.1 | 0.0 | -2.34714 | 18 | 0.03 |
| Variables | Paired t-test | ||||||||
| Mean (m) | SD (m) | n | Difference (m) | SE (m) | t | df | P value | 95%CI | |
| 6MWT-1 (m) | 445.6 | 120.5 | 19 | 14.4 | 26.7 | 2.347138 | 18 | 0.03 | 1.5-27.2 |
| 6MWT-2 (m) | 431.3 | 115.6 | |||||||
Although the gold standard for measuring functional capacity is the maximal oxygen consumption during ergometric testing on a treadmill or cycle ergometer, the 6MWT is a safe, practical, simple, inexpensive, and easy-to-administer test that provides a global assessment of the cardiovascular and respiratory systems[7]. In addition to these characteristics mentioned in the scientific literature, the results obtained in this study allow us to add another: The 6MWT is reliable for measuring the functional capacity of patients with LC.
Of the 20 participants who performed the 6MWT, only one had a difference of -105 m between the distances walked at the first and second timepoints. This distance was outside the Bland-Altman limits of agreement of -83.5 m to 45.7 m (Figure 1). The mean distances walked in the 6MWT were 445.6 ± 120.5 m at the first timepoint and 431.3 ± 115.6 m at the second. The difference between these two means was small (14.4 m) but significant (P = 0.03; Table 5). The correlation between the distances walked at the first and second timepoints was 0.98 (P = 0.0001) showing that the participants’ rankings in the first and second moments of the 6MWT were very similar (Figure 2). This small but significant difference in means and the strong, significant correlation between distances walked conferred an intraclass correlation coefficient of 0.97 (95% confidence internal: 0.90-0.99; Table 6), characterizing the 6MWT as having excellent reliability for measuring functional capacity in people with LC[15].
The typical measurement error of the 6MWT in people with LC was 18.9 m. Despite this small error, the 6MWT did not show absolute agreement between the distances walked at the first and second timepoints (t = -2.35, P = 0.03; Table 4). A decrease in the distance walked during the 6MWT as a marker of impaired functional capacity is associated with liver dysfunction[3,5]. Additionally, the distance walked and the percentage of predicted distance in the 6MWT can act as independent predictors of mortality, making it an important tool as part of the assessment of the risk of severe complications and death in LC[3,16].
The 6MWT is a test with excellent reliability, safety, ease of administration, and low cost. It can be introduced into routine practice without loss of diagnostic precision in estimating the functional capacity of patients with LC.
| 1. | Gutteling JJ, de Man RA, van der Plas SM, Schalm SW, Busschbach JJ, Darlington AS. Determinants of quality of life in chronic liver patients. Aliment Pharmacol Ther. 2006;23:1629-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Cichoż-Lach H, Michalak A. A Comprehensive Review of Bioelectrical Impedance Analysis and Other Methods in the Assessment of Nutritional Status in Patients with Liver Cirrhosis. Gastroenterol Res Pract. 2017;2017:6765856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Henrique DMN, Mourao-Junior CA, Pace F, Oliveira TMD, Malaguti C, Chebli J. Six-minute walk test predicts future decompensation in patients with compensated liver cirrhosis. Rev Assoc Med Bras (1992). 2022;68:991-994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Galant LH, Forgiarini Junior LA, Dias AS, Marroni CA. Condição funcional, força muscular respiratória e qualidade de vida em pacientes cirróticos. Rev Bras Fisioter. 2012;16:30-34. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Alameri HF, Sanai FM, Al Dukhayil M, Azzam NA, Al-Swat KA, Hersi AS, Abdo AA. Six Minute Walk Test to assess functional capacity in chronic liver disease patients. World J Gastroenterol. 2007;13:3996-4001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | McArdle WD, Katch FI, Katch VL. Fisiologia do Exercício - Nutrição, Energia e Desempenho Humano. Guanabara Koogan, 2024. |
| 7. | ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6981] [Cited by in RCA: 8649] [Article Influence: 360.4] [Reference Citation Analysis (0)] |
| 8. | Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed). 1982;284:1607-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 1120] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 9. | Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119:256-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 657] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 10. | Savci S, Inal-Ince D, Arikan H, Guclu-Gunduz A, Cetisli-Korkmaz N, Armutlu K, Karabudak R. Six-minute walk distance as a measure of functional exercise capacity in multiple sclerosis. Disabil Rehabil. 2005;27:1365-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Meys R, Janssen SMJ, Franssen FME, Vaes AW, Stoffels AAF, van Hees HWH, van den Borst B, Klijn PH, Burtin C, van 't Hul AJ, Spruit MA. Test-retest reliability, construct validity and determinants of 6-minute walk test performance in adult patients with asthma. Pulmonology. 2023;29:486-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 12. | Uszko-Lencer NHMK, Mesquita R, Janssen E, Werter C, Brunner-La Rocca HP, Pitta F, Wouters EFM, Spruit MA. Reliability, construct validity and determinants of 6-minute walk test performance in patients with chronic heart failure. Int J Cardiol. 2017;240:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Cano-de-la-Cuerda R, Jiménez-Antona C, Melián-Ortiz A, Molero-Sánchez A, Gil-de Miguel Á, Lizcano-Álvarez Á, Hernández-Barrera V, Varillas-Delgado D, Laguarta-Val S. Construct Validity and Test-Retest Reliability of a Free Mobile Application to Evaluate Aerobic Capacity and Endurance in Post-COVID-19 Syndrome Patients-A Pilot Study. J Clin Med. 2022;12:131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | The American College of Sports Medicine. Colégio Americano de Medicina Esportiva e Associação Americana de Diabetes - posicionamento official diabetes mellitus e exercício. [cited 21 May 2025]. Available from: https://www.acsm.org/wp-content/uploads/2025/01/Diabetes-Mellitus-e-Exercicio.pdf. |
| 15. | Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9979] [Cited by in RCA: 18057] [Article Influence: 1805.7] [Reference Citation Analysis (1)] |
| 16. | Pimentel CFMG, Amaral ACC, Gonzalez AM, Lai M, Mota DO, Ferraz MLG, Junior WM, Kondo M. Six-minute walking test performance is associated with survival in cirrhotic patients. World J Hepatol. 2021;13:1791-1801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/