©The Author(s) 2025.
World J Hepatol. Oct 27, 2025; 17(10): 110026
Published online Oct 27, 2025. doi: 10.4254/wjh.v17.i10.110026
Published online Oct 27, 2025. doi: 10.4254/wjh.v17.i10.110026
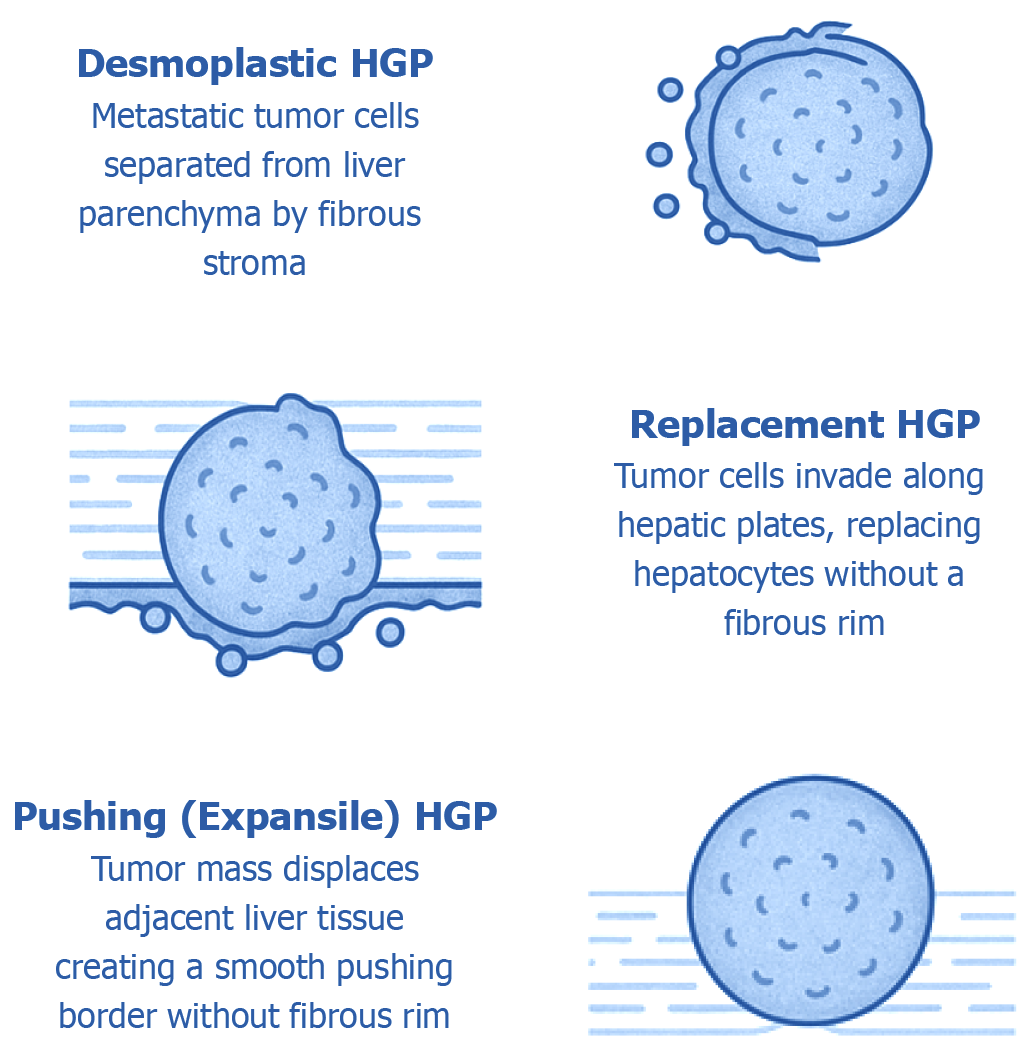
Figure 1 Histological growth patterns at the tumor–liver interface in hepatic metastases.
This diagram depicts the three major histopathological growth patterns (HGPs) that characterize how metastatic tumors interact with the surrounding liver parenchyma, each representing a distinct tumor–microenvironment relationship: (1) Desmoplastic pattern: Defined by the presence of a dense fibrotic stromal band encircling the tumor, effectively separating malignant cells from the adjacent hepatic tissue. This interface is commonly enriched with immune cell infiltrates and newly formed blood vessels, reflecting active stromal remodeling; (2) Replacement pattern: In this growth mode, tumor cells infiltrate the liver parenchyma by replacing hepatocytes along existing sinusoidal structures. The architecture of the liver is largely preserved, and no intervening fibrotic stroma is present. Tumor cells often hijack the native hepatic vasculature to support their growth; and (3) Pushing (expansile) pattern: Characterized by a sharply defined tumor boundary that displaces, rather than infiltrates, the surrounding liver tissue. This interface is smooth and lacks both fibrous stroma and invasion into adjacent hepatocytes. These histological patterns are not only morphologically distinct but also carry significant prognostic and biological implications, influencing metastatic progression, response to therapy, and overall clinical outcomes. HGP: Histopathological growth patterns.
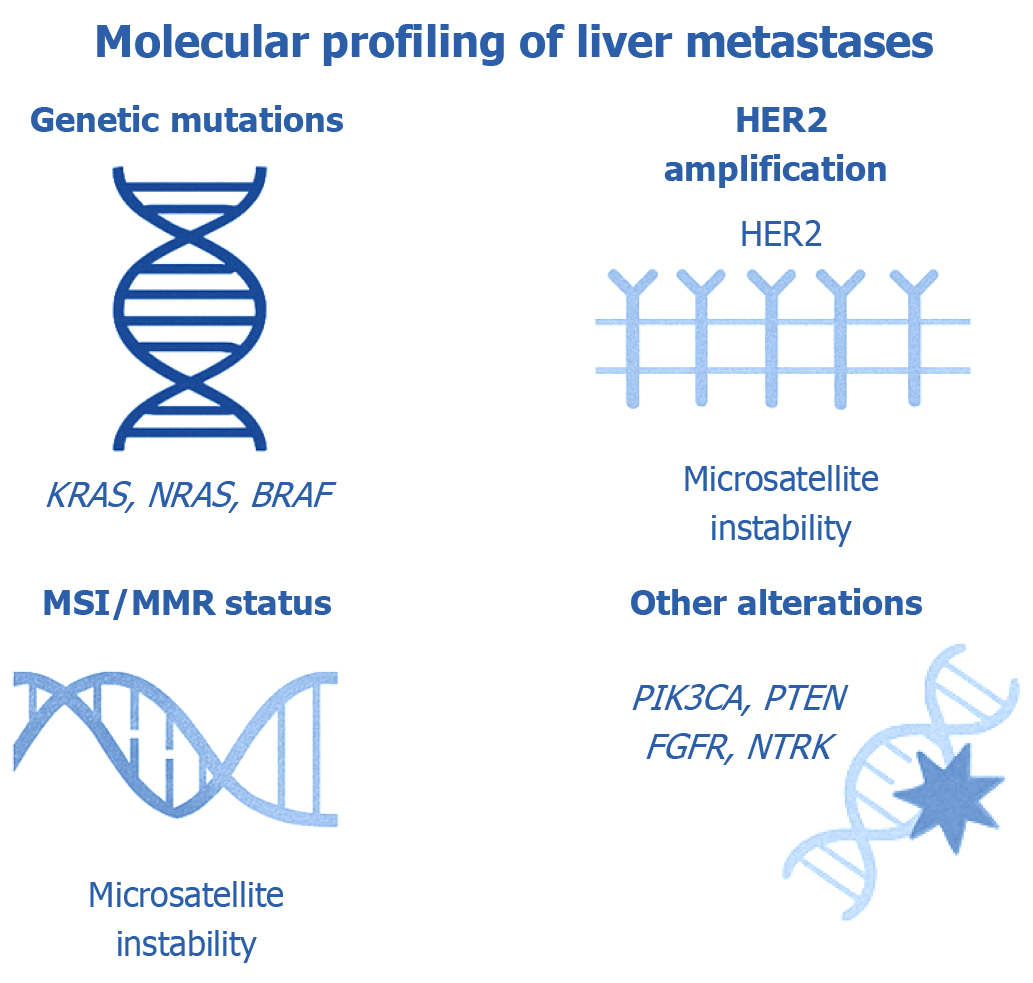
Figure 2 Molecular biomarkers relevant to the evaluation of liver metastases.
This illustration highlights key molecular biomarkers routinely investigated in liver metastases to support both prognostic assessment and therapeutic planning. Mutations in KRAS, NRAS, and BRAF are among the most frequently tested alterations, as they significantly influence eligibility for anti-epidermal growth factor receptor therapies and are associated with disease progression. Human epidermal growth factor receptor-2 (HER2) (ERBB2) amplification is recognized as a targetable alteration in certain subsets of gastrointestinal and breast cancers, offering opportunities for HER2-directed therapies. Analysis of microsatellite instability and mismatch repair status provides important predictive value for identifying patients likely to benefit from immunotherapy. Additional molecular events–such as mutations in PIK3CA (coding for the catalytic subunit of phosphoinositide 3-kinase), phosphatase and tensin homolog (PTEN) loss, alterations in fibroblast growth factor receptor, and neurotrophic tyrosine receptor kinase gene rearrangements-also have clinical relevance and may inform targeted treatment approaches. The integration of molecular profiling into standard clinical workflows enhances precision oncology, enabling more effective and personalized care for patients with metastatic involvement of the liver. FGFR: Fibroblast growth factor receptor; HER2: Human epidermal growth factor receptor-2; NTRK: Neurotrophic tyrosine receptor kinase; PTEN: Phosphatase and tensin homolog.
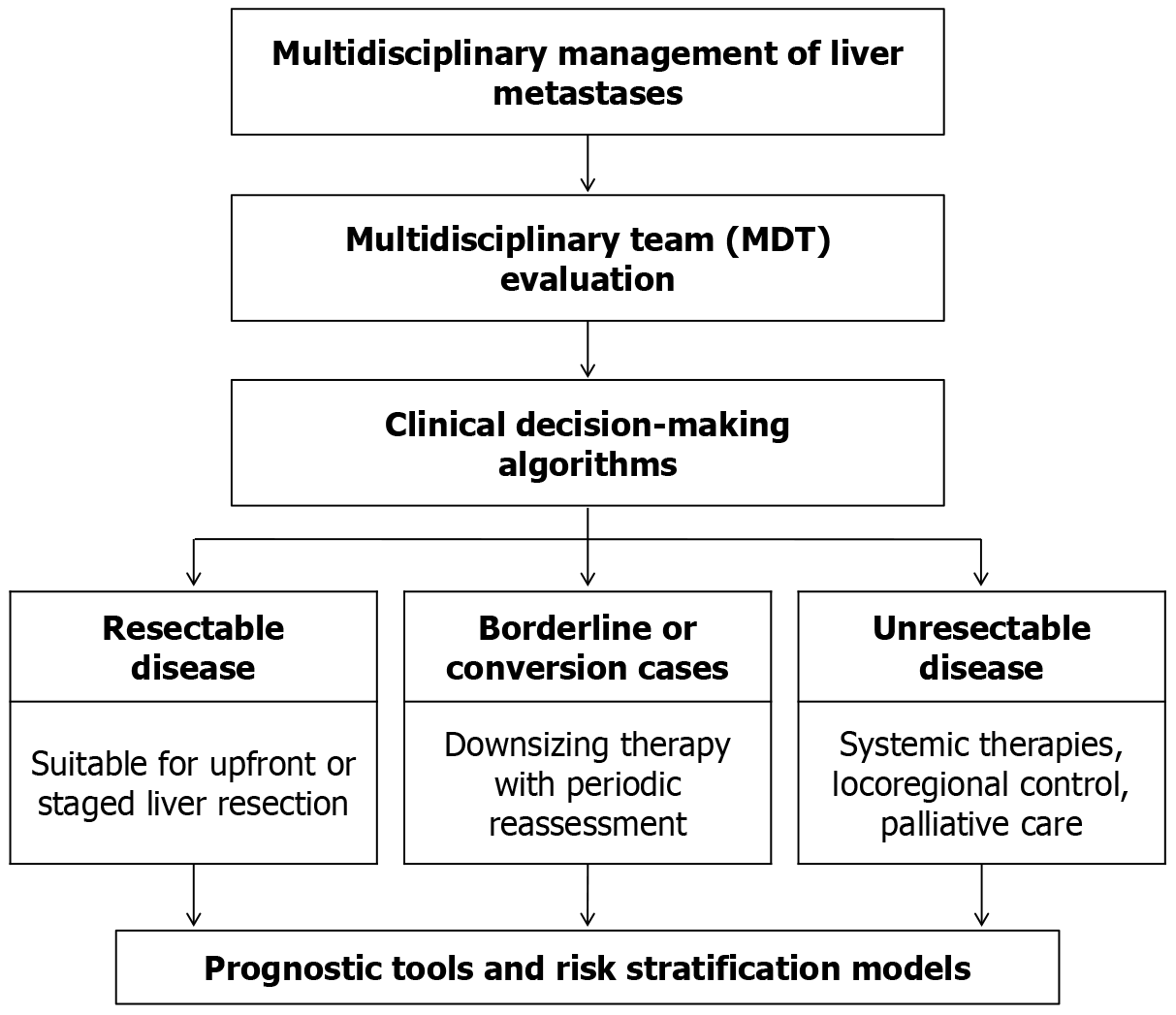
Figure 3 Integrated multidisciplinary strategy for managing liver metastases.
The process initiates with a comprehensive evaluation by a multidisciplinary team, leading to the application of structured clinical algorithms. Based on this assessment, patients are stratified into three clinical categories: (1) Those with respectable disease who may undergo primary or staged liver resection; (2) Borderline or potentially convertible cases, where tumor reduction strategies and ongoing evaluation are implemented; and (3) Patients with unrespectable disease, who are considered for systemic treatments, locoregional interventions, or palliative care. Prognostic assessment tools and risk stratification frameworks are employed throughout to support individualized treatment decisions and predict clinical outcomes. MDT: Multidisciplinary team.
- Citation: Ebrahim NAA, Farghaly TA, El-Sherif AA, Fahmy AM, Othman MO, Tahoun NS, Korany OM, Arafat A, Oreaba R, Soliman SMA. Clinicopathological insights and management of liver metastases: Current advances and future perspectives. World J Hepatol 2025; 17(10): 110026
- URL: https://www.wjgnet.com/1948-5182/full/v17/i10/110026.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i10.110026