Published online Feb 26, 2016. doi: 10.4252/wjsc.v8.i2.22
Peer-review started: September 6, 2015
First decision: November 27, 2015
Revised: December 17, 2015
Accepted: January 8, 2016
Article in press: January 11, 2016
Published online: February 26, 2016
Processing time: 178 Days and 9.2 Hours
Kidney disease is an escalating global health problem, for which the formulation of therapeutic approaches using stem cells has received increasing research attention. The complexity of kidney anatomy and function, which includes the diversity of renal cell types, poses formidable challenges in the identification of methods to generate replacement structures. Recent work using the zebrafish has revealed their high capacity to regenerate the integral working units of the kidney, known as nephrons, following acute injury. Here, we discuss these findings and explore the ways that zebrafish can be further utilized to gain a deeper molecular appreciation of renal stem cell biology, which may uncover important clues for regenerative medicine.
Core tip: The emergence of regeneration-based options for kidney disease has the potential to reduce the growing worldwide health burden of these heterogenous conditions. Research into the mechanisms of renal regeneration in vertebrates like the zebrafish may provide knowledge about fundamental principles that could be useful for cellular reprogramming or endogenous modulation of kidney cells in humans.
- Citation: Drummond BE, Wingert RA. Insights into kidney stem cell development and regeneration using zebrafish. World J Stem Cells 2016; 8(2): 22-31
- URL: https://www.wjgnet.com/1948-0210/full/v8/i2/22.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v8.i2.22
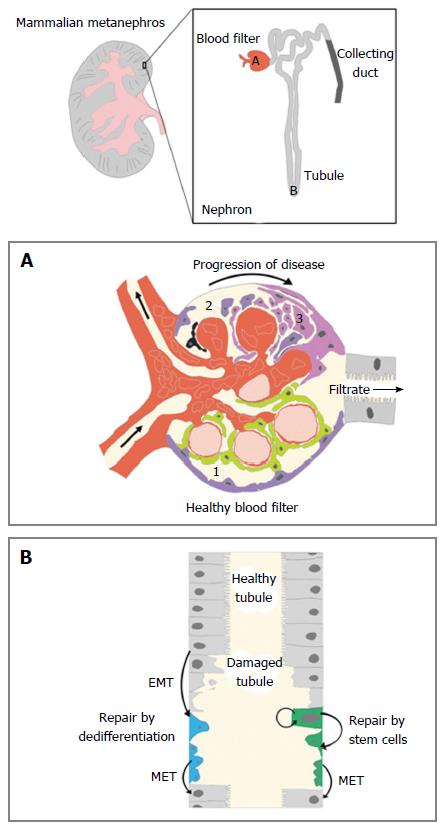
Kidneys are organs that are responsible for excreting metabolic wastes and maintaining fluid homeostasis in the body. The functional subunit of the kidney is the nephron, which is composed of different segments of epithelial cells that can be characterized by their expression profile of unique solute transporters[1,2]. Each human kidney contains approximately 1 million nephrons that are situated in an arborized fashion around a centralized collecting duct system (Figure 1). The nephron segments constitute three major functional regions within each nephron, and these regions act as a blood filter, a tubule that reabsorbs and secretes solutes, and a duct that fine-tunes water and electrolyte levels (Figure 1).
In the event of injury, the mammalian nephron has limited potential for self-repair[3]. After a nephron dies, fibrosis occurs which can cause a chain reaction of additional nephron stress and death, which eventually decreases organ functionality[4-6]. This is the case in acute kidney injury (AKI), chronic kidney disease, and end stage renal disease as the kidney suffers an initial injury, advanced fibrosis, and total loss of function, respectively[7-10]. Finding treatments for these conditions is no easy task for regenerative medicine due to the intricate architecture of the kidney and its rich diversity of cell types[11-14]. This has caused researchers to look for answers to renal regeneration queries in less obvious places[15,16]. One of those places is in the zebrafish, a vertebrate model organism with the uncanny ability to regenerate entire nephrons throughout its lifetime[17].
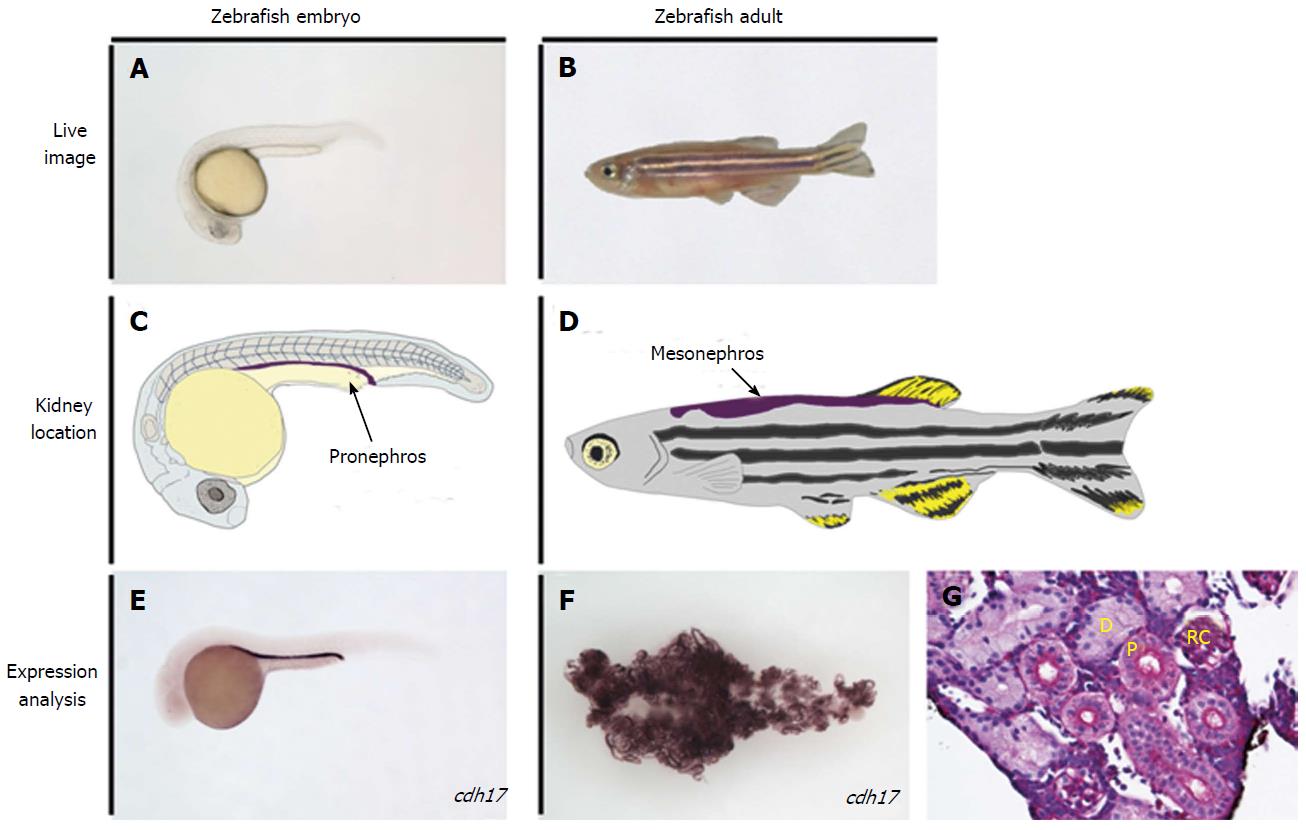
Danio rerio, commonly known as zebrafish, are small, tropical freshwater fish that have been used in biological research since the 1960’s[18,19] (Figure 2). They spawn large clutches of transparent eggs that allow the growing embryo to be studied non-invasively in vivo and phenotypically sorted[20]. They are also logistically favorable for experimental studies as they undergo rapid development ex utero, and the adults can be kept in high densities, which is space and cost effective[20-22]. Most importantly, modern molecular technology is applicable to experiments with zebrafish and extensive genetic mapping has shown they exhibit considerable conservation with other higher vertebrates, including humans[21,22].
It is no surprise that since zebrafish are an excellent animal model they have also been utilized in studying the kidney[23-25]. To date, zebrafish have been used to advance our knowledge of renal development as well as aspects of several kidney disease phenotypes[26-32]. In particular, mutagenesis screens in zebrafish have been used to identify genes that cause kidney malformations, which led to the discovery of ciliogenesis genes that cause renal cyst formation when disrupted[33-38]. Zebrafish have also been useful in chemical screens, such as the application of candidate and market-based pharmaceuticals to embryos, to examine influences to nephrogenesis and identify methods to alter disease states[39-41].
These various avenues of research have significance because of the fundamental similarities in the development and morphology of zebrafish nephrons compared to their mammalian counterparts. The embryonic zebrafish renal progenitor cell field is derived from the intermediate mesoderm, as in mammals[42,43], and gives rise to the first kidney iteration known as the pronephros[34]. The mesenchymal renal progenitors can be identified during ontogeny based on their gene expression profile using whole mount in situ hybridization (WISH) with markers such as lhx1a, osr1, pax2, and pax8[34,44-48]. Analogous to other vertebrates, these precursors in zebrafish are arranged in bilateral fields[34]. These renal progenitors undergo convergence and extension movements during late somitogenesis to form a pronephros composed of two nephrons, which involves a mesenchymal to epithelial transition to form the tubule and duct regions of the nephron[49,50]. The nephrons become fully functional after morphogenesis of the blood filter during the first few days of development[51-57]. Approximately 11-13 d after fertilization, clusters of basophilic cells appear on the pronephros that give rise to additional nephrons that make up a more advanced kidney, the mesonephros[58]. Mesonephros formation continues through the larval stages, with the penultimate organ becoming comprised of several hundred nephrons in total that are centralized into a single adult organ located on the dorsal wall of the abdominal cavity[59]. The mesonephros is the most mature kidney the zebrafish will have, unlike higher vertebrates that include reptiles, birds and mammals who undergo an additional stage of kidney development to make the metanephros, an elaborate kidney form comprised of thousands to millions of nephrons in different species[60]. Another interesting distinction is that the pronephros in higher vertebrates is a vestigial kidney that exists for only a short time during ontogeny[60]. Despite these differences, it has been shown that the segment pattern in the nephrons of the zebrafish pronephros[61] and mesonephros[62-64] is remarkably similar to the segment pattern in nephrons of other vertebrates, supporting the notion that nephron composition is the evolutionary link across species with diverse forms that inhabit a wide range of environments[60]. This has spurred a number of segmentation studies utilizing the zebrafish pronephros[65-70], which further suggest nephrogenesis mechanisms have been conserved during evolution[60]. The zebrafish pronephros and mesonephros are therefore useful for studying developmental and regenerative pathways.
It has been known for the last 25 years that some fish are capable of robust renal regeneration. Two phenomena have been observed: (1) replacement of epithelial populations within existing nephrons; and (2) the formation of new nephrons, also known as neonephrogenesis, during adult growth or in response to catastrophic organ damage[15]. The latter phenomenon was first observed when goldfish that had been treated with the nephrotoxin hexachlorobutadiene formed new nephrons after several weeks[15,71-73]. This was observed by an increase in DNA replication, as indicated by the incorporation of the nucleotide analog bromodeoxyuridine, followed by an increase in the percentage of volume of the kidneys of injured goldfish[15,71-73]. By comparison in mammals, if the basement membrane of the nephron remains intact, renal regeneration occurs by just the first phenomenon listed above, through the formation of a wave of flattened mesenchymal cells that differentiate into the required specialized epithelia[15]. Thus, mammals only exhibit nephron genesis during gestation and sometimes during early post-natal stages (the latter being a trait which varies across species), and never form new nephrons during adulthood[14,15]. However, both of these forms of renal regeneration have now been reported in a variety of other fish that include the zebrafish, catfish, trout, medaka and tilapia[15,62,63,74-76].
Amongst these possible animal models, the advancement of sophisticated genetic tools and methodologies in the zebrafish offers a particularly appealing avenue to delineate the mechanisms of renal regeneration events[77,78]. Indeed, a bevy of recent studies have provided an initial molecular framework to track and decipher such phenomena[78,79]. For example, in zebrafish new nephrons first appear as clusters of renal progenitors near existing mesonephros tubules[62,63]. These clusters express early markers of renal development such as lhx1a, pax2a, wt1b and pax8, which suggests that developmental pathways mirror pathways of regeneration[62,63,79]. These clusters then expand into S-shaped bodies that mature into nephrons that fuse with preexisting nephrons[62,63,79]. Understanding the molecular attributes of these cells is an attractive option to identify cell features that could allow for similar events to be induced in humans. In the following sections we further explore the utility of zebrafish for regeneration research in different renal cell types.
The first segment in the nephron is the blood filter, a structure also known as the renal corpuscle[77] (Figure 1A). The main components of the corpuscle are the glomerulus, a ball of capillaries that contain circulating blood, is surrounded by a space where the filtrate is collected, known as Bowman’s capsule, that enables liquid to be passed into the proximal tubule of the nephron[77] (Figure 1A). The integrity of the blood filter remains relatively constant in humans despite clinical observations that cells from this structure can be detected in the urine of healthy individuals[80]. Though long-term homeostasis has been largely attributed to hypertrophy of resident blood filter cells[80-82], there has also been a series of investigations into the regenerative nature of these cell types.
One of the cell types that make up the glomerulus are podocytes. Podocytes are crucial for the effectiveness of the glomerulus because they form an intricate sieve network with specialized extensions known as foot processes that wrap around the capillary bundle[77]. Low numbers of podocyte cells are a common cause and effect of glomerular diseases[80,81]. Whether podocytes can be replaced in the mature mammalian metanephros has received substantial research attention. One theory was that another glomerular cell type called parietal epithelial cells (PECs) act as a progenitor to replace lost podocytes[83-86]. This was suggested after observations in studies that PECs were positive for stem-cell markers CD24 and CD133, showed the capacity for self-renewal and directed differentiation in culture, contributed to damaged tubules and restored kidney function in SCID mice, and contributed to damaged glomeruli, tubules and restored kidney function in mice with adriamycin nephropathy[83-85]. It was also posed that PECs, acting as progenitors that over-proliferate after injury, could be the cause of glomerular lesions and scars[86]. The first theory lost traction after a subsequent study showed that adult mice with genetically labeled PECs did not produce genetically labeled podocytes in the context of injury[87]. These studies highlight that there is still much to be learned about the mechanisms behind glomerular disease and injury in mammals.
Contrary to the mammalian system, there are multiple studies that have shown that zebrafish are able to generate new podocytes. Zhou et al[88] created a conditional podocyte ablation transgenic line to observe podocyte regeneration. This line was created by inserting a bacterial nitroreductase (NTR) gene into the genome, which encodes a product that is converted into a cytotoxin when the drug metronidazole is added to the system. To induce podocyte-specific ablation, the NTR was subcloned downstream of the promoter for the gene podocin, and to visualize NTR+ podocytes, they were also engineered to simultaneously co-express the red fluorescent protein mCherry. This made the cytotoxic damage podocyte specific and allowed the injury to be viewed efficiently. A novel method of measuring proteinuria was also utilized in this study by marking vitamin D-binding protein (a similar protein to mammalian albumin which is how proteinuria is typically measured) with green fluorescent protein. After metronidazole treatment, there was evident edema, apoptosis and proteinuria in both the embryonic pronephros and adult mesonephros. In addition, the expression level of wt1b, which marks the developing nephron[62], increased and extended into the Bowman’s capsule. Huang et al[89] (2013) independently created a similar NTR-podocyte line and also reported observations of podocyte replacement after injury as seen by a recovery in the expression levels of podocyte markers podocin and nephrin. Collectively, this indicates that there could be podocyte-specific repair processes that occur in zebrafish. This gives hope to the notion that parallel processes might exist in the adult mammalian kidney, or could be exacerbated with the appropriate stimuli, though such possibilities have yet to be discovered.
The most extensive portion of the nephron is the tubule, which is composed of numerous discrete epithelial segments[1,2]. After the blood has been filtered by the glomerulus, the resulting filtrate is passed through the segments of the tubule[1,2]. These segments are uniquely identified by their expression profiles of distinct solute transporters that function to reabsorb and/or secrete molecules during urine production[2]. The epithelial cells of the tubule are sensitive to chemical damage, particularly in the proximal segments that perform bulk reabsorption of amino acids and sugars[78] (Figure 1B). Administering the antibiotic gentamicin in zebrafish embryos creates tubule damage that mimics AKI in humans, but leads to embryonic lethality[90]. In analogous studies performed in the zebrafish mesonephros, gentamicin injury was shown to cause a significant drop in expression of the proximal tubule marker slc20a1a in existing nephrons and a decrease in kidney functionality as indicated by an inability to uptake dextran[63,79]. However, the expression of slc20a1a is returned to near normal levels after 15 d post injection[63,79], while the reabsorptive functionality is broadly restored after approximately 3 wk[79]. Since these reports, laser ablation is another method of targeted injury that has also been used in zebrafish larvae to study renal regeneration. The laser ablation methodology provides the benefit of injuring a small region within the nephron tubule, which can be non-invasively imaged in real time to capture regenerative events[91-93].
While it is clear that regeneration occurs in the pre-existing nephron tubules of both the zebrafish mesonephros and the pronephros, the source of these cells has not been resolved (Figure 1B). Based on parallel studies in mammals, it is likely that intratubular cells replenish damaged regions, however the cellular mechanism remains controversial[14]. The two competing models are currently the dedifferentiation model and the stem/progenitor model[14]. In the dedifferentiation model, neighboring cells that are left intact in a damaged tubule undergo an epithelial to mesenchymal transformation to become immature, replicating cells that can replace the lost cells. After a sufficient number of cells have been produced, the mesenchymal cells then convert into differentiated epithelial cells to reconstitute the nephron. The alternative theory is that a residential, self-sustaining group of stem cells is responsible for replacing damaged epithelial cells.
In contrast to the controversy about the mechanics of nephron epithelial regeneration, the process of neonephrogenesis has been attributed to renal progenitors within the adult kidney organ[62,63]. A study by Zhou et al[62] first characterized the process of neonephrogenesis after gentamicin-induced AKI in adult zebrafish. They found that wt1b expression increased as soon as 48 h after injury followed by the appearance of wt1b aggregates within 4 d that gave rise to new nephrons. In a subsequent study, Diep et al[63] provided evidence for the extensive proliferative potential of these amazing renal progenitors in zebrafish. A transplantation assay was conducted by moving whole-kidney marrow cells expressing either red or green fluorescent protein into fish that had been injected with gentamicin. They found that 100% of recipient fish had grown fluorescent, donor-derived nephrons within three weeks after transplantation. Evidence that these nephrons were functional came from dextran uptake assays in which the nephrons were shown to be capable of absorbing sugar moieties circulating in the bloodstream. Interestingly, when an equal number of red fluorescent and green fluorescent donor cells were transplanted into an injured fish, mosaic nephrons containing both cell types were found which demonstrated that more than one nephron progenitor can contribute to the formation of a single nephron. When cells from these donor-derived nephrons were transplanted into a second and third fish, the progenitor cells showed continued proliferative potential.
Another major aim of the Diep et al[63] study involved the characterization of cellular aggregates that give rise to nephron tubules. In injected fish, there were multiple types of aggregates that formed from three to four fluorescent donor cells. Some were groups of 10-30 cells that were positive for the renal progenitor marker lhx1a and others were larger aggregates that expressed wt1b. These aggregates were positive for the expression of early acting renal markers and negative for mature nephron markers. Furthermore, only cells expressing the lhx1a reporter, when transplanted in aggregate form, had the potential to form new nephrons. These cells were found to express six2a and wt1a, similar to mammalian cells that possess nephrogenic potential during metanephros development. Overall, these and other studies suggested that wt1b is a marker for developing nephrons, and that lhx1a is a marker for renal progenitor cells[62,63]. Future research is needed to uncover the origins and developmental regulation of these potent renal progenitors in the zebrafish.
The knowledge that has been gained about renal regeneration in zebrafish has been shown to be useful and translatable to experiments using the mammalian model. A study by de Groh et al[40] used zebrafish embryos to screen thousands of commercially available pharmaceuticals to see how they impacted the renal progenitor field. The expression of markers pax2a, pax8, and lhx1a were examined in drug treated 14-h-old embryos using WISH and Tg:(lhx1a:GFP) transgenic embryos. It was found that the histone deactylase inhibitor PTBA increases the number of cells expressing lhx1a, pax2, and pax8. Although there was significant edema and curvature in drug treated embryos at 48 hpf, the expression of cdh17 appeared to be thicker, which marks the entire nephron. Furthermore, the expression of segment markers wt1a, slc4a4, slc12a1 were also increased. This indicated that increasing the number of renal progenitors could lead to an increase in the number of differentiated cells in the nephron.
A follow-up to this study by Cosentino et al[41] administered PTBA analog m4PTB to adult zebrafish after gentamicin-induced injury. Using the nucleotide 5-ethynyl-2’deoxyuridine to assess the proliferation index, the authors found that proliferation was increased in chemically injured fish treated with m4PTB compared to fish that had been chemically injured alone. Treatment with m4PTB also caused increased survival after gentamicin injections. m4PTB was also administered to mice with induced moderate ischemia-reperfusion (IR) induced AKI. This compound improved renal function and recovery in IR-AKI mice as indicated by a decrease in levels of serum creatine and markers for renal fibrosis. There was also a decrease in the number of cells in arrest, which indicates an activation of cells participating in repair.
A more recent study by Chiba et al[94] is another example of how chemical genetic studies in the zebrafish kidney are translatable to the mammalian model. In this study, the group first used transgenic retinoic reporter zebrafish and mice to establish that retinoic acid (RA) signaling increases in response to kidney injury as visualized by an elevation of fluorescently labeled RA expressing cells compared to controls. They found that this was most likely due to an increase of RA-synthesizing macrophages that are recruited to the tubules in the first few days after injury. Inhibiting RA signaling before and after injury increased the extremity of injury and reduced the effectiveness of repair mechanisms. The activation of RA signaling in mice using the compound all-trans-RA reduced levels of serum creatinine, M1 macrophage expression and fibrosis. These studies are proof-of-concept that compounds that improve renal regeneration and repair in zebrafish could also be useful to mammals.
Kidney diseases continue to be a considerable medical problem in our society with few avenues of treatment. Regenerative medicine aims to expand these avenues by directing the human body to repair itself. As zebrafish are already masters at regeneration in the kidney, understanding the mechanisms behind these processes may hold the key to understanding it in humans.
Moving forward, there are several essential areas of research. One major aspect of zebrafish kidney regeneration to study is the developmental origin of the renal progenitors that drive neonephrogenesis. Several reports have now provided substantial support to the notion that there is a residential pool of renal progenitors that exists in the mature kidney, which are likely to be self-renewing[62,63,79], but their developmental origins are obscure. Genetic fate mapping is necessary to appreciate where these cells arise and to help understand their self-renewal capacity[95]. Future studies that ascertain the molecular features of these cells may be needed to support genetic fate mapping with existing markers, such as lhx1a.
Studies are also needed to understand the source(s) of epithelial regeneration in damaged nephrons. In the nephrology field, this has only been addressed in mammals, which has indicated that intratubular populations are vital. As discussed in this review, whether the intratubular source is a resident stem/progenitor cell or is a dedifferentiated cell will be important to establish[96]. To further complicate this debate, the complexity of the nephron may allow for a scenario where both sources of regeneration are possible[97].
In summary, future studies need to better characterize the cellular basis of nephron repair and nephron neogenesis, and the zebrafish model provides a genetically tractable model to pursue these topics. Recently, a transgenic zebrafish line has been created by Wang et al[98] (2014) that utilizes green fluorescent protein to specifically label cells of the proximal tubule. This is an important advance for pursuing research about nephrogenesis in this segment during development and regeneration. Additional tools and transgenic lines such as this are needed to advance our ability to visualize renal regeneration, as they could be combined with chemical genetics to identify signals that modulate regeneration and the development of renal progenitors during embryogenesis[99]. Processes that are happening in regeneration that we cannot “see” must also be evaluated, such as renal cell signaling, gene activation, and cell fate decisions. Another mystery of kidney health that needs to be investigated in all vertebrates is why some subjects advance from initial injury to chronic conditions and even death while others fully recover. Factors that limit regenerative potential in humans may include genetic background, ethnicity, overall health, and age. While ongoing research in the reprogramming field shows promise for creating alternative sources of replacement kidney cells[100], clues from the zebrafish show promise for defining additional factors to advance renal regenerative therapies in the years to come.
We thank the staffs of the University of Notre Dame Department of Biological Sciences, Center for Zebrafish Research and Center for Stem Cells and Regenerative Medicine for their administrative support. The authors thank K.K. McCampbell for the images in Figure 2E-G, and all the members of our research lab for helpful discussions.
| 1. | Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 451] [Article Influence: 22.6] [Reference Citation Analysis (1)] |
| 2. | Wingert RA, Davidson AJ. The zebrafish pronephros: a model to study nephron segmentation. Kidney Int. 2008;73:1120-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Lazzeri E, Romagnani P, Lasagni L. Stem cell therapy for kidney disease. Expert Opin Biol Ther. 2015;15:1455-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Guo JK, Cantley LG. Cellular maintenance and repair of the kidney. Annu Rev Physiol. 2010;72:357-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210-4221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1541] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 6. | Berger K, Moeller MJ. Mechanisms of epithelial repair and regeneration after acute kidney injury. Semin Nephrol. 2014;34:394-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005;365:331-340. [PubMed] |
| 8. | Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 612] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 9. | Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1078-F1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 408] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 10. | Kline J, Rachoin JS. Acute kidney injury and chronic kidney disease: it’s a two-way street. Ren Fail. 2013;35:452-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Little MH. Regrow or repair: potential regenerative therapies for the kidney. J Am Soc Nephrol. 2006;17:2390-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Sagrinati C, Ronconi E, Lazzeri E, Lasagni L, Romagnani P. Stem-cell approaches for kidney repair: choosing the right cells. Trends Mol Med. 2008;14:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Pleniceanu O, Harari-Steinberg O, Dekel B. Concise review: Kidney stem/progenitor cells: differentiate, sort out, or reprogram? Stem Cells. 2010;28:1649-1660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | McCampbell KK, Wingert RA. Renal stem cells: fact or science fiction? Biochem J. 2012;444:153-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Reimschuessel R. A fish model of renal regeneration and development. ILAR J. 2001;42:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Sanz AB, Sanchez-Niño MD, Martín-Cleary C, Ortiz A, Ramos AM. Progress in the development of animal models of acute kidney injury and its impact on drug discovery. Expert Opin Drug Discov. 2013;8:879-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | McCampbell KK, Wingert RA. New tides: using zebrafish to study renal regeneration. Transl Res. 2014;163:109-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1552] [Cited by in RCA: 1578] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 19. | Phillips JB, Westerfield M. Zebrafish models in translational research: tipping the scales toward advancements in human health. Dis Model Mech. 2014;7:739-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8500] [Cited by in RCA: 9337] [Article Influence: 301.2] [Reference Citation Analysis (0)] |
| 21. | Lawson ND, Wolfe SA. Forward and reverse genetic approaches for the analysis of vertebrate development in the zebrafish. Dev Cell. 2011;21:48-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2735] [Cited by in RCA: 3621] [Article Influence: 278.5] [Reference Citation Analysis (0)] |
| 23. | Ebarasi L, Oddsson A, Hultenby K, Betsholtz C, Tryggvason K. Zebrafish: a model system for the study of vertebrate renal development, function, and pathophysiology. Curr Opin Nephrol Hypertens. 2011;20:416-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Gerlach GF, Wingert RA. Kidney organogenesis in the zebrafish: insights into vertebrate nephrogenesis and regeneration. Wiley Interdiscip Rev Dev Biol. 2013;2:559-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Drummond IA. Kidney development and disease in the zebrafish. J Am Soc Nephrol. 2005;16:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Swanhart LM, Cosentino CC, Diep CQ, Davidson AJ, de Caestecker M, Hukriede NA. Zebrafish kidney development: basic science to translational research. Birth Defects Res C Embryo Today. 2011;93:141-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Hentschel DM, Mengel M, Boehme L, Liebsch F, Albertin C, Bonventre JV, Haller H, Schiffer M. Rapid screening of glomerular slit diaphragm integrity in larval zebrafish. Am J Physiol Renal Physiol. 2007;293:F1746-F1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Cianciolo Cosentino C, Roman BL, Drummond IA, Hukriede NA. Intravenous microinjections of zebrafish larvae to study acute kidney injury. J Vis Exp. 2010;42:pii: 2079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Rider SA, Tucker CS, del-Pozo J, Rose KN, MacRae CA, Bailey MA, Mullins JJ. Techniques for the in vivo assessment of cardio-renal function in zebrafish (Danio rerio) larvae. J Physiol. 2012;590:1803-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Hanke N, Staggs L, Schroder P, Litteral J, Fleig S, Kaufeld J, Pauli C, Haller H, Schiffer M. “Zebrafishing” for novel genes relevant to the glomerular filtration barrier. Biomed Res Int. 2013;2013:658270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Christou-Savina S, Beales PL, Osborn DP. Evaluation of zebrafish kidney function using a fluorescent clearance assay. J Vis Exp. 2015;96:e52540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Kamei CN, Liu Y, Drummond IA. Kidney Regeneration in Adult Zebrafish by Gentamicin Induced Injury. J Vis Exp. 2015;e51912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Chen JN, Haffter P, Odenthal J, Vogelsang E, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development. 1996;123:293-302. [PubMed] |
| 34. | Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, Neuhauss SC, Stemple DL, Zwartkruis F, Rangini Z. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development. 1998;125:4655-4667. [PubMed] |
| 35. | Golling G, Amsterdam A, Sun Z, Antonelli M, Maldonado E, Chen W, Burgess S, Haldi M, Artzt K, Farrington S. Insertional mutagenesis in zebrafish rapidly identifies genes essential for early vertebrate development. Nat Genet. 2002;31:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 425] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 36. | Sun Z, Hopkins N. vhnf1, the MODY5 and familial GCKD-associated gene, regulates regional specification of the zebrafish gut, pronephros, and hindbrain. Genes Dev. 2001;15:3217-3229. [PubMed] |
| 37. | Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development. 2004;131:4085-4093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 426] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 38. | Zhao C, Malicki J. Genetic defects of pronephric cilia in zebrafish. Mech Dev. 2007;124:605-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Poureetezadi SJ, Wingert RA. Congenital and Acute Kidney Disease: Translational Research Insights from Zebrafish Chemical Genetics. Gen Med (Los Angel). 2013;1:112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | de Groh ED, Swanhart LM, Cosentino CC, Jackson RL, Dai W, Kitchens CA, Day BW, Smithgall TE, Hukriede NA. Inhibition of histone deacetylase expands the renal progenitor cell population. J Am Soc Nephrol. 2010;21:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Cianciolo Cosentino C, Skrypnyk NI, Brilli LL, Chiba T, Novitskaya T, Woods C, West J, Korotchenko VN, McDermott L, Day BW. Histone deacetylase inhibitor enhances recovery after AKI. J Am Soc Nephrol. 2013;24:943-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 42. | Saxen L. Organogenesis of the kidney. Cambridge University Press: Cambridge, UK 1987; . |
| 43. | Saxén L, Sariola H. Early organogenesis of the kidney. Pediatr Nephrol. 1987;1:385-392. [PubMed] |
| 44. | Krauss S, Johansen T, Korzh V, Fjose A. Expression of the zebrafish paired box gene pax[zf-b] during early neurogenesis. Development. 1991;113:1193-1206. [PubMed] |
| 45. | Picker A, Scholpp S, Böhli H, Takeda H, Brand M. A novel positive transcriptional feedback loop in midbrain-hindbrain boundary development is revealed through analysis of the zebrafish pax2.1 promoter in transgenic lines. Development. 2002;129:3227-3239. [PubMed] |
| 46. | Toyama R, Dawid IB. lim6, a novel LIM homeobox gene in the zebrafish: comparison of its expression pattern with lim1. Dev Dyn. 1997;209:406-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 47. | Swanhart LM, Takahashi N, Jackson RL, Gibson GA, Watkins SC, Dawid IB, Hukriede NA. Characterization of an lhx1a transgenic reporter in zebrafish. Int J Dev Biol. 2010;54:731-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | Pfeffer PL, Gerster T, Lun K, Brand M, Busslinger M. Characterization of three novel members of the zebrafish Pax2/5/8 family: dependency of Pax5 and Pax8 expression on the Pax2.1 (noi) function. Development. 1998;125:3063-3074. [PubMed] |
| 49. | Gerlach GF, Wingert RA. Zebrafish pronephros tubulogenesis and epithelial identity maintenance are reliant on the polarity proteins Prkc iota and zeta. Dev Biol. 2014;396:183-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | McKee R, Gerlach GF, Jou J, Cheng CN, Wingert RA. Temporal and spatial expression of tight junction genes during zebrafish pronephros development. Gene Expr Patterns. 2014;16:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 51. | Majumdar A, Drummond IA. Podocyte differentiation in the absence of endothelial cells as revealed in the zebrafish avascular mutant, cloche. Dev Genet. 1999;24:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 52. | Majumdar A, Lun K, Brand M, Drummond IA. Zebrafish no isthmus reveals a role for pax2.1 in tubule differentiation and patterning events in the pronephric primordia. Development. 2000;127:2089-2098. [PubMed] |
| 53. | Majumdar A, Drummond IA. The zebrafish floating head mutant demonstrates podocytes play an important role in directing glomerular differentiation. Dev Biol. 2000;222:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Serluca FC, Fishman MC. Pre-pattern in the pronephric kidney field of zebrafish. Development. 2001;128:2233-2241. [PubMed] |
| 55. | Serluca FC, Drummond IA, Fishman MC. Endothelial signaling in kidney morphogenesis: a role for hemodynamic forces. Curr Biol. 2002;12:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Hsu HJ, Lin G, Chung BC. Parallel early development of zebrafish interrenal glands and pronephros: differential control by wt1 and ff1b. Development. 2003;130:2107-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 57. | Kramer-Zucker AG, Wiessner S, Jensen AM, Drummond IA. Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev Biol. 2005;285:316-329. [PubMed] |
| 58. | Diep CQ, Peng Z, Ukah TK, Kelly PM, Daigle RV, Davidson AJ. Development of the zebrafish mesonephros. Genesis. 2015;53:257-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | Gerlach GF, Schrader LN, Wingert RA. Dissection of the adult zebrafish kidney. J Vis Exp. 2011;pii: 2839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Romagnani P, Lasagni L, Remuzzi G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat Rev Nephrol. 2013;9:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 61. | Wingert RA, Selleck R, Yu J, Song HD, Chen Z, Song A, Zhou Y, Thisse B, Thisse C, McMahon AP. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 2007;3:1922-1938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 274] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 62. | Zhou W, Boucher RC, Bollig F, Englert C, Hildebrandt F. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am J Physiol Renal Physiol. 2010;299:F1040-F1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 63. | Diep CQ, Ma D, Deo RC, Holm TM, Naylor RW, Arora N, Wingert RA, Bollig F, Djordjevic G, Lichman B. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature. 2011;470:95-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 64. | McCampbell KK, Springer KN, Wingert RA. Analysis of nephron composition and function in the adult zebrafish kidney. J Vis Exp. 2014;90:e51644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 65. | Wingert RA, Davidson AJ. Zebrafish nephrogenesis involves dynamic spatiotemporal expression changes in renal progenitors and essential signals from retinoic acid and irx3b. Dev Dyn. 2011;240:2011-2027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 66. | O‘Brien LL, Grimaldi M, Kostun Z, Wingert RA, Selleck R, Davidson AJ. Wt1a, Foxc1a, and the Notch mediator Rbpj physically interact and regulate the formation of podocytes in zebrafish. Dev Biol. 2011;358:318-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 67. | Naylor RW, Przepiorski A, Ren Q, Yu J, Davidson AJ. HNF1β is essential for nephron segmentation during nephrogenesis. J Am Soc Nephrol. 2013;24:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 68. | Li Y, Cheng CN, Verdun VA, Wingert RA. Zebrafish nephrogenesis is regulated by interactions between retinoic acid, mecom, and Notch signaling. Dev Biol. 2014;386:111-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 69. | Cheng CN, Wingert RA. Nephron proximal tubule patterning and corpuscles of Stannius formation are regulated by the sim1a transcription factor and retinoic acid in zebrafish. Dev Biol. 2015;399:100-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 70. | Cheng CN, Verdun VA, Wingert RA. Recent advances in elucidating the genetic mechanisms of nephrogenesis using zebrafish. Cells. 2015;4:218-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Reimschuessel R, Bennett RO, May EB, Lipsky MM. Renal histopathological changes in the golfish (Carassius auratus) after sublethal exposure to hexachlorobutadiene. Aquat Toxicol. 1989;15:169-180. [DOI] [Full Text] |
| 72. | Reimschuessel R, Williams D. Development of new nephrons in adult kidneys following gentamicin-induced nephrotoxicity. Ren Fail. 1995;17:101-106. [PubMed] |
| 73. | Salice CJ, Rokous JS, Kane AS, Reimschuessel R. New nephron development in goldfish (Carassius auratus) kidneys following repeated gentamicin-induced nephrotoxicosis. Comp Med. 2001;51:56-59. [PubMed] |
| 74. | Augusto J, Smith B, Smith S, Robertson J, Reimschuessel R. Gentamicin-induced nephrotoxicity and nephroneogenesis in Oreochromis nilotica, a tilapian fish. Dis Aquatic Org. 1996;26:49-58. [DOI] [Full Text] |
| 75. | Elger M, Hentschel H, Litteral J, Wellner M, Kirsch T, Luft FC, Haller H. Nephrogenesis is induced by partial nephrectomy in the elasmobranch Leucoraja erinacea. J Am Soc Nephrol. 2003;14:1506-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 76. | Watanabe N, Kato M, Suzuki N, Inoue C, Fedorova S, Hashimoto H, Maruyama S, Matsuo S, Wakamatsu Y. Kidney regeneration through nephron neogenesis in medaka. Dev Growth Differ. 2009;51:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 77. | Kroeger PT, Wingert RA. Using zebrafish to study podocyte genesis during kidney development and regeneration. Genesis. 2014;52:771-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 78. | McKee RA, Wingert RA. Zebrafish Renal Pathology: Emerging Models of Acute Kidney Injury. Curr Pathobiol Rep. 2015;3:171-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 79. | McCampbell KK, Springer KN, Wingert RA. Atlas of Cellular Dynamics during Zebrafish Adult Kidney Regeneration. Stem Cells Int. 2015;2015:547636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 80. | Vogelmann SU, Nelson WJ, Myers BD, Lemley KV. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol. 2003;285:F40-F48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 253] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 81. | Kriz W. Glomerular diseases: podocyte hypertrophy mismatch and glomerular disease. Nat Rev Nephrol. 2012;8:618-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 82. | Petermann AT, Pippin J, Durvasula R, Pichler R, Hiromura K, Monkawa T, Couser WG, Shankland SJ. Mechanical stretch induces podocyte hypertrophy in vitro. Kidney Int. 2005;67:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 83. | Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443-2456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 528] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 84. | Lazzeri E, Crescioli C, Ronconi E, Mazzinghi B, Sagrinati C, Netti GS, Angelotti ML, Parente E, Ballerini L, Cosmi L. Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J Am Soc Nephrol. 2007;18:3128-3138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 85. | Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 423] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 86. | Lasagni L, Romagnani P. Glomerular epithelial stem cells: the good, the bad, and the ugly. J Am Soc Nephrol. 2010;21:1612-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 87. | Berger K, Schulte K, Boor P, Kuppe C, van Kuppevelt TH, Floege J, Smeets B, Moeller MJ. The regenerative potential of parietal epithelial cells in adult mice. J Am Soc Nephrol. 2014;25:693-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 88. | Zhou W, Hildebrandt F. Inducible podocyte injury and proteinuria in transgenic zebrafish. J Am Soc Nephrol. 2012;23:1039-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 89. | Huang J, McKee M, Huang HD, Xiang A, Davidson AJ, Lu HA. A zebrafish model of conditional targeted podocyte ablation and regeneration. Kidney Int. 2013;83:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 90. | Hentschel DM, Park KM, Cilenti L, Zervos AS, Drummond I, Bonventre JV. Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol. 2005;288:F923-F929. [PubMed] |
| 91. | Johnson CS, Holzemer NF, Wingert RA. Laser ablation of the zebrafish pronephros to study renal epithelial regeneration. J Vis Exp. 2011;pii: 2845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Palmyre A, Lee J, Ryklin G, Camarata T, Selig MK, Duchemin AL, Nowak P, Arnaout MA, Drummond IA, Vasilyev A. Collective epithelial migration drives kidney repair after acute injury. PLoS One. 2014;9:e101304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Fogelgren B, Zuo X, Buonato JM, Vasilyev A, Baek JI, Choi SY, Chacon-Heszele MF, Palmyre A, Polgar N, Drummond I. Exocyst Sec10 protects renal tubule cells from injury by EGFR/MAPK activation and effects on endocytosis. Am J Physiol Renal Physiol. 2014;307:F1334-F1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 94. | Chiba T, Skrypnyk NI, Skvarca LB, Penchev R, Zhang KX, Rochon ER, Fall JL, Paueksakon P, Yang H, Alford CE. Retinoic Acid Signaling Coordinates Macrophage-Dependent Injury and Repair after AKI. J Am Soc Nephrol. 2015;pii:ASN.2014111108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 95. | Romagnani P, Rinkevich Y, Dekel B. The use of lineage tracing to study kidney injury and regeneration. Nat Rev Nephrol. 2015;11:420-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 96. | Romagnani P. Of mice and men: the riddle of tubular regeneration. J Pathol. 2013;229:641-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 97. | Li Y, Wingert RA. Regenerative medicine for the kidney: stem cell prospects & amp; challenges. Clin Transl Med. 2013;2:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 98. | Wang Y, Sun ZH, Zhou L, Li Z, Gui JF. Grouper tshβ promoter-driven transgenic zebrafish marks proximal kidney tubule development. PLoS One. 2014;9:e97806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 99. | Poureetezadi SJ, Donahue EK, Wingert RA. A manual small molecule screen approaching high-throughput using zebrafish embryos. J Vis Exp. 2014;93:e52063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 100. | Morales EE, Wingert RA. Renal stem cell reprogramming: Prospects in regenerative medicine. World J Stem Cells. 2014;6:458-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Fukuda S, Singh SR S- Editor: Ji FF L- Editor: A E- Editor: Li D