Published online Jun 26, 2015. doi: 10.4252/wjsc.v7.i5.883
Peer-review started: October 20, 2014
First decision: December 17, 2014
Revised: March 6, 2015
Accepted: April 1, 2015
Article in press: April 7, 2015
Published online: June 26, 2015
Processing time: 255 Days and 17.7 Hours
AIM: To facilitate close contacts between transplanted cardiomyocytes and host skeletal muscle using cell fusion mediated by hemagglutinating virus of Japan envelope (HVJ-E) and tissue maceration.
METHODS: Cardiomyocytes (1.5 × 106) from fetal rats were first cultured. After proliferation, some cells were used for fusion with adult muscle fibers using HVJ-E. Other cells were used to create cardiomyocyte sheets (area: about 3.5 cm2 including 2.1 × 106 cells), which were then treated with Nile blue, separated, and transplanted between the latissimus dorsi and intercostal muscles of adult rats with four combinations of HVJ-E and/or NaOH maceration: G1: HVJ-E(+), NaOH(+), Cardiomyocytes(+); G2: HVJ-E(-), NaOH(+), Cardiomyocytes(+); G3: HVJ-E(+), NaOH(-), Cardiomyocytes(+); G4: HVJ-E(-), NaOH(-), Cardiomyocytes(-). At 1 and 2 wk after transplantation, the four groups were compared by detection of beating domains, motion images using moving target analysis software, action potentials, gene expression of MLC-2v and Mesp1 by reverse transcription-polymerase chain reaction, hematoxylin-eosin staining, and immunostaining for cardiac troponin and skeletal myosin.
RESULTS: In vitro cardiomyocytes were fused with skeletal muscle fibers using HVJ-E. Cardiomyocyte sheets remained in the primary transplanted sites for 2 wk. Although beating domains were detected in G1, G2, and G3 rats, G1 rats prevailed in the number, size, motion image amplitudes, and action potential compared with G2 and G3 rats. Close contacts were only found in G1 rats. At 1 wk after transplantation, the cardiomyocyte sheets showed adhesion at various points to the myoblast layer in the latissimus dorsi muscle. At 2 wk after transplantation, close contacts were seen over a broad area. Part of the skeletal muscle sarcoplasma seemed to project into the myocardiocyte plasma and some nuclei appeared to share both sarcoplasmas.
CONCLUSION: The present results show that close contacts were acquired and facilitated the beating function, thereby providing a new cellular transplantation method using HVJ-E and NaOH maceration.
Core tip: It is very important to produce close contacts between transplanted cell sheets and host cells without fibrous invasion for tissue reconstruction and function. Unfortunately, cellular transplantation research has not focused on this problem and convenient methods have not yet been developed. In this study using hemagglutinating virus of Japan envelope, transplanted cardiomyocyte sheets were placed in direct contact with host skeletal muscles. By adding NaOH maceration of connective tissues, rich, largely-movable, beating domains were observed. Histological observations revealed successful formation of close contacts between the two cell types. This paper proposes a new idea of introducing artificial cell fusion into cellular transplantation methods.
- Citation: Takahashi Y, Tomotsune D, Takizawa S, Yue F, Nagai M, Yokoyama T, Hirashima K, Sasaki K. New model for cardiomyocyte sheet transplantation using a virus-cell fusion technique. World J Stem Cells 2015; 7(5): 883-893
- URL: https://www.wjgnet.com/1948-0210/full/v7/i5/883.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i5.883
Advances in regenerative medicine have realized clinical applications of pluripotent stem cell-derived differentiated cells[1], and have simultaneously allowed recognition of the importance and potential of cellular transplantation and the necessity for its further improvement.
One of the issues is how to establish and maintain morphological, functional junctions or dense contacts without fibrous invasion between the transplanted and host cells. Regarding cardiomyocyte transplantation into the heart in particular, if direct electrical coupling through gap junctions is not established between the transplanted and host cells, the transplanted area becomes a source of arrhythmia and/or disordered contractions[2,3].
Simple cellular injection does not always lead to the formation of electrical coupling between the transplanted and host cells. Instead, it can produce scarring, granulation, or connective tissue formation through tissue reactions such as phagocytosis of necrotic host cells, ingrowth of fibroblasts and capillaries[4], and interposition of a layer of non-cardiomyocyte cells between the graft and host cardiomyocytes[5], which may interrupt direct cell-cell interactions, electrical coupling, and integration. To overcome these disadvantages, there are two requirements: removal of the matrix and connective tissues surrounding the host tissue cells before transplantation to facilitate direct contacts between the transplanted and host cells, and close cellular contacts or fusion at the interface between the transplanted and host cells.
Another issue is how to obtain sufficiently large numbers of purified differentiated cardiomyocytes. Many methods using pluripotent stem cells have proposed improvements for maintaining cellular function for transplantation[6]. However, if a method can be established that allows the production of more effective systolic function with fewer pluripotent stem cell-derived cardiomyocytes, it will be preferable and more ideal.
The notion, trialing, and practice of using skeletal muscles or myoblasts instead cardiac muscles have repeated vicissitudes. The latissimus dorsi muscle has been used as a cardiac bioassist in clinical settings, but requires continuous external stimulation[7]. Some difficulties may remain in harmonizing the contraction of the muscle with the host heart. Skeletal myoblast transplantation has also been focused upon[8,9] and clinically applied[10]. However, other papers reported that myoblast transplantation caused arrhythmogenicity compared with human stem cell-derived cardiomyocytes (SC-CMs) because of the lack of gap junctions and presence of islet-like localized cell-clusters resulting in disturbance of the electrical conduction[11], leading to questions regarding its value. Recently, a new therapy involving epicardial placement of generated myoblast sheets in temperature-responsive dishes was developed and prevented arrhythmogenic substrate production through paracrine effects without islet-like cell-cluster formation causing electrical conduction disturbances[12,13]. However, it remains unknown how long its effectiveness continues or whether it continues to function until complete recovery.
If cardiomyocytes can fuse directly with skeletal muscles, integrated systolic forces may be retained and produce tough contractions without requiring large numbers of differentiated cells from pluripotent stem cells. A previous report in vitro showed the potential of spontaneous fusion between mouse neonatal cardiomyocytes and primary neonatal skeletal myoblasts[14]. Furthermore, mouse fetal cardiomyocytes and mouse embryonic stem cells were fused using polyethylene glycol, and survived to become chimeric cells[15].
On the basis of the above descriptions, we planned to establish a new transplantation model using a safe virus-cell fusion technique of cardiomyocytes with the latissimus dorsi muscle in addition to maceration of the cellular matrix and connective tissue of the host tissues.
Pregnant ICR mice (SLC, Hamamatsu, Japan) at embryonic day 14 and 6-wk-old ICR mice were used for in vitro experiments. In addition, 6-wk-old BALB/cSLc-nu/nu (SLC) were used as recipients for in vivo experiments. This study was approved by the Animal Care Committee of Shinshu University.
Preparation of fetal cardiomyocytes: Pregnant female mice were euthanized by cervical dislocation. After opening the abdomen under sterile conditions, the uterus was removed and washed three times with PBS containing 50 U/mL penicillin (Wako Pure Chemical Industries Ltd., Osaka, Japan) and 50 μg/mL streptomycin (Wako Pure Chemical Industries Ltd.). Fetuses were removed from the uterus and transferred to D-Hanks solution. After opening the thorax, the fetal heart was removed under a SZ-ST stereo microscope (Olympus, Tokyo, Japan), cut into small pieces, and centrifuged at 1200 rpm for 3 min. The supernatant was aspirated, and the pellet was resuspended with PBS and centrifuged again. This process was repeated three times. Subsequently, the pellet in a 15-mL tube was immersed in 5 mL of 0.05% trypsin/0.01% EDTA and shaken in a thermostatic bath at 37 °C for 10 min. The digested tissue was diluted with 5 mL of DMEM containing 10% FBS (Equitech-Bio Inc., Kerrville, TX, United States) and centrifuged as described above. After aspiration of the supernatant, the pellet was resuspended with the medium and filtered through a 70-μm Nylon Cell Strainer (BD, Franklin Lakes, NJ, United States). The cells were adjusted to 1.5 × 106 cells/mL and transferred into 60-mm gelatin-coated dishes.
Preparation of skeletal muscle fibers: Six-week-old ICR mice were euthanized by cervical dislocation. After disinfection with 70% ethanol, the skin of the leg was cut and separated. The muscles surrounding the legs were removed en bloc, almost completely cleared of connective tissue and fat tissue in D-Hanks solution under a stereo-microscope, cut into small pieces, and centrifuged at 1200 rpm for 3 min. The supernatant was aspirated, and the pellet was resuspended with PBS and centrifuged again. This process was repeated three times. Subsequently, 10 g of the pellet was immersed in 0.6 g/L collagenase type 1 (Wako Pure Chemical Industries Ltd.) including 0.05% trypsin/0.01% EDTA in 30 mL, divided into 5-mL aliquots in 15-mL tubes, and shaken in a thermostatic bath for 10 min at 37 °C. The digested tissues were diluted with 5 mL of DMEM containing 10% FBS and centrifuged under the same conditions. After aspiration of the supernatant, the pellet was resuspended with 10 mL of the medium, filtered through a 70-μm Nylon Cell Strainer, transferred into a 100-mm gelatin-coated dish, and preincubated at 37 °C under 5% CO2 for 3 h. After pipetting, the supernatant was transferred into another dish and preincubated for 2 h. This process was repeated twice. The final cell numbers were adjusted to 70 cells/mm2, transferred into 60-mm dishes, and incubated. The medium was exchanged for fresh DMEM containing 10% FBS every day.
Fusion of the two types of muscle cells using an HVJ envelope cell fusion kit: Cultured skeletal muscle fibers were washed three times with PBS, resuspended in Vybrant DiI Cell-labeling Solution (5 μL/mL) (Molecular Probes, Eugene, OR, United States), and incubated for 15 min. After aspiration, the solution was exchanged for DMEM containing 10% FBS. Cultured cardiomyocytes were treated with Vybrant DiO Cell-labeling Solution (Molecular Probes) using the same process.
The treated cardiomyocytes were separated as a sheet using a TPP Cell Spatula (TPP-US, St. Louis, MO, United States) and transferred into 60-mm dishes containing cultured skeletal muscle fibers. Subsequently, 5 μL/mL of the fusogen (HVJ Envelope Cell Fusion Kit GenomONE-CF: Ishihara Sangyo Kaisha Ltd., Osaka, Japan) was added to them. After then, the dishes were incubated for 15 min. The medium was then changed and the dishes were observed at 6, 12, 24, 36, and 48 h using an Axio Observer Z1 (Carl Zeiss, Oberkochen, Germany). For negative controls, the fusogen was omitted.
Preparation of transplanted cardiomyocytes: At 2 d before transplantation, cardiomyocytes were cultured in medium containing 2.5 × 10-5% Nile blue (Wako Pure Chemical Industries Ltd.) for staining. Alternatively, they were stained with DiO at 1 d before transplantation as described in in vitro experiments.
Transplantation: Depending on the treatments, the animals were divided into four groups as shown in Table 1. After peritoneal anesthesia (0.25 mL of 1% Nembutal Solution; Dainihon Sumitomo Pharma Co. Ltd., Osaka, Japan), the animals were fixed in a propped position. The dorsal skin was cut along the spine and separated to expose the latissimus dorsi muscle. After confirming the internal edge, the muscle was separated from the deeper intercostal muscle and its abdominal aspect was placed in contact with cotton wool immersed in 5 mol/L NaOH for a few seconds until the color of the muscle changed slightly. To inhibit any NaOH-associated damage to the intercostal muscle, a nylon film was inserted between the two muscles before maceration. The area was washed immediately with saline after the maceration. In control animals, no NaOH maceration was performed.
| G/T | HJV-E | NaOH | CMT |
| G1 | + | + | + |
| G2 | − | + | + |
| G3 | + | − | + |
| G4 | − | − | − |
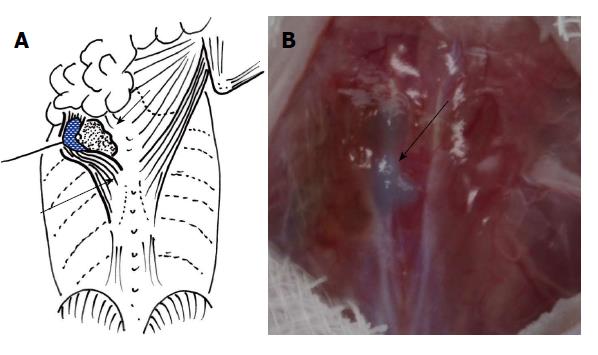
Nile blue-stained cardiomyocytes were separated from dishes with a scraper. A cell sheet was inserted between the latissimus dorsi muscle and intercostal muscle and expanded as extensively as possible with or without addition of HVJ-E (Figure 1). The incised skin was closed. At 1 and 2 wk after transplantation, the rats were deeply anesthetized, and the transplanted domains were analyzed by the following cell biological methods. As controls, the domains on the opposite side were evaluated.
Observation with a fluorescence stereo-microscope: To confirm that the cardiomyocytes survived and remained in the transplanted domain, cardiomyocytes were treated with DiO, transplanted under the latissimus dorsi muscle, and observed under a fluorescence stereo-microscope SZX12 after 2 wk.
Motion image analysis: To compare the beating strengths among groups, the variation distances followed by contraction seen on the latissimus dorsi muscle were estimated and computationally analyzed. After anesthesia, the dorsal skin was longitudinally cut and separated toward both sides to expose the latissimus dorsi muscle. A motion image of the transplanted domain was photographed with an AE·AF Digital Camera including a P-TTL stroboscope (Ricoh Imaging Company Ltd., Tokyo, Japan). Motion image analyses were performed using the moving target analysis software VW-H1MA (Keyence, Osaka, Japan).
Electrophysiological analysis: To assess whether each motion was caused by an action potential, electrophysiological analyses were performed. Two probes were separately placed at each end of the motional domain of the latissimus dorsi muscle, but not directly over the transplanted cardiomyocytes. The action potentials were recorded with a PowerLab SP (AD Instruments Japan Inc., Nagoya, Japan).
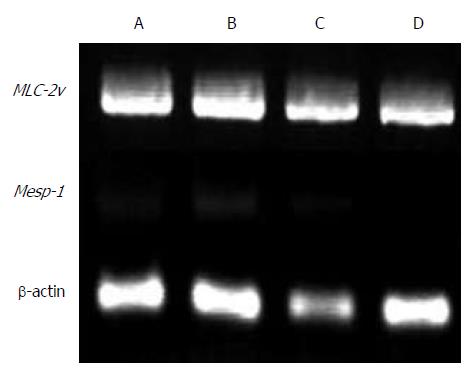
Reverse transcription-polymerase chain reaction: To confirm that the transplanted cardiomyocytes survived and did not lose their features, the gene expression in samples removed from the transplanted areas was confirmed by reverse transcription-polymerase chain reaction (RT-PCR) for cardiomyocyte markers (MLC-2v, marker of the ventricle; Mesp1, early marker of cardiac development). The primer sets, annealing temperatures, and predicted product sizes were as follows: MLC-2v: 5′-cctcgaactctccagaggtg-3′ and 5′-cttcctgtttatttgcgcac-3′, 53 °C, 612 bp; Mesp1: 5′-cctgggatgctgtttctgcg-3′ and 5′-acctgaccaagatcgagacg-3′, 60 °C, 291 bp; β-actin: 5′-ttccttcttgggtatggaat-3′ and 5′-gagcaatgatcttgatcttc-3′, 55 °C, 200 bp. Total RNA was extracted using TRIzol reagent (Invitrogen Japan, Tokyo, Japan). DNase-treated total RNA was used to prepare the first-strand cDNA with SuperScript II (Invitrogen Japan), following the manufacturer’s protocol. The cDNA samples were subjected to PCR amplification with the specific primer sets under linear conditions to approximate the original amounts of the specific transcripts. The amplification conditions included denaturation at 94 °C for 1 min, annealing at the temperature specified for each primer for 30 s, and elongation at 72 °C for 30 s. A total of 30 cycles were performed. The PCR products were size-fractioned by 1.5% agarose gel electrophoresis and visualized by ethidium bromide staining.
Hematoxylin-eosin staining:En bloc removed samples from the beating domains were fixed with 4% PFA in 0.1 mol/L phosphate buffer (pH 7.4) overnight. After dehydration through a graded ethanol series, the samples were prepared for hematoxylin-eosin (HE) staining using standard methods.
PFA-fixed tissue sections on slides were deparaffinized, rehydrated, washed three times with 0.02 mol/L PBS (pH 7.4), and treated with 20 μg/mL Proteinase K solution (Wako Pure Chemical Industries Ltd.) for 25 min at 37 °C. After three washes with PBS, the sections were immersed in 0.1% Triton X-100 (Nakalai Chemical Ltd., Kyoto, Japan) at room temperature for 1 h. After another three washes with PBS, the sections were immersed in blocking solution I (5% goat normal serum in 0.02 mol/L PBS) at room temperature for 1 h. The treated cells were stained with a mixture of primary antibodies comprising mouse anti-cardiac troponin (1:100; Chemicon, Billerica, MA, United States) and rabbit anti-skeletal myosin (1:500; Sigma-Aldrich Japan, Tokyo, Japan) at 4 °C for 12 h. After washing, the sections were reacted with one secondary antibody (1:1000; Alexa Fluor 568-conjugated goat anti-rabbit IgG; Molecular Probes) in 0.02 mol/L PBS containing 5% goat normal serum at room temperature for 1 h. After washing, the sections were immersed in blocking solution II (5% rabbit normal serum in 0.02 mol/L PBS) at room temperature for 1 h and then reacted with another secondary antibody (1:1000; Alexa Fluor 488-conjugated rabbit anti-mouse IgG; Molecular Probes) at room temperature for 1 h. After washing with PBS and reaction with Prolong Gold antifade reagent containing 4,6-diamidino-2-phenylindole dihydrochloride (DAPI; Invitrogen Japan), the sections were observed under an Axio Observer Z1 fluorescence microscope (Carl Zeiss).
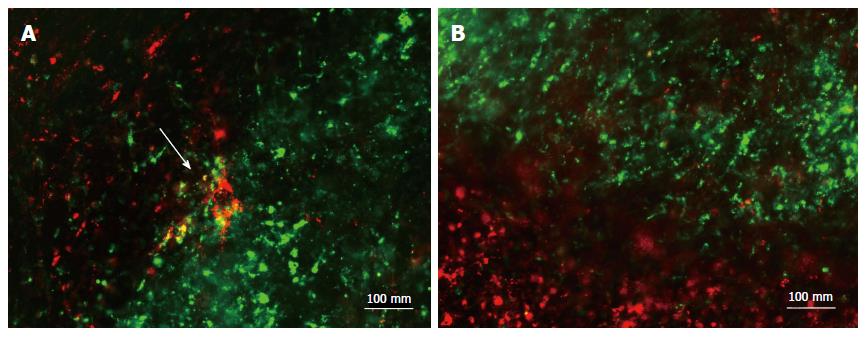
Cell fusion: To confirm the effectiveness of HVJ-E, DiO-stained cardiomyocytes were transferred onto DiI-stained muscle fibers in dishes, and HVJ-E was added. After 24 h, merged areas of DiO and DiI appeared (Figure 2A). In control dishes, no merged areas were observed (Figure 2B).
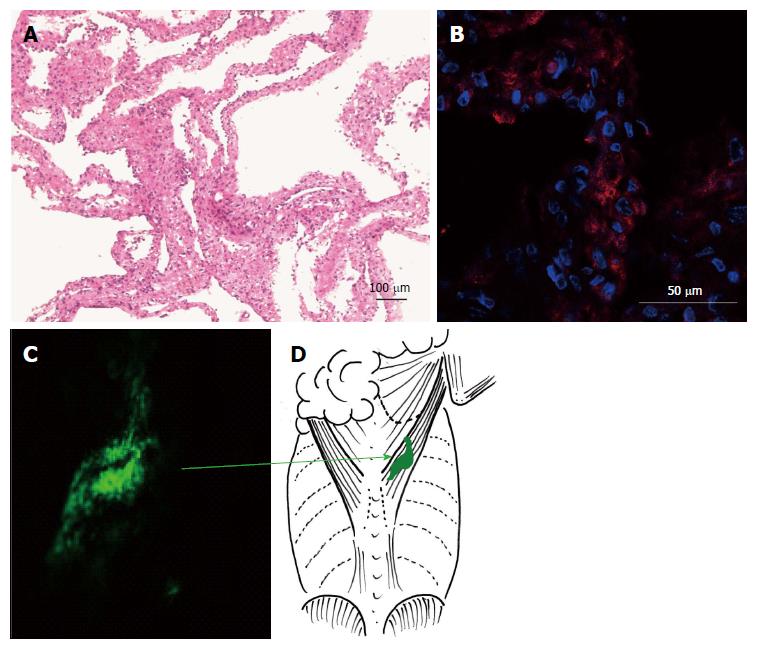
Before transplantation, the prepared cardiomyocyte sheets were peeled directly from the dishes using a scraper. The sheets were 50-100 μm in thickness and included rich dotted nuclei and eosinophilic cytoplasm. The nucleus had a spindle-type shape and showed a strong basophilic appearance with heterochromatin (Figure 3A). Immunostaining revealed cardiac troponin-positive cells (Figure 3B).
DiO-treated cardiomyocytes occupied the same position under the latissimus dorsi muscle as the first transplanted domain at 2 wk after transplantation, and did not extend or move into other areas around the muscle (Figure 3C and D).
Under four different experimental conditions (Table 1), cardiomyocyte sheets were inserted between the latissimus dorsi and intercostal muscles, and examined by motion image, action potential, RT-PCR, fluorescent dye, HE staining, and immunostaining analyses at 1 and 2 wk after transplantation.
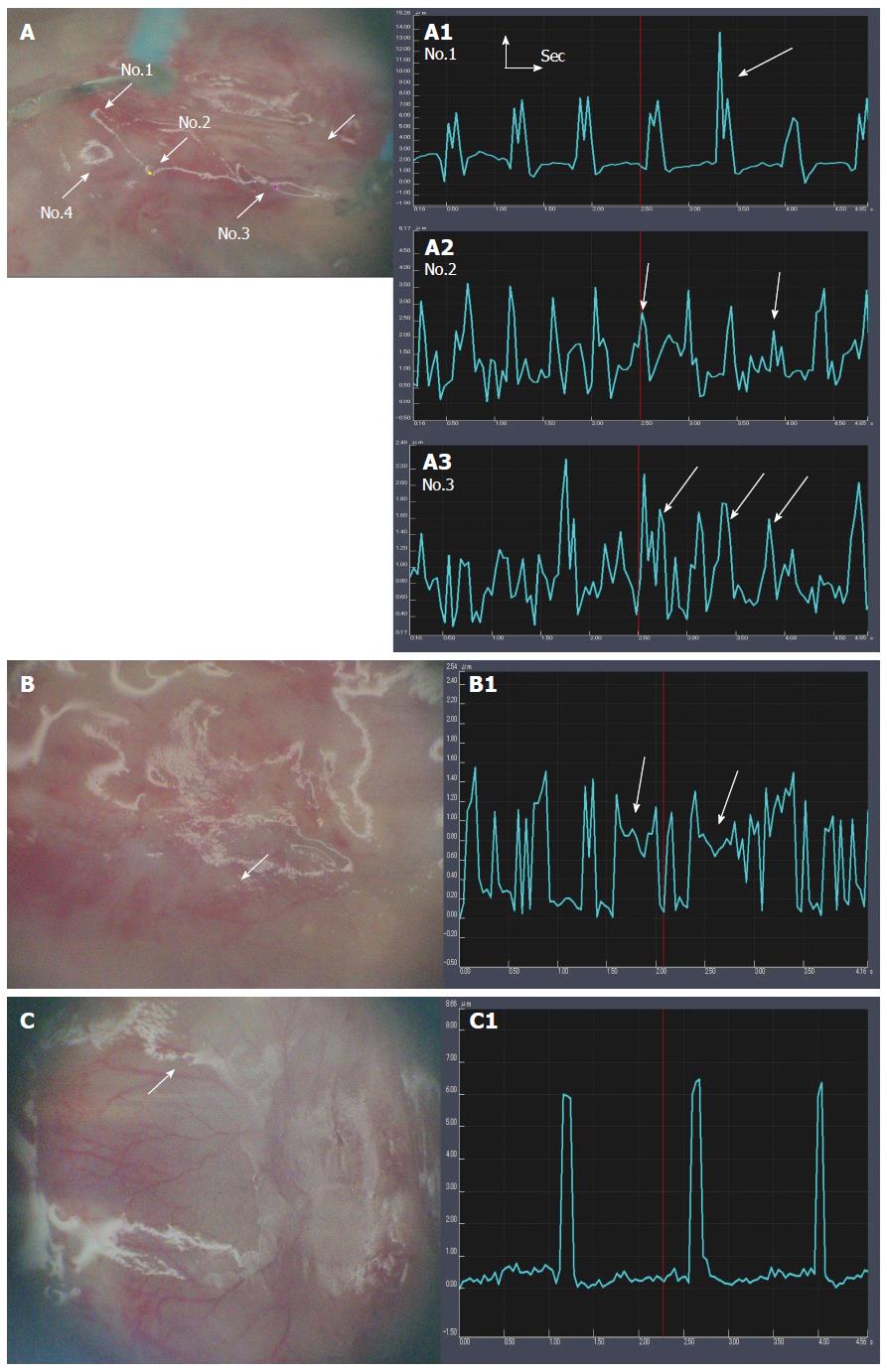
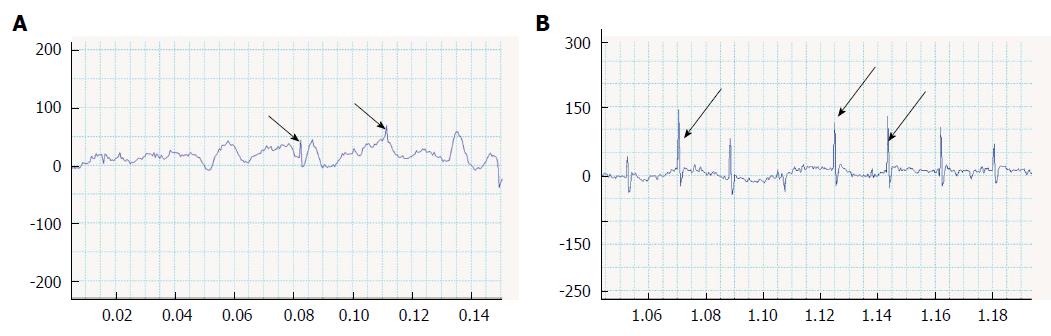
After confirming arrest of the host heart beats, the motion variation seen in the transplanted domains was analyzed computationally. The effectiveness of NaOH maceration and HVJ-E addition was compared in different conditions (Table 1). The G1 [HVJ-E(+), NaOH(+), Cardiomyocytes(+)], G2 [HVJ-E(-), NaOH(+), Cardiomyocytes(+)], and G3 [HVJ-E(+), NaOH(-), Cardiomyocytes(+)] rats all showed a beating-like motion (Figure 4A-C). However, the G1 rats showed the most numerous motion areas (Figure 4A), with a larger amplitude (2.5 ± 1.80 μm) compared with the other groups of rats. In G3 rats, the small amplitudes were seen more frequently (Figure 4B). The motion had a particular feature, in that some amplitudes did not return the basal line. The mean amplitude was 0.73 ± 0.55 μm. In G2 rats, no motion was found in the transplanted area at 1 wk after transplantation, while a few small motion areas were found at 2 wk after transplantation (Figure 4C). The amplitude was small (0.62 ± 0.36).
The action potentials were compared between G1 and G3 rats at 2 wk after transplantation. Consistent with the low fluttering motion amplitudes in G3 rats, their action potentials were also disordered (Figure 5A). On the other hand, the action potentials in G1 rats were relatively ordered and larger, but contained varied patterns (Figure 5B).
After transplantation, the motion samples under the latissimus dorsi muscle clearly expressed MLC-2v in G1 and G3 rats (Figure 6). Mesp1 showed weak expression at 2 wk after transplantation compared with 1 wk after transplantation.
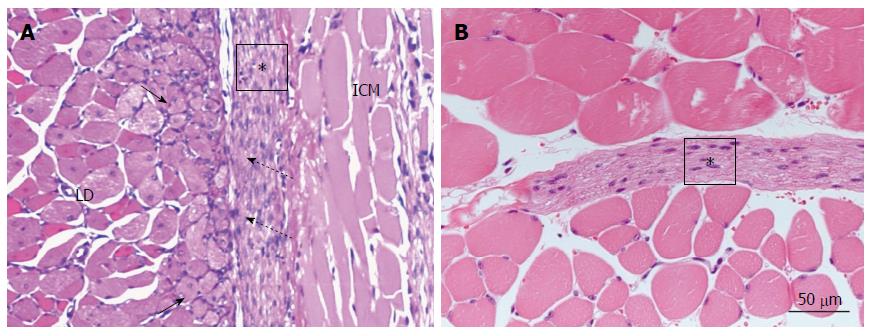
The interfaces between the NaOH-macerated skeletal muscle fibers and transplanted cardiomyocyte sheets showed marked differences. In the case of HVJ-E addition in particular, the inserted sheet showed close contacts with the myoblast areas. NaOH maceration destroyed the skeletal muscle fibers and brought about marked myoblast differentiation (Figure 7A). Myoblasts were observed as small round cells with a round clear nucleus, typical nucleolus, and paler cytoplasm than skeletal muscle fibers. The transplanted cells closely adhered to this active zone at several points. At 2 wk after transplantation, myoblasts were rarely found, but the sheet extended among the skeletal muscle fibers. Without NaOH maceration, the inserted cardiomyocyte sheet still survived, but the interface area was quiet without myoblast differentiation and showed definite separation (Figure 7B).
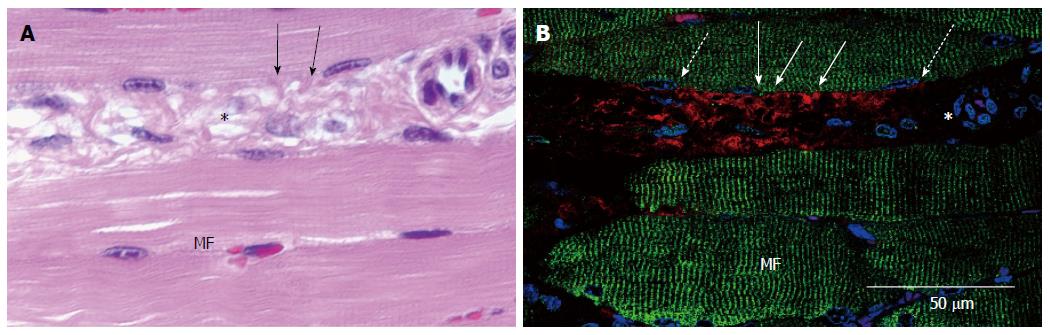
At 2 wk after transplantation, HE staining showed close contacts between the inserted cardiomyocyte sheet and the skeletal muscle fibers, in which collagenous fibers and fibroblasts were not found (Figure 8A). Immunostaining of the same area revealed that, although the interface was extremely close, cardiac troponin and skeletal muscle actinin did not merge (Figure 8B).
Cell transplantation is different from organ transplantation in that the transplanted cells interact with the surrounding host tissues and need to restructure themselves. The environments encircling transplanted cells are not always preferable for this restructuring. Transplanted cells are always close to cell death, because they cannot rapidly achieve a blood supply. If such cells are not in a sheet-like form, they need to conjugate with one another through a junctional apparatus, while fibroblasts or other free cells can invade the cells and inhibit their interactions. Meanwhile, it is even more difficult for transplanted cells to create new structures with host tissue cells, because basement membranes demarcate both the host and transplanted cells or tissues as the first barrier, and fibroblasts, collagenous fibers, elastic fibers, fat cells, and other free immunopotent cells can invade the interface easily as a second barrier. Although injected neonatal or pluripotent stem cell-derived cardiomyocytes conjugate with host heart cardiomyocytes[11], it remains uncertain whether these findings hold true, because extremely small domains were demonstrated by immunostaining.
In this study, cardiomyocytes were transplanted as sheets to avoid the cardiomyocyte restructuring process. Tough cardiomyocyte sheets can easily be acquired, because cardiomyocytes conjugate strongly with one another through intercalated disks and the sheets are easily peeled off using a scraper without requiring enzymatic digestion or other new techniques such as sheet engineering[13]. The peeled sheets do not become broken and the cells remain alive.
In addition, NaOH was used to remove the connective tissues and basement membranes. This procedure was derived from a method based on the morphology, which involved maceration of basement membranes, collagenous fibers, and elastic fibers applied to fixed specimens[16,17]. The myoblasts in the NaOH-macerated skeletal muscle fibers showed marked differentiation. The scrab of muscles alone did not produce as much reaction (data not shown). Myoblasts originate from satellite cells, which are self-renewing stem cells that become activated by products such as growth factors produced by crushed muscles to migrate to the injured areas, differentiate into myoblasts, and fuse with each other and the damaged cells to restore skeletal muscle cells[18]. Although it is well known clinically that NaOH devastates the tissue, penetrates easily followed by an acute necrotic phase, coagulation of intracellular proteins, and intense inflammatory reactions, and causes serious complications[19], no definite invasion of immunopotent cells and no fibroblasts, at least, were observed in this study for 2 wk after transplantation. While the marked myoblast recruitment and few invading cells created a better environment for cardiomyocyte transplantation, they were not sufficient because, although the cardiomyocyte sheets showed beating, their domains were smaller and they did not adhere closely to the latissimus dorsi muscle.
The procedure for cardiomyocyte sheet transplantation also required some improvements in this study. First, when the sheet was overlaid on the latissimus dorsi muscle under the skin, i.e., between the skin and the muscle, the procedure failed, in that no beating was observed with or without NaOH maceration (data not shown). This site under the skin, as opposed to under the muscle, may not be as good probably because of the poor vessel supply. Therefore, for subsequent survival, use of cardiomyocyte sheets necessitates some suitable conditions. Although the sheets constantly showed beating in NaOH maceration, when they were inserted between the latissimus dorsi and intercostal muscles, the beating sites were smaller and less frequent, the motion amplitude was smaller, and the action potentials were disordered. Moreover, close contacts between the transplanted sheets and the host muscular tissues were not formed despite the fact that the sheets were packed like a sandwich between the two muscular tissues. Thus, the space between the two muscles is good for transplantation, but not sufficient for close contact formation.
To obtain close contacts between a sheet and the latissimus dorsi muscle, artificial fusion of the membranes of cardiomyocytes and skeletal muscle fibers by HVJ-E was investigated. HVJ-E is composed of the envelope of Sendai virus and causes high fusion potency between cellular membranes[20]. It is easier and safer to handle compared with other viruses. After its effectiveness was confirmed in vitro, the fusogen was applied to transplantation in vivo. Its addition alone was not effective, as the motion amplitude was smaller, and the interspaces were histologically wider. This result was within our expectation, because the lack of removal of fibroblasts, collagenous fibers, and other connective tissues would have interrupted any close contact formation between the transplanted and host cells.
The combination of HVJ-E and NaOH maceration brought about dramatic changes, i.e., increased beating sites, larger amplitudes, albeit varied, and ordered action potentials. Although there were areas where the interfaces were separated, close contacts between cardiomyocytes and skeletal muscle cells were often found at various points, in which collagenous fibers and fibroblasts were not observed. This morphological feature, i.e., close contacts between skeletal muscle fibers and cardiomyocytes, may bring about complicated motion variance.
Cell fusion produces chimeric cells that include features of both cell types, as seen in hybridomas[15,21]. Intracellular organelles, including the nucleus, pass back and forth between both cell types after the formation of weak cytoplasmic bridges[22]. Although the close contact areas observed in this study seemed to be maintained as fused membranes, because both the cytoplasm shared one nucleus and seemed to be partially mingled with each other, while both cytoskeletal systems maintained independent forms from one another and did not undergo reconstruction. However, to prove the interrelationship between the membrane fusion and the cytoskeleton, other cellular morphological methods, such as transmission electron microscopy and deep-etching, may be required. This complicated cell biological theme exceeds the present study and needs to be further examined in future studies.
In conclusion, the combined method involving NaOH and HVJ-E treatments is effective for cellular transplantation as a new technique.
We thank Dr. Kametani K, Ms Suzuki K, and Ms Yoshitome A for technical support and Ms Maruyama J for secretarial assistance.
Advances in regenerative medicine requires further improvements of a cellular transplanted technique to realized clinical applications, because it is important to establish and maintain functional junctions or dense contacts without fibrous invasion between the transplanted and host cells.
In particular regarding cardiomyocyte transplantation into the heart, if direct electrical coupling is not established between the transplanted and host cells, the transplanted area becomes a source of arrhythmia and/or disordered contractions. It is challenging to overcome this issue, which has been performed in this research.
The most important innovations of the research propose a new invention of myocardiocyte sheet transplantation, which is to fuse face-to face areas between two tissues, after NaOH maceration, using virus envelops and not to intervene connective tissues between them. This method will protect a host heart from conduction disturbance.
This transplantation method using cell fusion will be applied clinically as a new one to establish cell biological function between host and donor tissues without intervention of connective tissues.
HVJ-E is a simplified form of hemagglutinating virus of Japan envelope. This virus is usually murine parainfluenza virus type 1 and is known as Sendai virus. The most novel feature is that it has an ability to fuse cells, which is a useful tool in the scientific world to produce hybridoma. In this experiment, its envelope is used, which grantees safety as well as effectiveness.
The current study provided the evidence that a new method using HVJ-E and NaOH may facilitate the cell transplantation into the tissues.
| 1. | Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 988] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 2. | Zhang YM, Hartzell C, Narlow M, Dudley SC. Stem cell-derived cardiomyocytes demonstrate arrhythmic potential. Circulation. 2002;106:1294-1299. [PubMed] |
| 3. | Pillekamp F, Halbach M, Reppel M, Pfannkuche K, Nazzal R, Nguemo F, Matzkies M, Rubenchyk O, Hannes T, Khalil M. Physiological differences between transplanted and host tissue cause functional decoupling after in vitro transplantation of human embryonic stem cell-derived cardiomyocytes. Cell Physiol Biochem. 2009;23:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Reinecke H, Zhang M, Bartosek T, Murry CE. Survival, integration, and differentiation of cardiomyocyte grafts: a study in normal and injured rat hearts. Circulation. 1999;100:193-202. [PubMed] |
| 5. | Halbach M, Krausgrill B, Hannes T, Wiedey M, Peinkofer G, Baumgartner S, Sahito RG, Pfannkuche K, Pillekamp F, Reppel M. Time-course of the electrophysiological maturation and integration of transplanted cardiomyocytes. J Mol Cell Cardiol. 2012;53:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Lundy SD, Gantz JA, Pagan CM, Filice D, Laflamme MA. Pluripotent stem cell derived cardiomyocytes for cardiac repair. Curr Treat Options Cardiovasc Med. 2014;16:319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Chachques JC, Jegaden O, Mesana T, Glock Y, Grandjean PA, Carpentier AF. Cardiac bioassist: results of the French multicenter cardiomyoplasty study. Asian Cardiovasc Thorac Ann. 2009;17:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Smits PC, van Geuns RJ, Poldermans D, Bountioukos M, Onderwater EE, Lee CH, Maat AP, Serruys PW. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: clinical experience with six-month follow-up. J Am Coll Cardiol. 2003;42:2063-2069. [PubMed] |
| 9. | Menasché P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 622] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 10. | Murry CE, Field LJ, Menasché P. Cell-based cardiac repair: reflections at the 10-year point. Circulation. 2005;112:3174-3183. [PubMed] |
| 11. | Gepstein L, Ding C, Rahmutula D, Wilson EE, Yankelson L, Caspi O, Gepstein A, Huber I, Olgin JE. In vivo assessment of the electrophysiological integration and arrhythmogenic risk of myocardial cell transplantation strategies. Stem Cells. 2010;28:2151-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Narita T, Shintani Y, Ikebe C, Kaneko M, Harada N, Tshuma N, Takahashi K, Campbell NG, Coppen SR, Yashiro K. The use of cell-sheet technique eliminates arrhythmogenicity of skeletal myoblast-based therapy to the heart with enhanced therapeutic effects. Int J Cardiol. 2013;168:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Matsuura K, Haraguchi Y, Shimizu T, Okano T. Cell sheet transplantation for heart tissue repair. J Control Release. 2013;169:336-340. [PubMed] |
| 14. | Reinecke H, Minami E, Poppa V, Murry CE. Evidence for fusion between cardiac and skeletal muscle cells. Circ Res. 2004;94:e56-e60. [PubMed] |
| 15. | Takei S, Yamamoto M, Cui L, Yue F, Johkura K, Ogiwara N, Iinuma H, Okinaga K, Sasaki K. Phenotype-specific cells with proliferative potential are produced by polyethylene glycol-induced fusion of mouse embryonic stem cells with fetal cardiomyocytes. Cell Transplant. 2005;14:701-708. [PubMed] |
| 16. | Ohtani O, Ushiki T, Taguchi T, Kikuta A. Collagen fibrillar networks as skeletal frameworks: a demonstration by cell-maceration/scanning electron microscope method. Arch Histol Cytol. 1988;51:249-261. [PubMed] |
| 17. | Zhang L, Ina K, Kitamura H, Campbell GR, Shimada T. The intercalated disc of monkey myocardial cells and Purkinje fibers as revealed by scanning electron microscopy. Arch Histol Cytol. 1996;59:453-465. [PubMed] |
| 18. | Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol. 2003;35:1151-1156. [PubMed] |
| 19. | Aksu B, Durmus-Altun G, Ustun F, Torun N, Kanter M, Umit H, Sut N. A new imaging modality in detection of caustic oesophageal injury: Technetium-99m pyrophosphate scintigraphy. Int J Pediatr Otorhinolaryngol. 2009;73:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Zhang Q, Li Y, Shi Y, Zhang Y. HVJ envelope vector, a versatile delivery system: its development, application, and perspectives. Biochem Biophys Res Commun. 2008;373:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. 1975. J Immunol. 2005;174:2453-2455. [PubMed] |
| 22. | Schneeberger EE, Harris H. An ultrastructural study of inter-specific cell fusion induced by inactivated Sendai virus. J Cell Sci. 1966;1:401-406. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Wada J S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Wang CH