Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.448
Peer-review started: July 28, 2014
First decision: August 28, 2014
Revised: September 17, 2014
Accepted: October 28, 2014
Article in press: October 29, 2014
Published online: March 26, 2015
Processing time: 235 Days and 17.9 Hours
Cell therapy is a promising treatment for diseases that are caused by cell degeneration or death. The cells for clinical transplantation are usually obtained by culturing healthy allogeneic or exogenous tissue in vitro. However, for diseases of the eye, obtaining the adequate number of cells for clinical transplantation is difficult due to the small size of tissue donors and the frequent needs of long-term amplification of cells in vitro, which results in low cell viability after transplantation. In addition, the transplanted cells often develop fibrosis or degrade and have very low survival. Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPS) are also promising candidates for cell therapy. Unfortunately, the differentiation of ESCs can bring immune rejection, tumorigenicity and undesired differentiated cells, limiting its clinical application. Although iPS cells can avoid the risk of immune rejection caused by ES cell differentiation post-transplantation, the low conversion rate, the risk of tumor formation and the potentially unpredictable biological changes that could occur through genetic manipulation hinder its clinical application. Thus, the desired clinical effect of cell therapy is impaired by these factors. Recent research findings recognize that the reason for low survival of the implanted cells not only depends on the seeded cells, but also on the cell microenvironment, which determines the cell survival, proliferation and even reverse differentiation. When used for cell therapy, the transplanted cells need a specific three-dimensional structure to anchor and specific extra cellular matrix components in addition to relevant cytokine signaling to transfer the required information to support their growth. These structures present in the matrix in which the stem cells reside are known as the stem cell microenvironment. The microenvironment interaction with the stem cells provides the necessary homeostasis for cell maintenance and growth. A large number of studies suggest that to explore how to reconstruct the stem cell microenvironment and strengthen its combination with the transplanted cells are key steps to successful cell therapy. In this review, we will describe the interactions of the stem cell microenvironment with the stem cells, discuss the importance of the stem cell microenvironment for cell-based therapy in ocular diseases, and introduce the progress of stem cell-based therapy for ocular diseases.
Core tip: Cell therapy is a promising treatment for the diseases caused by cell degeneration or death. However, the transplanted cells often develop fibrosis or are absorbed and cannot survive long. It is not simply because of seed cells, but also due to the cell microenvironment. How to reconstruct the stem cell microenvironment and strengthen its combination with the transplanted cells is the key to successful cell therapy. We will discuss the importance of the stem cell microenvironment for cell-based therapy in ocular diseases and introduce the progress of cell therapy for ocular diseases.
- Citation: Wan PX, Wang BW, Wang ZC. Importance of the stem cell microenvironment for ophthalmological cell-based therapy. World J Stem Cells 2015; 7(2): 448-460
- URL: https://www.wjgnet.com/1948-0210/full/v7/i2/448.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i2.448
Limbal stem cell deficiency, corneal endothelial decompensation, corneal grafts endothelial decompensation, retinitis pigmentosa, age-related macular degeneration, Stargardt disease and other hereditary retinal diseases are all caused by cell degeneration or death. There is no effective clinical treatment currently available. Unlike traditional medicine or surgical therapy, cell therapy is a promising treatment and can treat the abnormal cells most directly and efficiently.
The road to cell therapy has been a long and tortuous process. In the early days, most of the efforts to obtain sufficient therapeutic seed cells were based on establishing cell lines or improving tissue culture methods with the expectation that they will provide therapeutic effects after the cell transplantation. The former long-term passaged cell lines were mainly obtained by transgenic or nuclear atypia. Because of biosecurity risks and allograft immune rejection, cells obtained by this method are mainly used for basic research[1]. The cells for clinical transplantation are usually obtained by culturing healthy allogeneic or exogenous tissue in vitro. It is hard to get large tissue from the eye to obtain a sufficient amount of cells and the culture often needs to go through long-term amplification in vitro, resulting in low cell viability after transplantation. Cells often develop fibrosis or are absorbed and cannot survive long. Thus, the desired clinical effect of the cell therapy cannot be accessed and so it was shelved for a while.
Considerable progress has been made in the induction and differentiation of embryonic stem cells (ESCs) since the last century and it brought new vitality to cell therapy. However, the differentiation of ESCs can bring immune rejection, tumorigenicity and differentiation of uncertainty, which limits its clinical application. Although induced pluripotent stem cells (iPS) can avoid the risk of immune rejection caused by ESC differentiation post-transplantation, the low conversion rate and the risk of tumor formation still exists and the potentially unpredictable biological danger achieved through genetic manipulation hinders its clinical application. Thus, cell therapy returns to the transplantation of the cultured autologous cells, especially the enriched stem cells and the methods and the results have been greatly improved.
With the development of research, it is increasingly recognized that the reason the implanted cells cannot survive long-term in cell therapy is not simply because of seed cells, but also because of the cell microenvironment which determines cell survival, proliferation and even reverse differentiation. The birth of Dolly the sheep is the best example. In a good embryo environment, mature breast cells can be re-developed into a new individual sheep. From a biological point of view, whether it is a cell or an organism, it must exist in its surroundings with exchange material, energy and information. In terms of cell therapy, the transplanted cells need a specific three-dimensional structure to anchor and specific extra cellular matrix (ECM) components and cytokine biological information transfer to support their growth. These structures are present in the matrix in which the stem cells located and are called the stem cell microenvironment. The microenvironment interacts with the stem cells and they are interdependent, mutually promote and complement each other, working together to maintain the stem cell homeostasis[2-5]. A large number of studies suggest that exploring how to strengthen the organic combination of the transplanted cells and the stem cell niche and reconstructing the stem cell microenvironment is the key to successful cell therapy.
In this review, we will recount the interactions of the stem cell microenvironment with the stem cells, discuss the importance of the stem cell microenvironment for cell-based therapy in ocular diseases, and introduce the progress of stem cell treatment for ocular diseases.
In the process of inducing embryonic stem cells and iPS cell differentiation, it was discovered that the adult cells can induce embryonic stem cells to differentiate and the embryonic stem cells can promote somatic cell proliferation, repair the defects of co-cultured cells, or even reverse the differentiation state of somatic cells[6-9] by improving the microenvironment via secreting a variety of factors and cell interactions, etc.
We began to work on the induction and differentiation of embryonic stem cells and epidermal stem cells[10-12] in 1997 and found that when the adult cells were treated with supernatant from cultured embryonic stem cells or co-cultured with the embryonic stem cells, the aging process of adult cells can be slowed down or even reversed into progenitor cells and their self-renewal and proliferation capacity can be increased significantly[13-18], whereas the adult cells can also induce embryonic stem cells to differentiate. We found a phenomenon that the corneal epithelial cells maintain long-term proliferative capacity and tissue-specific cell phenotype by factors secreted from murine ESCs. Rabbit corneal epithelial cells, cat corneal endothelial cells, rabbit skin epithelial cells and rabbit conjunctiva epithelial cells grew very well in culture medium with addition of ESC conditioned medium. These corneal epithelial cells were serially subcultured for more than 20 passages and maintained high cell purity, cobblestone-like morphology, enhanced colony forming efficiency, normal diploid and capacity to regenerate a functional stratified corneal epithelial equivalent. The rabbit corneal epithelial cells cultured in the embryonic stem cell microenvironment can be continuously passaged over 55 generations in 22 wk, gradually restoring its precursor characteristics, such as: decreased corneal epithelial cell specific differentiation markers K3/K12 expression, increased corneal epithelial precursor cell markers P63 and ABCG2 expression, but the expression of Oct-4 was not detected, indicating that the embryonic stem cell microenvironment treated cells obtained a strong proliferative capacity without the potential tumorigenicity and uncertain differentiation[13-18]. Zhang et al[13] also found that the proliferation and maturation of the dendrite cells co-cultured with the bone marrow mesenchymal cells were able to be significantly promoted, with the enhanced precursor cell marker expression and reduced expression of differentiation markers, so that the mature dendritic cells were reversed to the original progenitor cell stage. Pearton et al[9] reported that mouse embryonic skin can induce the terminal rabbit central corneal epithelial cells to reverse to the limbal stem cells by gradually losing specific marker K12 and K3. These results strongly suggest that the stem cell microenvironment can significantly regulate adult cell proliferation. It has the potential to become a more effective and safe method to access autologous seed cells with high proliferative activity which are close to pluripotent stem cells or transient amplifying cells without uncertain differentiation direction or tumorigenicity and render them more suitable for clinical use.
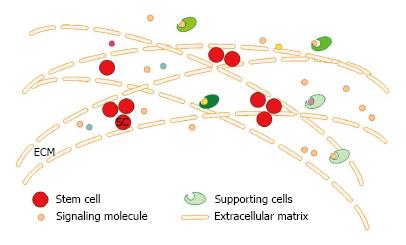
Stem cell microenvironment is the general term of the three-dimensional structure and a variety of signaling molecules (growth factors and their receptors, hormones and signaling molecules) present in the stroma where the stem cells reside and it can regulate the fate (proliferation/differentiation) of the stem cells. Because of its specific three-dimensional structure, it is vividly called niches (niche), which consists of three components: the extracellular matrix (ECM), niche cells (supporting cells, stem cells) and soluble factors derived from the niche cells (Figure 1). The proliferation and differentiation of stem cells are pre-programmed by themselves and are also affected by the microenvironment where they are residing. The stem cell microenvironment can anchor stem cells in vivo and regulate the self-renewal and production of their progeny cells through cell-cell, cell-ECM and cytokine-cell interactions. The different macromolecules or properties of the cells and ECM interact with each other in a complex and dynamic network[19,20]. Nowadays, there is increasing evidence showing that the ECM is not only the supportive scaffold but also plays a fundamental role in cell biology. It plays important roles in the development of the cells and can regulate their behavior[21] by the production, degradation of its components and the remodeling of the structure[22,23] and the direct and indirect signaling properties[21]. The polarity, division and migration of the cells can be influenced by the physical properties of the ECM, such as rigidity, porosity, topography and insolubility[24]. Cytokines play an important role in exchanging information from cell-cell and cell-ECM. The changes of the extracellular matrix components also affect the differentiation of the stem cells and the induced differentiation in vitro is accomplished by mimicking the cell microenvironment. So, it is difficult to obtain a long lasting therapeutic effect in cell-based therapy without the support of a good stem cell microenvironment, even when excellent cells are transplanted.
The importance of stem cell microenvironment in tissue engineering has also been verified. How to rebuild the stem cell microenvironment becomes the biggest challenge currently for constructing tissue engineered tissues and organs. In the past, because the scaffolds for tissue engineering of organs and tissues had no such sophisticated stem cell microenvironment, the desired therapeutic effect could not be achieved and the structure and function could not be completely recovered after transplantation. In 2009, we introduced phospholipase A2, which can specifically hydrolyze the phospholipids of the corneal stroma cell membrane, can destroy the cell structure and be used to prepare acellular porcine corneal stroma acellular porcine corneal stroma (APCS) for biological tissue engineering cornea[25]. The natural corneal collagen structure and 80% of the extracellular matrix components can be retained by this means. This APCS not only has good biocompatibility and biomechanical strength, but also keeps the limbal stem cells microenvironment necessary for their long-term proliferation. The grafts of APCS maintained good biomechanical strength and high transparency post-transplantation in animal experiments and it can help to rebuild the limbal stem cell microenvironment[26-30]. Nakayama et al[31], Ott et al[32], Petersen et al[33], Uygun et al[34] and Conrad et al[35] have also reported the use of acellular matrix as a scaffold in tissue engineering in kidneys, lungs, heart, trachea and bladder. As Song et al[36] stated, using an acellular matrix scaffold with natural extracellular matrix to build tissue engineering products can mediate organ development, reparation and regeneration which cannot be accessed by synthetic materials. There are further experiments that proved that the acellular materials with extracellular matrix play an important role in the regulation of stem cells[37]. These studies showed that a decellularized tissue with the natural ECM scaffold can induce the stem cells to differentiate into cell types present in certain tissue[31] and thus the decellularized organ has already been used in tissue engineering and cell therapy[31,36].
The microenvironment has a great effect on the fate of stem cells. In recombination experiments, hair follicle stem cells were induced to differentiate into corneal epithelial cells when they were cultured in a limbus-specific-like niche[38]. These cells grow into stratified epithelium and express the cornea-specific markers K12 and Pax6 while the epidermal specific K10 obviously down-regulated[38]. While in other studies the rabbit epithelial cells from the central cornea differentiated into epidermal keratinocytes when recombined with mouse embryonic dermis, it lost corneal-specific marker K3/K12 together with the down-regulation of Pax6 and expressed keratinocyte marker K5/K14[9]. All of these changes showed that the microenvironment has an important regulation role in the fate of the stem cells.
On the other hand, evidence in vivo showed the relevance of the ECM and the stem cell behavior in that the altered properties or the aged niches’ ability of maintaining stem cells’ stemness can be reduced[39] and it further affected stem cell proliferation and updated, which created a vicious cycle. Scientists from Harvard University attempted to study the relationship between human aging and microenvironmental changes between stem cells via the stem cell microenvironment (niche) and they found that altering the aged microenvironment with the young microenvironment can improve the ability of the proliferation and renewal of the stem cells. The study also found that adding “insulin-like growth factor-1” (IGF-1) can also improve the state of the aging stem cell microenvironment.
Cell receptors can directly mediate the interactions between the stem cells and the ECM where they are located. It was also found that integrins, a large family of heterodimeric transmembrane receptors, are important receptors for the ECM-stem cell interactions and can regulate cell survival, migration, proliferation and differentiation by connecting the ECM to the intracellular cytoskeleton[40] as well as the adhesion, anchorage and homing of the stem cells. Different kinds of integrins bind to different ECM components or different cell surface adhesion molecules and receptors[41-43]. Integrins can regulate the self-renewal and proliferation of the stem cells by directly activating focal adhesion kinase (FAK) and the phosphoinositide 3-kinase (PI3K) signaling pathway[40,44,45]. The α6β1 integrin can bind to the ECM protein laminin and help the spermatogonial stem cells home in to the testicular niche[46] and NSCs (neural stem cells) adhere to the vascular niche[47]. The α9 integrin is essential for the proliferation of the HSC[48] and NSC microenvironment[49] via binding protein tenascin-C in the ECM. The α4, α6, α9 and β1 integrin chains are also important to the HSCs in their homing in to the bone marrow niche[50-53]. HSC homing and proliferation are also regulated by αvβ3 integrin[54-56]. Furthermore, β1 integrins can control the balance between symmetric and asymmetric divisions in skin and brain, as well as the differentiation and self-renewal of the stem cells[57-60] by regulating the activity of the Notch pathway and EGF receptor[61,62]. They are also important for the proliferation of intestinal stem cells (ISCs) via the Hedgehog signaling pathway[63]. Signaling pathways of the growth factors and cytokines, such as IL-3 and TGF-β[43,64-67], can also be regulated by integrins and these signaling pathways can regulate the expression of integrins conversely[46]. Therefore, the receptors have specific activities in a certain kind of stem cell microenvironment.
Some ECM components can regulate their availability by setting a biochemical gradient and binding growth factors[21]. On the one hand, the ECM can also make the growth factors insoluble, unavailable or not bioactive and serve as the reservoir for them, like proteoglycans, collagens, fibronectin and vitronectin, which bind VEGFs, FGFs, HGFs, TGF-β and BMPs. On the other hand, ECM proteins and proteoglycans can be induced to be soluble and remodeled to release and distribute growth factors under the action of enzymes, such as metalloproteinase[21]. The function NSC can be favored by the ECM components in its niche by promoting growth factor activity via capturing FGF-2 from it[68,69]. Similarly, in the procedure of the regulation of muscle satellite cells, all kinds of growth factors bind to the cell surface or the basal lamina proteoglycan and then they can be activated in a certain signaling pathway[70].
The biophysical properties of the ECM can determine the behavior of stem cells. The balance of the internal forces generated by cell cytoskeleton tension and the external forces from the compression of the neighboring cells and the stiffness of the surrounding ECM maintains the shape of the cells and makes them stay in their anatomical localization[71], which can regulate the cell behavior finally[72-74]. Recently, the YAP/TAZ transcriptional factors were found to have key biological effects on the ECM elasticity, cell geometry and cytoskeletal modulation[71,74,75] among the mechanotransduction pathways (Wnt/β-catenin, PI3K/Akt, TGF-β, Ras/MAPK, and RhoA/ROCK pathways). In fact, ECM organization and composition can regulate tissue stiffness and then have an influence on the stem cell behavior[72,76]. The human mesenchymal stem cells can express organ-specific transcription factors and differentiate into myoblasts, neurons and osteoblasts[77] when they are cultured on ECMs with similar stiffness of the muscle, brain or bone respectively. Culture on hydrogel with the same elastic modulus as the bone marrow can increase the self-renewal ability and maintain multipotency of the hMSCs (human mesenchymal stem cells) compared to those cultured on stiffer substrates[78]. NSCs cultured on hydrogel with similar stiffness to the brain tissue gain the neuronal differentiation potential, while the stiffer gels make them differentiate into glial cells[79]. That the stiffness gradients of the hippocampus regulate NSC behavior in vivo confirmed that ECM and its biomechanical properties play important roles in the fate of the stem cell[80]. This opens new insights in to the role of ECM mechanical properties in the stem cell microenvironment.
Stem cells in ocular tissues: Several studies showed the conjunctiva epithelial stem cell niche located in the fornix by growth potential assays, label-retention analyses and keratin expression detection[81-84]. They have bipotential to differentiate into both epithelial cells and goblet cells[85].
Corneal epithelial stem cells resident at the basal limbal epithelium and called limbal stem cells were first applied to clinical use in ophthalmology. Tung-Tien Sun’s group did immunostaining with monoclonal antibodies against the corneal-specific K3[86] and showed the negative expression of K3/K12 in the limbal basal layer, which gave rise to a number of experiments that verified that the corneal stem cells were located in the limbus[86-88]. These label-retaining limbal cells[89] have a higher proliferative potential[90] and colony-forming ability[91] compared with central ones.
People made efforts to find specific molecules markers of the limbal stem cells. p63[92], vimentin[93-95], α-enolase[96,97], α9β1 integrin[98], tenascin-C and EMILIN1[99],ABCG2[100-103] and ABCB5[104] are all highly expressed in the basal layer of limbal epithelium but none of them are the specific markers of the limbal stem cells as expected. This is because the early differentiating cells[105,106] still have the stem cell markers and show intermediate profiles between stem and differentiated cells until the stem cell markers are down-regulated with the expression of the differentiated phenotype[107]. Thus, we can only enrich the stem cells while separating them with these molecules markers[108].
Since it is complex and difficult to characterize the corneal stem cells, people try to identify them by analyzing their niches and the regulatory functions of the niche.
The limbus is a specific region which is characterized by the palisades of Vogt with the papillae-like projections and the vascular net in the peripheral cornea[109]. It enables the epithelial cells to interact with ECM and chemical signals diffused from the vascular network[110]. Some studies showed that the limbus has a specific anatomic structure such as the niche, the LEC (limbal epithelial crypt) or LC (limbal crypt), which consists of a cord or finger of cells that are located between the palisades of the limbal stroma and extends radically to the conjunctiva stroma[92,93]. The high expression of K14[111], ABCG2[112] and p63[92,93] in cells at the LEC[112]/LC[113] suggest that they are the microenvironment of the limbal stem cells but the LEC/LC structure has not been found in other species besides humans and pigs[94].
This tissue with unique cellular properties can synthesize different kinds of ECM substrates. Several studies on the ECM components of the cornea were performed regarding the aspect of the biochemical and immunological characteristics. Corneal stroma comprises collagen type I-VI[114-117], glycosaminoglycans (chondroitin, heparin, dermatan) and keratan sulfates[118-122], fibronectin and laminin[105,123] and hyaluronic acid[124] and the limbal epithelial cells are more likely to adhere to a rougher surface than those in the central cornea[125]. To further study the interaction between corneal stem cells and their microenvironment and the different functions between the central cornea and the limbus, the corneal basement membrane components were analyzed by several studies. These studies found that the conjunctiva, limbal and central corneal epithelia have a heterogeneous composition of the basal membrane (BM)[126]. Some studies reported that there was no collagen IV in the central cornea BM[127], while others had the controversial results that collagen IV presented both in the limbal and the central corneal BM[126]. Later, people found that collagen IV α1 (IV) and α2 (IV) chains show more intense staining at the corneal limbus and the α3 (IV) chain shows an abrupt decrease at the limbus[128,129], while collagen types IV (α3-α4 chains) and XII are only expressed in the central cornea[128]. Then, other components of the limbal BM were studied further. Laminin α2-α5, β1-β3, γ1-γ3, nidogen-1, -2, SPARC/BM-40, as well as agrin are preferentially expressed in the limbal BM[128], which colocalized with the ABCG2/p63/K19-positive and K3/Cx43/desmoglein/integrin-a2-negative stem cells and early progenitor cell clusters[128,129]. The BM components, such as type XVI collagen, fibulin-2, tenascin-C/R, vitronectin, bamacan, chondroitin sulfate and versican, are colocalized with the putative vimentin-positive late progenitor cells[128-130] at the limbus. On the contrary, type V collagen, fibrillin-1 and 2, and thrombospondin-1 were almost only found in the corneal BM[128]; others, such as type IV collagen α5 and α6 chains, collagen types VII, XV, XVII and XVIII, laminin-111, laminin-332, laminin chains α3, β3 and γ2, fibronectin, matrilin-2 and 4, and perlecan, were expressed throughout the epithelial layer on the ocular surface[129,130]. All these studies showed that the BM at the limbus has a specific ECM composition which is different from that in the peripheral or central cornea. This suggested that the EMC at the LEC/LC created a microenvironment that regulates stem cells and their progeny by supporting stemness while inhibiting the differentiation and preserving the proliferative abilities in limbal cells.
The stem cells of the corneal endothelium and the trabecular meshwork are believed to be located at the transition zone between the peripheral corneal endothelium and the anterior non-filtering portion of the trabecular meshwork[131]. Corneal stroma stem cells are located in the limbal stroma, play roles in visualization and have corresponded to the limbal niche cells[132].
At the early stage, the stem cells of the lens were assumed to be the label-retaining cells which are located at the anterior central region of the lens[133]. Yamamoto et al[134] concluded that the germinative zone of the lens epithelium contains transient amplifying cells with the positive expression of proliferation markers, such as A1, B1, C and D1 cyclins and PCNA (proliferating cell nuclear antigen), and can be labeled by BrdU (5-bromo-2’-deoxyuridine). On the other hand, other studies showed that they were probably located in the region anterior to the germinative zone. However, Remington et al[135] assumed that lens stem cells resided in the ciliary body because the lens is non-vascular, its epithelium does not have the morphology of other stem cells and no type of tumors are derived from the lens. Thus, the existence of the lens stem cells remains controversial and needs to be elucidated.
Previously, it was believed that there were no stem cells in the mammalian retina since it cannot regenerate[136] but von Leithner et al[137] found retinal precursors in the peripheral retinal pigment epithelium later. However, the cells from the pigmented ciliary margin were later found to have the ability to form spherical colonies and produce various types of differentiated retinal cell[138]. These results gave the evidence that retinal stem cells are located in the pigmented ciliary margin epithelium.
Limbal stem cell deficiency can be caused by a myriad of insults that present with the following pathological states: damaged corneal barrier function, persistent corneal epithelium defects or recurrent corneal erosions, chronic inflammation associated with corneal stromal scarring, visualization, conjunctivalization and eventually blindness. Some researchers speculate that this occurs due to gradual deterioration of the limbal stromal niches[139]. Thus, limbal epithelial stem cell transplantation and the reestablishment of the limbal stem cell microenvironment are necessary for ocular surface reconstruction in these diseases[139].
Autologous or allogenic limbal transplantation has achieved good clinical efficiency in the treatment of limbal stem cell deficiency. However, in successful cases post limbal transplantation, there is a doubt as to whether there is a causal relationship between the survival of the donor limbal stem cells and the clinical effect. Shimazaki et al[140] detected the presence of donor-derived epithelial cells in 60% of cases (10 eyes/9 cases) post human limbal transplantation with fluorescence in situ hybridization assay, with 77.8% by RFLP analysis. At the same time, the authors stated that there was no difference in the postoperative clinical outcomes whether the presence of survived donor-derived cells was detected. Thus, more studies should be carried out to confirm the clinical significance of the survival of donor cells. Furthermore, it was reported that donor-derived cells were undetected in cases with objective clinical improvement after three to five years post limbal transplantation, even with DNA fingerprinting analysis[141]. This suggested that the survival of donor-derived cells is not essential for improvements of the clinical symptoms. These results indicated that limbal transplantation might simply serve as the corneal stroma transplantation. Presumably, limbal transplantation improves the residual stoma stem cell microenvironment, making residual stem cells in the patient regenerate on the ocular surface and allowing for improvement of the ocular surface[142]. Therefore, some scholars believe that the essence of limbal transplantation may be the restoration of the limbal stroma resulting in increased stability of the limbal stem cell “niche”[143]. So, limbal epithelial stem cell deficiency treatment lies in the application of various methods to restore the normal limbus matrix.
Although traditional corneal transplantation, limbal transplantation, has already achieved good results in ocular surface reconstruction for corneal diseases, there still several problems that have hindered clinical application, such as the shortage of donors, the immune rejection post-transplantation and other issues. Therefore, the construction of tissue engineering cornea in vitro with appropriate biomaterials and synthetic materials is the hope for solving these questions. How to get a sufficient amount of high activity seed cells for tissue engineering has been the challenge for tissue engineering product construction and cell therapy. The microenvironment plays an important role in the survival and development of cells and tissues. The microenvironment or simulated microenvironment can effectively induce ES and iPS differentiation in a certain direction and the embryonic microenvironment also has the effect of reverse differentiation of the adult cells. We have considered two aspects in our studies: (1) cellular microenvironment: embryonic stem cell microenvironment culture systems can make the differentiated corneal epithelial cells, conjunctiva epithelial cells[15] and even human corneal endothelial cells[17] obtain a strong proliferation ability and can be passaged long-term with de-differentiation cell marker expression, normal cell morphology and karyotype, but no tumorigenicity. Our preliminary findings showed that the ES microenvironment may inhibit the apoptosis of the cells by activating telomerase via integrin the b1-FAK-PI3K/Akt, telomerase-p21-mitochondrial axis and FAK/Wnt signaling pathway[18,144]; and (2) stromal microenvironment: the APCS limbal produced by lipase (not existing protease digestion)[25,30] can retain the normal extracellular matrix, collagen lamellar micro ultrastructure. It can repair and maintain the stemness and proliferation ability of the limbal stem cells after it is transplanted to the limbal stem cell deficiency rabbit model. In short, the microenvironment can be used to obtain sufficiently pure seed cells with strong proliferation capability but no immunogenicity. The microenvironment plays an important role in cell therapy and is the basis for the long-term efficacy of the treatment. Establishment of seed cells using the microenvironment will make cell therapy return to autologous cell transplantation. Maintaining the specific three-dimensional structure and the extracellular matrix to promote the proliferation and long-term survival of the transplanted cells[145] has great meaning. Professor Ott et al[32] stated that acellular matrix has a natural extracellular matrix which can mediate and guide organ development and mediate repair and regeneration. The key steps for the best clinical efficacy of cell transplantation are the long-term survival of the seed cells and the reconstruction of the stem cell niche.
Retinal stem cells and optic nerve repair: Retinal diseases such as age-related macular degeneration, Leber congenital amaurosis and cone rod dystrophy are caused by lesions of retinal neuronal cells, which have an irreversible pathological process of degeneration and damage of the retinal neuronal cells, causing serious visual impairment or even blindness which currently cannot be effectively treated. Since the stem cells have self-renewal and multi-differentiation potential abilities, using stem cells as donor cells for retinal diseases treatment has become a hot topic.
In 2000, Wirtschafter et al[83] found that groups of self-renewing cells in the ciliary epithelium of the adult mice can form neurospheres when they were cultured in vitro. They can be induced to differentiate into specific types of neuronal cells in the retina, such as the rod cells, bipolar cells and glial cells, indicating that retinal stem cells exist in adult mammalian eyes. Ballios et al[146] found that retinal stem cells (retinal stem cell, RSC) also exist in the ciliary margin zone in people of different ages. These cells have proliferative capacity in vitro and can be induced to differentiate into different retinal neurons. Some of these stem cells can migrate and integrate into the host retina and can even differentiate into photoreceptor cells. These studies suggested that RSCs from different sources of animals or humans can survive and migrate to the host retina layers after transplantation. Although the implanted RSCs can migrate into the retina and differentiate into a variety of retinal cells, most transplanted RSCs remain in the subretinal space or the vitreous body, while less can be integrated and induced to differentiation, so the efficiency is not ideal.
Meyer et al[147] transplanted GFP-labeled ESCs into mouse vitreous after induced differentiation and found that the transplanted cells can migrate into the entire retina layers and differentiate into retinal neurons cells with the expression of markers such as NeuN, calretinin and cPKC-a. In 2010, Parameswaran et al[148], using a similar method as embryonic stem cell differentiation, completed the differentiation of mouse iPS cells into retinal ganglion cells, which not only highly express the retinal ganglion cells regulation gene Ath5, Wtl, Brn3b, Rpfl and lrx2, but also can specifically project upwards to the superior colliculus with the synapse structure formation which has a sensitive tetrodotoxin voltage-dependent sodium current. This fully proved that iPS cells can differentiate into retinal ganglion cells. In glaucoma and traumatic optic neuropathy, the regeneration of retinal ganglion cells may be the only way to restore vision. The finding that iPS cells can differentiate into retinal ganglion cells provides a new method for the treatment of such diseases.
Arnhold et al[149] found that the cones cannot survive without healthy rod cells. Therefore, it is difficult to achieve the aim of the treatment by simply transplanting the induced iPS cells into the retina of patients with retinitis pigmentosa because the rod cells will eventually die.
Stem cell treatment for retinitis pigmentosa: Retinal pigment epithelium (RPE) is a single layer of epithelium with polarity and a rich pigment that is located between the neural retina and choriocapillaris layer. It can support the metabolism and activities of the retinal photoreceptor cells and phagocytose the outer segment photoreceptors. Recent studies show that the retinal pigment epithelium also has self-renewing stem cells that can be induced to form other cell types under suitable conditions.
RPE degeneration diseases caused by age-related macular degeneration (AMD), hereditary retinal degeneration, macular dystrophy and Stargardt disease result in the death of photoreceptors and neural retina and eventually cause blindness. There is currently no effective treatment for retinitis pigmentosa. The transplantation of the induced differentiated stem cells to establish the retinal pigment epithelium membrane in vivo has been studied extensively but it is still far from functional reconstruction. Thus, looking for new ways to stimulate the repair of RPE is an important research direction for the future. With the rise of regenerative medicine research, stem cell transplantation as a regenerative therapy becomes a hotspot for research. In the past decade, scholars induced bone marrow mesenchymal stem cells, iPS cells and embryonic stem cells to differentiate into retinal pigment epithelial cells but the process of differentiation into RPE cells is not clear and only a small fraction of cells can be differentiated into RPE cells. So, the research of finding the desired factors to directly induce cell differentiation is still very popular.
In 2010, Geron biopharmaceutical company (Geron, United States) sponsored the world’s first clinical trial on using hESC to repair damaged nerves. Currently, the United States Food and Drug Administration has approved the ACT company (Advanced Stem Cell Technologies) to carry out two Phase II clinical trials on using embryonic stem cells to treat macular degeneration in the United States, dry AMD and juvenile macular dystrophy (Stargardt disease). This time, they induced hESCs to differentiate into retinal epithelial cells with purity over 99%. Approximately 50000 retinal pigment epithelial cells were isolated and injected into the retina of two patients. Four months later, the researchers found that RPE had been completely replaced by the injected retinal epithelial cells. They measured the visual acuity of the two female patients and the data confirmed that the injected cells survived and largely improved their vision. Early data suggest that hESC therapy is not only safe but also efficient. The research paper was published in the world’s oldest and most respected peer-reviewed medical journal, “The Lancet”[150]. These results from ACT have brought new optimistic hope for stem cell research. This same study found that it is crucial that the transplanted cells can attach to the Bruch’s membrane and integrate into the host RPE layer and survive to have a successful therapeutic effect[149].
In summary, recent studies show that real cell therapy is not a simple supplement of cells. The interaction, interdependence, mutual promotion and supplementation between the transplanted cells and the microenvironment are more important. The microenvironment of the recipient can regulate the transplanted cell behavior and decide their fate. The transplanted cells cannot survive long-term without the support from the microenvironment of the recipient. The survived transplanted cells can not only completely replace the recipient’s cells, but also supplement the sufficient quantity and function of the recipient’s cells. More importantly, they are involved in the cellular microenvironment reconstruction so the stem cells obtain a stronger self-renewal capacity and proliferation ability. Therefore, in order to improve clinical cell therapy, we should pay more attention to the characteristics and components of each stem cell microenvironment and put efforts into understanding the regulation mechanisms of the stem cell microenvironment[151].
P- Reviewer: Navas A, Shih YF S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | Liu J, Song G, Wang Z, Huang B, Gao Q, Liu B, Xu Y, Liang X, Ma P, Gao N. Establishment of a corneal epithelial cell line spontaneously derived from human limbal cells. Exp Eye Res. 2007;84:599-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673-1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2247] [Cited by in RCA: 1920] [Article Influence: 112.9] [Reference Citation Analysis (0)] |
| 3. | Peerani R, Zandstra PW. Enabling stem cell therapies through synthetic stem cell-niche engineering. J Clin Invest. 2010;120:60-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Pera MF, Tam PP. Extrinsic regulation of pluripotent stem cells. Nature. 2010;465:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 223] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | Watt FM, Fujiwara H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb Perspect Biol. 2011;3:a005124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 280] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 6. | Chou YF, Chen HH, Eijpe M, Yabuuchi A, Chenoweth JG, Tesar P, Lu J, McKay RD, Geijsen N. The growth factor environment defines distinct pluripotent ground states in novel blastocyst-derived stem cells. Cell. 2008;135:449-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Guo Y, Graham-Evans B, Broxmeyer HE. Murine embryonic stem cells secrete cytokines/growth modulators that enhance cell survival/anti-apoptosis and stimulate colony formation of murine hematopoietic progenitor cells. Stem Cells. 2006;24:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120-4126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 992] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 9. | Pearton DJ, Yang Y, Dhouailly D. Transdifferentiation of corneal epithelium into epidermis occurs by means of a multistep process triggered by dermal developmental signals. Proc Natl Acad Sci USA. 2005;102:3714-3719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Wang Z, Ge J, Chen J, Huang B. [Preliminary experimental study on commitment differentiation of embryonic stem cells induced by corneal limbal stroma in vitro]. Yanke Xuebao. 1999;15:195-198. [PubMed] |
| 11. | Wang Z, Ge J, Huang B, Gao Q, Liu B, Wang L, Yu L, Fan Z, Lu X, Liu J. Differentiation of embryonic stem cells into corneal epithelium. Sci China C Life Sci. 2005;48:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Gao N, Wang Z, Huang B, Ge J, Lu R, Zhang K, Fan Z, Lu L, Peng Z, Cui G. Putative epidermal stem cell convert into corneal epithelium-like cell under corneal tissue in vitro. Sci China C Life Sci. 2007;50:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Zhang B, Liu R, Shi D, Liu X, Chen Y, Dou X, Zhu X, Lu C, Liang W, Liao L. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood. 2009;113:46-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 14. | Orkin SH, Hochedlinger K. Chromatin connections to pluripotency and cellular reprogramming. Cell. 2011;145:835-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 318] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 15. | Liu Y, Ding Y, Ma P, Wu Z, Duan H, Liu Z, Wan P, Lu X, Xiang P, Ge J. Enhancement of long-term proliferative capacity of rabbit corneal epithelial cells by embryonic stem cell conditioned medium. Tissue Eng Part C Methods. 2010;16:793-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Zhan W, Liu Z, Liu Y, Ke Q, Ding Y, Lu X, Wang Z. Modulation of rabbit corneal epithelial cells fate using embryonic stem cell extract. Mol Vis. 2010;16:1154-1161. [PubMed] |
| 17. | Lu X, Chen D, Liu Z, Li C, Liu Y, Zhou J, Wan P, Mou YG, Wang Z. Enhanced survival in vitro of human corneal endothelial cells using mouse embryonic stem cell conditioned medium. Mol Vis. 2010;16:611-622. [PubMed] |
| 18. | Zhou J, Chen F, Xiao J, Li C, Liu Y, Ding Y, Wan P, Wang X, Huang J, Wang Z. Enhanced functional properties of corneal epithelial cells by coculture with embryonic stem cells via the integrin β1-FAK-PI3K/Akt pathway. Int J Biochem Cell Biol. 2011;43:1168-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Ozbek S, Balasubramanian PG, Chiquet-Ehrismann R, Tucker RP, Adams JC. The evolution of extracellular matrix. Mol Biol Cell. 2010;21:4300-4305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 247] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 20. | Watt FM, Huck WT. Role of the extracellular matrix in regulating stem cell fate. Nat Rev Mol Cell Biol. 2013;14:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 629] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 21. | Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2554] [Cited by in RCA: 2522] [Article Influence: 148.4] [Reference Citation Analysis (0)] |
| 22. | Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221-233. [PubMed] |
| 23. | Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:a005058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1129] [Cited by in RCA: 1606] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 24. | Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2019] [Cited by in RCA: 2306] [Article Influence: 164.7] [Reference Citation Analysis (0)] |
| 25. | Wu Z, Zhou Y, Li N, Huang M, Duan H, Ge J, Xiang P, Wang Z. The use of phospholipase A(2) to prepare acellular porcine corneal stroma as a tissue engineering scaffold. Biomaterials. 2009;30:3513-3522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Zhou Y, Wu Z, Ge J, Wan P, Li N, Xiang P, Gao Q, Wang Z. Development and characterization of acellular porcine corneal matrix using sodium dodecylsulfate. Cornea. 2011;30:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Wu Z, Zhou Q, Duan H, Wang X, Xiao J, Duan H, Li N, Li C, Wan P, Liu Y. Reconstruction of auto-tissue-engineered lamellar cornea by dynamic culture for transplantation: a rabbit model. PLoS One. 2014;9:e93012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Xiao J, Duan H, Liu Z, Wu Z, Lan Y, Zhang W, Li C, Chen F, Zhou Q, Wang X. Construction of the recellularized corneal stroma using porous acellular corneal scaffold. Biomaterials. 2011;32:6962-6971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Liu Z, Zhou Q, Zhu J, Xiao J, Wan P, Zhou C, Huang Z, Qiang N, Zhang W, Wu Z. Using genipin-crosslinked acellular porcine corneal stroma for cosmetic corneal lens implants. Biomaterials. 2012;33:7336-7346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Huang M, Li N, Wu Z, Wan P, Liang X, Zhang W, Wang X, Li C, Xiao J, Zhou Q. Using acellular porcine limbal stroma for rabbit limbal stem cell microenvironment reconstruction. Biomaterials. 2011;32:7812-7821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Nakayama KH, Batchelder CA, Lee CI, Tarantal AF. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng Part A. 2010;16:2207-2216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 32. | Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 818] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 33. | Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1015] [Cited by in RCA: 855] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 34. | Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1162] [Cited by in RCA: 1013] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 35. | Conrad C, Schuetz C, Clippinger B, Vacanti JP, Markmann JF, Ott HC. Bio-engineered endocrine pancreas based on decellularized pancreatic matrix and mesenchymal stem cell/islet cell coculture. J Am Coll Surgeons. 2010;211:S62. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med. 2011;17:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 351] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 37. | Soto-Gutierrez A, Yagi H, Uygun BE, Navarro-Alvarez N, Uygun K, Kobayashi N, Yang YG, Yarmush ML. Cell delivery: from cell transplantation to organ engineering. Cell Transplant. 2010;19:655-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Blazejewska EA, Schlötzer-Schrehardt U, Zenkel M, Bachmann B, Chankiewitz E, Jacobi C, Kruse FE. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells. 2009;27:642-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 39. | Kurtz A, Oh SJ. Age related changes of the extracellular matrix and stem cell maintenance. Prev Med. 2012;54 Suppl:S50-S56. [PubMed] |
| 40. | Legate KR, Wickström SA, Fässler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 582] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 41. | Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673-687. [PubMed] |
| 42. | Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1074] [Cited by in RCA: 1238] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 43. | Brizzi MF, Tarone G, Defilippi P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr Opin Cell Biol. 2012;24:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 299] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 44. | Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209:139-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 835] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 45. | Buitenhuis M. The role of PI3K/protein kinase B (PKB/c-akt) in migration and homing of hematopoietic stem and progenitor cells. Curr Opin Hematol. 2011;18:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Kanatsu-Shinohara M, Takehashi M, Takashima S, Lee J, Morimoto H, Chuma S, Raducanu A, Nakatsuji N, Fässler R, Shinohara T. Homing of mouse spermatogonial stem cells to germline niche depends on beta1-integrin. Cell Stem Cell. 2008;3:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 47. | Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 862] [Cited by in RCA: 805] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 48. | Nakamura-Ishizu A, Okuno Y, Omatsu Y, Okabe K, Morimoto J, Uede T, Nagasawa T, Suda T, Kubota Y. Extracellular matrix protein tenascin-C is required in the bone marrow microenvironment primed for hematopoietic regeneration. Blood. 2012;119:5429-5437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 49. | Kazanis I, Belhadi A, Faissner A, Ffrench-Constant C. The adult mouse subependymal zone regenerates efficiently in the absence of tenascin-C. J Neurosci. 2007;27:13991-13996. [PubMed] |
| 50. | Potocnik AJ, Brakebusch C, Fässler R. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity. 2000;12:653-663. [PubMed] |
| 51. | Qian H, Tryggvason K, Jacobsen SE, Ekblom M. Contribution of alpha6 integrins to hematopoietic stem and progenitor cell homing to bone marrow and collaboration with alpha4 integrins. Blood. 2006;107:3503-3510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 52. | Grassinger J, Haylock DN, Storan MJ, Haines GO, Williams B, Whitty GA, Vinson AR, Be CL, Li S, Sørensen ES. Thrombin-cleaved osteopontin regulates hemopoietic stem and progenitor cell functions through interactions with alpha9beta1 and alpha4beta1 integrins. Blood. 2009;114:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 53. | Schreiber TD, Steinl C, Essl M, Abele H, Geiger K, Müller CA, Aicher WK, Klein G. The integrin alpha9beta1 on hematopoietic stem and progenitor cells: involvement in cell adhesion, proliferation and differentiation. Haematologica. 2009;94:1493-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 589] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 55. | Wang Z, Li G, Tse W, Bunting KD. Conditional deletion of STAT5 in adult mouse hematopoietic stem cells causes loss of quiescence and permits efficient nonablative stem cell replacement. Blood. 2009;113:4856-4865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 56. | Umemoto T, Yamato M, Ishihara J, Shiratsuchi Y, Utsumi M, Morita Y, Tsukui H, Terasawa M, Shibata T, Nishida K. Integrin-αvβ3 regulates thrombopoietin-mediated maintenance of hematopoietic stem cells. Blood. 2012;119:83-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Raymond K, Deugnier MA, Faraldo MM, Glukhova MA. Adhesion within the stem cell niches. Curr Opin Cell Biol. 2009;21:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 58. | Marthiens V, Kazanis I, Moss L, Long K, Ffrench-Constant C. Adhesion molecules in the stem cell niche--more than just staying in shape? J Cell Sci. 2010;123:1613-1622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 59. | Suh HN, Han HJ. Collagen I regulates the self-renewal of mouse embryonic stem cells through α2β1 integrin- and DDR1-dependent Bmi-1. J Cell Physiol. 2011;226:3422-3432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 60. | Chen S, Lewallen M, Xie T. Adhesion in the stem cell niche: biological roles and regulation. Development. 2013;140:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 61. | Campos LS, Decker L, Taylor V, Skarnes W. Notch, epidermal growth factor receptor, and beta1-integrin pathways are coordinated in neural stem cells. J Biol Chem. 2006;281:5300-5309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 62. | Brisken C, Duss S. Stem cells and the stem cell niche in the breast: an integrated hormonal and developmental perspective. Stem Cell Rev. 2007;3:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Jones RG, Li X, Gray PD, Kuang J, Clayton F, Samowitz WS, Madison BB, Gumucio DL, Kuwada SK. Conditional deletion of beta1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. J Cell Biol. 2006;175:505-514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 64. | Defilippi P, Rosso A, Dentelli P, Calvi C, Garbarino G, Tarone G, Pegoraro L, Brizzi MF. {beta}1 Integrin and IL-3R coordinately regulate STAT5 activation and anchorage-dependent proliferation. J Cell Biol. 2005;168:1099-1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Uberti B, Dentelli P, Rosso A, Defilippi P, Brizzi MF. Inhibition of β1 integrin and IL-3Rβ common subunit interaction hinders tumour angiogenesis. Oncogene. 2010;29:6581-6590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 529] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 67. | Ivaska J, Heino J. Cooperation between integrins and growth factor receptors in signaling and endocytosis. Annu Rev Cell Dev Biol. 2011;27:291-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 68. | Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, Mercier F. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 69. | Douet V, Kerever A, Arikawa-Hirasawa E, Mercier F. Fractone-heparan sulphates mediate FGF-2 stimulation of cell proliferation in the adult subventricular zone. Cell Prolif. 2013;46:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Cosgrove BD, Sacco A, Gilbert PM, Blau HM. A home away from home: challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation. 2009;78:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 71. | Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 772] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 72. | Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010;137:1407-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 619] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 73. | Mammoto A, Mammoto T, Ingber DE. Mechanosensitive mechanisms in transcriptional regulation. J Cell Sci. 2012;125:3061-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 293] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 74. | Hao J, Zhang Y, Wang Y, Ye R, Qiu J, Zhao Z, Li J. Role of extracellular matrix and YAP/TAZ in cell fate determination. Cell Signal. 2014;26:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 75. | Sun Y, Chen CS, Fu J. Forcing stem cells to behave: a biophysical perspective of the cellular microenvironment. Annu Rev Biophys. 2012;41:519-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 323] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 76. | DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12:308-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 728] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 77. | Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9969] [Cited by in RCA: 9927] [Article Influence: 496.4] [Reference Citation Analysis (14)] |
| 78. | Winer JP, Janmey PA, McCormick ME, Funaki M. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng Part A. 2009;15:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 272] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 79. | Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate modulus directs neural stem cell behavior. Biophys J. 2008;95:4426-4438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 790] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 80. | Keung AJ, de Juan-Pardo EM, Schaffer DV, Kumar S. Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells. 2011;29:1886-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 81. | Wei ZG, Wu RL, Lavker RM, Sun TT. In vitro growth and differentiation of rabbit bulbar, fornix, and palpebral conjunctival epithelia. Implications on conjunctival epithelial transdifferentiation and stem cells. Invest Ophthalmol Vis Sci. 1993;34:1814-1828. [PubMed] |
| 82. | Wei ZG, Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells are preferentially located in fornical epithelium: implications on conjunctival epithelial homeostasis. Invest Ophthalmol Vis Sci. 1995;36:236-246. [PubMed] |
| 83. | Wirtschafter JD, McLoon LK, Ketcham JM, Weinstock RJ, Cheung JC. Palpebral conjunctival transient amplifying cells originate at the mucocutaneous junction and their progeny migrate toward the fornix. Trans Am Ophthalmol Soc. 1997;95:417-29; discussion 429-32. [PubMed] |
| 84. | Harun MH, Sepian SN, Chua KH, Ropilah AR, Abd Ghafar N, Che-Hamzah J, Bt Hj Idrus R, Annuar FH. Human forniceal region is the stem cell-rich zone of the conjunctival epithelium. Hum Cell. 2013;26:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Wei ZG, Lin T, Sun TT, Lavker RM. Clonal analysis of the in vivo differentiation potential of keratinocytes. Invest Ophthalmol Vis Sci. 1997;38:753-761. [PubMed] |
| 86. | Liu CY, Zhu G, Westerhausen-Larson A, Converse R, Kao CW, Sun TT, Kao WW. Cornea-specific expression of K12 keratin during mouse development. Curr Eye Res. 1993;12:963-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 87. | Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 910] [Cited by in RCA: 995] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 88. | Wu RL, Zhu G, Galvin S, Xu C, Haseba T, Chaloin-Dufau C, Dhouailly D, Wei ZG, Lavker RM, Kao WY. Lineage-specific and differentiation-dependent expression of K12 keratin in rabbit corneal/limbal epithelial cells: cDNA cloning and northern blot analysis. Differentiation. 1994;55:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 989] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 90. | Pellegrini G, Golisano O, Paterna P, Lambiase A, Bonini S, Rama P, De Luca M. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145:769-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 530] [Cited by in RCA: 513] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 91. | Kruse FE, Tseng SC. Serum differentially modulates the clonal growth and differentiation of cultured limbal and corneal epithelium. Invest Ophthalmol Vis Sci. 1993;34:2976-2989. [PubMed] |
| 92. | Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98:3156-3161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1049] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 93. | Kasper M. Patterns of cytokeratins and vimentin in guinea pig and mouse eye tissue: evidence for regional variations in intermediate filament expression in limbal epithelium. Acta Histochem. 1992;93:319-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 94. | Lauweryns B, van den Oord JJ, De Vos R, Missotten L. A new epithelial cell type in the human cornea. Invest Ophthalmol Vis Sci. 1993;34:1983-1990. [PubMed] |
| 95. | Lauweryns B, van den Oord JJ, Missotten L. The transitional zone between limbus and peripheral cornea. An immunohistochemical study. Invest Ophthalmol Vis Sci. 1993;34:1991-1999. [PubMed] |
| 96. | Zieske JD, Bukusoglu G, Yankauckas MA. Characterization of a potential marker of corneal epithelial stem cells. Invest Ophthalmol Vis Sci. 1992;33:143-152. [PubMed] |
| 97. | Zieske JD, Bukusoglu G, Yankauckas MA, Wasson ME, Keutmann HT. Alpha-enolase is restricted to basal cells of stratified squamous epithelium. Dev Biol. 1992;151:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 98. | Pajoohesh-Ganji A, Ghosh SP, Stepp MA. Regional distribution of alpha9beta1 integrin within the limbus of the mouse ocular surface. Dev Dyn. 2004;230:518-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Høye AM, Couchman JR, Wewer UM, Fukami K, Yoneda A. The newcomer in the integrin family: integrin α9 in biology and cancer. Adv Biol Regul. 2012;52:326-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 100. | Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 313] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 101. | Watanabe K, Nishida K, Yamato M, Umemoto T, Sumide T, Yamamoto K, Maeda N, Watanabe H, Okano T, Tano Y. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004;565:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 149] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 102. | Budak MT, Alpdogan OS, Zhou M, Lavker RM, Akinci MA, Wolosin JM. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci. 2005;118:1715-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 103. | de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li DQ. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 236] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 104. | Ksander BR, Kolovou PE, Wilson BJ, Saab KR, Guo Q, Ma J, McGuire SP, Gregory MS, Vincent WJ, Perez VL. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014;511:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 105. | Noisa P, Ramasamy TS, Lamont FR, Yu JS, Sheldon MJ, Russell A, Jin X, Cui W. Identification and characterisation of the early differentiating cells in neural differentiation of human embryonic stem cells. PLoS One. 2012;7:e37129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 106. | Naujok O, Lenzen S. A critical re-evaluation of CD24-positivity of human embryonic stem cells differentiated into pancreatic progenitors. Stem Cell Rev. 2012;8:779-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 107. | Lehrer MS, Sun TT, Lavker RM. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J Cell Sci. 1998;111:2867-2875. [PubMed] |
| 108. | Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 637] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 109. | Townsend WM. The limbal palisades of Vogt. Trans Am Ophthalmol Soc. 1991;89:721-756. [PubMed] |
| 110. | Ecoiffier T, Yuen D, Chen L. Differential distribution of blood and lymphatic vessels in the murine cornea. Invest Ophthalmol Vis Sci. 2010;51:2436-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 111. | Meller D, Pires RT, Tseng SC. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol. 2002;86:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 175] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 112. | Dua HS, Shanmuganathan VA, Powell-Richards AO, Tighe PJ, Joseph A. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005;89:529-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 289] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 113. | Shortt AJ, Secker GA, Munro PM, Khaw PT, Tuft SJ, Daniels JT. Characterization of the limbal epithelial stem cell niche: novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells. 2007;25:1402-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 228] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 114. | Lee RE, Davison PF. The collagens of the developing bovine cornea. Exp Eye Res. 1984;39:639-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 115. | Linsenmayer TF, Bruns RR, Mentzer A, Mayne R. Type VI collagen: immunohistochemical identification as a filamentous component of the extracellular matrix of the developing avian corneal stroma. Dev Biol. 1986;118:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 116. | Linsenmayer TF, Fitch JM, Mayne R. Extracellular matrices in the developing avian eye: type V collagen in corneal and noncorneal tissues. Invest Ophthalmol Vis Sci. 1984;25:41-47. [PubMed] |
| 117. | Pratt BM, Madri JA. Immunolocalization of type IV collagen and laminin in nonbasement membrane structures of murine corneal stroma. A light and electron microscopic study. Lab Invest. 1985;52:650-656. [PubMed] |
| 118. | Funderburgh JL, Chandler JW. Proteoglycans of rabbit corneas with nonperforating wounds. Invest Ophthalmol Vis Sci. 1989;30:435-442. [PubMed] |
| 119. | Funderburgh JL, Caterson B, Conrad GW. Keratan sulfate proteoglycan during embryonic development of the chicken cornea. Dev Biol. 1986;116:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 120. | Funderburgh JL, Caterson B, Conrad GW. Distribution of proteoglycans antigenically related to corneal keratan sulfate proteoglycan. J Biol Chem. 1987;262:11634-11640. [PubMed] |
| 121. | Hyldahl L, Aspinall R, Watt FM. Immunolocalization of keratan sulphate in the human embryonic cornea and other human foetal organs. J Cell Sci. 1986;80:181-191. [PubMed] |
| 122. | Hart GW. Biosynthesis of glycosaminolgycans during corneal development. J Biol Chem. 1976;251:6513-6521. [PubMed] |
| 123. | Fitch JM, Birk DE, Linsenmayer C, Linsenmayer TF. Stromal assemblies containing collagen types IV and VI and fibronectin in the developing embryonic avian cornea. Dev Biol. 1991;144:379-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 124. | Toole BP, Trelstad RL. Hyaluronate production and removal during corneal development in the chick. Dev Biol. 1971;26:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 243] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 125. | Gipson IK. The epithelial basement membrane zone of the limbus. Eye (Lond). 1989;3:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 126. | Kolega J, Manabe M, Sun TT. Basement membrane heterogeneity and variation in corneal epithelial differentiation. Differentiation. 1989;42:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 127. | Cleutjens JP, Havenith MG, Kasper M, Vallinga M, Bosman FT. Absence of type IV collagen in the centre of the corneal epithelial basement membrane. Histochem J. 1990;22:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 128. | Schlötzer-Schrehardt U, Dietrich T, Saito K, Sorokin L, Sasaki T, Paulsson M, Kruse FE. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res. 2007;85:845-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 129. | Kabosova A, Azar DT, Bannikov GA, Campbell KP, Durbeej M, Ghohestani RF, Jones JC, Kenney MC, Koch M, Ninomiya Y. Compositional differences between infant and adult human corneal basement membranes. Invest Ophthalmol Vis Sci. 2007;48:4989-4999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 130. | Ljubimov AV, Burgeson RE, Butkowski RJ, Michael AF, Sun TT, Kenney MC. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest. 1995;72:461-473. [PubMed] |
| 131. | Yu WY, Sheridan C, Grierson I, Mason S, Kearns V, Lo AC, Wong D. Progenitors for the corneal endothelium and trabecular meshwork: a potential source for personalized stem cell therapy in corneal endothelial diseases and glaucoma. J Biomed Biotechnol. 2011;2011:412743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 132. | Li GG, Chen SY, Xie HT, Zhu YT, Tseng SC. Angiogenesis potential of human limbal stromal niche cells. Invest Ophthalmol Vis Sci. 2012;53:3357-3367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 133. | Zhou M, Leiberman J, Xu J, Lavker RM. A hierarchy of proliferative cells exists in mouse lens epithelium: implications for lens maintenance. Invest Ophthalmol Vis Sci. 2006;47:2997-3003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 134. | Yamamoto N, Majima K, Marunouchi T. A study of the proliferating activity in lens epithelium and the identification of tissue-type stem cells. Med Mol Morphol. 2008;41:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 135. | Remington SG, Meyer RA. Lens stem cells may reside outside the lens capsule: an hypothesis. Theor Biol Med Model. 2007;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 136. | Perron M, Harris WA. Retinal stem cells in vertebrates. Bioessays. 2000;22:685-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 137. | von Leithner PL, Ciurtin C, Jeffery G. Microscopic mammalian retinal pigment epithelium lesions induce widespread proliferation with differences in magnitude between center and periphery. Mol Vis. 2010;16:570-581. [PubMed] |
| 138. | Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D. Retinal stem cells in the adult mammalian eye. Science. 2000;287:2032-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 708] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 139. | Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78:433-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 242] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 140. | Shimazaki J, Kaido M, Shinozaki N, Shimmura S, Munkhbat B, Hagihara M, Tsuji K, Tsubota K. Evidence of long-term survival of donor-derived cells after limbal allograft transplantation. Invest Ophthalmol Vis Sci. 1999;40:1664-1668. [PubMed] |
| 141. | Williams KA, Brereton HM, Aggarwal R, Sykes PJ, Turner DR, Russ GR, Coster DJ. Use of DNA polymorphisms and the polymerase chain reaction to examine the survival of a human limbal stem cell allograft. Am J Ophthalmol. 1995;120:342-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 142. | Mills JC, Gordon JI. The intestinal stem cell niche: there grows the neighborhood. Proc Natl Acad Sci USA. 2001;98:12334-12336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 143. | Zeng Y, Yang J, Huang K, Lee Z, Lee X. A comparison of biomechanical properties between human and porcine cornea. J Biomech. 2001;34:533-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 220] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 144. | Liu Z, Wan P, Duan H, Zhou J, Tan B, Liu Y, Zhou Q, Zhou C, Huang Z, Tian B. ES micro-environment enhances stemness and inhibits apoptosis in human limbal stem cells via the maintenance of telomerase activity. PLoS One. 2013;8:e53576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 145. | Sellaro TL, Ravindra AK, Stolz DB, Badylak SF. Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ-specific extracellular matrix scaffolds. Tissue Eng. 2007;13:2301-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 163] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 146. | Ballios BG, Clarke L, Coles BL, Shoichet MS, Van Der Kooy D. The adult retinal stem cell is a rare cell in the ciliary epithelium whose progeny can differentiate into photoreceptors. Biol Open. 2012;1:237-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 147. | Meyer JS, Katz ML, Maruniak JA, Kirk MD. Embryonic stem cell-derived neural progenitors incorporate into degenerating retina and enhance survival of host photoreceptors. Stem Cells. 2006;24:274-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 148. | Parameswaran S, Balasubramanian S, Babai N, Qiu F, Eudy JD, Thoreson WB, Ahmad I. Induced pluripotent stem cells generate both retinal ganglion cells and photoreceptors: therapeutic implications in degenerative changes in glaucoma and age-related macular degeneration. Stem Cells. 2010;28:695-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 149. | Arnhold S, Absenger Y, Klein H, Addicks K, Schraermeyer U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch Clin Exp Ophthalmol. 2007;245:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 150. | Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 986] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 151. | Chen FM, Wu LA, Zhang M, Zhang R, Sun HH. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: Promises, strategies, and translational perspectives. Biomaterials. 2011;32:3189-3209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 272] [Article Influence: 18.1] [Reference Citation Analysis (0)] |