Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.418
Peer-review started: July 27, 2014
First decision: August 28, 2014
Revised: September 25, 2014
Accepted: October 31, 2014
Article in press: November 3, 2014
Published online: March 26, 2015
Processing time: 235 Days and 23.2 Hours
Cancer stem cells (CSCs) are maintained by their somatic stem cells and are responsible for tumor initiation, chemoresistance, and metastasis. Evidence for the CSCs existence has been reported for a number of human cancers. The CSC mitochondria have been shown recently to be an important target for cancer treatment, but clinical significance of CSCs and their mitochondria properties remain unclear. Mitochondria-targeted agents are considerably more effective compared to other agents in triggering apoptosis of CSCs, as well as general cancer cells, via mitochondrial dysfunction. Mitochondrial metabolism is altered in cancer cells because of their reliance on glycolytic intermediates, which are normally destined for oxidative phosphorylation. Therefore, inhibiting cancer-specific modifications in mitochondrial metabolism, increasing reactive oxygen species production, or stimulating mitochondrial permeabilization transition could be promising new therapeutic strategies to activate cell death in CSCs as well, as in general cancer cells. This review analyzed mitochondrial function and its potential as a therapeutic target to induce cell death in CSCs. Furthermore, combined treatment with mitochondria-targeted drugs will be a promising strategy for the treatment of relapsed and refractory cancer.
Core tip: This review is devoted to the analysis of mitochondrial function as a therapeutic target to induce cell death in cancer stem cells (CSCs). In particular, we focused on the differences in energy metabolism and features between CSC and non-CSC mitochondria, and between CSCs and normal stem cells. We described the roles of mitochondria that may make CSCs more susceptible to anti-cancer treatment and apoptosis, and how these may be useful to develop novel strategies for cancer treatment, such as through combined therapy with specific mitochondrial-targeting drugs.
- Citation: Song IS, Jeong JY, Jeong SH, Kim HK, Ko KS, Rhee BD, Kim N, Han J. Mitochondria as therapeutic targets for cancer stem cells. World J Stem Cells 2015; 7(2): 418-427
- URL: https://www.wjgnet.com/1948-0210/full/v7/i2/418.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i2.418
Over the last decade, cancer therapies have improved the quality of life of cancer patients. However, although almost all developed anti-cancer drugs are apparently successful following initial therapy, secondary tumors development and disease relapse is common. The limitation of classical anti-cancer therapies has been attributed recently to the existence of cancer stem cells (CSCs), which are quiescent, have relatively small population, and highly drug-resistant cells. CSCs act like stem cells (SCs) and are responsible for cancer growth and metastasis[1]. Through the continued effort of many researchers, CSCs features have been revealed, such as anti-cancer drug resistance, metastasis, proliferation, hypoxic tolerance, and the capacity for neovessel induction[2,3].
Mitochondria-targeted drugs may overcome potentially the drug-resistance mechanisms that have progressed toward conventional chemo-therapeutics in cancer[4-7]. Mitochondria produce ATP, but they also mediate cell death and produce reactive oxygen species (ROS). Although ROS are affected in the regulation of various cellular responses, excessive production may be harmful to the cell[8]. Cancer cells also exhibit extensive metabolic rearrangement that makes them more susceptible to alteration of mitochondria than normal cells[9,10]. However, mitochondrial properties of CSCs in tumors remain unknown.
This review analyzed the potential role of mitochondria as a therapeutic target for inducing cell death in CSCs. In particular, we focused on the differences in energy metabolism and mitochondrial features between CSCs and non-CSCs, as well as between CSCs and normal SCs, and how these unique features of CSCs may increase the susceptibility of CSCs to anti-cancer treatment and apoptosis induction. We described how CSC mitochondria may be useful targets for the development of novel cancer treatment strategies, such as targeting CSCs via combination therapy with specific mitochondrial-targeting drugs.
The concept of CSCs is many decades old[11]. In the middle of 1800s, the embryonal rest theory of cancer introduced the idea that cancer arises from SCs, but the existence of CSCs in tumors could not be verified due to a lack of techniques. Furth et al[12] first alluded to CSCs in 1937 when they showed that a single cell within a tumor initiates the generation of new tumor in a recipient mouse[12]. This finding was defined in the 1960s and 1970s by the development of quantitative methods to measure the tumorigenic ability able to sustain tumor growth in vivo. In the middle of 1900s, Radiolabeling permitted the measurements of cellular phenotype such as cell proliferation, lifespan, and hierarchical organizations within normal tissues[13]. Al-Hajj et al[14] and Singh et al[15] represented that a small subset of cells within breast and brain tumors can be isolated prospectively and can generate phenotypically heterogeneous tumor in vivo. Thus, these various evidences represent that diverse solid tumors are organized hierarchically and sustained by a distinct subpopulation of CSCs.
CSCs are classified according to several properties such as the presence of cell surface markers and their occupancy in the Fluorescence Activated Cell Sorting (FACS) analysis. Flow cytometry with antibodies against cell surface antigens has been the preferred method for characterizing and sorting normal stem cells. However, differences between CSC and normal SC markers are not well defined, and CSCs and normal SCs share some surface markers.
Most of CSCs studies isolate CSCs marker or a combination of markers, which is expressed heterogeneously in a certain tumor type. Based on this marker heterogeneity, subpopulations including CSCs are isolated from original tumors and injected into immuno-deficient mice, after which tumor growth is assessed several weeks or months later. Table 1 shows current CSC markers according to cancer types, as FACS markers allow for consistent sorting according to marker expression. For example, Al-Hajj et al[14] used a marker combination of the CD24 and CD44 as an indicator of breast CSC, and the CD133 marker has been shown to be both normal SC and CSC marker[16-20].
| Marker | Cancer origin | Marker properties | Ref. |
| ALDH1 | Breast | Catalyzes the oxidation of aliphatic and aromatic aldehydes | [81] |
| Converts retinol to retinoic acid | |||
| AdSC | |||
| ABC135 | Melanomas | ATP binding cassette family | [82] |
| Involved in transport of sterol and other lipids | |||
| Bmi-1 | Breast, prostate, leukemias, neuroblastomas | HSC, NSC, and AdSC marker | [83,84] |
| CD20 | Metastatic melanomas | Hematopoietic marker | [85] |
| CD29 | Breast, colon | AdSC marker | [86,87] |
| CD34 | Leukemias, sarcomas | HSC, MSC marker | [88-91] |
| CD44 | Breast, pancreas, colon, head and neck, prostate | Adhesion molecule related to metastasis | [91-96] |
| HSC and pluripotent stem cell marker | |||
| Normal prostate epithelial stem cell marker | |||
| CD49f | Prostate | Adhesion to extracellular matrix | [97] |
| CD90 | Liver, breast, glioblastomas | Glycoprotein, role in stem cell differentiation | [98-100] |
| MSC marker | |||
| CD113 | Lung, pancreas, colon, glioblastoma, melanomas, etc. | HSC, NSC AdSC (colon) marker | [16-18,101-104] |
| CD117 | Breast, ovarian, lung, glioblastoma | Progenitor cell marker | [105,106] |
| Oct4 | Many carcinomas | Embryonic stem cell and induced pluripotent stem cell marker | [107,108] |
| Sca-1 | Lung | Skin epithelial stem cell and HSC marker | [109] |
The first embryonic SC lines were developed from the inner cell mass of early embryos in 1998[21]. In 1999 and 2000, it was discovered that it could produce different cell types through manipulating adult mouse tissues, indicating that stem cell differentiation and proliferation could be controlled externally. Both somatic SCs and CSCs generate numerous daughter cells, differentiate into a variety of cell types, actively express telomerase, activate anti-apoptotic pathways, increase active membrane transports, and metastasize[22]. Moreover, SCs are induced to differentiate by niche signaling and outer environmental stimuli. Niche signaling keeps the undifferentiation of SCs until they are stimulated to generate new cells, suggesting a similarity with signaling pathways that govern normal SC proliferation. Local environment signaling can initiate CSC proliferation, and thus, trigger tumor initiation and growth[23]. Therefore, SC markers and features may not be effective therapeutic targets for inhibiting CSC growth.
As the main energy producers, mitochondria produce ATP using the tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS). However, they also generate ROS during this process, which are harmful to the cell if produced excessively. In addition, mitochondria play a crucial role for the regulation of cell death pathways and intracellular Ca2+ homeostasis. Mitochondria activate apoptosis by regulating the releasement of proapoptotic proteins space to the cytosol from the mitochondrial intermembrane[7], and they also play a crucial role in non-apoptotic cell death[24].
Key regulators related to cell death and other cellular processes in the mitochondria are frequently altered in cancer cells[8], as cancer cell mitochondria differ functionally and structurally compare with that of normal cells[25]. Fast growing tumors result in hypoxia because of an inadequate amount of oxygen from the local vasculature. In addition, cancer cells include the DNA mutation of mitochondria and nucleus, which affect the OXPHOS components and result in ROS overproduction, wasteful ATP production, and mitochondrial oxidative damage[25]. Warburg[26] pioneered research on the cancer-related alterations in mitochondrial respiration and suggested a mechanism to explain how they progress during the tumorigenesis. The proposed mechanism differs from that in non-malignant cells utilizing OXPHOS. Although aerobic glycolysis has been corroborated in cancer cells, the function of mitochondria has been controversial[27]. In cancer cells, the aerobic glycolysis generate glycolytic intermediates to the pentose phosphate pathway. Moreover, the glycolytic ATP generation is important for survival in hypoxic conditions[28]. In OXPHOS, the ATP synthesis requires much oxygen, which leads to continuous the ROS production such as superoxide anion, organic peroxide, and hydrogen peroxide[29]. If the generated ROS are not eliminated by redox regulating system, they may cause cellular damage.
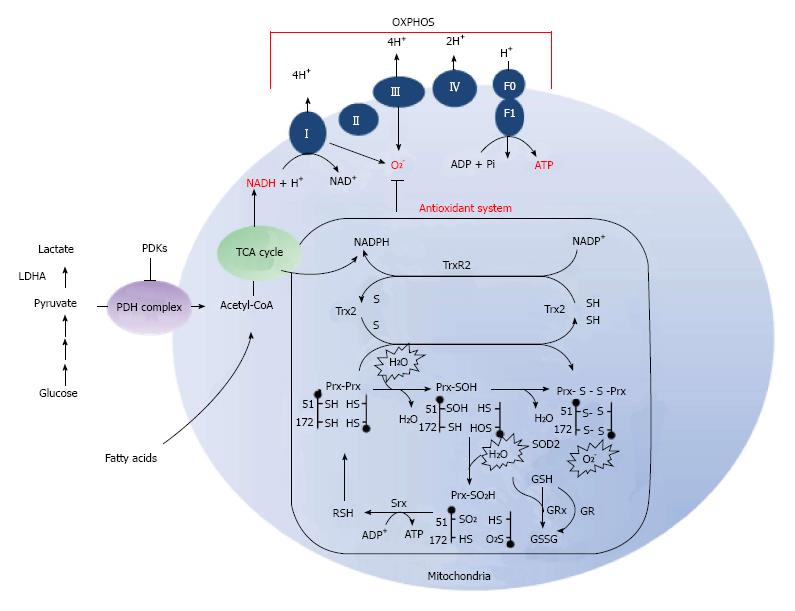
Mitochondria have a multi-level network of redox-defense systems for the elimination of hydrogen peroxide (Figure 1). Glutathione and glutathione peroxidases require nicotinamide adenine dinucleotide phosphate (NADPH) for the reduction of H2O2 and other peroxides generated in the mitochondria. Mitochondrial redox balances are also regulated by the mitochondrial inner membrane electrochemical gradient, which mitochondrial Complex V (ATP synthase) uses to produce ATP from ADP and inorganic phosphate (Pi).
Moreover, the physiological significance of mitochondrial redox balance has been highlighted by the antioxidant genes-deletion and over-expression. As antioxidant defense system, Peroxiredoxin (Prx) 3, Prx5, superoxide dismutase 2, and thioredoxin 2 eliminates ROS produced in mitochondria[30,31]. Knockout (KO) of Prx3 mice result in induction of oxidative damage[32], KO of thioredoxin 2 mice showed an embryonic lethal phenotype[33] and KO of superoxide dismutase 2 mice die within 3 wk of birth because of mitochondrial oxidative damage and severe neurodegeneration[34,35]. Therefore, the inhibition of antioxidant systems may provide a targeted therapy that leads to mitochondrial dysfunction and cell death.
Mitochondria harbor a robust mitochondrial transmembrane potential (ΔΨm), and the exchange of small metabolites between the mitochondrial matrix and the cytosol is induced by the low conductance of permeability transition pore complex (PTPC)[36]. The rupture of mitochondrial membranes leading to functional impairment result in the release of toxic mitochondrial intermembrane space proteins, such as apoptosis-inducing factor and cytochrome c, into the cytosol[37]. Under apoptotic conditions, including ROS and Ca2+ overload, the PTPC presumes a high conductance state allowing uncontrolled influx of small solutes into the matrix of mitochondria. This mitochondrial permeability transition (MPT) leads to osmotic swelling of the mitochondrial matrix and dissipation of the ΔΨm[38,39], and eventually cell death occurs due to mitochondrial outer membrane permeabilization[40]. The MPT is triggered by reagents increasing ROS generation, cytosolic Ca2+ concentrations, or acting on the PTPC. Therefore, the induction of mitochondrial membrane permeabilization are attractive targets to develop drug for cancer therapy.
As mentioned above, mitochondria play important role in apoptosis, but also trigger cell death through various mechanisms[41-43]. Various mitochondria-targeted strategies for cancer treatment have been developed over the last decade[6,44] that focused on the development of agents that regulate the MPT, Bcl-2 family proteins, and ROS production in cancer[6]. Numerous molecules, acting on mitochondria, are currently used or being tested in clinical trials[45]. Several experimental anti-cancer drugs, such as ceramide[46], CD437[47], and MKT077[48], and clinically approved anti-cancer drugs, such as etoposide[49], paclitaxel[50], and vinorelbine[51], induce apoptosis via mitochondria dysfunction. Furthermore, determining of pathophysiological differences of mitochondria between cancer cells and normal cells, will improve the selectivity of mitochondria-targeted anti-cancer agents.
Because mitochondria play a key role in the alteration of oxidative stress, energy status, and apoptotic stimuli, scientists have assumed that they are also involved in the regulation of stemness and differentiation in SCs. Researchers have attempted to employ mitochondrial properties in the selection of SCs[52]. Lonergan et al[53] and Bavister[54] suggested that functional mitochondrial characteristics, such as subcellular localization and metabolic activity could verify stemness, SC stability, and pluripotency. Mitochondria are localized in perinuclear sites in embryonic stem cells (ESCs) and have a more scattered distribution throughout the cytoplasm after differentiation and senescence[55].
Mitochondrial metabolic activity is also related to cell differentiation, as early passages of an adult primate stromal cell line have a higher oxygen consumption rate (OCR) and a low ATP/ mitochondrial DNA content compared with long-term cultured cells[53]. In CD34+ hematopoietic SCs, a low mitochondrial OCR and mitochondrial mass result in a predominantly perinuclear mitochondrial arrangement[56].
Antioxidant enzyme expression also shows a dramatic change during differentiation[57]. Moreover, ROS play an agonistic role in the differentiation of ESCs. Enhanced intracellular ROS as the differentiation stimulus may act on transplanted SCs into the cardiovascular lineage[58], indicating that mitochondrial redox metabolism act as a crucial regulator in cardiac differentiation of SCs. Furthermore, Plotnikov et al[59] suggest a correlation of the mitochondrial function and the status of neural SCs.
SC mitochondria play important roles in maintaining stemness and differentiation. However, whether the roles of CSC mitochondria are similar to SC mitochondria or cancer cells in general is uncertain. Two hypotheses on the origin of CSCs, both of which contribute to acute myeloid leukemia[1,60], have been proposed. One hypothesis of the origin of CSCs is that they are derivatives of SCs residing in various organs. Genetic mutations and epigenetic changes, which are crucial for initiation and progression of tumor growth, accumulate in long-lived stem cells, and the transformation of SCs into CSCs initiates carcinogenesis. CSCs may also have a greater differentiation potential than other SCs. (SCs can be divided into the following groups based on differentiation potential: the totipotent, pluripotent, multipotent, and unipotent group). Another hypothesis assumes the existence of ESC-like cells that convert into CSCs when they are exposed to damaging environmental factors. Additional differentiation and mutation of these cells may also contribute to development of CSCs[61]. Based on these reports, the CSCs may be more differentiated than normal SCs and likewise, the mitochondrial properties of CSCs are different from those of SCs or general cancer cells.
Recently, Ye et al[62] determined the mitochondrial features between lung CSCs and non-CSCs. As a results, it is showed a lower mtDNA contents, lower OCR, glucose consumption, intracellular ATP and ROS level in the lung CSCs compared to non-CSCs. Leukemia CSCs showed a low ROS level and reduced OXPHOS compared with that of non-CSCs[63]. However, Pastò et al[64] reported that CSCs exhibited over-expressed genes related to glucose uptake, oxidative phosphorylation, and fatty acid β-oxidation, indicating higher ability to direct pyruvate towards the TCA cycle. As reported, ovarian CSCs showed higher mitochondrial ROS production and ΔΨm than non-CSCs. In addition, targeting mitochondrial biogenetics induced caspase-independent cell death in ovarian CSCs[65]. In glioma CSCs, a higher mitochondrial reserve capacity was measured as compared to the differentiated cells[66]. Glioblastoma CSCs also depend on OXPHOS for their energy production and survival[67]. Besides, breast CSCs have higher ATP content compared to their differentiated progeny[68]. Based on these studies, CSCs mitochondria showed the different roles and features according to the cancer type. A summary of the mitochondrial features between CSCs and non-CSCs according to cancer origin is highlighted in Table 2. Although the mitochondrial features of CSCs in several cancers are not identical, CSCs mitochondria obviously differ from those of non-CSCs. Moreover, mitochondrial features of CSCs have not been clearly defined in other cancer types. Most importantly, little has been known about the mitochondrial features related to energy metabolism and the ROS/antioxidant enzyme system of CSCs in colon, stomach, liver, bone, and prostate cancer. Therefore, defining these features will be essential for developing a mitochondria-targeted therapeutic drug that induces death of CSCs, and therefore, reduces the risk of relapsed or refractory cancer.
| Cancer origin | Mitochondria features | Energy metabolism of CSC | Target/drug for CSCs | Ref. | ||
| Feature | CSC | Non-CSC | ||||
| Breast | Glucose uptake | High | Low | OXPHOS | [68] | |
| ATP contents | High | Low | ||||
| OCR | High | Low | ||||
| Lactate production | Low | High | ||||
| Membrane potential | High | Low | ||||
| Glioma | Glucose consumption | Low | High | OXPHOS | [66] | |
| ATP contents | High | Low | ||||
| Lactate production | Low | High | ||||
| OCR | High | Low | OXPHOS | IMP-2 | [67] | |
| ATP contents | High | Low | ||||
| Leukemia | ROS | Low | High | Low glycolysis | Bcl-2/ | [63] |
| Proliferation rate | Slow | Fast | Low OXPHOS | ABT263 | ||
| OCR | Low | High | ||||
| Lactate production | Low | High | ||||
| ATP contents | Low | High | ||||
| Lung | Glucose consumption | Low | High | [62] | ||
| OCR | Low | High | ||||
| ROS level | Low | High | ||||
| ATP contents | Low | High | ||||
| Membrane potential | High | Low | ||||
| Mitochondrial DNA | Low | High | ||||
| Ovarian | NV-128 | [65] | ||||
| ROS | High | Low | OXPHOS | [64] | ||
| Membrane potential | High | Low | ||||
| ATP contents | High | Low | ||||
| Glucose deprivation | Resist | Sensitive | ||||
Despite the recent surge of published studies on CSCs, the clinical significance of this population remains unclear and has been slow in progression of the development of clinical agents to eliminate CSCs. However, most experts agree that effective anti-cancer drugs should be targeted toward CSCs in addition to non-CSCs. Current cancer treatments such as conventional chemotherapy and radiotherapy target rapidly proliferating cells that make up the bulk of the tumor, but do not specifically target CSCs. Thus, the hypotheses on the origin of CSCs may explain the development of relapsed and metastatic cancer. In cancer therapy, the new paradigm requires development of novel anti-cancer drug molecules and drug targets to assess drug responses of CSCs.
Altered expression of genes involved in apoptosis, survival, and DNA repair machinery are among the multiple mechanisms responsible for the chemoresistance of leukemic[69], brain[70], pancreatic[71], breast[72], melanoma[73,74], and colon cancer[75] CSCs. Liu et al[23] reports that CD133+ glioblastoma cells isolated from patients have a high expression of genes in the Bcl-2 and inhibitor of apoptosis (IAP) families. Moreover, several types of CSCs have upregulated ATP binding cassette (ABC) pumps that make them resistant to various chemotherapeutics[73,74]. Therefore, finding targets that efficiently promote CSC cell death is important and a focus of intensive research. Dong and colleagues demonstrate that loss of fructose-1,6-biphosphatase in breast CSCs induces glycolysis, as well as inhibiting oxygen consumption and ROS generation, through the suppression of mitochondrial Complex I activity[76]. The report implies that overproduction of ROS and reduction in glucose metabolism may be effective against breast CSCs. Hirsch et al[77] showed that metformin, an AMPK activator and Complex I inhibitor often used as the first-line drug for treating diabetes, and selectively kills CSCs in breast cancer cell lines. The novel isoflavone derivative NV-128 significantly decreased mitochondrial function, as shown by a decreases in ATP, Complex I, and Complex IV levels, and induced cell death in ovarian CSCs[65]. These results demonstrate that specific mitochondrial targeted compounds can induce cell death in chemoresistant CSCs and may be a new venue for treating ovarian cancer patients with relapsed or metastatic cancer. The new-generation taxoid SB-T-1214 significantly inhibited stemness gene expression profiles and induced cell death in both CSCs and general cancer cells, indicating its promise in overcoming relapsed and refractory cancer due to CSCs[78]. Finally, mitochondria-targeted vitamin E succinate (MitoVES), which includes the positively charged triphenylphosphonium group, may be the most well-characterized toxic agent in its ability to induce apoptosis in breast CSCs[79]. Meanwhile, it was reported that a drug which inhibits the self-renewal of CSCs by targeting of Notch and Hedgehog pathway has been developed[80]. It was also reported that has been developed a drugs, which can eliminate CSCs by targeting cell surface markers such as CD133 and EpCAM. However, the use of these drugs increases the exposure to side effects due to the sharing of signaling pathway and cell surface marker with normal SCs. Thus, it is important to understand how CSCs differ from normal SCs and differentiated cells. Moreover, a full understanding of the mitochondrial function and energy metabolism in CSCs contributes to the development of the agents targeting mitochondrial functions (such as ROS overproduction, energy metabolism inhibition, and antioxidant protein inhibition), and presents a need to develop new strategies to target CSCs in the clinical field[80].
In summary, the mitochondria are an important tool to investigate CSCs properties and to develop anti-cancer drugs. However, the properties and clinical significance of mitochondria in CSCs have not been verified. Because mitochondria-targeted therapy may open new strategies for the treatment of relapsed and refractory cancer, mitochondrial properties unique to CSCs need to be defined. Furthermore, combined treatment with mitochondrial-targeted and anti-cancer drugs may specifically induce the death of both CSCs and general cancer cells and promises to be a novel cancer therapy.
P- Reviewer: Ker CG, T Kusmic C, O-Uchi J, Scatena R S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6844] [Cited by in RCA: 6975] [Article Influence: 279.0] [Reference Citation Analysis (0)] |
| 2. | Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2552] [Cited by in RCA: 2648] [Article Influence: 147.1] [Reference Citation Analysis (0)] |
| 3. | Li Z, Rich JN. Hypoxia and hypoxia inducible factors in cancer stem cell maintenance. Curr Top Microbiol Immunol. 2010;345:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Song IS, Kim HK, Lee SR, Jeong SH, Kim N, Ko KS, Rhee BD, Han J. Mitochondrial modulation decreases the bortezomib-resistance in multiple myeloma cells. Int J Cancer. 2013;133:1357-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Song IS, Jeong YJ, Jeong SH, Heo HJ, Kim HK, Lee SR, Ko TH, Youm JB, Kim N, Ko KS. Combination treatment with 2-methoxyestradiol overcomes bortezomib resistance of multiple myeloma cells. Exp Mol Med. 2013;45:e50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1285] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 7. | Song IS, Kim HK, Jeong SH, Lee SR, Kim N, Rhee BD, Ko KS, Han J. Mitochondrial peroxiredoxin III is a potential target for cancer therapy. Int J Mol Sci. 2011;12:7163-7185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol. 2008;18:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 487] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 9. | Bellance N, Lestienne P, Rossignol R. Mitochondria: from bioenergetics to the metabolic regulation of carcinogenesis. Front Biosci (Landmark Ed). 2009;14:4015-4034. [PubMed] |
| 10. | Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1553] [Cited by in RCA: 1737] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 11. | Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793-4807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 750] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 12. | Furth J, Kahn M C, Breedis C. The transmission of leukaemia of mice with a single cell. Am J Cancer. 1937;31:276-282. [DOI] [Full Text] |
| 13. | Clermont Y, Leblond CP. Renewal of spermatogonia in the rat. Am J Anat. 1953;93:475-501. [RCA] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 109] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983-3988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7830] [Cited by in RCA: 7794] [Article Influence: 338.9] [Reference Citation Analysis (0)] |
| 15. | Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5422] [Cited by in RCA: 5625] [Article Influence: 255.7] [Reference Citation Analysis (0)] |
| 16. | O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2977] [Cited by in RCA: 3067] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 17. | Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2952] [Cited by in RCA: 3051] [Article Influence: 152.6] [Reference Citation Analysis (0)] |
| 18. | Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821-5828. [PubMed] |
| 19. | Florek M, Haase M, Marzesco AM, Freund D, Ehninger G, Huttner WB, Corbeil D. Prominin-1/CD133, a neural and hematopoietic stem cell marker, is expressed in adult human differentiated cells and certain types of kidney cancer. Cell Tissue Res. 2005;319:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 219] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Mehra N, Penning M, Maas J, Beerepoot LV, van Daal N, van Gils CH, Giles RH, Voest EE. Progenitor marker CD133 mRNA is elevated in peripheral blood of cancer patients with bone metastases. Clin Cancer Res. 2006;12:4859-4866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147. [PubMed] |
| 22. | Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883-1890; discussion 1895-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 939] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 23. | Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Galluzzi L, Kroemer G. Necroptosis: a specialized pathway of programmed necrosis. Cell. 2008;135:1161-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 418] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 25. | Modica-Napolitano JS, Singh KK. Mitochondrial dysfunction in cancer. Mitochondrion. 2004;4:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 238] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA. 2010;107:8788-8793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1407] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 29. | Reed DJ. Glutathione: toxicological implications. Annu Rev Pharmacol Toxicol. 1990;30:603-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 504] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 30. | Rabilloud T, Heller M, Rigobello MP, Bindoli A, Aebersold R, Lunardi J. The mitochondrial antioxidant defence system and its response to oxidative stress. Proteomics. 2001;1:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Banmeyer I, Marchand C, Clippe A, Knoops B. Human mitochondrial peroxiredoxin 5 protects from mitochondrial DNA damages induced by hydrogen peroxide. FEBS Lett. 2005;579:2327-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Huh JY, Kim Y, Jeong J, Park J, Kim I, Huh KH, Kim YS, Woo HA, Rhee SG, Lee KJ. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Antioxid Redox Signal. 2012;16:229-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Nonn L, Williams RR, Erickson RP, Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003;23:916-922. [PubMed] |
| 34. | Lebovitz RM, Zhang H, Vogel H, Cartwright J, Dionne L, Lu N, Huang S, Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA. 1996;93:9782-9787. [PubMed] |
| 35. | Hinerfeld D, Traini MD, Weinberger RP, Cochran B, Doctrow SR, Harry J, Melov S. Endogenous mitochondrial oxidative stress: neurodegeneration, proteomic analysis, specific respiratory chain defects, and efficacious antioxidant therapy in superoxide dismutase 2 null mice. J Neurochem. 2004;88:657-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Bouchier-Hayes L, Muñoz-Pinedo C, Connell S, Green DR. Measuring apoptosis at the single cell level. Methods. 2008;44:222-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1272] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 38. | Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 778] [Cited by in RCA: 757] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 39. | Marzo I, Brenner C, Zamzami N, Jürgensmeier JM, Susin SA, Vieira HL, Prévost MC, Xie Z, Matsuyama S, Reed JC. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027-2031. [PubMed] |
| 40. | Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2548] [Cited by in RCA: 2742] [Article Influence: 144.3] [Reference Citation Analysis (3)] |
| 41. | Gulbins E, Dreschers S, Bock J. Role of mitochondria in apoptosis. Exp Physiol. 2003;88:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Hiendleder S, Schmutz SM, Erhardt G, Green RD, Plante Y. Transmitochondrial differences and varying levels of heteroplasmy in nuclear transfer cloned cattle. Mol Reprod Dev. 1999;54:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Waterhouse NJ, Goldstein JC, Kluck RM, Newmeyer DD, Green DR. The (Holey) study of mitochondria in apoptosis. Methods Cell Biol. 2001;66:365-391. [PubMed] |
| 44. | Fantin VR, Leder P. Mitochondriotoxic compounds for cancer therapy. Oncogene. 2006;25:4787-4797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Pathania D, Millard M, Neamati N. Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Adv Drug Deliv Rev. 2009;61:1250-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 46. | Stover TC, Sharma A, Robertson GP, Kester M. Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin Cancer Res. 2005;11:3465-3474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 47. | Holmes WF, Soprano DR, Soprano KJ. Elucidation of molecular events mediating induction of apoptosis by synthetic retinoids using a CD437-resistant ovarian carcinoma cell line. J Biol Chem. 2002;277:45408-45419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Propper DJ, Braybrooke JP, Taylor DJ, Lodi R, Styles P, Cramer JA, Collins WC, Levitt NC, Talbot DC, Ganesan TS. Phase I trial of the selective mitochondrial toxin MKT077 in chemo-resistant solid tumours. Ann Oncol. 1999;10:923-927. [PubMed] |
| 49. | Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Distinct pathways for stimulation of cytochrome c release by etoposide. J Biol Chem. 2000;275:32438-32443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Kidd JF, Pilkington MF, Schell MJ, Fogarty KE, Skepper JN, Taylor CW, Thorn P. Paclitaxel affects cytosolic calcium signals by opening the mitochondrial permeability transition pore. J Biol Chem. 2002;277:6504-6510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 51. | Chinnery PF, Taylor GA, Howell N, Andrews RM, Morris CM, Taylor RW, McKeith IG, Perry RH, Edwardson JA, Turnbull DM. Mitochondrial DNA haplogroups and susceptibility to AD and dementia with Lewy bodies. Neurology. 2000;55:302-304. [PubMed] |
| 52. | Bertoncello I, Hodgson GS, Bradley TR. Multiparameter analysis of transplantable hemopoietic stem cells: I. The separation and enrichment of stem cells homing to marrow and spleen on the basis of rhodamine-123 fluorescence. Exp Hematol. 1985;13:999-1006. [PubMed] |
| 53. | Lonergan T, Brenner C, Bavister B. Differentiation-related changes in mitochondrial properties as indicators of stem cell competence. J Cell Physiol. 2006;208:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 54. | Bavister BD. The mitochondrial contribution to stem cell biology. Reprod Fertil Dev. 2006;18:829-838. [PubMed] |
| 55. | Barnett DK, Kimura J, Bavister BD. Translocation of active mitochondria during hamster preimplantation embryo development studied by confocal laser scanning microscopy. Dev Dyn. 1996;205:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 56. | Piccoli C, Ria R, Scrima R, Cela O, D’Aprile A, Boffoli D, Falzetti F, Tabilio A, Capitanio N. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. Novel evidence of the occurrence of NAD(P)H oxidase activity. J Biol Chem. 2005;280:26467-26476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 57. | Rhee SG, Kang SW, Chang TS, Jeong W, Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001;52:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 502] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 58. | Sauer H, Wartenberg M. Reactive oxygen species as signaling molecules in cardiovascular differentiation of embryonic stem cells and tumor-induced angiogenesis. Antioxid Redox Signal. 2005;7:1423-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 59. | Plotnikov EY, Marei MV, Podgornyi OV, Aleksandrova MA, Zorov DB, Sukhikh GT. Functional activity of mitochondria in cultured neural precursor cells. Bull Exp Biol Med. 2006;141:142-146. [PubMed] |
| 60. | Miyamoto T, Weissman IL, Akashi K. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8; 21 chromosomal translocation. Proc Natl Acad Sci USA. 2000;97:7521-7526. [PubMed] |
| 61. | Kucia M, Ratajczak MZ. Stem cells as a two edged sword--from regeneration to tumor formation. J Physiol Pharmacol. 2006;57 Suppl 7:5-16. [PubMed] |
| 62. | Ye XQ, Li Q, Wang GH, Sun FF, Huang GJ, Bian XW, Yu SC, Qian GS. Mitochondrial and energy metabolism-related properties as novel indicators of lung cancer stem cells. Int J Cancer. 2011;129:820-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 63. | Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O’Dwyer KM. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 1081] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 64. | Pastò A, Bellio C, Pilotto G, Ciminale V, Silic-Benussi M, Guzzo G, Rasola A, Frasson C, Nardo G, Zulato E. Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget. 2014;5:4305-4319. [PubMed] [DOI] [Full Text] |
| 65. | Alvero AB, Montagna MK, Holmberg JC, Craveiro V, Brown D, Mor G. Targeting the mitochondria activates two independent cell death pathways in ovarian cancer stem cells. Mol Cancer Ther. 2011;10:1385-1393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 66. | Vlashi E, Lagadec C, Vergnes L, Matsutani T, Masui K, Poulou M, Popescu R, Della Donna L, Evers P, Dekmezian C. Metabolic state of glioma stem cells and nontumorigenic cells. Proc Natl Acad Sci USA. 2011;108:16062-16067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 439] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 67. | Janiszewska M, Suvà ML, Riggi N, Houtkooper RH, Auwerx J, Clément-Schatlo V, Radovanovic I, Rheinbay E, Provero P, Stamenkovic I. Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev. 2012;26:1926-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 388] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 68. | Vlashi E, Lagadec C, Vergnes L, Reue K, Frohnen P, Chan M, Alhiyari Y, Dratver MB, Pajonk F. Metabolic differences in breast cancer stem cells and differentiated progeny. Breast Cancer Res Treat. 2014;146:525-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 112] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | Essers MA, Trumpp A. Targeting leukemic stem cells by breaking their dormancy. Mol Oncol. 2010;4:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 70. | Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4467] [Cited by in RCA: 4834] [Article Influence: 241.7] [Reference Citation Analysis (0)] |
| 71. | Lonardo E, Hermann PC, Heeschen C. Pancreatic cancer stem cells - update and future perspectives. Mol Oncol. 2010;4:431-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 72. | McDermott SP, Wicha MS. Targeting breast cancer stem cells. Mol Oncol. 2010;4:404-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 73. | Frank NY, Pendse SS, Lapchak PH, Margaryan A, Shlain D, Doeing C, Sayegh MH, Frank MH. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem. 2003;278:47156-47165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 189] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 74. | Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, Sayegh MH, Sadee W, Frank MH. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320-4333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 415] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 75. | Boman BM, Huang E. Human colon cancer stem cells: a new paradigm in gastrointestinal oncology. J Clin Oncol. 2008;26:2828-2838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 76. | Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, Lin Y, Yao J, Shi J, Kang T. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23:316-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 661] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 77. | Hirsch HA, Iliopoulos D, Struhl K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci USA. 2013;110:972-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 355] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 78. | Botchkina GI, Zuniga ES, Das M, Wang Y, Wang H, Zhu S, Savitt AG, Rowehl RA, Leyfman Y, Ju J. New-generation taxoid SB-T-1214 inhibits stem cell-related gene expression in 3D cancer spheroids induced by purified colon tumor-initiating cells. Mol Cancer. 2010;9:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 79. | Biasutto L, Dong LF, Zoratti M, Neuzil J. Mitochondrially targeted anti-cancer agents. Mitochondrion. 2010;10:670-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 80. | Loureiro R, Mesquita KA, Oliveira PJ, Vega-Naredo I. Mitochondria in cancer stem cells: a target for therapy. Recent Pat Endocr Metab Immune Drug Discov. 2013;7:102-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 81. | Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3173] [Cited by in RCA: 3102] [Article Influence: 163.3] [Reference Citation Analysis (0)] |
| 82. | Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C. Identification of cells initiating human melanomas. Nature. 2008;451:345-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1140] [Cited by in RCA: 1044] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 83. | Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915-920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1017] [Cited by in RCA: 973] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 84. | Lukacs RU, Memarzadeh S, Wu H, Witte ON. Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell. 2010;7:682-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 211] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 85. | Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328-9337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 923] [Cited by in RCA: 938] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 86. | Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1496] [Cited by in RCA: 1522] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 87. | Pontier SM, Muller WJ. Integrins in mammary-stem-cell biology and breast-cancer progression--a role in cancer stem cells? J Cell Sci. 2009;122:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 88. | Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87:1-13. [PubMed] |
| 89. | Furness SG, McNagny K. Beyond mere markers: functions for CD34 family of sialomucins in hematopoiesis. Immunol Res. 2006;34:13-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 90. | Rongioletti F, Donati P, Amantea A, Ferrara G, Montinari M, Santoro F, Parodi A. Obesity-associated lymphoedematous mucinosis. J Cutan Pathol. 2009;36:1089-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1548] [Cited by in RCA: 1633] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 92. | Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158-10163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1678] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 93. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2377] [Cited by in RCA: 2453] [Article Influence: 129.1] [Reference Citation Analysis (0)] |
| 94. | Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946-10951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1998] [Cited by in RCA: 2045] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 95. | Günthert U, Hofmann M, Rudy W, Reber S, Zöller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1240] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 96. | Zöller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 885] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 97. | Burger PE, Xiong X, Coetzee S, Salm SN, Moscatelli D, Goto K, Wilson EL. Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc Natl Acad Sci USA. 2005;102:7180-7185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 209] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 98. | Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 936] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 99. | Augello A, Kurth TB, De Bari C. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater. 2010;20:121-133. [PubMed] |
| 100. | Salcido CD, Larochelle A, Taylor BJ, Dunbar CE, Varticovski L. Molecular characterisation of side population cells with cancer stem cell-like characteristics in small-cell lung cancer. Br J Cancer. 2010;102:1636-1644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 101. | Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1265] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 102. | Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1987] [Cited by in RCA: 2160] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 103. | Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1292] [Cited by in RCA: 1374] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 104. | Mizrak D, Brittan M, Alison M. CD133: molecule of the moment. J Pathol. 2008;214:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 406] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 105. | Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311-4320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1023] [Cited by in RCA: 1016] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 106. | Ponnusamy MP, Batra SK. Ovarian cancer: emerging concept on cancer stem cells. J Ovarian Res. 2008;1:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 107. | Monk M, Holding C. Human embryonic genes re-expressed in cancer cells. Oncogene. 2001;20:8085-8091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 268] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 108. | Carpenter MK, Rosler E, Rao MS. Characterization and differentiation of human embryonic stem cells. Cloning Stem Cells. 2003;5:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 211] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 109. | Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1571] [Cited by in RCA: 1619] [Article Influence: 77.1] [Reference Citation Analysis (0)] |