Published online Apr 26, 2011. doi: 10.4252/wjsc.v3.i4.34
Revised: January 10, 2011
Accepted: January 17, 2011
Published online: April 26, 2011
AIM: To determine the tissue and temporal distribution of human umbilical cord matrix stem (hUCMS) cells in severe combined immunodeficiency (SCID) mice.
METHODS: For studying the localization of hUCMS cells, tritiated thymidine-labeled hUCMS cells were injected in SCID mice and tissue distribution was quantitatively determined using a liquid scintillation counter at days 1, 3, 7 and 14. Furthermore, an immunofluorescence detection technique was employed in which anti-human mitochondrial antibody was used to identify hUCMS cells in mouse tissues. In order to visualize the distribution of transplanted hUCMS cells in H&E stained tissue sections, India Black ink 4415 was used to label the hUCMS cells.
RESULTS: When tritiated thymidine-labeled hUCMS cells were injected systemically (iv) in female SCID mice, the lung was the major site of accumulation at 24 h after transplantation. With time, the cells migrated to other tissues, and on day three, the spleen, stomach, and small and large intestines were the major accumulation sites. On day seven, a relatively large amount of radioactivity was detected in the adrenal gland, uterus, spleen, lung, and digestive tract. In addition, labeled cells had crossed the blood brain barrier by day 1.
CONCLUSION: These results indicate that peripherally injected hUCMS cells distribute quantitatively in a tissue-specific manner throughout the body.
- Citation: Maurya DK, Doi C, Pyle M, Rachakatla RS, Davis D, Tamura M, Troyer D. Non-random tissue distribution of human naïve umbilical cord matrix stem cells. World J Stem Cells 2011; 3(4): 34-42
- URL: https://www.wjgnet.com/1948-0210/full/v3/i4/34.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v3.i4.34
Although embryonic stem cells (ESCs) have significant potential in treatment of many medical disorders, there are moral/ethical issues surrounding their derivation which hamper their clinical use. In addition, the limited number of cells at initial harvest necessitates extensive in vitro expansion. There are fewer moral/ethical concerns in relation to postnatal stem cells, and it was found recently that umbilical cord matrix contains an inexhaustible, non-controversial source of stem cells[1-11]. These multipotent stem cells, called umbilical cord matrix stem (UCMS) or Wharton’s jelly stem cells, are isolated from the mesenchyme-like cushioning material called ‘Wharton’s jelly’ found between the vessels of the umbilical cord[7]. UCMS cells have several properties that encourage their development as therapeutic agents. Namely, they (1) are isolated in large numbers; (2) are negative for CD34 and CD45; (3) grow vigorously and can be frozen/ thawed; (4) can be clonally expanded; and (5) can easily be engineered to express exogenous proteins[9-11].
Over the past decade, stem cells have been the focus of investigations for the treatment of diseases ranging from chronic heart failure to diabetes and multiple sclerosis. The process of stem-cell-based repair of acute injury involves homing and engraftment of the stem cell of interest to the site of injury, followed by either differentiation of the stem cell to indigenous end-organ cells or liberation of paracrine factors that lead to preservation and/or optimization of organ function[12].
Tumors are composed of both tumor cells and nonmalignant benign cells. The “benign” tumor compartment includes blood vessels, stromal fibroblasts, and infiltrating inflammatory cells[13]. Stromal fibroblasts offer structural support for malignant cells and influence the behavior and aggressiveness of cancers[13]. The formation of tumor stroma resembles wound healing and scar formation[14]. Malignant cells induce de novo formation of connective tissue in order to provide enough stroma to support cancer growth[15,16]. The homing of stem cells to tumors and other areas of inflammation is well established[17-20]. There are also a number of reports showing that genetically engineered stem cells efficiently deliver therapeutic proteins to cancers and other sites of inflammation[7,19,21-24]. Wharton’s jelly umbilical cord stem cells have the ability to traffic selectively to tumors[20]. Our previous studies indicate that human umbilical cord matrix stem (hUCMS) cells were attracted toward SDF-1 and VEGF in vitro[19]. We also found that hUCMS cells alone or hUCMS cells engineered to secrete the cytokine interferon (IFN)-β (hUCMS-IFN-β) are capable of reducing the growth of MDA 231 human breast carcinoma cells[19,25] and H358 lung bronchioloalveolar carcinoma cells (our un-published results). These observations open new possibilities for the treatment of various cancers and other types of diseases using stem cells. The potential of stem cell-based therapies for treating a myriad of human and animal diseases led us to study the trafficking of UCMS cells in un-engineered severe combined immunodeficiency (SCID) mice over time and to evaluate whether some tissues in the animal preferentially recruit UCMS cells. To address these aims, we analyzed the tissue distribution of hUCMS cells using sensitive radioisotope tracking coupled with histochemistry and immunohistochemistry.
Human UCMS cells were harvested from term deliveries at the time of birth, with the mother’s consent. The methods used to isolate and culture hUCMS cells were previously described[11]. Human UCMS cells were maintained in low serum defined medium (DM), a mixture of 56% low glucose DMEM (Invitrogen, Carlsbad, CA), 37% MCBD 201 (Sigma, St. Louis, MO) and 2% fetal bovine serum (FBS, Atlanta Biologicals Inc, Norcross, GA) containing 1 × insulin transferring selenium-X (Invitrogen), 1 × ALBUMax1 (Invitrogen), 1 × Pen/Strep (Invitrogen), 10 nmol/L dexamethasone (Sigma), 100 μmol/L ascorbic acid 2-phosphate (Sigma), 10 ng/mL epidermal growth factor (R&D Systems, Minneapolis, MN), and 10 ng/mL platelet derived growth factor-BB (R&D Systems). Cells were incubated at 37°C in an incubator with 5% CO2 at saturating humidity. When cells had reached 70%-80% confluency, they were detached with 0.25% trypsin-EDTA (Invitrogen), the trypsin was inactivated with fresh media, and the cells were centrifuged at 250 ×g for 5 min and replated in a ratio of 1:3. Human UCMS cells from passages 6-8 were utilized for the present study.
To evaluate the homing of hUCMS cells, female SCID mice, 6-8 wk of age, with CB17 background were purchased from Charles River (Wilmington, MA). Mice were held for 10 d after arrival to allow them to acclimatize. All animal experiments were done under strict adherence with the Institutional Animal Care and Use Committee protocol as set by Kansas State University.
hUCMS cells were radiolabeled by incubating 1 × 106 cells with 1 μCi tritiated thymidine for 24 h. For measuring the retention of radioactivity, the cells were washed with 1 × PBS and fed with fresh medium without tritiated thymidine. Retention of radioactivity was measured on days 1, 3, 7 and 10 post-labeling. Cells were harvested at given time points and radioactivity incorporated into the cells was measured by a Packard liquid scintillation counter Tri-Carb 2100TR (Perkin Elmer Life Science Boston, MA).
For studying the localization of hUCMS cells, 1.5 × 106 cells were radiolabeled with 9 μCi tritiated thymidine (3H-hUCMS cells). The next day the cells were washed twice with 1 × PBS and lifted from the culture dish by trypsinization. Cells were counted and washed with PBS. Finally, cells (2.5 × 106/mL) were suspended in PBS. Each mouse was inoculated with 0.5 × 106 cells in 200 μL PBS through tail vein injection. Randomized individual animals were kept in a clean room in cages with HEPA filters. Feces and urine were collected daily during the experimental period. Mice were sacrificed on days 1, 3, 7 and 14 after 3H-hUCMS cell injection and various tissues were collected. All tissues were homogenized in 9-20 volumes of PBS (a larger volume of PBS was used for small tissues, such as adrenal glands) and 0.5 mL homogenate was used for liquid scintillation counting in duplicate.
Anti-human mitochondrial antibody was used to identify hUCMS cells in mouse tissues. For immunofluorescence staining, tissue sections (6 μm) were washed with phosphate buffered saline-0.2% Triton X-100 (PBS-TX) and fixed with 70% ethanol and acetone (1:1) mixture. This was followed by washing with three changes of PBS-TX. Tissue sections were blocked with 5% normal goat serum in PBS-TX for 30 min, and then incubated with anti-human mitochondrial antibody produced in mouse (1:500, Chemicon, Temecula, CA), in PBS-TX overnight. The tissues were washed three times with PBS-TX and incubated with Alexa Fluor 488 conjugated secondary antibody raised in goat (1:500, Molecular Probes, Carlsbad, CA) for 3 h. The tissues were incubated for 30 min in Hoechst 33342 (10 μg/mL, Sigma) as a nuclear counter-stain followed by a triple rinse with PBS-TX. The antigen localization was observed using epifluorescence microscopy (Nikon Eclipse). Images were captured using a Roper Cool Snap ES camera and Metamorph 7 software.
In order to visualize transplanted hUCMS cells in H&E stained tissue sections, we developed a method to label the cells with waterproof drawing ink, India Black ink 4415 (Sanford, Oak Brook, IL). The ink was diluted 1:10 in ultrapure water and sterilized by autoclaving. To label cells for injection, the diluted ink was added to DM at a dilution of 1:20, for a final ink concentration of 0.5% and cells were incubated for 24 h. After lifting labeled cells by trypsinization, excess India Black ink was washed away with 1 × PBS. The labeled cells suspended in PBS (0.5 × 106 cells/200 μL PBS) were intravenously injected through the tail vein. Mice were sacrificed by CO2 asphyxiation on days 1, 3, 7 and 14 after injection. The mice were perfused with 20 mL saline containing 0.05% heparin and then with 20 mL 10% formalin in saline. Tissues were dissected, fixed in 10% formalin, and paraffin-embedded. To determine the distribution of the hUCMS cells, tissue sections were stained with hematoxylin and eosin.
Data is represented as mean ± SE values from 5 animals.
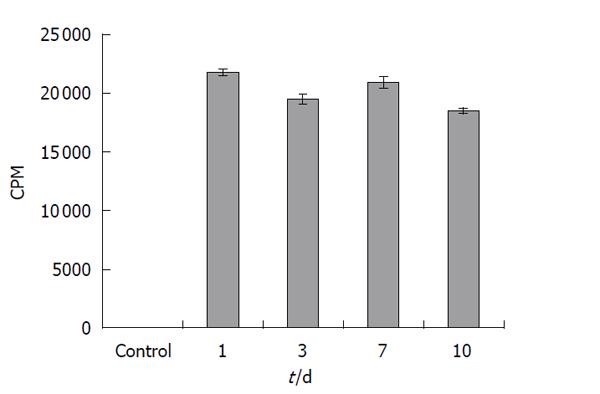
First, retention of the tritiated thymidine in hUCMS cells was evaluated using hUCMS cells in culture. Over 90% of the radioactivity was retained in the cells for at least 10 d (Figure 1). It is therefore suggested that tritiated thymidine labeled hUCMS cells can be utilized to determine their tissue localization.
Although a preliminary mouse study using 0.5 × 106 hUCMS cells labeled with 1.0 μCi tritiated thymidine distinctively identified major tissue distribution sites, the specific radioactivity per gram of tissue was not high. Therefore, 0.5 × 106 cells radiolabeled with 3.0 μCi tritiated thymidine were utilized for the tissue distribution study. The specific activity of the injected cells at the time of injection was determined to be 529 900 ± 33 470 c/min per 1 × 106 cells (0.53 c/min per cell). Use of higher radioactivity per cell also provides a longer period of observation and greater sensitivity.
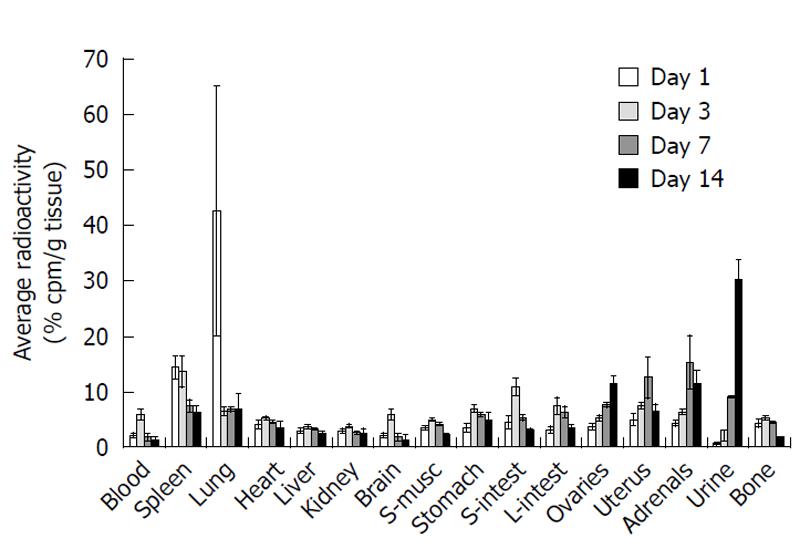
Observation of tissue radioactivity, human mitochondrial antigen, and India Black ink labeled cells made it clear that after administration of hUCMS cell via the tail vein, lung and spleen are the first target organs for engraftment of hUCMS cells. Tissue distributions of radioactivity throughout the body at various time points are presented in Figure 2. Considerable specific radioactivity was detected in the lung and spleen after 1 d, whereas little activity was detected in other tissues (Figure 2, white bars). However, hUCMS cells re-distributed to other tissues with time. On day 3, relatively large amounts of specific radioactivity were found in the spleen and digestive tract (Figure 2, light grey bars). On day 7, the highest specific radioactivity was detected in the adrenal glands (Figure 2, dark grey bars). It is noteworthy that on day 7, the second highest specific radioactivity was in the uterus. On day 14, specific radioactivity in various tissues was reduced in comparison to earlier days. The ovaries, adrenal glands and uterus showed the highest retention of radioactivity (Figure 2, black bars). The radioactivity retention in the lung and spleen were also relatively high.
Since the characteristics of gene expression in hUCMS cells are similar to those in bone marrow mesenchymal stem cells[10], and since recruitment of mesenchymal stem cells to bone marrow is possible, migration of UCMS cells to bone marrow was determined by measuring the radioactivity in whole femur homogenate. The maximum amount of radioactivity in femur homogenate was detected on day 1, and with time the amount of radioactivity decreased slowly (total radioactivity observed per gram femur bone at days 1, 3, 7 and 14 was 7.6%, 5.23%, 5.18% and 3.44%, respectively). This may suggest that bone marrow is an important distribution site for circulating mesenchymal stem cells, and that UCMS cells mimic behavior of bone marrow mesenchymal stem cells.
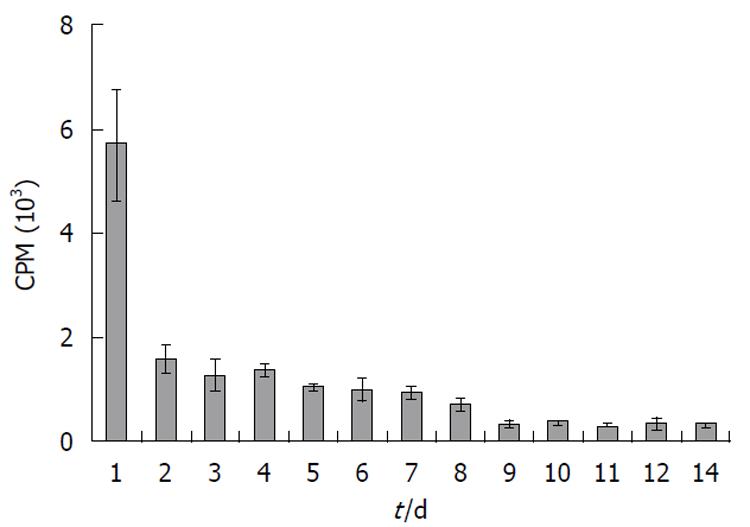
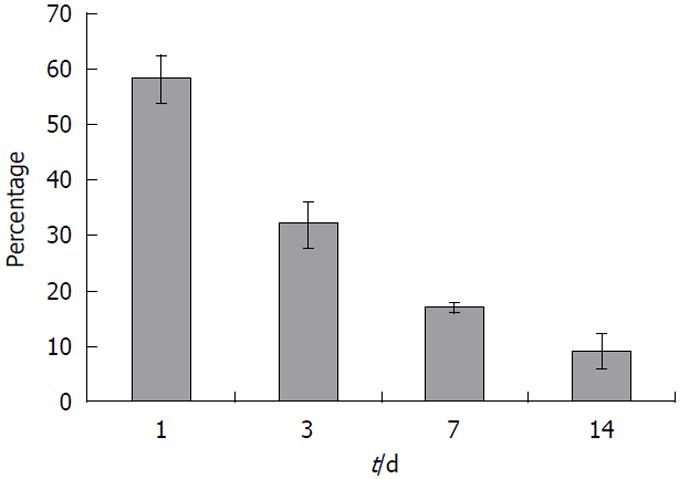
No radioactivity was detected in the feces (data not shown). Elimination of radioactivity by urinary excretion was significant. A large quantity of the radioactivity, approximately 2.75% of total recovered radioactivity, was excreted via this route within 24 h of injection (Figure 3). However, urinary excretion of the radioactivity gradually decreased with time (Figure 3). The total amount of radioactivity recovered from all tissues analyzed and from urine was calculated to be 58, 33, 17 and 10% of total injected radioactivity on days 1, 3, 7 and 14, respectively (Figure 4).
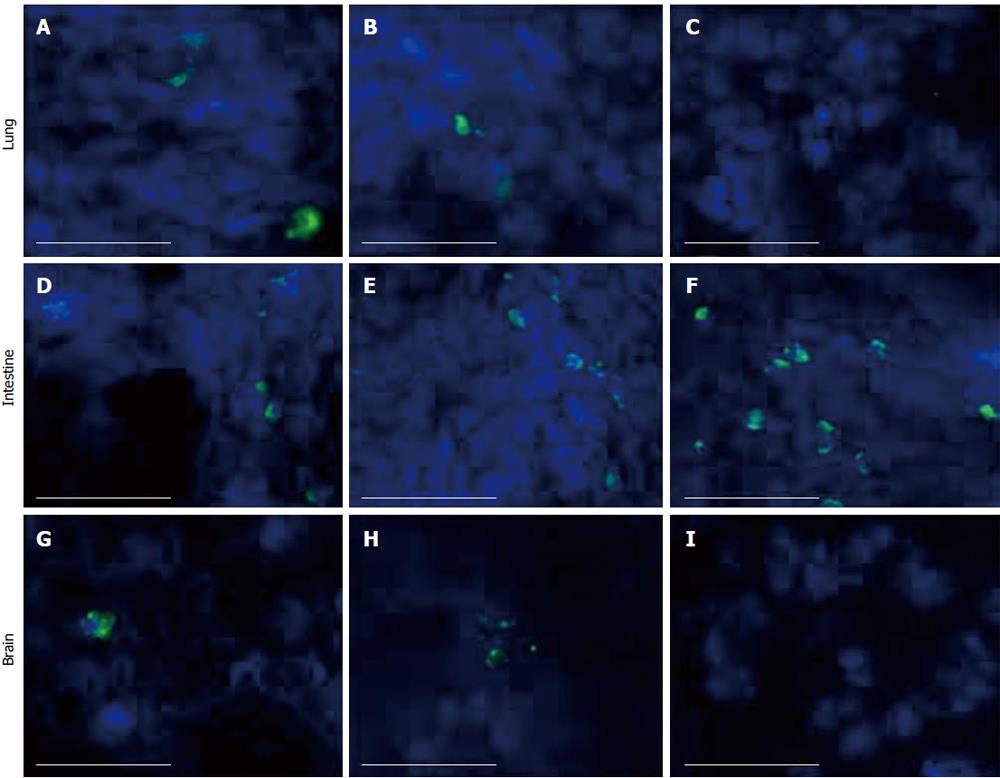
To further substantiate the radioactivity-based tissue distribution of hUCMS cells, immunofluorescence staining was applied to all tissues. Although hUCMS cells were detected in lung, intestine, and brain at days 1 and 3 (Figure 5),
they were undetectable by this method in most other tissues even 1 d after injection. However, we were able to detect hUCMS cells at day 7 in the intestine (Figure 5).
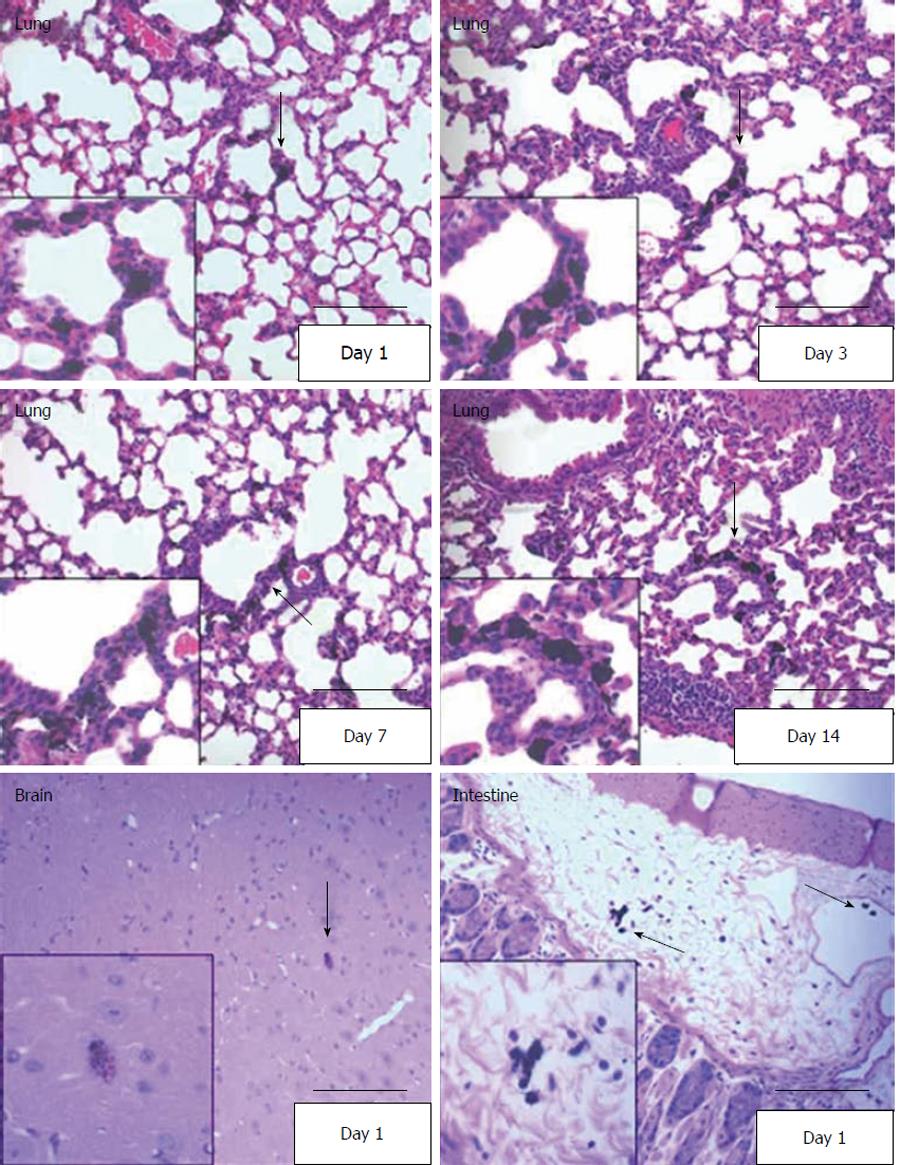
As another method to validate the radiolabeled cell distribution in various tissues, and to better observe morphology in paraffin embedded sections, we developed a technique in which cells were loaded with India Black ink before injection. In this method, hUCMS cells loaded with India Black ink were observed as black particle-loaded cells in H & E stained slides. By this method we were able to detect the cells in brain, lung, and stomach on day 1 (Figure 6). The India Black ink-loaded hUCMS cells were also detected on days 3, 7 and 14 in the lung, but not in the uterus, spleen, kidney, etc. (Figure 6).
Increasing evidence suggests that adult and postnatal stem cells are valuable resources for various medical therapies. Among many tissue-originated multipotent stem cells, UCMS cells are very usable due to their abundance, low immunogenicity[26], and simplicity of harvest and in vitro expansion[7,11]. However, the tissue distribution characteristics of transplanted UCMS cells in the normal body have not been studied previously. Many of the aforementioned therapies require systemic transplantation of stem cells. Therefore, their tissue distribution in physiological and pathological conditions is important. Here we report the distribution of hUCMS cells in SCID mice after systemic transplantation using the sensitive tracking method of radiolabeled cell detection combined with histochemical and immunohistochemical detection of hUCMS cells.
The lung and spleen were found to be the major distribution tissues at 24 h after transplantion (Figure 2, white bars). The spleen continued to be the highest distribution site even 3 d after transplantation (Figure 2, light grey bars). Since the spleen is the site for clearance of most foreign and damaged cells present in circulation, the splenic distribution may be associated with clearing of damaged or dead hUCMS cells. Splenic clearance may also be related to the initial clearance of radioactivity in the urine after transplantation (Figure 3).
On day three, in addition to the spleen, the gastrointestinal tract, including the stomach and small and large intestines, were the primary distribution organs (Figure 2, white grey bars). However, this distribution pattern was changed on day 7, when the highest distribution was found to be in the adrenal glands and uterus (Figure 2, dark grey bars). The lung, spleen and intestine show lesser amounts by day 7. This pattern was not changed at 14 d after transplantation (Figure 2, black bars) although the total radioactivity recovered was significantly decreased.
On the other hand, although a significant amount of radioactivity was distributed in skeletal muscle, the India Black ink labeled UCMS cells were not detected in skeletal muscle. This is perhaps due to the fact that the UCMS cells distrubuted through such a large amount of skeletal muscle. Therefore, although the total amount of calculated radioactivity in muscle is high, the specific activity (radioactivity per gram tissue) is low, and individual cells may have escaped detection. To study the distribution of hUCMS cells in bone marrow, radioactivity in whole femur homogenate was determined. A noticeable amount of radioactivity was detected in the femur homogenate. This may suggest that hUCMS cells localize in the bone marrow niche and potentially contribute to bone marrow function.
The primary distribution sites were visually confirmed by detection of hUCMS cells using immunohistochemical methods and India Black ink labeling of the hUCMS cells. Careful observation of the morphology of the transplanted cells in H&E tissue sections, immunohistochemical and India Black ink labeling and the time course study suggest that the majority of transplanted cells detected in various tissues are intact and were not engulfed by macrophages or other phagocytic cells. Transplanted UCMS cells remained as characteristically large cells and many of them were adjacent to the lung vasculature even 14 d after transplantation (Figure 6). Therefore, a significant portion of the transplanted UCMS cells appears to be intact for a lengthy period. However, a portion of the transplanted cells appears to be destroyed and removed from circulation, and free tritiated thymidine is excreted into the urine. Therefore, it is difficult to rule out the possibility that a small portion of radiolabeled hUCMS cells may have been engulfed by macrophages and redistributed with macrophage migration. However, all three detection methods were in good agreement and identified the lung, gastrointestinal tract, and brain as primary tissue distribution sites at days 1 and 3 after cell transplantation. This agreement of UCMSC distribution between the three methods supports the suggestion that India Black ink labeled cells behaved as unlabeled cells do in vivo, although it is difficult to completely exclude the possibility that India Black ink labeled cells may have been sequestered differently. The high level of radioactivity distribution to the adrenal glands may be misleading, since we were unable to detect the intact hUCMS cells in adrenal glands by immunohistochemical and histochemical methods. Since the adrenal glands and ovaries are small (approximately 30 mg/gland), a small artifact in the radioactive signal would be exaggerated mathematically. In addition, it is possible that metabolized or free tritiated thymidine may have been redistributed to these tissues. We expected to find that the lung was the primary distribution site in the early stages of transplantation because our previous studies indicated that systemically transplanted IFN-β-expressing hUCMS cells significantly reduce the growth of lung metastasized MDA 231 human breast carcinoma xenografts[19,25]. This efficient lung distribution indicates that UCMS cells may have substantial therapeutic potential in various lung diseases including cancer, asthma and pneumonia, when UCMS cells are used to deliver therapeutic molecules and/or express therapeutic genes.
The distribution of hUCMS cells in the uterus and ovaries is of interest. The present study clearly indicates that the major distribution site in the reproductive organs is the uterus rather than the ovaries. Since both organs are sites of extensive remodeling during the estrous cycle[27], the stem cells may be responding to chemoattractants similar to those associated with wound healing or tumors. The observation of relatively high distribution in the intestinal tract also correlates with localization to regions of rapid tissue turnover, since the epithelium is replaced every 4-7 d. Radiolabeled hUCMS cell distribution to uterus, ovaries, and digestive tract suggests that transplanted UCMS cells may play a role in tissue remodeling associated with aggressive cell division. Although the hUCMS cells have been shown to traffic to tumor tissues in a manner similar to bone marrow mesenchymal stem cells and neural stem cells[25], the present results suggest that a portion of systemically transplanted UCMS cells are likely to distribute to other tissues that contain actively dividing cells.
One of the most interesting findings is that there was radioactivity in the brain 1, 3, and 7 d after transplantation. This finding was confirmed by immunohistochemical detection and India Black ink labeling of hUCMS cells. Since the cells were not detected in the blood-brain barrier vasculature, they evidently crossed the blood brain barrier. Although this barrier is comprised primarily of tight junctions between endothelial cells and poses a formidable obstacle to the delivery of many therapeutic agents to the central nervous system[28], UCMS cells could cross this barrier and potentially be used as delivery vehicles for therapeutics to the brain. In support of this speculation, systemically administered neuronal stem cells engineered to express a pro-drug processing enzyme were found to migrate into the brain and to exhibit therapeutic properties[22]. Furthermore, bone marrow MSC or fetal stem cells physiologically cross blood-brain barrier and play an important role in blood vessel remodeling[29] and in healing of brain injury[30].
Since detection of the radiolabeled cells is very sensitive as compared to histochemical and immunohistochemical detection of the cells, it was possible to monitor the distribution of the hUCMS cells in most tissues via radiolabeling. Immunofluorescence and India Black ink labeling made it possible to clarify the exact location of the hUCMS cells within many organs, but were less sensitive than radiolabeling. A possible explanation for this sensitivity difference is that in the radioactive method we have homogenized whole tissues whereas the immunofluorescence and India ink labeling methods were carried out on sections only 5-10 μM thick, so it is possible that cells may have been present in higher numbers in other parts of the organ.
In conclusion, intravenously administered hUCMS cells exhibited time-specific tissue distribution in SCID mice. The lung was a major distribution site at 24 h after transplantation. With time, major distribution sites changed to the gastrointestinal tract at 3 and 7 d after transplantation. In addition to the gastrointestinal tract, both ovaries and uterus were found to be distribution sites. The tissue distributions were confirmed by immunohistochemical and histological observations of hUCMS cells in tissue sections. Morphological analysis revealed that systemically administered hUCMS cells can be efficently distributed in brain. These findings indicate that UCMS cells are potentially useful tools for the treatment of various diseases in the lung, gastrointestinal tract, and brain.
The authors recently found that human umbilical cord matrix stem (hUCMS) cells alone, or hUCMS cells transduced with interferon (IFN)-β, significantly reduce the growth of human breast carcinoma cells and human lung broncheoloalveolar carcinoma cells in vitro and in vivo. These findings open a new area for the therapeutic application of stem cells for multiple types of cancer.
Stem cells can be derived from a variety of sources such as embryos [embryonic stem cells (ESCs)], bone marrow stem cells, fetal tissues, cord blood, etc. ESCs have moral/ethical issues surrounding their derivation. Although postnatal stem cells offer fewer concerns, many postnatal stem cells derived from the sources listed above also have drawbacks which hamper their clinical use. these include invasive tissue collection and prolonged in vitro expansion times due to the very limited number of cells which can be initially harvested. Recently, it was found that umbilical cord matrix contains an inexhaustible, non-controversial source of stem cells. Therefore, studying quantitative tissue distribution of hUCMS cells in animals provides fundamentally important knowledge for the use of this stem cell type in the future.
Previous studies indicate that hUCMS cells were attracted toward SDF-1 and VEGF in vitro (Rachakatla et al, 2008). The authors also found that hUCMS cells alone or hUCMS cells engineered to secrete the IFN-β (hUCMS-IFN-β) are capable of reducing the growth of human breast carcinoma cells and lung broncheoloalveolar carcinoma cells. These observations open a new era in the treatment of various cancers and other types of diseases using stem cells. Intravenously administered hUCMS cells exhibited time-specific tissue distribution in severe combined immunodeficiency (SCID) mice. The lung was a major distribution site at 1 d after transplantation. Major distribution sites changed to the gastrointestinal tract at 3 and 7 d after transplantation. Morphological analysis revealed that systemically administered hUCMS cells can also be efficently distributed in the brain. These results suggest that hUCMS cells could be used as a therapeutic and/or therapeutic delivery tools for abnormalities in these tissues.
Since hUCMS cells are shown to be poorly immunogenic (Weiss et al, 2006; Cho et al, 2008), it is conceivable that hUCMS cells could be used as allogeneic therapeutics or therapeutic gene and/or protein delivery tools for inflammatory diseases and various types of cancer. Conveniently, hUCMS cells appear to be tropic to inflamatory tissues and tumors (Rachakatla et al, 2008; Ayuzawa et al, 2009; Matsuzuka et al, 2010).
In the presented manuscript authors showed the tissue distribution pattern of iv injected hUCMS cells into SCID mice. Their results indicated that the first site of engraftment is lung, a finding which may be anticipated since the route of administration was intravenous.
Peer reviewers: Alp Can, MD, PhD, Professor, Laboratory for Stem Cell Science, Department of Histology and Embryology, Ankara University, School of Medicine, Sihhiye, Ankara 06100, Turkey; Herman S Cheung, Professor, Department of Biomedical Engineering, University of Miami, 219 A McArthur Annex, 1251 Memorial Drive, Coral Gables, FL 33146, United States
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
| 1. | Campard D, Lysy PA, Najimi M, Sokal EM. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134:833-848. |
| 2. | Can A, Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25:2886-2895. |
| 3. | Friedman R, Betancur M, Boissel L, Tuncer H, Cetrulo C, Klingemann H. Umbilical cord mesenchymal stem cells: adjuvants for human cell transplantation. Biol Blood Marrow Transplant. 2007;13:1477-1486. |
| 4. | Hoynowski SM, Fry MM, Gardner BM, Leming MT, Tucker JR, Black L, Sand T, Mitchell KE. Characterization and differentiation of equine umbilical cord-derived matrix cells. Biochem Biophys Res Commun. 2007;362:347-353. |
| 5. | La Rocca G, Anzalone R, Corrao S, Magno F, Loria T, Lo Iacono M, Di Stefano A, Giannuzzi P, Marasà L, Cappello F. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol. 2009;131:267-282. |
| 6. | Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, Han ZB, Xu ZS, Lu YX, Liu D. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017-1026. |
| 7. | Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. 2003;21:50-60. |
| 8. | Seshareddy K, Troyer D, Weiss ML. Method to isolate mesenchymal-like cells from Wharton's Jelly of umbilical cord. Methods Cell Biol. 2008;86:101-119. |
| 9. | Troyer DL, Weiss ML. Wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26:591-599. |
| 10. | Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24:781-792. |
| 11. | Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2:155-162. |
| 12. | Penn MS, Khalil MK. Exploitation of stem cell homing for gene delivery. Expert Opin Biol Ther. 2008;8:17-30. |
| 14. | Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650-1659. |
| 15. | Hasebe T, Sasaki S, Sugitoh M, Ono M, Saitoh N, Ochiai A. Highly proliferative intratumoral fibroblasts and a high proliferative microvessel index are significant predictors of tumor metastasis in T3 ulcerative-type colorectal cancer. Hum Pathol. 2001;32:401-409. |
| 16. | Kuniyasu H, Abbruzzese JL, Cleary KR, Fidler IJ. Induction of ductal and stromal hyperplasia by basic fibroblast growth factor produced by human pancreatic carcinoma. Int J Oncol. 2001;19:681-685. |
| 17. | Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846-12851. |
| 18. | Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307-3318. |
| 19. | Rachakatla RS, Marini F, Weiss ML, Tamura M, Troyer D. Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Ther. 2007;14:828-835. |
| 20. | Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603-3608. |
| 21. | Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15:739-752. |
| 22. | Aboody KS, Najbauer J, Schmidt NO, Yang W, Wu JK, Zhuge Y, Przylecki W, Carroll R, Black PM, Perides G. Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro Oncol. 2006;8:119-126. |
| 23. | Kumar S, Chanda D, Ponnazhagan S. Therapeutic potential of genetically modified mesenchymal stem cells. Gene Ther. 2008;15:711-715. |
| 24. | Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593-1603. |
| 25. | Rachakatla RS, Pyle MM, Ayuzawa R, Edwards SM, Marini FC, Weiss ML, Tamura M, Troyer D. Combination treatment of human umbilical cord matrix stem cell-based interferon-beta gene therapy and 5-fluorouracil significantly reduces growth of metastatic human breast cancer in SCID mouse lungs. Cancer Invest. 2008;26:662-670. |
| 26. | Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D, McIntosh KR. Immune properties of human umbilical cord Wharton's jelly-derived cells. Stem Cells. 2008;26:2865-2874. |
| 27. | Zoubina EV, Fan Q, Smith PG. Variations in uterine innervation during the estrous cycle in rat. J Comp Neurol. 1998;397:561-571. |
| 28. | Karnovsky MJ. The ultrastructural basis of capillary permeability studied with peroxidase as a tracer. J Cell Biol. 1967;35:213-236. |
| 29. | Galimi F, Summers RG, van Praag H, Verma IM, Gage FH. A role for bone marrow-derived cells in the vasculature of noninjured CNS. Blood. 2005;105:2400-2402. |
| 30. | Tan XW, Liao H, Sun L, Okabe M, Xiao ZC, Dawe GS. Fetal microchimerism in the maternal mouse brain: a novel population of fetal progenitor or stem cells able to cross the blood-brain barrier? Stem Cells. 2005;23:1443-1452. |