Published online Aug 26, 2010. doi: 10.4252/wjsc.v2.i4.93
Revised: August 3, 2010
Accepted: August 10, 2010
Published online: August 26, 2010
Tissue engineering is an interdisciplinary field promising new therapeutic means for replacing lost or severely damaged tissues or organs. However, the fabrication of complex engineered tissues has been hampered due to the lack of vascularization to provide sufficient blood supply after implantation. In this article, we propose using rapid prototyping technology to prefabricate a scaffold with an inside hollowed vascular system including an arterial end, a venous end and capillary networks between them. The scaffold will be ''printed'' layer by layer. When printing every layer, a ''low-melting point'' material will be used to form a blood vessel network and a tissue-specific material will be used outside it. Hereafter the ‘low-melting point’ material will be evacuated by vaporization to ensure a hollowed vessel network. Then the inside hollowed capillary network can be endothelialized by using autologous endothelial cells in a cycling bioreactor while the outside material can be embedded with tissue-special cells. In the end, the new vascularized autologous grafts could be transferred to the defect site by using microsurgical techniques to connect the grafts with the host artery and vein. The strategy would facilitate construction of complex tissue engineering if the hypothesis proved to be practical.
- Citation: Liu JC. A novel strategy for engineering vascularized grafts in vitro. World J Stem Cells 2010; 2(4): 93-96
- URL: https://www.wjgnet.com/1948-0210/full/v2/i4/93.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v2.i4.93
Tissue engineering has tremendous potential for the treatment of a wide range of medical conditions. It represents a new concept that focuses on regeneration of neotissues from cells with the support of biomaterials and growth factors[1]. Many tissues have been engineered, including skin, bone, cartilage, tendon and periodontal tissues. But clinical use of engineered tissues and tissue substitutes is largely limited to thin or avascular tissues such as skin or cartilage because of the lack of transplant vascularization[2,3]. Fabrication of complex organs like heart or kidney still has a long way to go. Researchers have been working hard to overcome this limitation[4-6]. This research can be divided into four groups: scaffold design, angiogenic factor delivery, in vivo prevascularization and in vitro prevascularization[7]. Most of these strategies have their limitations:
in vivo prevascularization requires two separate surgeries and in vitro prevascularization[8] is associated with a complex in vitro culture period that might not be easy to perform in a standard hospital situation. Moreover, at present there is no convincing evidence that any of these strategies will be sufficient to sustain tissue-engineered constructs that are larger than several millimeters after implantation[7]. So, it is a priority to find a new way of vascularization in tissue engineering.
Small-diameter tissue engineering arteries (< 6 mm) have been successfully constructed in vitro but few studies have involved fabrication of capillary network in vitro. One reason is that a capillary is 6-8 um in diameter which is only 1/10 that of a single hair. On the other hand, a small tissue contains a great amount of capillaries. Development of advanced biomaterials and scaffold constructing technology give opportunity to accept the challenge. Rapid prototyping (RP) is a common name for several techniques which read in data from computer-aided design (CAD) drawings and manufacture automatically three-dimensional objects layer-by-layer according to the virtual design[9]. RP enables the control of the scaffold porosity, making it possible to fabricate applications with desired structural integrity. In this article, we present our hypothesis on the basis of the advanced material and RP technology, implying a new approach to prefabricate scaffolds with a hollowed vascular network and a new way of vascularization in tissue engineering.
To explore the possibility of fabrication of vascularized grafts, we hypothesize in this paper that, using computer-aided rapid prototyping technology and ‘low-melting point’ material, a scaffold with a hollowed vascular system of capillary networks with an arterial end and a venous end could be fabricated.
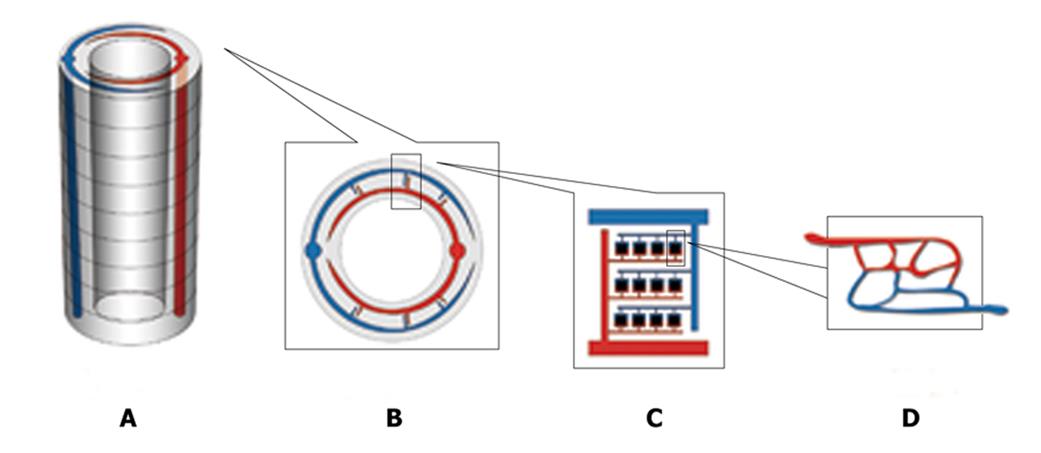
Firstly, two types of material will be used: the outside tissue-specific material and the inside ‘low-melting point’ material. The use of tissue-specific material depends on what kind of tissue is fabricated, for example, hydroxyapatite for bone engineering. The inside ‘low-melting point’ material will be shaped like a vascular network and will be eliminated in the end to form the inside hollowed vascular network. Secondly, rapid prototyping computer-aided 3D printing technology will be used to fabricate a particular 3D scaffold layer-by-layer[10]. Hundreds of layers of 200-um in thickness will be constructed layer by layer to form a special-shaped scaffold, for example a cylinder-shaped scaffold (Figure 1A). When constructing each layer, the outside tissue-specific material is deposited first and then the inside ‘low-melting point’ material is deposited to form a hierarchical vascular network under the control of a computer (Figure 1B, C and D). The basic unit (Figure 1D),
like the physiological microcirculation system, is 250 um in length and 250 um in width and the diameter of a ‘capillary’ is 10 um. When the bio-material is printed layer by layer, the artery end and the vein end will connect with its counterpart of the adjacent layer (Figure 1A). In the end, a main artery and vein are formed throughout it automatically in the scaffold. In every layer the main artery or vein will send out its branches. Thirdly, the scaffold is put inside a vacuum and its temperature is increased to reach its vaporization point. Then the vascular-formed material (low-melting point material) is eliminated from the scaffold and a hollowed network is formed. Fourthly, targeted tissue cells are seeded into the outside tissue-specific material and cultured in a static manner while endothelium cells are seeded into the inside hollow surface in a rotative and dynamic way until the hollowed vascular network is endothelialized[11].
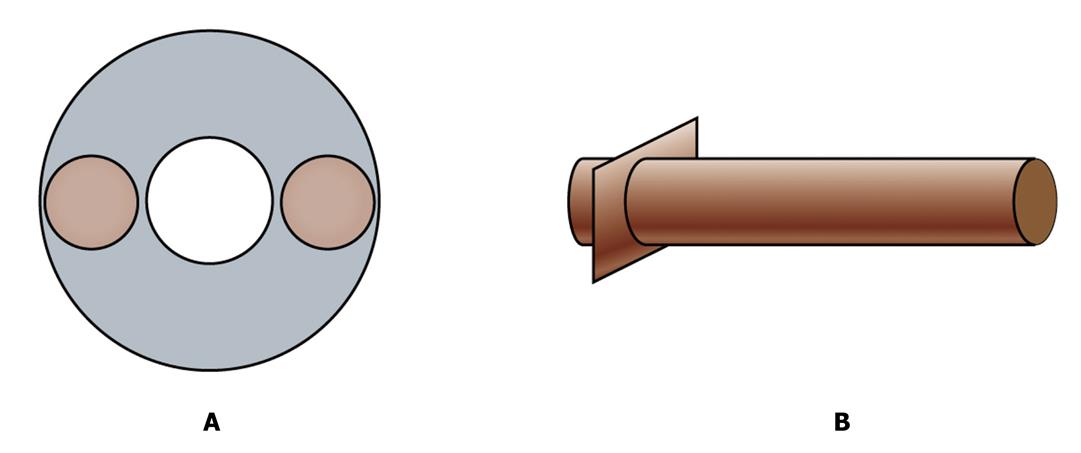
Now we have a vascularized graft. But how can we transplant the graft into a host and connect its vessels with host axial blood vessels? We need two connectors. They are two tissue engineered blood vessels and will be used as bridges to connect the fabricated graft with host artery and vein by microsurgery (Figure 2). Tissue engineered blood vessels have been successfully fabricated and applied clinically[12] and so, using similar methods, we will fabricate the vessels. Specifically, the scaffold of the connection blood vessels are prefabricated with rigid material in one end like hydroxyapatite (HA) and soft material in the other end like polyglycolic acids (PGA). The rigid end will be plugged in to the artery or vein end of the fabricated scaffold and the soft end will be sutured with the host’s vessels when the whole artificial graft is transplanted into a host.
Some important issues should be considered to evaluate the feasibility of the vascularized graft. Although rapid Prototyping technology, such as 3D Printing[13], offer great confidence to construct kinds of scaffolds prefabricated in the computer concisely and rapidly, fineness of the rapid prototyping machine should be considered. Is it possible to put a 10-um capillary-like deposition concisely in position? Advanced micro-nanoprocessing technologies may hold the promise to overcome the challenge[14]. Another consideration is the diameter of an endothelial cell which, as we know, is 10-50 um. Whether the endothelial cells would adhere to surfaces of the 10-um capillary-like tunnels or be obstructed before it is another problem. Endothelial cells have shown characteristics of preferring growth along micro-tunnels[15]. It is reasonable to speculate that endothelial cells could adhere to surface of the capillary-like tunnels in the above scaffold.
If the hypothesis is proved to be practical, the endothelialized graft could ensure the oxygen and nutrition supply once it is transplanted into a living host body and could improve the growth and differentiation of cells to form targeted tissues or organs. Considering the outside tissue-specific material and cells could be changed depending on our aim and the inside material and endothelialization could be used repeatedly, the method in this paper could be used as a model for complex tissue regeneration.
Vascularization remains one of the main obstacles that need to be overcome before large tissue-engineered constructs can be applied in clinical applications. We propose a new strategy to fabricate a vascularized graft in tissue engineering. Based on advanced materials and rapid prototyping technology, a prefabricated scaffold with a hollowed vascular-like network could be obtained. After endothelialization, the graft could be transplanted into a living host. The cells of the graft could proliferate and differentiate better and a complex and functional tissue or organ could be possibly constructed.
Peer reviewer: Alain Chapel, PhD, Departement of Man Radioprotection, Institute of Nuclear Safety and Radioprotection, IRSN DRPH SRBE, FAR 92262, France
S- Editor Wang JL L- Editor Roemmele A E- Editor Yang C
| 1. | Miller MJ, Patrick CW Jr. Tissue engineering. Clin Plast Surg. 2003;30:91-103, vii. |
| 3. | Mertsching H, Schanz J, Steger V, Schandar M, Schenk M, Hansmann J, Dally I, Friedel G, Walles T. Generation and transplantation of an autologous vascularized bioartificial human tissue. Transplantation. 2009;88:203-210. |
| 4. | Cassell OC, Morrison WA, Messina A, Penington AJ, Thompson EW, Stevens GW, Perera JM, Kleinman HK, Hurley JV, Romeo R. The influence of extracellular matrix on the generation of vascularized, engineered, transplantable tissue. Ann N Y Acad Sci. 2001;944:429-442. |
| 5. | Casabona F, Martin I, Muraglia A, Berrino P, Santi P, Cancedda R, Quarto R. Prefabricated engineered bone flaps: an experimental model of tissue reconstruction in plastic surgery. Plast Reconstr Surg. 1998;101:577-581. |
| 6. | Frerich B, Kurtz-Hoffmann J, Lindemann N, Müller S. [Tissue engineering of vascularized bone and soft tissue transplants]. Mund Kiefer Gesichtschir. 2000;4 Suppl 2:S490-S495. |
| 7. | Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol. 2008;26:434-441. |
| 8. | Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D'Amore PA. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879-884. |
| 9. | Peltola SM, Melchels FP, Grijpma DW, Kellomäki M. A review of rapid prototyping techniques for tissue engineering purposes. Ann Med. 2008;40:268-280. |
| 10. | Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21:157-161. |
| 11. | Zhang WJ, Liu W, Cui L, Cao Y. Tissue engineering of blood vessel. J Cell Mol Med. 2007;11:945-957. |
| 12. | Shin'oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med. 2001;344:532-533. |
| 13. | Leukers B, Gülkan H, Irsen SH, Milz S, Tille C, Schieker M, Seitz H. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J Mater Sci Mater Med. 2005;16:1121-1124. |