Published online Jul 26, 2025. doi: 10.4252/wjsc.v17.i7.107770
Revised: May 6, 2025
Accepted: July 3, 2025
Published online: July 26, 2025
Processing time: 118 Days and 0.3 Hours
Diet and nutrition significantly influence health, largely by regulating intestinal nutrient absorption. The intestinal epithelium, as the primary site for nutrient uptake, undergoes continuous renewal driven by precise regulation of intestinal stem cells (ISCs). Nutrient sensing and metabolism are key determinants of ISC fate, making ISCs a central link between nutrient metabolism and the regulation of intestinal tissue renewal and homeostasis. Understanding how ISCs respond or make adaptations to nutritional signals is therefore vital for maintaining intestinal homeostasis. Recent studies have spotlighted the origin and identity of ISCs and broadened our insight into the plasticity and function of ISCs under different conditions. Mitochondria, the central hubs of energy production and metabolic signals provided by dietary components and metabolic substrates, such as gluco
Core Tip: Diet and nutrition play a critical role in health by regulating intestinal nutrient absorption, with intestinal stem cells serving as the central link between nutrient metabolism and intestinal tissue renewal. Nutrient sensing, metabolic regulation, and mitochondrial function are key determinants of intestinal stem cell fate, balancing self-renewal and differentiation. Understanding these mechanisms offers insights into stem cell-based therapies for gastrointestinal diseases and dietary interventions to promote health.
- Citation: Li WH, Yuan XY, Wang Z, Lin R. Nutrient sensing in intestinal stem cell: Linking dietary nutrients to cellular metabolic regulation. World J Stem Cells 2025; 17(7): 107770
- URL: https://www.wjgnet.com/1948-0210/full/v17/i7/107770.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i7.107770
The mammalian intestinal epithelium constitutes the largest mucosal barrier and serves as the primary site for nutrient absorption[1,2]. Dietary components regulate physiological processes by triggering metabolic and sensory signaling pathways directly at the intestinal lining prior to their systemic dissemination. The nutritional microenvironment can influence the differentiation and self-renewal activities of intestinal stem cells (ISCs), which reside at the base of crypts and orchestrate epithelial turnover[3,4]. This adaptive mechanism ensures the maintenance of functional and structural integrity of the intestine under varying nutritional conditions, potentially positioning ISCs as central integrators coupling luminal nutrient sensing with tissue homeostasis.
The proliferation and differentiation of ISCs are tightly regulated by nutritional factors. Macronutrients, including saccharides, lipids, and amino acids, mediate the mitotic expansion of ISCs into transit-amplifying cells through two major signaling pathways: Wnt/β-catenin and Notch. Among these, the Wnt/β-catenin pathway primarily regulates ISCs self-renewal and proliferation, while the Notch pathway governs cell fate determination and differentiation. Following migration from the crypt base toward the intestinal lumen, transit-amplifying cells undergo cell cycle exit and lineage commitment, ultimately differentiating into two principal epithelial lineages: Absorptive cells, primarily enterocytes responsible for nutrient absorption, and secretory cells, including goblet cells, Paneth cells, enteroendocrine cells (EECs), and Tuft cells[3,5]. Notably, ISCs and their differentiated progeny exhibit distinct metabolic profiles throughout their lineage commitment. During differentiation, ISCs alter their metabolic modes [e.g., oxidative phosphorylation (OXPHOS), fatty acid oxidation and glycolysis] through nutrient-sensing pathways regulated by mammalian target of rapamycin, peroxisome proliferator-activated receptor (PPAR), AMP-activated protein kinase and other related signaling[6,7]. If abnormalities in nutrient-sensing pathways occur due to factors such as malnutrition, they may lead to intestinal inflammation and barrier dysfunction[8]. Deciphering the precise regulatory mechanisms through which dietary components modulate ISC plasticity represents a crucial research topic essential for understanding intestinal homeostasis, developing nutritional interventions, and holds translational promise for precision nutrition strategies targeting mucosal regeneration disorders.
This review summarizes the importance of nutrient sensing, metabolic regulation, and mitochondrial function in determining ISC fate and their impact on intestinal health, aiming to elucidate the complex interactions between diet, metabolism, and stem cell biology. By mapping the nutrient sensing-metabolic regulation axis, we lay a theoretical foundation for developing stem cell-based therapeutic strategies for gastrointestinal diseases and innovative dietary interventions to promote intestinal health.
Previous studies have shown that the crypts harbor two distinct ISC populations: Crypt base columnar (CBC) cells, characterized by high expression of leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5), which drive constitutive epithelial renewal through active proliferation[9]; and a quiescent population of cells located at the fourth position above the crypt base, characteristically expressing homeodomain only protein, Bmi1, mTert, and leucine-rich repeats and immunoglobulin-like domains containing protein 1, and serves as a reserve regenerative pool activated during mucosal injury[10-14].
Emerging studies challenge the exclusivity of Lgr5+ ISCs in lineage specification, revealing fibrinogen-binding protein 1+ capable of regenerating crypt-villus structures (including Lgr5+ CBC populations) through biphasic regenerative trajectories[15,16]. Single-cell transcriptomic and chromatin accessibility analyses further identified the Lgr4+ proliferative cells within the crypt isthmus (+4 to +13 cell positions from the crypt base). These cells exhibit functional plasticity to replenish Lgr5+ pools post-depletion[17]. Notably, co-expression of fibrinogen-binding protein 1 in Lgr4+ cells implies potential cellular overlap, yet whether these populations represent distinct states, transitional intermediates, or spatially adjacent subsets remains unresolved.
Intestinal repair following injury fundamentally differs from homeostatic renewal by engaging dedifferentiation of specialized cells, which reveal context-dependent stem cell adaptations. Dextran sodium sulfate-induced colitis triggers fetal-like epithelial reprogramming through extracellular matrix remodeling, FAK/Src activation, and YAP/TAZ nuclear translocation[18]. Radiation-resistant tuft cells respond to interleukin (IL)-4/IL-13 signaling by acquiring multipotent differentiation capacity[19], while Bmi1+Prox1+ EECs demonstrate injury-activated stem potential[20]. LY6A (SCA-1) fetal-like stem cells demonstrate organoid-forming capacity and regenerative functionality post-injury[21]. Single-cell analyses further identify clustering rare revival stem cells marked by clustering expression that generate all major intestinal lineages through temporal differentiation hierarchies[22]. Lineage tracing confirms enhanced differentiation potential of LGR5+p27+ cells following 5-fluorouracil-induced injury[23]. These findings reveal that facultative stem cell states following injury are contextually mobilized, yet the molecular logic governing their hierarchical interactions and niche-specific activation thresholds remains incompletely defined.
Despite the identification of diverse subpopulations with regenerative potential, current research on dietary regulation of stem cell fate remains disproportionately focused on LGR5+ ISCs. Future investigations should systematically elucidate how nutrient-sensing pathways and dietary components modulate the context-dependent activation of these heterogeneous regenerative populations, particularly facultative progenitors to unravel their metabolic dependencies and therapeutic potential.
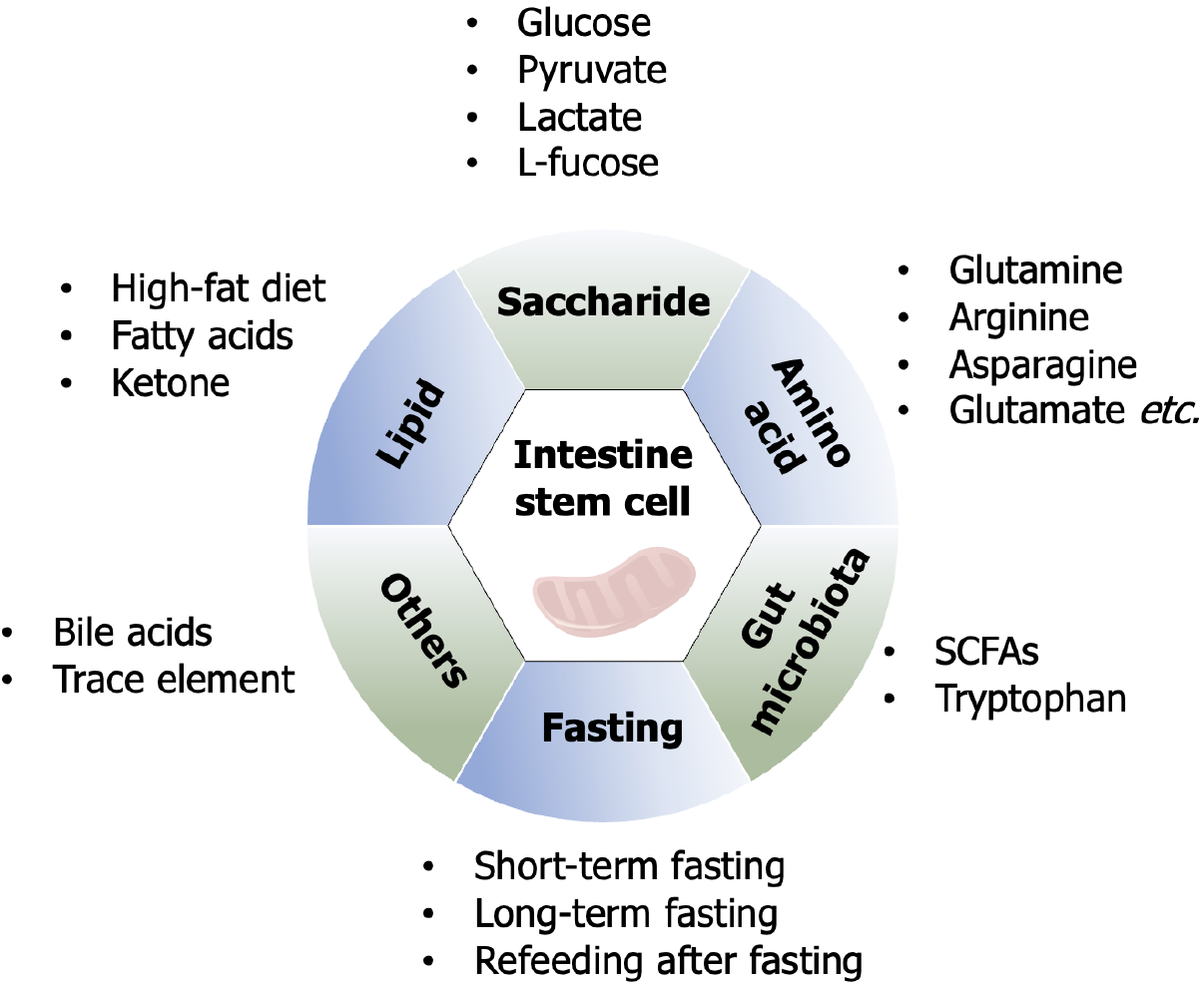
Dietary nutrients have a wide range of effects on health and disease[24]. A deeper understanding of the regulatory relationship of dietary factors on ISCs is not only conducive to further understanding the biological properties of ISCs, but will also strongly contribute to the clinical translation of dietary interventions toward targeting ISCs for the treatment of intestinal diseases (Figure 1).
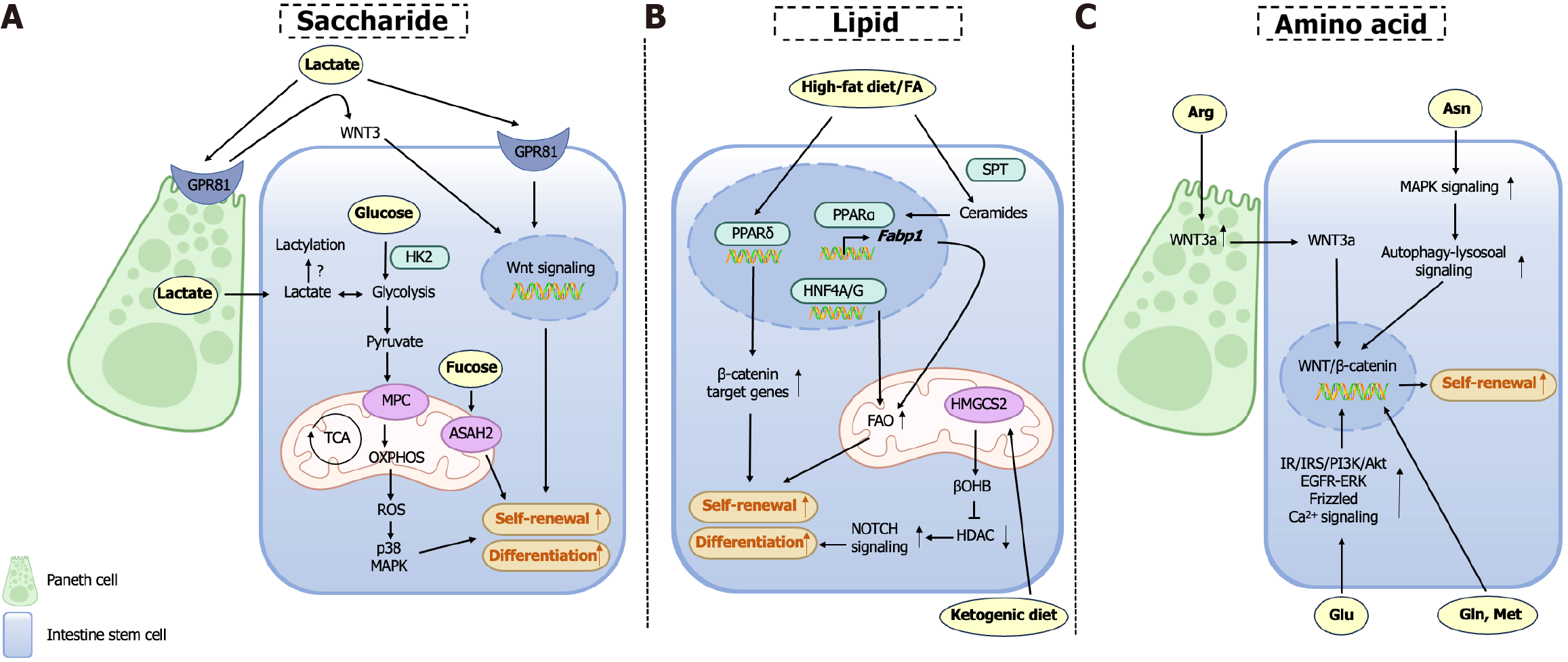
Glucose functions as the primary energetic substrate governing ISC homeostasis, with metabolic plasticity between glycolysis and OXPHOS critically determining ISCs fate. ISCs predominantly rely on glycolytic metabolism to sustain proliferative self-renewal, while metabolic reprogramming toward OXPHOS drives secretory lineage commitment, as demonstrated by impaired differentiation upon OXPHOS inhibition[25]. Pharmacological suppression of glycolysis (e.g., 2-deoxyglucose) or genetic ablation of hexokinase 2, the rate-limiting glycolytic enzyme, robustly attenuates ISC proliferation, confirming their obligate dependence on glycolytic flux[26,27]. Paradoxically, chronic hyperglycemia disrupts ISC homeostasis through metabolic stress overload. In Drosophila models, high-sugar diets induce ISC dysfunction via oxidative stress-mediated activation of the c-Jun N-terminal kinase pathway and suppression of regenerative Janus kinase-signal transducer of activation (STAT) signaling, collectively skewing ISC fate toward premature differentiation and depleting the stem cell pool[28,29]. These findings underscore the necessity for precise regulation of glycolytic activity to balance ISC self-renewal with lineage specification, revealing glucose as both a metabolic substrate and a critical modulator of stem cell plasticity.
Pyruvate, a key metabolic node generated from glucose via glycolysis in the cytoplasm, regulates ISC fate. Impaired function of the mitochondrial pyruvate carrier (MPC) disrupts mitochondrial pyruvate import, enhancing ISC self-renewal, amplifying stemness-associated transcriptional networks, and promoting proliferation, while MPC overexpression suppresses ISC expansion, establishing MPC as a bidirectional rheostat of stem cell dynamics[30-32]. Interestingly, terminally differentiated intestinal epithelial cells can regulate ISCs activity, as MPC deficiency in epithelial cells non-autonomously stimulates ISC proliferation[33]. A recent breakthrough study has structurally revealed the molecular architecture of MPC, substrate recognition motifs, transmembrane translocation pathway, and pharmacological inhibition mechanisms[34]. These structural insights have laid a foundation for developing MPC-targeted therapeutics to modulate ISCs functionality.
Lactate, the terminal glycolytic metabolite, serves dual roles as both a metabolic substrate (via pyruvate conversion for OXPHOS) and a signaling molecule in ISCs. Paneth cell-derived lactate sustains elevated ISC OXPHOS via mitochondrial substrate channeling, where reactive oxygen species (ROS) hormesis activates p38 mitogen-activated protein kinases (MAPK)-mediated morphogenic programs essential for crypt-villus patterning activity, which paradoxically avoids oxidative damage through controlled ROS generation. Mechanistically, this redox balance activates p38 MAPK-mediated differentiation programs essential for crypt-villus axis formation[35]. Furthermore, lactogenic probiotics (Bifidobacterium, Lactobacillus) enhance ISC proliferation and mucosal repair regeneration via Gi-protein-coupled receptor 81 (GPR81)-dependent coordination of Wnt3 secretion from co-activation in Paneth cells and stromal niches, driving β-catenin-mediated regeneration in chemotherapy/radiation injury models[36]. Notably, Lactobacillus amylovorus specifically potentiates ISC function through this lactate-GPR81-Wnt/β-catenin axis, suggesting strain-specific therapeutic applications for mucosal homeostasis[37]. Histone lactylation - a lactate-derived epigenetic modification - regulates stemness in other stem systems[38,39], yet its role in ISCs remains unstudied, pointing to an unexplored gap in metabolic-epigenetic crosstalk during intestinal repair regeneration. Numerous lactic acid-producing bacterial strains have been documented to enhance gut health, and the specific species and their applications are summarized in Table 1[36,37,40-48]. Future research should focus on determining whether the health-promoting effects of these bacterial strains across multiple human physiological systems are exclusively mediated by lactic acid, while exploring the potential of developing dietary strategies involving lactic acid supplementation as alternatives to probiotic interventions for optimizing ISC functionality (Table 1).
| Bacterial strains | Mechanism/pathway | Biological effects | Ref. |
| Bifidobacterium spp. | GPR81 signaling | Promoting ISCs proliferation | [36] |
| Lactobacillus amylovorus | GPR81-Wnt/β-catenin axis | Enhancing ISCs proliferation | [37] |
| Lactobacillus salivarius | SUCNR1-mitochondria axis | Activating ISCs activity | [40] |
| Lactobacillus paracasei VL8 | Tryptophan microbial metabolism | Inducing ISCs differentiation | [41] |
| Lactobacillus delbrueckii | - | Expanding ISCs population | [42] |
| Lactobacillus rhamnosus, Lactobacillus acidophilus, Bifidobacterium lactis | - | Augmenting innate and adaptive immunity | [43] |
| Lactobacillus casei | - | Ameliorating intestinal injury | [44] |
| Lactobacillus reuteri | Wnt/β-catenin axis | Mitigating intestinal mucosal damage | [45] |
| Lactipianibacillus plantarum | - | Alleviating constipation | [46] |
| Lactipianibacillus plantarum HFY11 | - | Suppressing colitis | [47] |
| Lacticaseibacillus paracasei JY06E, Lactobacillus gasseri JM1 | - | Relieving constipation | [48] |
L-fucose, a dietary monosaccharide with therapeutic potential for intestinal disorders, mediates mucosal repair through multi-layered mechanisms[49,50]. L-fucose regulates the unfolded protein response by direct fucosylation of ISCs to defend against inflammatory injury[51]. Concurrently, L-fucose enriches Akkermansia-derived propionate biosynthesis to indirectly drive ISC proliferation[52]. In addition, L-fucose activates the aryl hydrocarbon receptor (AHR)/IL-22 axis in lamina propria monocytes, establishing an immune-metabolic niche that promotes ISC self-renewal through paracrine signaling[53]. L-fucose orchestrates intestinal homeostasis through coordinated mechanisms involving ISCs fucosylation modification, microbial metabolite crosstalk, and immune niche modulation, reflecting its systemic regulatory capacity across biological compartments.
Dietary carbohydrates and their metabolites exert pleiotropic control over ISC fate, including energy metabolism reprogramming, epigenetic regulation, post-translational modifications, and symbiotic microbiota crosstalk. Importantly, moderate carbohydrate intake, strategic lactate supplementation, and consumption of beneficial dietary monosaccharides may enhance ISC-mediated epithelial regeneration and intestinal homeostasis by optimizing metabolic flexibility.
High-fat diet (HFD) induces aberrant ISC hyperproliferation, thereby accelerating colorectal tumorigenesis and metabolic syndrome. Mechanistically, HFD enhances the proliferative capacity of LGR5+ ISCs through PPARδ activation, which directly links dietary lipid overload to oncogenic transformation[7]. This synergizes with HFD-induced de novo lipo
Fatty acids play indispensable roles in ISC maintenance. Hepatic nuclear factors hepatocyte nuclear factor 4 alpha and hepatocyte nuclear factor 4 gamma orchestrate fatty acid β-oxidation gene networks essential for ISC self-renewal[54]. Ceramides, sphingolipid derivatives generated via serine palmitoyltransferase-mediated biosynthesis, act as pro-stemness signals by activating PPARα and fatty acid binding protein 1. This ceramide-PPARα axis enhances lipid utilization and intestinal progenitor proliferation, with sphingolipid biosynthetic enzymes (including serine palmitoyltransferase) upregulated in human intestinal adenomas, underscoring their conserved role in tumorigenesis[55].
Ketone bodies are of significance as intermediates in the oxidation of fatty acids, and have emerged as important regulators of ISC functionality. The ketogenic diet, a high-fat, low-carbohydrate regimen mimicking fasting metabolism, induces ketogenesis via hepatic and intestinal 3-hydroxy-3-methylglutaryl-CoA synthase 2. 3-hydroxy-3-methylglutaryl-CoA synthase 2 is highly expressed in Lgr5+ ISCs, and its genetic ablation impairs intestinal stemness and regenerative capacity. Mechanistically, β-hydroxybutyrate, a predominant ketone body, enhances ISC post-injury regeneration through histone deacetylase (HDAC) inhibition-mediated to stimulate Notch activation[56]. Furthermore, β-hydroxybutyrate supplementation has been demonstrated to suppress colorectal tumorigenesis[57]. These findings suggest that targeted metabolite supplementation may circumvent the need for complex dietary interventions, offering a dual therapeutic strategy to enhance ISC functionality while inhibiting oncogenic progression.
These findings collectively delineate a dynamic lipid-metabolic network wherein PPAR isoforms (δ/α) and ketone body signaling serve as central nodes integrating dietary inputs, β-oxidation, and sphingolipid metabolism to balance ISC self-renewal, differentiation, and oncogenic risk. The convergence of HFD- and ketogenic diet-mediated effects on ISC plasticity underscores the profound influence of lipid metabolism on epithelial regeneration and disease susceptibility. While lipid metabolites enhance ISC proliferation, their therapeutic application remains limited due to oncogenic risks and metabolic comorbidities. Future research must delineate context-specific mechanisms to safely harness fatty acids for ISC expansion.
Rapid renewal of the intestinal epithelium, a process demanding substantial amino acids for protein synthesis, is tightly regulated by specific amino acids that orchestrate ISC functionality through conserved metabolic and signaling networks.
Glutamine enhances ISC-mediated regeneration by amplifying Wnt/β-catenin signaling in a dose-dependent manner, while fueling glutaminolysis-dependent energy metabolism[58,59]. Glutamate (Glu), the most abundant amino acid in luminal proteins, modulates calcium signaling to adapt ISC proliferation and differentiation rates to dietary fluctuations in Drosophila[60]. While in porcine models, extracellular Glu was utilized to activate mechanistic target of rapamycin complex 1 (mTORC1) via insulin receptor/insulin receptor substrates/phosphatidylinositol-3-kinase/Akt, epidermal growth factor receptor/extracellular signal-regulated kinase, and further β-catenin/frizzled7 pathways, synergistically driving epithelial renewal[61-63]. Asparagine, a conditionally non-essential amino acid, maintains intestinal homeostasis by suppressing senescent ISC hyperproliferation via autophagy activation[64].
L-arginine stimulates ISC proliferation through dual niche signaling: Wnt2b derived from CD90+ stromal cells and Wnt3a derived from Paneth cells are both critical for epithelial barrier integrity[65,66]. Methionine reactivates Wnt/β-catenin signaling through metabolic-epigenetic crosstalk, potentiating ISC self-renewal[67]. Evolutionarily conserved Sestrins emerge as central nutrient sensors, suppressing target of rapamycin complex 1 and augmenting autophagy to fine-tune ISC turnover. Sestrin overexpression extends intestinal longevity by balancing anabolic and catabolic processes under dietary amino acid fluctuations[68].
Collectively, amino acids coordinate ISC dynamics through interconnected pathways encompassing Wnt/β-catenin, mTORC1, and autophagy, to align epithelial renewal with metabolic demands. Glutamine/Glu drive proliferative outputs and asparagine/Sestrin enforce quality control via proteostatic mechanisms. L-arginine/methionine fine-tune niche signaling to sustain stemness. Future therapeutic strategies may focus on modulating amino acid availability in ISCs through dietary interventions or targeted regulation of endogenous metabolic pathways, contingent upon precise characterization of amino acid-specific impacts on ISC fate determination.
The gut microbiota, a complex ecosystem, critically regulates ISCs dynamics through intertwined immune and metabolic pathways to sustain epithelial homeostasis and host health[69,70]. Gut microbiota-derived components orchestrate ISC-immune crosstalk through pattern recognition receptor activation. For instance, Lactobacillus rhamnosus GG stimulates Toll-like receptor 2 on macrophages via lipoteichoic acid, inducing chemokine secretion and migration of prostaglandin E2-producing mesenchymal ISCs to shield ISCs from radiation-induced damage[71]. Crypt-specific microbiota-derived lipopolysaccharide maintains the proliferation-differentiation balance of colonic epithelium[72], while muramyl dipep
Microbial metabolites exert compartmentalized control over ISCs dynamics. Short-chain fatty acids, produced via anaerobic fermentation of dietary fibers, act as ligands for G-protein-coupled receptors and HDAC inhibitors. Acetate supports organoid growth by suppressing β-oxidation under low acetyl-CoA conditions[75], while propionate restores Lgr5+ ISCs in dextran sodium sulfate-injured organoids via GPR41/GPR43-dependent signaling[52,76]. Of note, butyrate exhibits dual roles: It preserves Lgr5+ ISC stemness through HDAC inhibition[77], yet suppresses ISCs proliferation via forkhead box O3 activation at physiological concentrations[78].
Tryptophan metabolism bridges microbiota-ISC crosstalk through divergent signaling axes. Lactobacillus murinus-derived indole-3-acetic acid converted from tryptophan suppresses ISC differentiation via mitochondrial bioenergetics disruption[79], while indole-3-aldehyde activates group 3 innate lymphoid cells via AHR to secrete IL-22, promoting ISC proliferation through STAT3 signaling[80]. Host-derived serotonin, regulated by microbiota metabolites like valeric acid, enhances prostaglandin E2 production in macrophages via 5-HT(2A) serotonin receptor/5-HT(3A) serotonin receptor receptors, activating Wnt/β-catenin signaling in ISCs to drive self-renewal[81].
The gut microbiota as a metabolic-immune nexus, integrating microbial ligands (e.g., muramyl dipeptide, short-chain fatty acids) and host pathways (Toll-like receptor 2/NOD2, AHR/STAT3) to balance ISC proliferation, differentiation, and stress adaptation. Future therapeutic strategies targeting microbiota-derived metabolites or immune signaling of ISCs niche could restore epithelial integrity in inflammatory bowel disease, radiation injury, and metabolic disorders.
Research has shown that various fasting strategies can significantly extend the lifespan of different organisms and enhance their tissue regenerative capabilities[82-84]. Under caloric restriction (a 40% reduction in caloric intake), the signaling of mTORC1 in Paneth cells is suppressed, leading to an increase in the expression of bone marrow stromal cell antigen-1[85]. Bone marrow stromal cell antigen-1 is an ectoenzyme that generates the paracrine factor cyclic ADP ribose, which effectively enhances the self-renewal capacity of ISCs[86]. Short-term activation of mTORC1 signaling aids in the physiological regulation of ISCs, whereas sustained mTORC1 signaling may impair ISCs function[87,88]. Therefore, by rationally modulating this mechanism - such as through controlled caloric restriction or the use of drugs like rapamycin and cyclic ADP ribose - it may become a potential strategy for improving ISCs function and treating related diseases.
It has been reported that short-term fasting (24-hour) enhances ISC function via fatty acid oxidation activation[89]. Unlike caloric restriction, short-term fasting-refeeding cycles enhance ISCs function primarily mediated by refeeding-induced activation of phosphatidylinositol-3-kinase/AKT/mTORC1 signaling and the promotion of global protein translation, which is independent of the Paneth cell niche[90]. However, this diet may also lead to increased polyamine metabolism and protein synthesis, thereby elevating the risk of tumor formation[91]. Given the different effects of fasting and fasting-refeeding on ISCs function and carcinogenesis, future studies are needed to further clarify the roles of fasting-feeding timing, total calorie intake, and meal content during refeeding, as well as whether repetitive cycles of fasting and refeeding (such as asynchronous intermittent fasting regimens, e.g., 2-day fasting per week) play significant roles in fasting-related ISCs regulation and carcinogenesis. These investigations will facilitate the development of optimized fasting-refeeding strategies for tissue regeneration that do not elevate cancer risk.
There are other factors that can influence the functional state of ISCs. Bile acids, not only participate in lipid digestion[92], but also act as pleiotropic signaling modulators to regulate physiological responses[93]. The release of endogenous bile acids activates the Takeda G protein-coupled receptor 5 receptor in ISCs, which supports ISC self-renewal by activating the SRC/YAP regeneration mechanism[94].
Trace elements such as iron also impact ISCs fate determination. Iron overload can inhibit the proliferation of ISCs by suppressing the Notch signaling pathway[95,96], promoting their differentiation into secretory mature cells while inhibiting absorptive lineages[97]. In addition, iron overload may lead to ferroptosis in the intestinal epithelium[98]. These findings point to the multifactorial regulatory framework governing ISC behavior. Further exploration of undiscovered modulators and their mechanistic interplay will advance our understanding of intestinal regeneration (Figure 2).
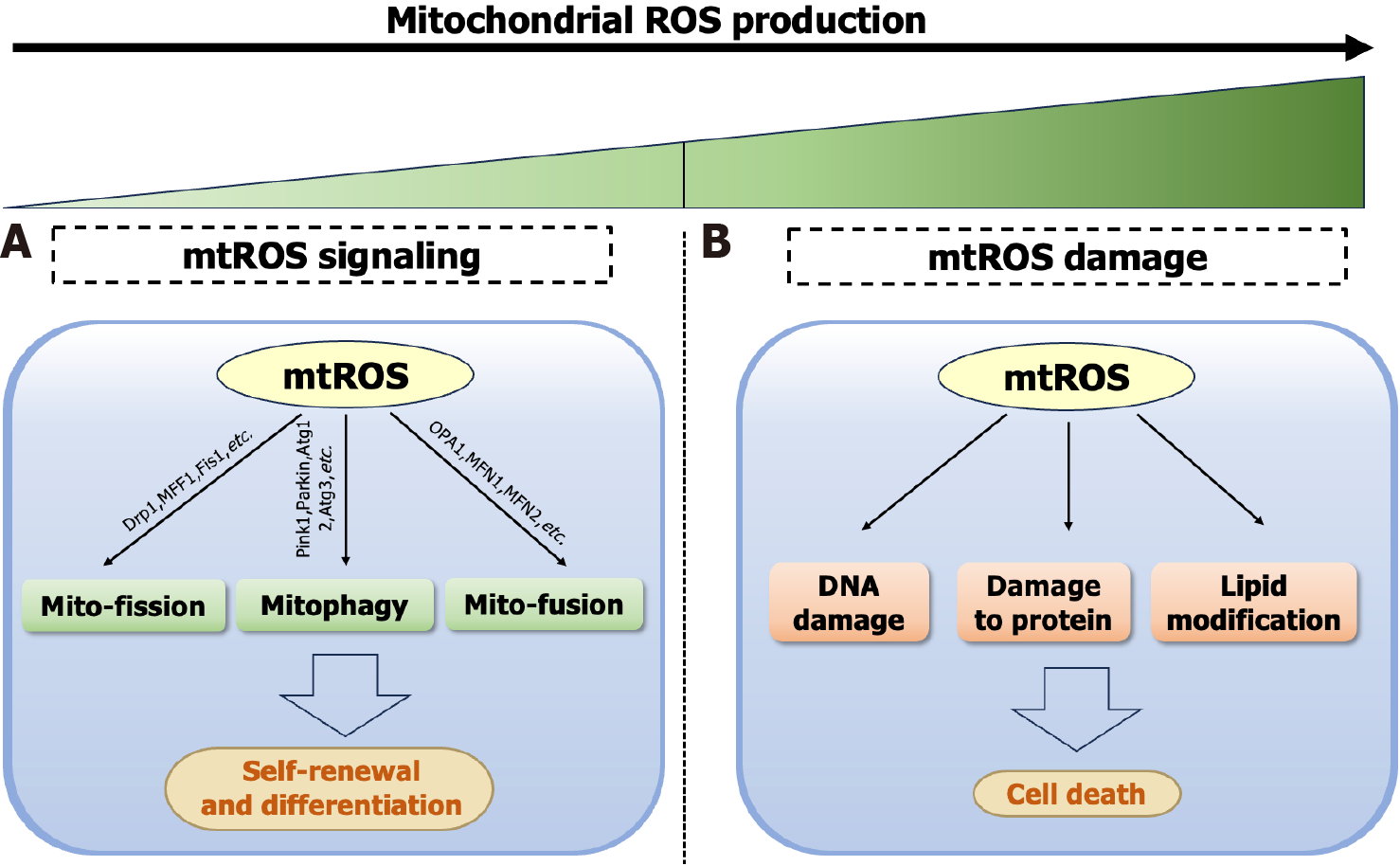
Mitochondria serve as central bioenergetic and biosynthetic hubs in ISCs, fueling ISC function and epithelial renewal[99]. The synthesis of amino acids[100], nucleotides, and lipids[101] depends on mitochondria to meet cellular biosynthetic demands. Moreover, mitochondrial dynamics can regulate the differentiation of ISCs and maintain the structural and morphological integrity of mitochondria[102]. ISCs exhibit metabolic heterogeneity based on functional states: Quiescent ISCs exhibit lower energy consumption with fewer mitochondria. While activated Lgr5+ CBCs undergo mitochondrial biogenesis and morphological elongation to adopt OXPHOS-dominant metabolism, meeting heightened biosynthetic demands[35]. This metabolic flexibility enables ISCs to dynamically adjust their metabolic patterns and mitochondrial dynamics in response to physiological demands[30,103,104].
Changes in metabolic patterns, such as during the differentiation of ISCs into Paneth cells, are accompanied by downregulation of forkhead box O3/Notch signaling and a shift toward glycolysis for energy production[105]. Addi
Mitochondrial dysfunction emerges as a pathogenic nexus across intestinal pathologies, where impaired bioenergetics and redox imbalance converge to disrupt epithelial homeostasis[110-112]. During the development of colorectal tumors, aerobic glycolysis in ISCs is enhanced, accompanied by weakened OXPHOS[31], and abnormal pyruvate metabolism[113]. Ulcerative colitis manifests mitochondrial electron transport chain defects that drive pathogenic ROS cascades[114,115], while fucosyltransferase 2-deficient ISCs can impair respiratory chain complexes and mitophagy, compromising ISCs function[116]. As the hub and energy factory of metabolism, in-depth research into mitochondrial function will provide critical insights into further elucidating the metabolic regulation mechanisms of ISCs.
Stem cells have demonstrated vast potential in regenerative medicine, with nutritional status playing a crucial regulatory role in these processes. The stem cell niche is a highly specialized and dynamic microenvironment surrounding ISCs[117], which not only provides structural support but also regulates stem cell behavior through signaling mediated by environmental nutrients[118,119]. Human intestinal organoids transcend 2D culture limitations by recapitulating crypt-villus architectures and niche interactions[120,121]. By adjusting the nutrient composition of the culture medium, the effects of nutrition on ISCs behavior can be easily manifested.
Multiple gastrointestinal organoid platforms are being utilized for high-throughput drug and nutrient screening. Notably, jejunal organoid-derived monolayers permit direct quantification of nutrient and electrolyte absorption, highlighting the potential utility of organoid models in nutritional screening[122-124]. Studies have demonstrated that multi-phenotypic screening, using miniaturized organoid models and single-cell RNA sequencing, can identify small molecules that regulate ISC differentiation[125]. These methods can also be applied to screen for nutrients or factors with the potential to modulate stem cell differentiation. Microfluidic organoids have recently been utilized for drug screening or fundamental research[126]. In the future, large-scale screening may uncover novel nutrients or metabolites and reveal their regulatory mechanisms on stem cell behavior. As organoid technology continues to advance and find broader applications, it is expected to uncover additional unknown nutritional factors that regulate ISC behavior, and engineer personalized nutritional matrices for regenerative therapies.
Intestinal epithelial regeneration is governed by ISCs, whose self-renewal and differentiation are dynamically regulated by niche signals, dietary nutrients, and metabolic adaptations. This review highlights how glucose, lipids, amino acids, and microbial metabolites modulate ISC fate via pathways such as glycolysis-OXPHOS transitions, PPAR signaling, and Wnt/β-catenin activation. Mitochondrial dynamics integrate energy production, redox balance, and biosynthesis to sustain stemness, while dietary interventions and microbiota-derived metabolites fine-tune regenerative plasticity. We still do not fully understand the metabolic dependency mechanisms of facultative progenitors or whether they increase cancer risk in specific environments. Advancing organoid-based nutrient screening and resultant therapies may unlock precision strategies to enhance mucosal repair while circumventing metabolic trade-offs in gastrointestinal pathologies.
| 1. | Tabula Muris Consortium; Overall coordination; Logistical coordination; Organ collection and processing; Library preparation and sequencing; Computational data analysis; Cell type annotation; Writing group; Supplemental text writing group; Principal investigators. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature. 2018;562:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1738] [Cited by in RCA: 2018] [Article Influence: 252.3] [Reference Citation Analysis (0)] |
| 2. | Wang Y, Song W, Wang J, Wang T, Xiong X, Qi Z, Fu W, Yang X, Chen YG. Single-cell transcriptome analysis reveals differential nutrient absorption functions in human intestine. J Exp Med. 2020;217:e20191130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 285] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 3. | Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol. 2019;16:19-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 725] [Article Influence: 103.6] [Reference Citation Analysis (0)] |
| 4. | Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 996] [Article Influence: 76.6] [Reference Citation Analysis (2)] |
| 5. | Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 318] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 6. | Ochocki JD, Simon MC. Nutrient-sensing pathways and metabolic regulation in stem cells. J Cell Biol. 2013;203:23-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong SJ, Bauer-Rowe KE, Xifaras ME, Akkad A, Arias E, Pinello L, Katz Y, Shinagare S, Abu-Remaileh M, Mihaylova MM, Lamming DW, Dogum R, Guo G, Bell GW, Selig M, Nielsen GP, Gupta N, Ferrone CR, Deshpande V, Yuan GC, Orkin SH, Sabatini DM, Yilmaz ÖH. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature. 2016;531:53-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 592] [Cited by in RCA: 647] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 8. | Patterson GT, Osorio EY, Peniche A, Dann SM, Cordova E, Preidis GA, Suh JH, Ito I, Saldarriaga OA, Loeffelholz M, Ajami NJ, Travi BL, Melby PC. Pathologic Inflammation in Malnutrition Is Driven by Proinflammatory Intestinal Microbiota, Large Intestine Barrier Dysfunction, and Translocation of Bacterial Lipopolysaccharide. Front Immunol. 2022;13:846155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Date S, Sato T. Mini-gut organoids: reconstitution of the stem cell niche. Annu Rev Cell Dev Biol. 2015;31:269-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, Breault DT. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 444] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 11. | Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, Guo Y, Shyr Y, Aronow BJ, Haigis KM, Franklin JL, Coffey RJ. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 582] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 12. | Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915-920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1017] [Cited by in RCA: 975] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 13. | Breault DT, Min IM, Carlone DL, Farilla LG, Ambruzs DM, Henderson DE, Algra S, Montgomery RK, Wagers AJ, Hole N. Generation of mTert-GFP mice as a model to identify and study tissue progenitor cells. Proc Natl Acad Sci U S A. 2008;105:10420-10425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 961] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 15. | Leung C, Tan SH, Barker N. Recent Advances in Lgr5(+) Stem Cell Research. Trends Cell Biol. 2018;28:380-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 16. | Capdevila C, Miller J, Cheng L, Kornberg A, George JJ, Lee H, Botella T, Moon CS, Murray JW, Lam S, Calderon RI, Malagola E, Whelan G, Lin CS, Han A, Wang TC, Sims PA, Yan KS. Time-resolved fate mapping identifies the intestinal upper crypt zone as an origin of Lgr5+ crypt base columnar cells. Cell. 2024;187:3039-3055.e14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 59] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 17. | Malagola E, Vasciaveo A, Ochiai Y, Kim W, Zheng B, Zanella L, Wang ALE, Middelhoff M, Nienhüser H, Deng L, Wu F, Waterbury QT, Belin B, LaBella J, Zamechek LB, Wong MH, Li L, Guha C, Cheng CW, Yan KS, Califano A, Wang TC. Isthmus progenitor cells contribute to homeostatic cellular turnover and support regeneration following intestinal injury. Cell. 2024;187:3056-3071.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 61] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 18. | Yui S, Azzolin L, Maimets M, Pedersen MT, Fordham RP, Hansen SL, Larsen HL, Guiu J, Alves MRP, Rundsten CF, Johansen JV, Li Y, Madsen CD, Nakamura T, Watanabe M, Nielsen OH, Schweiger PJ, Piccolo S, Jensen KB. YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell Stem Cell. 2018;22:35-49.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 514] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 19. | Huang L, Bernink JH, Giladi A, Krueger D, van Son GJF, Geurts MH, Busslinger G, Lin L, Begthel H, Zandvliet M, Buskens CJ, Bemelman WA, López-Iglesias C, Peters PJ, Clevers H. Tuft cells act as regenerative stem cells in the human intestine. Nature. 2024;634:929-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 20. | Yan KS, Gevaert O, Zheng GXY, Anchang B, Probert CS, Larkin KA, Davies PS, Cheng ZF, Kaddis JS, Han A, Roelf K, Calderon RI, Cynn E, Hu X, Mandleywala K, Wilhelmy J, Grimes SM, Corney DC, Boutet SC, Terry JM, Belgrader P, Ziraldo SB, Mikkelsen TS, Wang F, von Furstenberg RJ, Smith NR, Chandrakesan P, May R, Chrissy MAS, Jain R, Cartwright CA, Niland JC, Hong YK, Carrington J, Breault DT, Epstein J, Houchen CW, Lynch JP, Martin MG, Plevritis SK, Curtis C, Ji HP, Li L, Henning SJ, Wong MH, Kuo CJ. Intestinal Enteroendocrine Lineage Cells Possess Homeostatic and Injury-Inducible Stem Cell Activity. Cell Stem Cell. 2017;21:78-90.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 284] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 21. | Bues J, Biočanin M, Pezoldt J, Dainese R, Chrisnandy A, Rezakhani S, Saelens W, Gardeux V, Gupta R, Sarkis R, Russeil J, Saeys Y, Amstad E, Claassen M, Lutolf MP, Deplancke B. Deterministic scRNA-seq captures variation in intestinal crypt and organoid composition. Nat Methods. 2022;19:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 22. | Ayyaz A, Kumar S, Sangiorgi B, Ghoshal B, Gosio J, Ouladan S, Fink M, Barutcu S, Trcka D, Shen J, Chan K, Wrana JL, Gregorieff A. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature. 2019;569:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 405] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 23. | Ishikawa K, Sugimoto S, Oda M, Fujii M, Takahashi S, Ohta Y, Takano A, Ishimaru K, Matano M, Yoshida K, Hanyu H, Toshimitsu K, Sawada K, Shimokawa M, Saito M, Kawasaki K, Ishii R, Taniguchi K, Imamura T, Kanai T, Sato T. Identification of Quiescent LGR5(+) Stem Cells in the Human Colon. Gastroenterology. 2022;163:1391-1406.e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 24. | Shay JES, Yilmaz ÖH. Dietary and metabolic effects on intestinal stem cells in health and disease. Nat Rev Gastroenterol Hepatol. 2025;22:23-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 25. | Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014;15:243-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 813] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 26. | Zhou W, Ramachandran D, Mansouri A, Dailey MJ. Glucose stimulates intestinal epithelial crypt proliferation by modulating cellular energy metabolism. J Cell Physiol. 2018;233:3465-3475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Li C, Zhou Y, Wei R, Napier DL, Sengoku T, Alstott MC, Liu J, Wang C, Zaytseva YY, Weiss HL, Wang Q, Evers BM. Glycolytic Regulation of Intestinal Stem Cell Self-Renewal and Differentiation. Cell Mol Gastroenterol Hepatol. 2023;15:931-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 917] [Cited by in RCA: 813] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 29. | Zhang X, Jin Q, Jin LH. High sugar diet disrupts gut homeostasis though JNK and STAT pathways in Drosophila. Biochem Biophys Res Commun. 2017;487:910-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Schell JC, Wisidagama DR, Bensard C, Zhao H, Wei P, Tanner J, Flores A, Mohlman J, Sorensen LK, Earl CS, Olson KA, Miao R, Waller TC, Delker D, Kanth P, Jiang L, DeBerardinis RJ, Bronner MP, Li DY, Cox JE, Christofk HR, Lowry WE, Thummel CS, Rutter J. Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nat Cell Biol. 2017;19:1027-1036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 256] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 31. | Bensard CL, Wisidagama DR, Olson KA, Berg JA, Krah NM, Schell JC, Nowinski SM, Fogarty S, Bott AJ, Wei P, Dove KK, Tanner JM, Panic V, Cluntun A, Lettlova S, Earl CS, Namnath DF, Vázquez-Arreguín K, Villanueva CJ, Tantin D, Murtaugh LC, Evason KJ, Ducker GS, Thummel CS, Rutter J. Regulation of Tumor Initiation by the Mitochondrial Pyruvate Carrier. Cell Metab. 2020;31:284-300.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 32. | Aliluev A, Tritschler S, Sterr M, Oppenländer L, Hinterdobler J, Greisle T, Irmler M, Beckers J, Sun N, Walch A, Stemmer K, Kindt A, Krumsiek J, Tschöp MH, Luecken MD, Theis FJ, Lickert H, Böttcher A. Diet-induced alteration of intestinal stem cell function underlies obesity and prediabetes in mice. Nat Metab. 2021;3:1202-1216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 33. | Wisidagama DR, Thummel CS. Regulation of Drosophila Intestinal Stem Cell Proliferation by Enterocyte Mitochondrial Pyruvate Metabolism. G3 (Bethesda). 2019;9:3623-3630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Liang J, Shi J, Song A, Lu M, Zhang K, Xu M, Huang G, Lu P, Wu X, Ma D. Structures and mechanism of the human mitochondrial pyruvate carrier. Nature. 2025;641:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 35. | Rodríguez-Colman MJ, Schewe M, Meerlo M, Stigter E, Gerrits J, Pras-Raves M, Sacchetti A, Hornsveld M, Oost KC, Snippert HJ, Verhoeven-Duif N, Fodde R, Burgering BM. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature. 2017;543:424-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 404] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 36. | Lee YS, Kim TY, Kim Y, Lee SH, Kim S, Kang SW, Yang JY, Baek IJ, Sung YH, Park YY, Hwang SW, O E, Kim KS, Liu S, Kamada N, Gao N, Kweon MN. Microbiota-Derived Lactate Accelerates Intestinal Stem-Cell-Mediated Epithelial Development. Cell Host Microbe. 2018;24:833-846.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 341] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 37. | Wu H, Mu C, Li X, Fan W, Shen L, Zhu W. Breed-Driven Microbiome Heterogeneity Regulates Intestinal Stem Cell Proliferation via Lactobacillus-Lactate-GPR81 Signaling. Adv Sci (Weinh). 2024;11:e2400058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 38. | Wu J, Hu M, Jiang H, Ma J, Xie C, Zhang Z, Zhou X, Zhao J, Tao Z, Meng Y, Cai Z, Song T, Zhang C, Gao R, Cai C, Song H, Gao Y, Lin T, Wang C, Zhou X. Endothelial Cell-Derived Lactate Triggers Bone Mesenchymal Stem Cell Histone Lactylation to Attenuate Osteoporosis. Adv Sci (Weinh). 2023;10:e2301300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 71] [Reference Citation Analysis (0)] |
| 39. | Pan L, Feng F, Wu J, Fan S, Han J, Wang S, Yang L, Liu W, Wang C, Xu K. Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacol Res. 2022;181:106270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 269] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 40. | Luo D, Zou M, Rao X, Wei M, Zhang L, Hua Y, Yu L, Cao J, Ye J, Qi S, Wang H, Mi Y, Zhang C, Li J. Lactobacillus salivarius metabolite succinate enhances chicken intestinal stem cell activities via the SUCNR1-mitochondria axis. Poult Sci. 2025;104:104754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 41. | Zhang T, Cheng T, Geng S, Mao K, Li X, Gao J, Han J, Sang Y. Synbiotic Combination between Lactobacillus paracasei VL8 and Mannan-Oligosaccharide Repairs the Intestinal Barrier in the Dextran Sulfate Sodium-Induced Colitis Model by Regulating the Intestinal Stem Cell Niche. J Agric Food Chem. 2024;72:2214-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 42. | Wang M, Ren Y, Guo X, Ye Y, Zhu H, Zhang J, Huang Z, Yu K. Postbiotics from Lactobacillus delbrueckii Alleviate Intestinal Inflammation by Promoting the Expansion of Intestinal Stem Cells in S. Typhimurium-Induced Mice. Foods. 2024;13:874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Gill HS, Rutherfurd KJ, Prasad J, Gopal PK. Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019). Br J Nutr. 2000;83:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 242] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 44. | Watanabe T, Nishio H, Tanigawa T, Yamagami H, Okazaki H, Watanabe K, Tominaga K, Fujiwara Y, Oshitani N, Asahara T, Nomoto K, Higuchi K, Takeuchi K, Arakawa T. Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: involvement of lactic acid. Am J Physiol Gastrointest Liver Physiol. 2009;297:G506-G513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 45. | Ding X, Tang R, Zhao J, Xu Y, Fu A, Zhan X. Lactobacillus reuteri alleviates LPS-induced intestinal mucosal damage by stimulating the expansion of intestinal stem cells via activation of the Wnt/β-catenin signaling pathway in broilers. Poult Sci. 2024;103:104072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 46. | Tan F, Kong CS. Inhibitory Effect of Lactiplantibacillus plantarun HFY11 on Compound Diphenoxylate-Induced Constipation in Mice. Biomolecules. 2025;15:358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 47. | Tan F, Zhou X, Ren L, Kong CS. Effect of Lactiplantibacillus plantatum HFY11 on Colitis in Mice. Foods. 2024;13:1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 48. | Cheng S, Li B, Ding Y, Hou B, Hung W, He J, Jiang Y, Zhang Y, Man C. The probiotic fermented milk of Lacticaseibacillus paracasei JY062 and Lactobacillus gasseri JM1 alleviates constipation via improving gastrointestinal motility and gut microbiota. J Dairy Sci. 2024;107:1857-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 49. | Yao H, Shi H, Jiang C, Fan M, Zhang Y, Qian W, Lin R. L-Fucose promotes enteric nervous system regeneration in type 1 diabetic mice by inhibiting SMAD2 signaling pathway in enteric neural precursor cells. Cell Commun Signal. 2023;21:273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 50. | He R, Li Y, Han C, Lin R, Qian W, Hou X. L-Fucose ameliorates DSS-induced acute colitis via inhibiting macrophage M1 polarization and inhibiting NLRP3 inflammasome and NF-kB activation. Int Immunopharmacol. 2019;73:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 51. | Wang Z, Tan C, Duan C, Wu J, Zhou D, Hou L, Qian W, Han C, Hou X. FUT2-dependent fucosylation of HYOU1 protects intestinal stem cells against inflammatory injury by regulating unfolded protein response. Redox Biol. 2023;60:102618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 52. | Duan C, Wu J, Wang Z, Tan C, Hou L, Qian W, Han C, Hou X. Fucose promotes intestinal stem cell-mediated intestinal epithelial development through promoting Akkermansia-related propanoate metabolism. Gut Microbes. 2023;15:2233149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 53. | Tan C, Hong G, Wang Z, Duan C, Hou L, Wu J, Qian W, Han C, Hou X. Promoting Effect of L-Fucose on the Regeneration of Intestinal Stem Cells through AHR/IL-22 Pathway of Intestinal Lamina Propria Monocytes. Nutrients. 2022;14:4789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Chen L, Vasoya RP, Toke NH, Parthasarathy A, Luo S, Chiles E, Flores J, Gao N, Bonder EM, Su X, Verzi MP. HNF4 Regulates Fatty Acid Oxidation and Is Required for Renewal of Intestinal Stem Cells in Mice. Gastroenterology. 2020;158:985-999.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 55. | Li Y, Chaurasia B, Rahman MM, Kaddai V, Maschek JA, Berg JA, Wilkerson JL, Mahmassani ZS, Cox J, Wei P, Meikle PJ, Atkinson D, Wang L, Poss AM, Playdon MC, Tippetts TS, Mousa EM, Nittayaboon K, Anandh Babu PV, Drummond MJ, Clevers H, Shayman JA, Hirabayashi Y, Holland WL, Rutter J, Edgar BA, Summers SA. Ceramides Increase Fatty Acid Utilization in Intestinal Progenitors to Enhance Stemness and Increase Tumor Risk. Gastroenterology. 2023;165:1136-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 56. | Cheng CW, Biton M, Haber AL, Gunduz N, Eng G, Gaynor LT, Tripathi S, Calibasi-Kocal G, Rickelt S, Butty VL, Moreno-Serrano M, Iqbal AM, Bauer-Rowe KE, Imada S, Ulutas MS, Mylonas C, Whary MT, Levine SS, Basbinar Y, Hynes RO, Mino-Kenudson M, Deshpande V, Boyer LA, Fox JG, Terranova C, Rai K, Piwnica-Worms H, Mihaylova MM, Regev A, Yilmaz ÖH. Ketone Body Signaling Mediates Intestinal Stem Cell Homeostasis and Adaptation to Diet. Cell. 2019;178:1115-1131.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 297] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 57. | Dmitrieva-Posocco O, Wong AC, Lundgren P, Golos AM, Descamps HC, Dohnalová L, Cramer Z, Tian Y, Yueh B, Eskiocak O, Egervari G, Lan Y, Liu J, Fan J, Kim J, Madhu B, Schneider KM, Khoziainova S, Andreeva N, Wang Q, Li N, Furth EE, Bailis W, Kelsen JR, Hamilton KE, Kaestner KH, Berger SL, Epstein JA, Jain R, Li M, Beyaz S, Lengner CJ, Katona BW, Grivennikov SI, Thaiss CA, Levy M. β-Hydroxybutyrate suppresses colorectal cancer. Nature. 2022;605:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 240] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 58. | Moore SR, Guedes MM, Costa TB, Vallance J, Maier EA, Betz KJ, Aihara E, Mahe MM, Lima AA, Oriá RB, Shroyer NF. Glutamine and alanyl-glutamine promote crypt expansion and mTOR signaling in murine enteroids. Am J Physiol Gastrointest Liver Physiol. 2015;308:G831-G839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 59. | Tian J, Li Y, Bao X, Yang F, Tang X, Jiang Q, Yang C, Yin Y, Yao K. Glutamine boosts intestinal stem cell-mediated small intestinal epithelial development during early weaning: Involvement of WNT signaling. Stem Cell Reports. 2023;18:1451-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 60. | Deng H, Gerencser AA, Jasper H. Signal integration by Ca(2+) regulates intestinal stem-cell activity. Nature. 2015;528:212-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 61. | Zhu M, Qin YC, Gao CQ, Yan HC, Li XG, Wang XQ. Extracellular Glutamate-Induced mTORC1 Activation via the IR/IRS/PI3K/Akt Pathway Enhances the Expansion of Porcine Intestinal Stem Cells. J Agric Food Chem. 2019;67:9510-9521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 62. | Zhu M, Qin YC, Gao CQ, Yan HC, Wang XQ. l-Glutamate drives porcine intestinal epithelial renewal by increasing stem cell activity via upregulation of the EGFR-ERK-mTORC1 pathway. Food Funct. 2020;11:2714-2724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 63. | Qin YC, Zhou JY, Zhu M, Zan GX, Gao CQ, Yan HC, Li XG, Wang XQ. L-glutamate requires β-catenin signalling through Frizzled7 to stimulate porcine intestinal stem cell expansion. Cell Mol Life Sci. 2022;79:523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 64. | Luo T, Zhao L, Feng C, Yan J, Yuan Y, Chen H. Asparagine prevents intestinal stem cell aging via the autophagy-lysosomal pathway. Aging Cell. 2025;24:e14423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 65. | Hou Q, Dong Y, Huang J, Liao C, Lei J, Wang Y, Lai Y, Bian Y, He Y, Sun J, Sun M, Jiang Q, Wang B, Yu Z, Guo Y, Zhang B. Exogenous L-arginine increases intestinal stem cell function through CD90+ stromal cells producing mTORC1-induced Wnt2b. Commun Biol. 2020;3:611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Hou Q, Dong Y, Yu Q, Wang B, Le S, Guo Y, Zhang B. Regulation of the Paneth cell niche by exogenous L-arginine couples the intestinal stem cell function. FASEB J. 2020;34:10299-10315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Zhou JY, Wang Z, Zhang SW, Lin HL, Gao CQ, Zhao JC, Yang C, Wang XQ. Methionine and Its Hydroxyl Analogues Improve Stem Cell Activity To Eliminate Deoxynivalenol-Induced Intestinal Injury by Reactivating Wnt/β-Catenin Signaling. J Agric Food Chem. 2019;67:11464-11473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 68. | Lu J, Temp U, Müller-Hartmann A, Esser J, Grönke S, Partridge L. Sestrin is a key regulator of stem cell function and lifespan in response to dietary amino acids. Nat Aging. 2021;1:60-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 69. | de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71:1020-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 1531] [Article Influence: 382.8] [Reference Citation Analysis (0)] |
| 70. | Wu H, Mu C, Xu L, Yu K, Shen L, Zhu W. Host-microbiota interaction in intestinal stem cell homeostasis. Gut Microbes. 2024;16:2353399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 71. | Riehl TE, Alvarado D, Ee X, Zuckerman A, Foster L, Kapoor V, Thotala D, Ciorba MA, Stenson WF. Lactobacillus rhamnosus GG protects the intestinal epithelium from radiation injury through release of lipoteichoic acid, macrophage activation and the migration of mesenchymal stem cells. Gut. 2019;68:1003-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 162] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 72. | Yi H, Patel AK, Sodhi CP, Hackam DJ, Hackam AS. Novel role for the innate immune receptor Toll-like receptor 4 (TLR4) in the regulation of the Wnt signaling pathway and photoreceptor apoptosis. PLoS One. 2012;7:e36560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Nigro G, Rossi R, Commere PH, Jay P, Sansonetti PJ. The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe. 2014;15:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 74. | Levy A, Stedman A, Deutsch E, Donnadieu F, Virgin HW, Sansonetti PJ, Nigro G. Innate immune receptor NOD2 mediates LGR5(+) intestinal stem cell protection against ROS cytotoxicity via mitophagy stimulation. Proc Natl Acad Sci U S A. 2020;117:1994-2003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 75. | Stine RR, Sakers AP, TeSlaa T, Kissig M, Stine ZE, Kwon CW, Cheng L, Lim HW, Kaestner KH, Rabinowitz JD, Seale P. PRDM16 Maintains Homeostasis of the Intestinal Epithelium by Controlling Region-Specific Metabolism. Cell Stem Cell. 2019;25:830-845.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 76. | Bajic D, Niemann A, Hillmer AK, Mejias-Luque R, Bluemel S, Docampo M, Funk MC, Tonin E, Boutros M, Schnabl B, Busch DH, Miki T, Schmid RM, van den Brink MRM, Gerhard M, Stein-Thoeringer CK. Gut Microbiota-Derived Propionate Regulates the Expression of Reg3 Mucosal Lectins and Ameliorates Experimental Colitis in Mice. J Crohns Colitis. 2020;14:1462-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 77. | Yin X, Farin HF, van Es JH, Clevers H, Langer R, Karp JM. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods. 2014;11:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 458] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 78. | Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, Pearce EL, Oltz EM, Stappenbeck TS. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell. 2016;165:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 506] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 79. | Wei W, Liu Y, Hou Y, Cao S, Chen Z, Zhang Y, Cai X, Yan Q, Li Z, Yuan Y, Wang G, Zheng X, Hao H. Psychological stress-induced microbial metabolite indole-3-acetate disrupts intestinal cell lineage commitment. Cell Metab. 2024;36:466-483.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 59] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 80. | Hou Q, Ye L, Liu H, Huang L, Yang Q, Turner JR, Yu Q. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018;25:1657-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 303] [Article Influence: 37.9] [Reference Citation Analysis (18)] |
| 81. | Zhu P, Lu T, Wu J, Fan D, Liu B, Zhu X, Guo H, Du Y, Liu F, Tian Y, Fan Z. Gut microbiota drives macrophage-dependent self-renewal of intestinal stem cells via niche enteric serotonergic neurons. Cell Res. 2022;32:555-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 82. | Cheng CW, Yilmaz ÖH. 100 Years of Exploiting Diet and Nutrition for Tissue Regeneration. Cell Stem Cell. 2021;28:370-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, Anderson RM. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 470] [Cited by in RCA: 632] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 84. | Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, Da Sacco S, Mirisola M, Quinn DI, Dorff TB, Kopchick JJ, Longo VD. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14:810-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 355] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 85. | Yilmaz ÖH, Katajisto P, Lamming DW, Gültekin Y, Bauer-Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, Nielsen GP, Mino-Kenudson M, Zukerberg LR, Bhan AK, Deshpande V, Sabatini DM. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 609] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 86. | Mattila J, Viitanen A, Fabris G, Strutynska T, Korzelius J, Hietakangas V. Stem cell mTOR signaling directs region-specific cell fate decisions during intestinal nutrient adaptation. Sci Adv. 2024;10:eadi2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 87. | Amcheslavsky A, Ito N, Jiang J, Ip YT. Tuberous sclerosis complex and Myc coordinate the growth and division of Drosophila intestinal stem cells. J Cell Biol. 2011;193:695-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 88. | Lengefeld J, Cheng CW, Maretich P, Blair M, Hagen H, McReynolds MR, Sullivan E, Majors K, Roberts C, Kang JH, Steiner JD, Miettinen TP, Manalis SR, Antebi A, Morrison SJ, Lees JA, Boyer LA, Yilmaz ÖH, Amon A. Cell size is a determinant of stem cell potential during aging. Sci Adv. 2021;7:eabk0271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 89. | Mihaylova MM, Cheng CW, Cao AQ, Tripathi S, Mana MD, Bauer-Rowe KE, Abu-Remaileh M, Clavain L, Erdemir A, Lewis CA, Freinkman E, Dickey AS, La Spada AR, Huang Y, Bell GW, Deshpande V, Carmeliet P, Katajisto P, Sabatini DM, Yilmaz ÖH. Fasting Activates Fatty Acid Oxidation to Enhance Intestinal Stem Cell Function during Homeostasis and Aging. Cell Stem Cell. 2018;22:769-778.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 334] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 90. | Imada S, Khawaled S, Shin H, Meckelmann SW, Whittaker CA, Corrêa RO, Alquati C, Lu Y, Tie G, Pradhan D, Calibasi-Kocal G, Nascentes Melo LM, Allies G, Rösler J, Wittenhofer P, Krystkiewicz J, Schmitz OJ, Roper J, Vinolo MAR, Ricciardiello L, Lien EC, Vander Heiden MG, Shivdasani RA, Cheng CW, Tasdogan A, Yilmaz ÖH. Short-term post-fast refeeding enhances intestinal stemness via polyamines. Nature. 2024;633:895-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 91. | Brunner S, Janssen KP, Ecker J. Post-fast refeeding: rise of intestinal stemness and mutagen-induced cancer risk through polyamine metabolism. Signal Transduct Target Ther. 2024;9:317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 92. | Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1081] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 93. | Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 2024] [Article Influence: 202.4] [Reference Citation Analysis (0)] |
| 94. | Sorrentino G, Perino A, Yildiz E, El Alam G, Bou Sleiman M, Gioiello A, Pellicciari R, Schoonjans K. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology. 2020;159:956-968.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 284] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 95. | Takahashi T, Shiraishi A. Stem Cell Signaling Pathways in the Small Intestine. Int J Mol Sci. 2020;21:2032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 96. | Vooijs M, Liu Z, Kopan R. Notch: architect, landscaper, and guardian of the intestine. Gastroenterology. 2011;141:448-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 97. | Zhao J, Ma W, Wang S, Zhang K, Xiong Q, Li Y, Yu H, Du H. Differentiation of intestinal stem cells toward goblet cells under systemic iron overload stress are associated with inhibition of Notch signaling pathway and ferroptosis. Redox Biol. 2024;72:103160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 98. | Zhao J, Xu B, Xiong Q, Feng Y, Du H. Erastininduced ferroptosis causes physiological and pathological changes in healthy tissues of mice. Mol Med Rep. 2021;24:713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 99. | Flavell RB. Mitochondrion as a multifunctional organelle. Nature. 1971;230:504-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 100. | Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, Sabatini DM. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell. 2015;162:540-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 1095] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 101. | Horvath SE, Daum G. Lipids of mitochondria. Prog Lipid Res. 2013;52:590-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 710] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 102. | Dai Z, Li D, Du X, Ge Y, Hursh DA, Bi X. Drosophila Caliban preserves intestinal homeostasis and lifespan through regulating mitochondrial dynamics and redox state in enterocytes. PLoS Genet. 2020;16:e1009140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 103. | Wei M, Nurjanah U, Li J, Luo X, Hosea R, Li Y, Zeng J, Duan W, Song G, Miyagishi M, Kasim V, Wu S. YY2-DRP1 Axis Regulates Mitochondrial Fission and Determines Cancer Stem Cell Asymmetric Division. Adv Sci (Weinh). 2023;10:e2207349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 104. | Praharaj PP, Patra S, Mishra SR, Mukhopadhyay S, Klionsky DJ, Patil S, Bhutia SK. CLU (clusterin) promotes mitophagic degradation of MSX2 through an AKT-DNM1L/Drp1 axis to maintain SOX2-mediated stemness in oral cancer stem cells. Autophagy. 2023;19:2196-2216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 105. | Stringari C, Edwards RA, Pate KT, Waterman ML, Donovan PJ, Gratton E. Metabolic trajectory of cellular differentiation in small intestine by Phasor Fluorescence Lifetime Microscopy of NADH. Sci Rep. 2012;2:568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 194] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 106. | McCauley HA, Riedman AM, Enriquez JR, Zhang X, Watanabe-Chailland M, Sanchez JG, Kechele DO, Paul EF, Riley K, Burger C, Lang RA, Wells JM. Enteroendocrine Cells Protect the Stem Cell Niche by Regulating Crypt Metabolism in Response to Nutrients. Cell Mol Gastroenterol Hepatol. 2023;15:1293-1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 107. | Palma FR, Gantner BN, Sakiyama MJ, Kayzuka C, Shukla S, Lacchini R, Cunniff B, Bonini MG. ROS production by mitochondria: function or dysfunction? Oncogene. 2024;43:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 266] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 108. | Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 966] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 109. | Morris O, Jasper H. Reactive Oxygen Species in intestinal stem cell metabolism, fate and function. Free Radic Biol Med. 2021;166:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 110. | Ho GT, Aird RE, Liu B, Boyapati RK, Kennedy NA, Dorward DA, Noble CL, Shimizu T, Carter RN, Chew ETS, Morton NM, Rossi AG, Sartor RB, Iredale JP, Satsangi J. MDR1 deficiency impairs mitochondrial homeostasis and promotes intestinal inflammation. Mucosal Immunol. 2018;11:120-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 111. | Jackson DN, Panopoulos M, Neumann WL, Turner K, Cantarel BL, Thompson-Snipes L, Dassopoulos T, Feagins LA, Souza RF, Mills JC, Blumberg RS, Venuprasad K, Thompson WE, Theiss AL. Mitochondrial dysfunction during loss of prohibitin 1 triggers Paneth cell defects and ileitis. Gut. 2020;69:1928-1938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 112. | Sünderhauf A, Hicken M, Schlichting H, Skibbe K, Ragab M, Raschdorf A, Hirose M, Schäffler H, Bokemeyer A, Bettenworth D, Savitt AG, Perner S, Ibrahim S, Peerschke EI, Ghebrehiwet B, Derer S, Sina C. Loss of Mucosal p32/gC1qR/HABP1 Triggers Energy Deficiency and Impairs Goblet Cell Differentiation in Ulcerative Colitis. Cell Mol Gastroenterol Hepatol. 2021;12:229-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 113. | Sandoval IT, Delacruz RG, Miller BN, Hill S, Olson KA, Gabriel AE, Boyd K, Satterfield C, Van Remmen H, Rutter J, Jones DA. A metabolic switch controls intestinal differentiation downstream of Adenomatous polyposis coli (APC). Elife. 2017;6:e22706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 114. | Sifroni KG, Damiani CR, Stoffel C, Cardoso MR, Ferreira GK, Jeremias IC, Rezin GT, Scaini G, Schuck PF, Dal-Pizzol F, Streck EL. Mitochondrial respiratory chain in the colonic mucosal of patients with ulcerative colitis. Mol Cell Biochem. 2010;342:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 115. | Hsieh SY, Shih TC, Yeh CY, Lin CJ, Chou YY, Lee YS. Comparative proteomic studies on the pathogenesis of human ulcerative colitis. Proteomics. 2006;6:5322-5331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 116. | Duan C, Wang Z, Wu J, Tan C, Fang F, Qian W, Han C, Hou X. Fut2 Deficiency Promotes Intestinal Stem Cell Aging by Damaging Mitochondrial Functions via Down-Regulating α1,2-Fucosylation of Asah2 and Npc1. Research (Wash D C). 2024;7:0343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 117. | Farahzadi R, Valipour B, Montazersaheb S, Fathi E. Targeting the stem cell niche micro-environment as therapeutic strategies in aging. Front Cell Dev Biol. 2023;11:1162136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 118. | Voog J, Jones DL. Stem cells and the niche: a dynamic duo. Cell Stem Cell. 2010;6:103-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 291] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 119. | Fujii M, Matano M, Toshimitsu K, Takano A, Mikami Y, Nishikori S, Sugimoto S, Sato T. Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell. 2018;23:787-793.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 394] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 120. | Dutta D, Heo I, Clevers H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol Med. 2017;23:393-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 608] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 121. | Takahashi T, Fujishima K, Kengaku M. Modeling Intestinal Stem Cell Function with Organoids. Int J Mol Sci. 2021;22:10912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 122. | Zhao Y, Li S, Zhu L, Huang M, Xie Y, Song X, Chen Z, Lau HC, Sung JJ, Xu L, Yu J, Li X. Personalized drug screening using patient-derived organoid and its clinical relevance in gastric cancer. Cell Rep Med. 2024;5:101627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 123. | Mao Y, Wang W, Yang J, Zhou X, Lu Y, Gao J, Wang X, Wen L, Fu W, Tang F. Drug repurposing screening and mechanism analysis based on human colorectal cancer organoids. Protein Cell. 2024;15:285-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 84] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 124. | Haynes J, Palaniappan B, Tsopmegha E, Sundaram U. Regulation of nutrient and electrolyte absorption in human organoid-derived intestinal epithelial cell monolayers. Transl Res. 2022;248:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 125. | Mead BE, Hattori K, Levy L, Imada S, Goto N, Vukovic M, Sze D, Kummerlowe C, Matute JD, Duan J, Langer R, Blumberg RS, Ordovas-Montanes J, Yilmaz ÖH, Karp JM, Shalek AK. Screening for modulators of the cellular composition of gut epithelia via organoid models of intestinal stem cell differentiation. Nat Biomed Eng. 2022;6:476-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 126. | Carvalho MR, Yan LP, Li B, Zhang CH, He YL, Reis RL, Oliveira JM. Gastrointestinal organs and organoids-on-a-chip: advances and translation into the clinics. Biofabrication. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/