Published online May 26, 2025. doi: 10.4252/wjsc.v17.i5.106547
Revised: April 1, 2025
Accepted: May 7, 2025
Published online: May 26, 2025
Processing time: 87 Days and 1.1 Hours
Knee osteoarthritis (KOA), characterized by heterogeneous arthritic manifestations and complex peripheral joint disorder, is one of the leading causes of disability worldwide, which has become a high burden due to the multifactorial nature and the deficiency of available disease-modifying treatments. The app
Core Tip: Knee osteoarthritis is one of the leading causes of disability worldwide and has become a high burden due to the multifactorial nature and the deficiency of available disease-modifying treatments. Herein, we review the state-of-the-art literatures of the quality by design strategy of mesenchymal stem/stromal cell drug products for knee osteoarthritis treatment, together with discuss the accompanied principles as well, which will collectively help to reduce the gap between compliant products and the production of compliant good manufacturing practice.
- Citation: Yu H, Zhang F, He YC, Zhang LS. Quality by design strategy of human mesenchymal stem/stromal cell drug products for the treatment of knee osteoarthritis. World J Stem Cells 2025; 17(5): 106547
- URL: https://www.wjgnet.com/1948-0210/full/v17/i5/106547.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i5.106547
Knee osteoarthritis (KOA) is the most common form of arthritis with high incidence, which affects approximately 650 million people worldwide, with a prevalence of 14%-38% in women and 4%-14% in men aged 40 and older[1]. The quality of life of KOA patients is significantly reduced due to the limitation of efficacy by current treatments, including lifestyle changes (e.g., exercise, weight loss, and smoking cessation) and injectable interventions [e.g., corticosteroids[2], hyaluronic acid (HA), and injections of platelet-rich plasma (PRP) with short-to medium-term analgesic effects[3]].
Human mesenchymal stem/stromal cells (MSCs) are heterogeneous cell populations with splendid immunosuppressive and hematopoietic-supporting properties, which have been extensively explored in both preclinical and clinical investigations for the illumination of pathogenesis and treatment of refractory and recurrent diseases[4,5]. For instance, our colleagues and we have demonstrated the feasibility of MSC-based regimens for a wide range of disease treatment such as osteoarthritis[6], acquired aplastic anemia[7,8], cerebral infarction[9], acute lung injury[10,11], Crohn’s-like enterocutaneous fistula[12], infected anal fistula[13], acute-on-chronic liver failure[14], acute graft-versus-host disease[4,15], aplastic anemia[7], critical limb ischemia[16], cerebral infarction[9], radiation-induced colorectal fibrosis[17], and even the coronavirus disease 2019 pneumonia-associated acute respiratory distress syndrome[18,19]. Unlike other stem cell subtypes that require matching, MSCs meet the demand of allogeneic transplantation and thus serve as the most rapidly developing stem cell type in clinical trials and play critical roles in bone cells as well[20,21].
State-of-the-art literatures have highlighted MSCs as an attractive candidate for the treatment of KOA attributes to the multi-modal mode of action. For instance, MSCs are adequate to suppress the pro-inflammatory cytokines (e.g., tumor necrosis factor-α, interleukin-6) and immune cell responses (e.g., macrophages for cell clearance) in the knee-joint microenvironment via modulating diverse paracrine factors and extracellular vesicles[22,23]. To date, numerous clinical trials of MSCs-based regimens upon KOA have been registered and launched globally, and in particular, in South Korea and India[24]. However, no commercial MSC product expect for Mesoblast’s Ryoncil (remestemcel-L-rknd) have been approved in the United States, which further indicates the bottlenecks in the production of high-quality MSCs for industrial manufacturing and clinical application. Therefore, the introduction of new tools and methods including the quality by design (QbD) method will provide an important basis for the development of MSC products for KOA treatment.
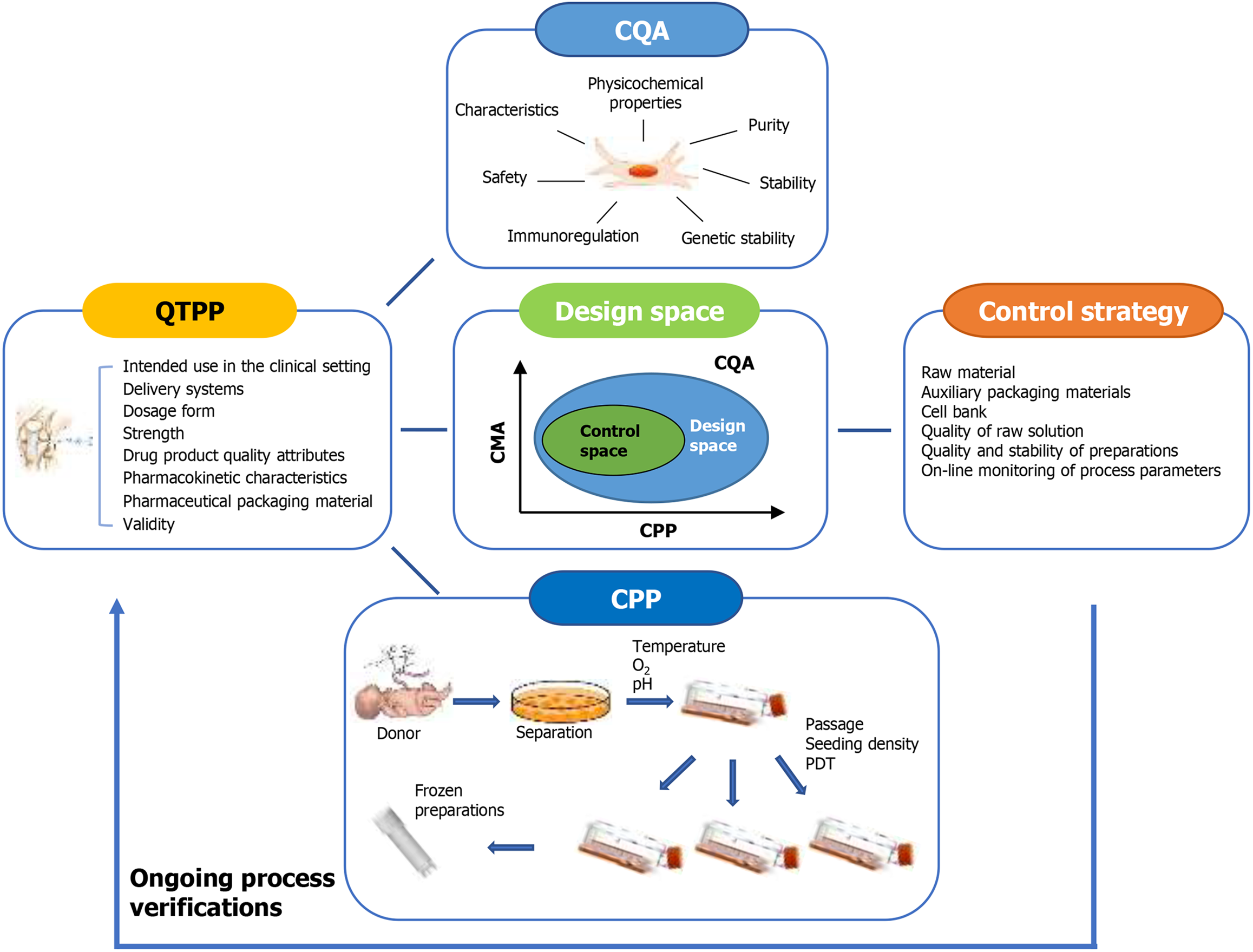
QbD is a scientific and risk-based framework for process design based on relating product and process attributes to product quality. As a systematic development approach that begins with predefined objectives, QbD places an emphasis on understanding the process control of products, which is grounded in scientific principles and quality risk ma
The QbD manufactured by CTP is composed of four functional modules, including cell culture equipment module, cell proliferation module, scale and control module. QbD is a product development and life cycle management framework promoted by regulatory agencies, which plays a very important role in the traditional pharmaceutical field. Considering that QbD is a common management framework, it can be applied to the field of cell therapy in combination with new specific technical solutions. Connecting measurable molecular and cellular characteristics of cell populations with final product quality through QbD is a key step to achieve large-scale cell products. To be more effective, cell therapy bioprocess design and optimization need to include several basic criteria: (1) Evaluation of relevant cell properties; (2) Determination and control of key parameters; (3) Robust prediction strategies for interrogating and evaluating many parameters that may affect cell culture output; and (4) Methods to test many different parameters that may affect cell output in a high-throughput and large-scale relevant manner[28]. In brief, QbD first describes the Quality Target Product Profile (QTPP), identifies the critical quality attributes (CQAs) that directly affect the safety and effectiveness of products, verifies the critical process parameters (CPPs) that affect these attributes, and develops a design space to quantify the impact of parameter variability on quality attributes. Subsequently, a control strategy is developed to maintain the process parameters to ensure that the product quality is within a certain range, and the process is verified on a scale. Once CTP manufacturing is implemented, QbD allows the production process to be monitored and repeatedly optimized with the increase of process knowledge (Figure 1)[29].
QTPP describes the use, safety and efficacy of the target product and is the starting point of QbD. Determining strict and easy to measure criteria is essential for establishing QTPP. The purpose of MSC products is to treat diseases (clinical-grade cell products), and its safety depends on: (1) Cell safety, that is, the genomic stability of MSC during in vitro culture and expansion; and (2) MSC preparation safety, that is, the cell injection cannot be contaminated by microorganisms, and the excipients meet the requirements of the Pharmacopoeia. Cell cultures are susceptible to many types of contamination, especially microbial (e.g., bacteria, fungi, and mycoplasma), endotoxin, and cell line cross contamination. In addition, non-cellular particles (including plastic debris, residual microcarriers and fibers) produced by manufacturing equipment and materials must be supervised, which might impact the expansion performances and differentiation potential of MSCs[30-32].
The production of CTPs usually requires the mixing of bioactive chemicals, including cytokines, small molecules, serum and carriers, etc. These auxiliary substances must be fully removed to avoid being regarded as drugs themselves. Therefore, QTPP must describe the maximum residue level of these auxiliary materials in the final MSC product to ensure the safety, and additional treatments that may affect the final cell yield. In additional, the potency of MSCs includes strength and effectiveness, that is, the effect achieved at the same dose (cell concentration)[33-35]. Therefore, the discussion of the quality or biological efficacy of MSCs is inseparable from the specific indications[36]. For example, MSCs are adequate for simultaneously immunosuppression and vascular regeneration acceleration, which thus re
The definition of CQAs is a physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality. CQAs are generally linked to drug substances, excipients, intermediates (in-process materials), and final drug products. CQAs directly determine product quality, while CPP and material attributes indirectly affect product quality by influencing CQAs[38-40]. Therefore, a main goal of the manufacturing process is to ensure that the CQAs of the final cell product (identity, purity, efficacy, and safety) are maintained at every step of the entire process until entering the market, and can be evaluated with the aid of specific feature tools and analytical methods[41-43].
The CQAs of MSC products should include cellular characteristics, such as dosage, cell quantity, cell viability, therapeutic potency, in vitro differentiation capacity, expected in vivo effect, product quality, genetic stability, and cell purity (Table 1). Commonly, MSCs involved in clinical applications should satisfy four types of quality attributes, including basic biological attributes, microbiological safety, biological safety, and biological efficacy. Among them, the biological efficacy of MSCs refers to the biological functions that correspond to or can be used to predict the clinical efficacy of MSCs during the preclinical stage[44], which serves as an important quality attribute that determines the clinical outcomes of MSCs at present and in future.
| QTPP | CQA | Quality control | Acceptable standards | Ref. |
| Cell characteristics | Morphology | Microscopic visual inspection | Shuttle shaped in wall attached state | [90] |
| Cell viability | Cell count and viability detection | Cell viability ≥ 90% before product distribution | [91] | |
| MSC identity | Specific positive antigen expression ≥ 95% | Flow cytometry: CD105+, CD73+, CD90+ | [92] | |
| Specific negative antigen expression ≤ 2% | Flow cytometry: CD45-, CD34-, CD14- or CD11b-, CD79α- or CD19-, HLA-DR- | [92] | ||
| PDT | PDT calculation formula | 8 hours < PDT < 48 hours | [93] | |
| Cell differentiation | Osteogenic differentiation | Color reaction with Alizarin Red S | [94] | |
| Adipogenic differentiation | Color reaction with Oil Red O | [95] | ||
| Chondrogenic differentiation | Color reaction with Alcian Blue | [96] | ||
| Cell cycle | PI staining | G0/G1% > 70% | ||
| Product quality | Purity | Sterility testing for each lot | No detectable | [97] |
| Mycoplasma testing for each lot | No detectable | [98] | ||
| Endotoxin testing for each lot | ≤ 0.5 CFU/mL | [98] | ||
| Genetic stability | Karyotype | 23 pairs of chromosomes | [99] | |
| STR | Each cell passage is from the same source | |||
| Biological safety | Human telomerase activity | No or weak expression | [100] | |
| MSC soft agar cloning | No detectable | [101] | ||
| Therapeutic potency | In vivo effect | Co-culture with T cells | Inhibition of T cell growth | [102] |
| Paracrine action | The secretion of bioactive molecules | PGE-2, IL-10 | [103] |
Ensuring high-quality standards in production facilities necessitates a thorough understanding of process parameter interactions and their potential impact on CQAs. The culture parameters that affect the cell yield can be divided into two categories: (1) The “traditional” physicochemical bioprocess engineering parameters that are important for the op
Since their first identification in bone marrow in the 1960s, MSCs of diverse origins have been consecutively isolated from adult tissues (e.g., adipose tissue, bone marrow, muscle tendon, uterine blood, dental pulp and sac) and perinatal tissues
Compared to the invasive collection of MSCs from bone marrow and adipose tissue, umbilical cord-derived MSCs are more suitable for large-scale preparation due to their non-invasive collection, cost-effectiveness, and accessibility[50]. To ensure the sustainability of industrial production and the stability of quality, the large-scale cell bank construction based on standardized processes is the core steps for “seed cells” separation and the subsequent ex vivo amplification, together with the cryopreservation of MSC products[14,15,51]. Of note, current updates have verified that the traditional cr
Overall, the most important issue at this stage is the considerable donor-to-donor and intra-population heterogeneity[53-55]. Due to the limitations in the detection of diverse infection or functional abnormalities, each donor carries a “one to many” related risk for MSC-based products upon KOA treatment. For example, the age of the donor is an important factor affecting cell quality[56], including the number of primary isolated cells[57], cell differentiation ability[58], cell doubling level[59] and increasing genetic instability[60].
For cell amplification, the traditional methods are usually based on two-dimensional cell culture flasks, and the introduction of personnel operation greatly increases the risk of contamination. Currently, common strategies to mitigate these risks include limiting the number of cell containers[61] or utilizing fully enclosed production system[62]. In most clinical trials, MSCs have been prepared by utilizing the basal culture medium containing L-glutamine, non-essential amino acid and specific ions, together with 5%-20% fetal bovine serum to ensure cell expansion[63]. It’s of note that the addition of ingredients will directly affect the functional performance of MSCs[64-66]. Relevant process parameters are usually measured by dynamic detection, such as cell number and viability, micronutrient concentration and physicochemical variables[67]. The advantage of physicochemical biological process is that most engineering parameters can be measured and controlled in real time, which helps guide cell output of the MSC products. It is worth noting that oxygen has been considered to play an important role in the maintenance and differentiation of MSCs, together with the regulation of cell fate in culture expansion system[68].
In the process of subculture, telomere length will gradually decrease with the increase of passages, resulting in gradual senescence of cells[69]. Aging is irreversible once it occurs, which will not only lead to the loss of pluripotency[70], but also increase the risk of tumorigenicity[71] and reduce the ability of immunosuppression[72]. However, according to karyotype analysis, the proportion of stem cells with abnormal karyotype is high in the two generations of cells just after primary isolation[73]. Therefore, a better solution for in vitro culture as a clinical application is selecting seed cells at passages ranging from 4 to 8 for the subsequent large-scale generations of MSCs.
Currently, evidence on the clinical mechanism of MSC treatment remains limited, yet it is crucial for the large-scale application of MSCs in disease intervention, including KOA. For instance, the Food and Drug Administration’s previous rejection of Mesoblast’s allogeneic bone marrow MSC (M) product as an allogeneic product for the treatment of steroid-refractory acute graft-versus-host disease, is partly due to the insufficient CQAs used by sponsors[74,75]. In detail, the investigators didn’t demonstrate a clear relationship between the product’s CQAs and the concomitant clinical efficacy[42]. Therefore, a better understanding of the underlying mechanism of action will be beneficial in the following aspects: (1) This will aid the design of novel and improved MSC therapies to realize the anticipated effects of KOA; (2) This will assist in the selection of CQAs for MSC products, which are associated with the mechanism of action and can serve as release criteria for these products; (3) This will facilitate the identification of patients who are more likely to respond to MSCs intervention based on the use of precision medicine methods; and (4) This will enhance the communication of expected clinical outcomes based on MSCs intervention.
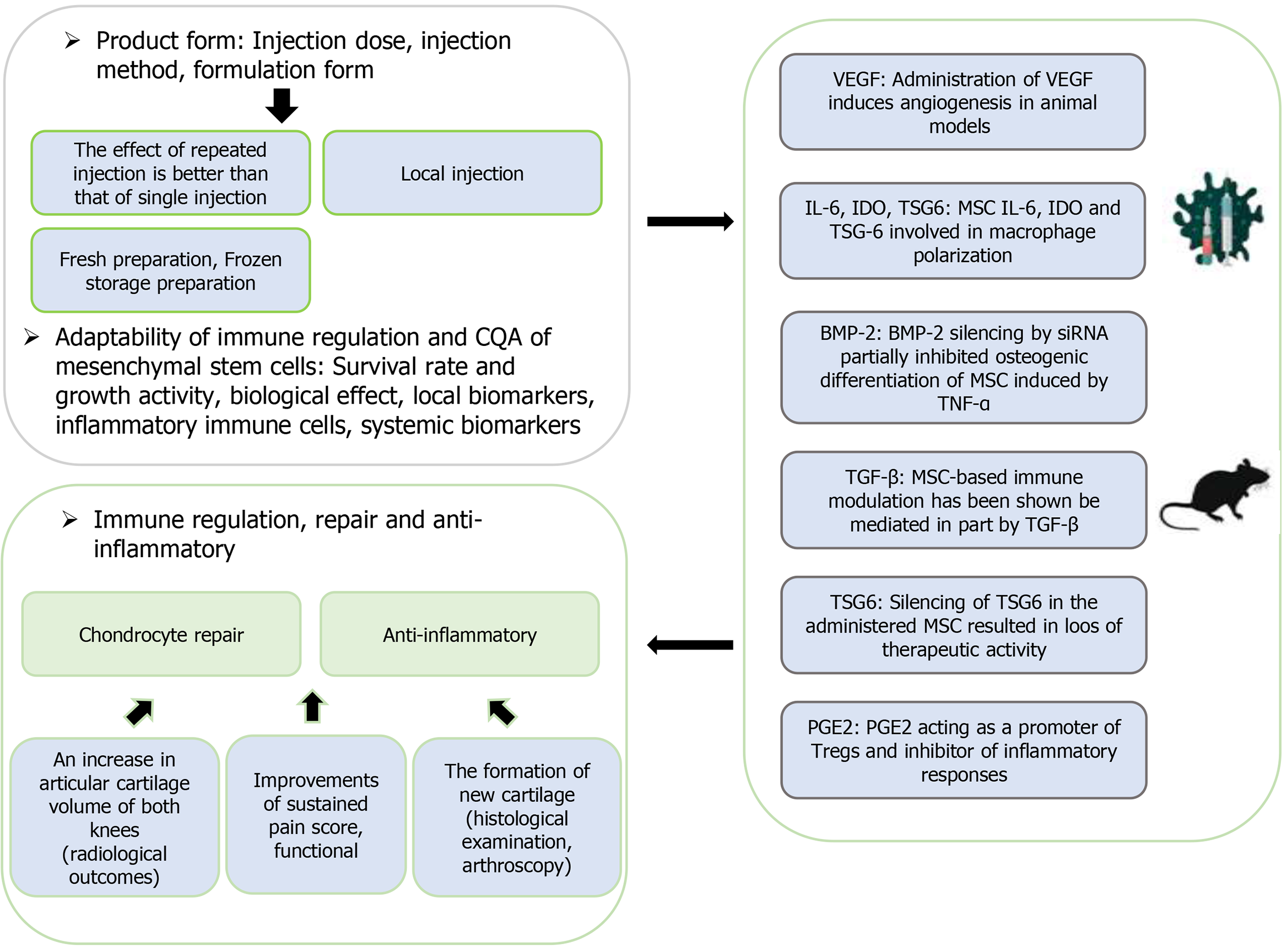
Clinical trials show that MSCs can directly exert cartilage repair and/or cartilage protection effects on KOA by releasing cartilage protective factors or growth factors, and indirectly by regulating the joint microenvironment[76]. At the same time, more joint tissues should be taken into consideration, including the infrapatellar fat pad, subchondral bone, and ligaments. Meanwhile, it’s important to carefully consider the donor factors, process parameters, and delivery strategies of MSCs, which collectively contribute to the mode of action and the overall therapeutic efficacy (Figure 2).
The dose of MSCs in the treatment of KOA is a key issue that needs to be focused on. Clinical data indicate that low-dose MSCs do not show significant effects on pain relief and cartilage repair in KOA patients. Meta-analysis results suggest that a dose of (40-60) × 106 MSCs is effective for treating osteoarthritis, which can significantly improve the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and function subscale scores[24]. However, it should be noted that high doses of MSCs (> 100 × 106) may not provide better outcomes, but rather have a more pronounced protective effect on cartilage tissue[77]. As shown in a published randomized controlled trial (RCT) by Matas et al[78], repeat umbilical cord-derived MSCs intraarticular injections revealed superiority in ameliorating KOA over the single MSC dose. According to the WOMAC pain and function subscale scores in a first-in-human study, Anil et al[79] reported that KOA patients with repeat injection of infrapatellar fat pad-derived MSCs showed significant improvement compared to the control group. However, those with single MSC injection showed sustained improvement up to 9 months, but the WOMAC score returned to control group level at the 12-month follow-up visit. Therefore, longer follow-up visit would have afforded a better evaluation of the synergistic or additive effects of repeat MSC injections.
The main biological function of MSCs is immune regulation, which is also an important mechanism in alleviating KOA. To confirm the immunoregulatory mechanism of MSCs in KOA, Matas et al[78] investigated the relationship between the baseline immune regulatory fitness of MSCs in vitro and its clinical efficacy. They measured the interferon gamma induced immune regulatory gene expression [including prostaglandin E2, interleukin-10, CD274, indoleamine 2,3-dioxygenase (IDO), hepatocyte growth factor] and tumor necrosis factor-α induced tumor necrosis factor-stimulated gene 6 protein expression by MSCs in vitro, and found correlations with changes in WOMAC and knee injury and os
Furthermore, Chen et al[80] found that the expression level of intracellular IDO protein in stimulated MSCs was significantly correlated with the inhibition of T cell proliferation in vitro and the improvement of knee injury and osteoarthritis outcome score sports, visual analogue scale, and international knee documentation committee scores in KOA patients after intraarticular injection of cells. Interestingly, Leijs et al[81] turned to enzyme-linked immunosorbent assay and relative methods to detect IDO protein expression by interferon-γ-stimulated MSCs, which could effectively evaluate the immunomodulatory effects of MSCs triggered by pro-inflammatory cytokines in arthritic synovial fluids. Therefore, it would be of great help to complete a satisfactory method validation and then incorporated into the CQAs for MSCs in the treatment of KOA. As a differentiation test for MSCs, the multi-lineage differentiation potential of MSCs under the conditioned medium also provides an in vitro source of drug quality control for the treatment of KOA with MSCs.
To better verify the therapeutic potential of MSCs in treating KOA, numerous literatures have compared with other conventional treatment methods, including HA injection, PRP injection, arthroscopic debridement, high tibial osteotomy, and conservative treatment. For instance, Naja et al[82] conducted a meta-analysis based on recent RCTs to investigate the efficacy of commonly used non-surgical interventions in patients with mild to moderate KOA, and a 19 RCTs with diverse treatment options were included (e.g., bone marrow MSCs, PRP, HA, corticosteroids, non-steroidal anti-inflammatory drugs). They found the total WOMAC score of the MSC and PRP intervention groups significantly decreased from baseline to 12 months, and MSC treatment showed the greatest improvement in pain reduction and functional enhancement according to the meta-analysis. A systematic review further demonstrated the dynamics of MSC therapy upon KOA patients with significant improvement in every subsequent follow-up visit[83]. Compared to 18 months, the WOMAC score slightly decreased at 24 months, highlighting that the first 18-24 months post-treatment were expected to yield the most significant clinical benefits. In recent years, an increasing body of clinical evidence points to the superior effectiveness of MSC therapy for KOA. With the advancement of the cell therapy industry, cell therapy-derived entities such as exosomes are demonstrating their potential in alleviating KOA symptoms by modulating macrophage polarization, promoting angiogenesis, enhancing osteogenesis, and inhibiting tunnels osteolysis.
Unlike most small molecule drugs or biopharmaceuticals, stem cell-based therapies are typically produced in unique manufacturing environments, including equipment and processes for cultivating, testing, and packaging. The collected raw materials are processed in a good manufacturing practice environment and then applied in clinical practice. A key distinction between stem cell-based therapies and traditional pharmaceuticals is the complexity and quantity of raw materials used in manufacturing. For manufacturers, determining which components are most critical to quality is crucial. When adding raw materials (such as cytokines and growth factors) during the manufacturing process of cellular products, they can affect the growth, differentiation, or function of cells[7]. Some of stem cell-based therapy products face manufacturing process challenges after amplification (culture amplification), resulting in high cost of goods and high variability in production preparation processes[7,84,85]. Use planar techniques such as T-flasks or multi-layer cell engineering, or perform cell expansion via a single use of 3D microcarrier-based culture in a bioreactor. After enzyme treatment (e.g., trypsin) releases adherent cells, the downstream process stage includes removal of microcarriers and volume reduction for cell concentration and washing. The next step is to place the cells in a low-temperature preservation buffer for low-temperature preservation. Therefore, the understanding of how cells interact with their environment is currently deepening, and bioreactor systems that can control the cellular environment are collecting data that is increasingly focused on molecular and cellular information. At the same time, deepen the understanding of the molecular basis of stem cell state, including adhesion dependence, metabolic network state, clonality, and proliferation control, which is important for KOA efficacy but different from traditional medicine (e.g., icariin) to a large extent[86-89]. The QbD principle is in the ascendant and highly valued in the pharmaceutical industry. The development of the stem cell therapy industry is bound to combine the QbD principle to develop its own production preparation process and management system.
The co-authors thank the members in Faculty of Life Sciences and Medicine, Kunming University of Science and Technology, The Fourth People’s Hospital of Jinan Affiliated to Shandong Second Medical University, Gansu Provincial Hospital, and Nankai University School of Medicine. We also thank the support from Shandong Provincial Key Medical and Health Laboratory of Blood Ecology and Biointelligence, Jinan Key Laboratory of Medical Cell Bioengineering, and Shandong Health Youth Science and Technology Innovation Team.
| 1. | Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020;29-30:100587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 850] [Article Influence: 141.7] [Reference Citation Analysis (0)] |
| 2. | Billesberger LM, Fisher KM, Qadri YJ, Boortz-Marx RL. Procedural Treatments for Knee Osteoarthritis: A Review of Current Injectable Therapies. Pain Res Manag. 2020;2020:3873098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 3. | Lohmander LS, Roos EM. Clinical update: treating osteoarthritis. Lancet. 2007;370:2082-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Zhao Q, Zhang L, Wei Y, Yu H, Zou L, Huo J, Yang H, Song B, Wei T, Wu D, Zhang W, Zhang L, Liu D, Li Z, Chi Y, Han Z, Han Z. Systematic comparison of hUC-MSCs at various passages reveals the variations of signatures and therapeutic effect on acute graft-versus-host disease. Stem Cell Res Ther. 2019;10:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Zhang L, Chi Y, Wei Y, Zhang W, Wang F, Zhang L, Zou L, Song B, Zhao X, Han Z. Bone marrow-derived mesenchymal stem/stromal cells in patients with acute myeloid leukemia reveal transcriptome alterations and deficiency in cellular vitality. Stem Cell Res Ther. 2021;12:365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Zhang L, Wei Y, Chi Y, Liu D, Yang S, Han Z, Li Z. Two-step generation of mesenchymal stem/stromal cells from human pluripotent stem cells with reinforced efficacy upon osteoarthritis rabbits by HA hydrogel. Cell Biosci. 2021;11:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Wei Y, Zhang L, Chi Y, Ren X, Gao Y, Song B, Li C, Han Z, Zhang L, Han Z. High-efficient generation of VCAM-1(+) mesenchymal stem cells with multidimensional superiorities in signatures and efficacy on aplastic anaemia mice. Cell Prolif. 2020;53:e12862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Huo J, Zhang L, Ren X, Li C, Li X, Dong P, Zheng X, Huang J, Shao Y, Ge M, Zhang J, Wang M, Nie N, Jin P, Zheng Y. Multifaceted characterization of the signatures and efficacy of mesenchymal stem/stromal cells in acquired aplastic anemia. Stem Cell Res Ther. 2020;11:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | Zhang X, Sang X, Chen Y, Yu H, Sun Y, Liang X, Zheng X, Wang X, Yang H, Bi J, Zhang L, Wang P. VCAM-1(+) hUC-MSCs Exert Considerable Neuroprotection Against Cerebral Infarction in Rats by Suppression of NLRP3-Induced Pyroptosis. Neurochem Res. 2023;48:3084-3098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2771] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 11. | Zhang L, Zhuo Y, Yu H. Spatio-temporal metabolokinetics and therapeutic effect of CD106(+) mesenchymal stem/stromal cells upon mice with acute lung injury. Cell Biol Int. 2023;47:720-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Hou H, Zhang L, Duan L, Liu Y, Han Z, Li Z, Cao X. Spatio-Temporal Metabolokinetics and Efficacy of Human Placenta-Derived Mesenchymal Stem/Stromal Cells on Mice with Refractory Crohn's-like Enterocutaneous Fistula. Stem Cell Rev Rep. 2020;16:1292-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Zhao M, Wang A, Zhang L, Yu H. Establishment of a novel experimental model of infected anal fistula in rat. Lab Anim Res. 2022;38:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 14. | Yu H, Feng Y, Du W, Zhao M, Jia H, Wei Z, Yan S, Han Z, Zhang L, Li Z, Han Z. Off-the-shelf GMP-grade UC-MSCs as therapeutic drugs for the amelioration of CCl4-induced acute-on-chronic liver failure in NOD-SCID mice. Int Immunopharmacol. 2022;113:109408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 15. | Wang A, Zhang L, Zhao M, Yu H. Quality Control Analysis of Mesenchymal Stem/Stromal Cells During Investigational New Drug Application for GvHD Administration in China. Curr Stem Cell Res Ther. 2023;18:1032-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Cressman A, Le B, Morales D, Yen WS, Wu FJ, Perotti NH, Fury B, Nolta JA, Fierro FA. Investigational New Drug-enabling studies to use genetically modified mesenchymal stromal cells in patients with critical limb ischemia. Stem Cells Transl Med. 2025;14:szae094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Thandar M, Yang X, Zhu Y, Huang Y, Zhang X, Huang S, Zhang L, Chi P. Mesenchymal stem cells derived from adipose tissue and umbilical cord reveal comparable efficacy upon radiation-induced colorectal fibrosis in rats. Am J Cancer Res. 2024;14:1594-1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Aitong W, Leisheng Z, Hao Y. Visualized Analyses of Investigations Upon Mesenchymal Stem/stromal Cell-based Cytotherapy and Underlying Mechanisms for COVID-19 Associated ARDS. Curr Stem Cell Res Ther. 2022;17:2-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Zhang LS, Yu Y, Yu H, Han ZC. Therapeutic prospects of mesenchymal stem/stromal cells in COVID-19 associated pulmonary diseases: From bench to bedside. World J Stem Cells. 2021;13:1058-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 1107] [Article Influence: 100.6] [Reference Citation Analysis (1)] |
| 21. | Li X, Peng B, Zhu X, Wang P, Xiong Y, Liu H, Sun K, Wang H, Ou L, Wu Z, Liu X, He H, Mo S, Peng X, Tian Y, Zhang R, Yang L. Changes in related circular RNAs following ERβ knockdown and the relationship to rBMSC osteogenesis. Biochem Biophys Res Commun. 2017;493:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Song Y, Jorgensen C. Mesenchymal Stromal Cells in Osteoarthritis: Evidence for Structural Benefit and Cartilage Repair. Biomedicines. 2022;10:1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, Ra JC, Oh S, Yoon KS. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32:1254-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 666] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 24. | Robb KP, Fitzgerald JC, Barry F, Viswanathan S. Mesenchymal stromal cell therapy: progress in manufacturing and assessments of potency. Cytotherapy. 2019;21:289-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 25. | Wagner W, Bork S, Horn P, Krunic D, Walenda T, Diehlmann A, Benes V, Blake J, Huber FX, Eckstein V, Boukamp P, Ho AD. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS One. 2009;4:e5846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 380] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 26. | Sawada R, Kusakawa S, Kusuhara M, Tanaka K, Miura T, Yasuda S, Sato Y. Increasing robustness of in vitro assay for immnosuppressive effect of mesenchymal stromal/stem cells: The role of inflammatory cytokine production by peripheral blood mononuclear cells. Regen Ther. 2025;28:321-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Hoang DM, Nguyen QT, Phan TTK, Ngo ATL, Pham PT, Bach TQ, Le PTT, Bui HTP, Thanh LN. Advanced cell-based products generated via automated and manual manufacturing platforms under the quality by design principle: Are they equivalent or different? Heliyon. 2023;9:e15946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 28. | Rathore AS, Winkle H. Quality by design for biopharmaceuticals. Nat Biotechnol. 2009;27:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 532] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 29. | Mitchell M. Determining criticality-process parameters and quality attributes part ii: Design of experiments and data-driven criticality. BioPharm Int. 2014;27:32-40. |
| 30. | Loubière C, Sion C, De Isla N, Reppel L, Guedon E, Chevalot I, Olmos E. Impact of the type of microcarrier and agitation modes on the expansion performances of mesenchymal stem cells derived from umbilical cord. Biotechnol Prog. 2019;35:e2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Krutty JD, Dias AD, Yun J, Murphy WL, Gopalan P. Synthetic, Chemically Defined Polymer-Coated Microcarriers for the Expansion of Human Mesenchymal Stem Cells. Macromol Biosci. 2019;19:e1800299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Lin YM, Lim JF, Lee J, Choolani M, Chan JK, Reuveny S, Oh SK. Expansion in microcarrier-spinner cultures improves the chondrogenic potential of human early mesenchymal stromal cells. Cytotherapy. 2016;18:740-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Zhao Y, Ding Y, Wang Z, Wang Q, Ye D, Luan Z. Therapeutic and continuative effects of human umbilical cord-derived mesenchymal stromal cells in food-allergic mice. Cell Transplant. 2025;34:9636897251326899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Spoerer TM, Larey AM, Asigri W, Daga KR, Marklein RA. High throughput morphological screening identifies chemically defined media for mesenchymal stromal cells that enhances proliferation and supports maintenance of immunomodulatory function. Stem Cell Res Ther. 2025;16:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Samsonraj RM, Rai B, Sathiyanathan P, Puan KJ, Rötzschke O, Hui JH, Raghunath M, Stanton LW, Nurcombe V, Cool SM. Establishing criteria for human mesenchymal stem cell potency. Stem Cells. 2015;33:1878-1891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 36. | Lipsitz YY, Timmins NE, Zandstra PW. Quality cell therapy manufacturing by design. Nat Biotechnol. 2016;34:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 204] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 37. | Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion. 2014;54:1418-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 314] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 38. | Tee CA, Roxby DN, Othman R, Denslin V, Bhat KS, Yang Z, Han J, Tucker-Kellogg L, Boyer LA. Metabolic modulation to improve MSC expansion and therapeutic potential for articular cartilage repair. Stem Cell Res Ther. 2024;15:308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | López-Fernández A, Codinach M, Coca MI, Prat-Vidal C, Castaño J, Torrents S, Aran G, Rodríguez L, Querol S, Vives J. Comparability exercise of critical quality attributes of clinical-grade human mesenchymal stromal cells from the Wharton's jelly: single-use stirred tank bioreactors versus planar culture systems. Cytotherapy. 2024;26:418-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Hogrebe NJ, Ishahak M, Millman JR. Developments in stem cell-derived islet replacement therapy for treating type 1 diabetes. Cell Stem Cell. 2023;30:530-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 41. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 13048] [Article Influence: 686.7] [Reference Citation Analysis (12)] |
| 42. | Robb KP, Galipeau J, Shi Y, Schuster M, Martin I, Viswanathan S. Failure to launch commercially-approved mesenchymal stromal cell therapies: what's the path forward? Proceedings of the International Society for Cell & Gene Therapy (ISCT) Annual Meeting Roundtable held in May 2023, Palais des Congrès de Paris, Organized by the ISCT MSC Scientific Committee. Cytotherapy. 2024;26:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 43. | Robb KP, Audet J, Gandhi R, Viswanathan S. Putative critical quality attribute matrix identifies mesenchymal stromal cells with potent immunomodulatory and angiogenic "fitness" ranges in response to culture process parameters. Front Immunol. 2022;13:972095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 44. | Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, Rogers AJ, Levitt J, Wiener-Kronish J, Bajwa EK, Leavitt A, McKenna D, Thompson BT, Matthay MA. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 595] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 45. | Muñoz-Domínguez N, Carreras-Sánchez I, López-Fernández A, Vives J. Optimisation of processing methods to improve success in the derivation of human multipotent mesenchymal stromal cells from cryopreserved umbilical cord tissue fragments. Cryobiology. 2022;108:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 46. | Maillot C, Sion C, De Isla N, Toye D, Olmos E. Quality by design to define critical process parameters for mesenchymal stem cell expansion. Biotechnol Adv. 2021;50:107765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Dunn CM, Kameishi S, Grainger DW, Okano T. Strategies to address mesenchymal stem/stromal cell heterogeneity in immunomodulatory profiles to improve cell-based therapies. Acta Biomater. 2021;133:114-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 48. | Bijonowski BM, Fu Q, Yuan X, Irianto J, Li Y, Grant SC, Ma T. Aggregation-induced integrated stress response rejuvenates culture-expanded human mesenchymal stem cells. Biotechnol Bioeng. 2020;117:3136-3149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Wang L, Zhang L, Liang X, Zou J, Liu N, Liu T, Wang G, Ding X, Liu Y, Zhang B, Liang R, Wang S. Adipose Tissue-Derived Stem Cells from Type 2 Diabetics Reveal Conservative Alterations in Multidimensional Characteristics. Int J Stem Cells. 2020;13:268-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Margossian T, Reppel L, Makdissy N, Stoltz JF, Bensoussan D, Huselstein C. Mesenchymal stem cells derived from Wharton's jelly: comparative phenotype analysis between tissue and in vitro expansion. Biomed Mater Eng. 2012;22:243-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Ikebe C, Suzuki K. Mesenchymal stem cells for regenerative therapy: optimization of cell preparation protocols. Biomed Res Int. 2014;2014:951512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 52. | François M, Copland IB, Yuan S, Romieu-Mourez R, Waller EK, Galipeau J. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-γ licensing. Cytotherapy. 2012;14:147-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 279] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 53. | Christ B, Franquesa M, Najimi M, van der Laan LJW, Dahlke MH. Cellular and Molecular Mechanisms of Mesenchymal Stem Cell Actions. Stem Cells Int. 2017;2017:2489041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Kang I, Lee BC, Choi SW, Lee JY, Kim JJ, Kim BE, Kim DH, Lee SE, Shin N, Seo Y, Kim HS, Kim DI, Kang KS. Donor-dependent variation of human umbilical cord blood mesenchymal stem cells in response to hypoxic preconditioning and amelioration of limb ischemia. Exp Mol Med. 2018;50:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 55. | Phinney DG. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem. 2012;113:2806-2812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 334] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 56. | Baker N, Boyette LB, Tuan RS. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone. 2015;70:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 228] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 57. | Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1405] [Cited by in RCA: 1361] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 58. | Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 472] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 59. | Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014;12:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 390] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 60. | Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 957] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 61. | Fekete N, Rojewski MT, Fürst D, Kreja L, Ignatius A, Dausend J, Schrezenmeier H. GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC. PLoS One. 2012;7:e43255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 62. | Zanini C, Severina F, Lando G, Fanizza C, Cesana E, Desidera D, Bonifacio M. Good design practices for an integrated containment and production system for advanced therapies. Biotechnol Bioeng. 2020;117:2319-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Zhang L, Wang H, Liu C, Wu Q, Su P, Wu D, Guo J, Zhou W, Xu Y, Shi L, Zhou J. MSX2 Initiates and Accelerates Mesenchymal Stem/Stromal Cell Specification of hPSCs by Regulating TWIST1 and PRAME. Stem Cell Reports. 2018;11:497-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 64. | Abdelrazik H, Spaggiari GM, Chiossone L, Moretta L. Mesenchymal stem cells expanded in human platelet lysate display a decreased inhibitory capacity on T- and NK-cell proliferation and function. Eur J Immunol. 2011;41:3281-3290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 65. | Ferro AP, de Jesus Guirro RR, Ferraresi C, Celli J, Orellana MD, de Santis GC, Junior JAF, de Oliveira Guirro EC. Influence of Different Photobiomodulation Parameters on Multi-Potent Adipose Tissue Mesenchymal Cells In Vitro. Photobiomodul Photomed Laser Surg. 2024;42:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 66. | Silva-Cote I, Cruz-Barrera M, Cañas-Arboleda M, Correa-Araujo L, Méndez L, Jagielska J, Camacho B, Salguero G. Strategy for the Generation of Engineered Bone Constructs Based on Umbilical Cord Mesenchymal Stromal Cells Expanded with Human Platelet Lysate. Stem Cells Int. 2019;2019:7198215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Attaelmanan GA, Khalil HB. Assessment of Umbilical Cord Mesenchymal Stem Cell Cultivation Using Fetal Bovine Serum or Platelet Lysate. Cureus. 2025;17:e78044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 68. | Hoch AI, Leach JK. Concise review: optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications. Stem Cells Transl Med. 2014;3:643-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 69. | Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 572] [Cited by in RCA: 637] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 70. | Bork S, Pfister S, Witt H, Horn P, Korn B, Ho AD, Wagner W. DNA methylation pattern changes upon long-term culture and aging of human mesenchymal stromal cells. Aging Cell. 2010;9:54-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 333] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 71. | Kim J, Kang JW, Park JH, Choi Y, Choi KS, Park KD, Baek DH, Seong SK, Min HK, Kim HS. Biological characterization of long-term cultured human mesenchymal stem cells. Arch Pharm Res. 2009;32:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 72. | Nasef A, Mathieu N, Chapel A, Frick J, François S, Mazurier C, Boutarfa A, Bouchet S, Gorin NC, Thierry D, Fouillard L. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation. 2007;84:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 259] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 73. | Binato R, de Souza Fernandez T, Lazzarotto-Silva C, Du Rocher B, Mencalha A, Pizzatti L, Bouzas LF, Abdelhay E. Stability of human mesenchymal stem cells during in vitro culture: considerations for cell therapy. Cell Prolif. 2013;46:10-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 74. | Kurtzberg J, Prockop S, Chaudhury S, Horn B, Nemecek E, Prasad V, Satwani P, Teira P, Hayes J, Burke E; MSB-275 Study Group. Study 275: Updated Expanded Access Program for Remestemcel-L in Steroid-Refractory Acute Graft-versus-Host Disease in Children. Biol Blood Marrow Transplant. 2020;26:855-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 75. | Blanc KL, Dazzi F, English K, Farge D, Galipeau J, Horwitz EM, Kadri N, Krampera M, Lalu MM, Nolta J, Patel NM, Shi Y, Weiss DJ, Viswanathan S. ISCT MSC committee statement on the US FDA approval of allogenic bone-marrow mesenchymal stromal cells. Cytotherapy. 2025;27:413-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 76. | Buzaboon N, Alshammary S. Clinical Applicability of Adult Human Mesenchymal Stem Cell Therapy in the Treatment of Knee Osteoarthritis. Stem Cells Cloning. 2020;13:117-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 77. | Lamo-Espinosa JM, Mora G, Blanco JF, Granero-Moltó F, Nuñez-Córdoba JM, Sánchez-Echenique C, Bondía JM, Aquerreta JD, Andreu EJ, Ornilla E, Villarón EM, Valentí-Azcárate A, Sánchez-Guijo F, Del Cañizo MC, Valentí-Nin JR, Prósper F. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II). J Transl Med. 2016;14:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 242] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 78. | Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI, Alcayaga-Miranda F, González PL, Muse E, Khoury M, Figueroa FE, Espinoza F. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl Med. 2019;8:215-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 279] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 79. | Anil U, Markus DH, Hurley ET, Manjunath AK, Alaia MJ, Campbell KA, Jazrawi LM, Strauss EJ. The efficacy of intra-articular injections in the treatment of knee osteoarthritis: A network meta-analysis of randomized controlled trials. Knee. 2021;32:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 80. | Chen HH, Chen YC, Yu SN, Lai WL, Shen YS, Shen PC, Lin SH, Chang CH, Lee SM. Infrapatellar fat pad-derived mesenchymal stromal cell product for treatment of knee osteoarthritis: a first-in-human study with evaluation of the potency marker. Cytotherapy. 2022;24:72-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 81. | Leijs MJ, van Buul GM, Lubberts E, Bos PK, Verhaar JA, Hoogduijn MJ, van Osch GJ. Effect of Arthritic Synovial Fluids on the Expression of Immunomodulatory Factors by Mesenchymal Stem Cells: An Explorative in vitro Study. Front Immunol. 2012;3:231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Naja M, Fernandez De Grado G, Favreau H, Scipioni D, Benkirane-Jessel N, Musset AM, Offner D. Comparative effectiveness of nonsurgical interventions in the treatment of patients with knee osteoarthritis: A PRISMA-compliant systematic review and network meta-analysis. Medicine (Baltimore). 2021;100:e28067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 83. | Agarwal N, Mak C, Bojanic C, To K, Khan W. Meta-Analysis of Adipose Tissue Derived Cell-Based Therapy for the Treatment of Knee Osteoarthritis. Cells. 2021;10:1365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 84. | Fazzina R, Iudicone P, Fioravanti D, Bonanno G, Totta P, Zizzari IG, Pierelli L. Potency testing of mesenchymal stromal cell growth expanded in human platelet lysate from different human tissues. Stem Cell Res Ther. 2016;7:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Valencia J, Yáñez RM, Muntión S, Fernández-García M, Martín-Rufino JD, Zapata AG, Bueren JA, Vicente Á, Sánchez-Guijo F. Improving the therapeutic profile of MSCs: Cytokine priming reduces donor-dependent heterogeneity and enhances their immunomodulatory capacity. Front Immunol. 2025;16:1473788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 86. | Chen G, Wang C, Wang J, Yin S, Gao H, Xiang LU, Liu H, Xiong Y, Wang P, Zhu X, Yang LI, Zhang R. Antiosteoporotic effect of icariin in ovariectomized rats is mediated via the Wnt/β-catenin pathway. Exp Ther Med. 2016;12:279-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 87. | Liu H, Xiong Y, Zhu X, Gao H, Yin S, Wang J, Chen G, Wang C, Xiang L, Wang P, Fang J, Zhang R, Yang L. Icariin improves osteoporosis, inhibits the expression of PPARγ, C/EBPα, FABP4 mRNA, N1ICD and jagged1 proteins, and increases Notch2 mRNA in ovariectomized rats. Exp Ther Med. 2017;13:1360-1368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 88. | Wu Z, Ou L, Wang C, Yang L, Wang P, Liu H, Xiong Y, Sun K, Zhang R, Zhu X. Icaritin induces MC3T3-E1 subclone14 cell differentiation through estrogen receptor-mediated ERK1/2 and p38 signaling activation. Biomed Pharmacother. 2017;94:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 89. | Liu H, Xiong Y, Wang H, Yang L, Wang C, Liu X, Wu Z, Li X, Ou L, Zhang R, Zhu X. Effects of water extract from epimedium on neuropeptide signaling in an ovariectomized osteoporosis rat model. J Ethnopharmacol. 2018;221:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 90. | Garikipati VN, Jadhav S, Pal L, Prakash P, Dikshit M, Nityanand S. Mesenchymal stem cells from fetal heart attenuate myocardial injury after infarction: an in vivo serial pinhole gated SPECT-CT study in rats. PLoS One. 2014;9:e100982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | Belotti D, Gaipa G, Bassetti B, Cabiati B, Spaltro G, Biagi E, Parma M, Biondi A, Cavallotti L, Gambini E, Pompilio G. Full GMP-compliant validation of bone marrow-derived human CD133(+) cells as advanced therapy medicinal product for refractory ischemic cardiomyopathy. Biomed Res Int. 2015;2015:473159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Sun Y, Wang TE, Hu Q, Zhang W, Zeng Y, Lai X, Zhang L, Shi M. Systematic comparation of the biological and transcriptomic landscapes of human amniotic mesenchymal stem cells under serum-containing and serum-free conditions. Stem Cell Res Ther. 2022;13:490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Marcu R, Hawkins BJ, Fryer BH, Lee Y, Chou C, Tsai E, Lee J. Identification of a unique source of human mesenchymal stromal cells with significantly extended population doubling capacity. Cytotherapy. 2020;22:S86-S87. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 94. | Rodríguez JP, González M, Ríos S, Cambiazo V. Cytoskeletal organization of human mesenchymal stem cells (MSC) changes during their osteogenic differentiation. J Cell Biochem. 2004;93:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 218] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 95. | Lee K, Chen Y, Yoshitomi T, Kawazoe N, Yang Y, Chen G. MSC Differentiation: Osteogenic and Adipogenic Differentiation of Mesenchymal Stem Cells in Gelatin Solutions of Different Viscosities (Adv. Healthcare Mater. 23/2020). Adv Healthc Mater. 2020;9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 96. | Beane OS, Fonseca VC, Cooper LL, Koren G, Darling EM. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS One. 2014;9:e115963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 97. | Steigman SA, Armant M, Bayer-Zwirello L, Kao GS, Silberstein L, Ritz J, Fauza DO. Preclinical regulatory validation of a 3-stage amniotic mesenchymal stem cell manufacturing protocol. J Pediatr Surg. 2008;43:1164-1169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Alany RG, Wen J. Pharmaceutical manufacturing handbook: Production and processes. United States: John Wiley & Sons, Inc., 2008. |
| 99. | Dominina AP, Fridliandskaia II, Zemel'ko VI, Pugovkina NA, Kovaleva ZV, Zenin VV, Grinchuk TM, Nikol'skiĭ NN. [Mesenchymal stem cells of human endometrium do not undergo spontaneous transformation during long-term cultivation]. Tsitologiia. 2013;55:69-74. [PubMed] |
| 100. | Tichon A, Gowda BK, Slavin S, Gazit A, Priel E. Telomerase activity and expression in adult human mesenchymal stem cells derived from amyotrophic lateral sclerosis individuals. Cytotherapy. 2009;11:837-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 101. | Qin X, Zhang W, Li J, Wei J, Kan W, Li R. [Tumorigenicity of human mesenchymal stem cells from adipose tissue]. Tianjin Yiyao. 2013;41:152-153. [DOI] [Full Text] |
| 102. | Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 1027] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 103. | Ghannam S, Pène J, Moquet-Torcy G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185:302-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 444] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/