Published online Dec 26, 2025. doi: 10.4252/wjsc.v17.i12.114076
Revised: October 12, 2025
Accepted: November 11, 2025
Published online: December 26, 2025
Processing time: 105 Days and 13.5 Hours
Tendon-bone healing remains a significant clinical challenge due to the high risk of re-rupture following injury. While mesenchymal stem cells (MSCs) show great potential in enhancing tendon-bone healing, their clinical application is limited by issues such as low delivery efficiency, restricted differentiation potential, and potential immunogenicity. Recently, various strategies combining MSCs with other approaches, such as preconditioning, biomaterial integration, gene modi
Core Tip: This review synthesized advances optimizing mesenchymal stem cell-based therapy for tendon-bone healing, spanning metabolic or mechanical preconditioning, instructive biomaterials (aligned fibers, gradient mineralization, controlled release), gene/cargo engineering, and exosome-centered paracrine modulation. These strategies target persistent hurdles (poor homing/engraftment, lineage commitment at the fibrocartilaginous interface, hostile inflammatory milieu, and immunogenicity) while improving zonal enthesis regeneration and mechanical integration. We also highlighted scalable manufacturing and safety/readout standardization as key enablers to translate robust efficacy from preclinical models to rigorous clinical trials.
- Citation: Li H, Li ZP, Zhu MT, Lan CH, Wang YX, Liao P, Chen Z, Wang P, Sun JK, Shi Z, Lu PY, Lou C, Xu GH. Optimizing mesenchymal stem cell therapy for tendon-bone healing: Multifaceted approaches and future directions. World J Stem Cells 2025; 17(12): 114076
- URL: https://www.wjgnet.com/1948-0210/full/v17/i12/114076.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i12.114076
Tendon-bone healing is a complex process that transitions tendon tissue to bone and is crucial for joint stability and mobility. However, after tendon-bone injuries, natural healing often fails to restore the original structure, leading to fibrosis, functional impairment, and high re-rupture rates[1]. Tendon-bone injuries typically require surgery, but postoperative outcomes often do not fully restore function, leaving patients at risk of poor healing and re-injury. For example, failure rates for rotator cuff tear (RCT) repair range from 20% to 94%, and anterior cruciate ligament re
Stem cell therapy, especially with mesenchymal stem cells (MSCs), has gained attention for tissue repair and regeneration[3]. MSCs are widely accessible, have immunomodulatory properties, and can differentiate into osteoblasts, chondrocytes, fibroblasts, and tenocytes, all essential for tendon-bone healing[4,5]. Moreover, MSCs secrete bioactive factors that accelerate tissue regeneration. However, challenges like low engraftment efficiency, limited differentiation potential, and possible immunogenicity hinder the broader application of MSCs[2,6,7].
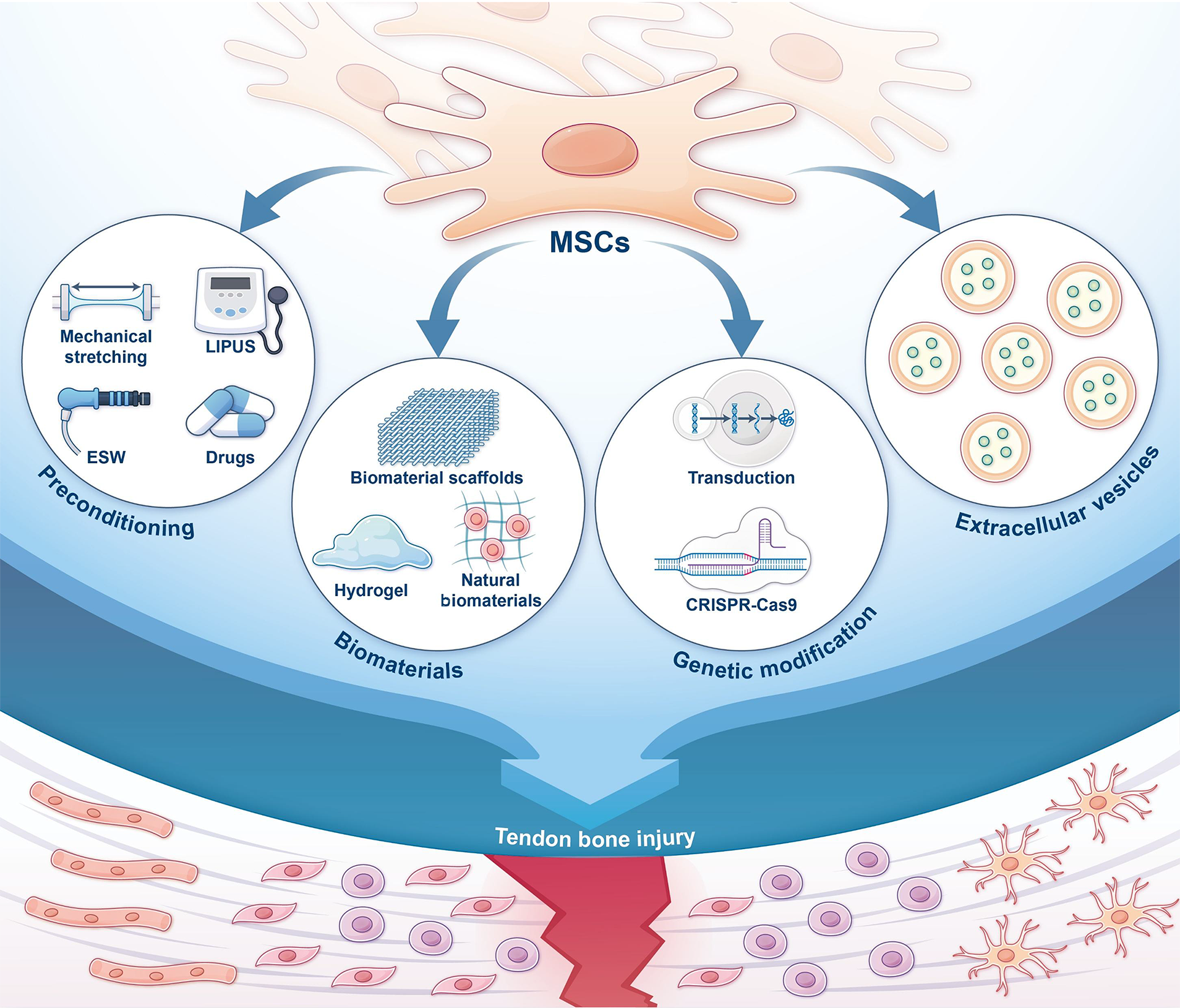
To address these limitations several strategies have been developed to enhance MSC efficacy. These include physical and pharmacological preconditioning, MSC-biomaterial combinations for improved delivery, gene modifications, and novel cell-free therapies using MSC derivatives. These approaches show promise in enhancing MSC effectiveness and provide new insights for tendon-bone healing research and clinical applications (Figure 1).
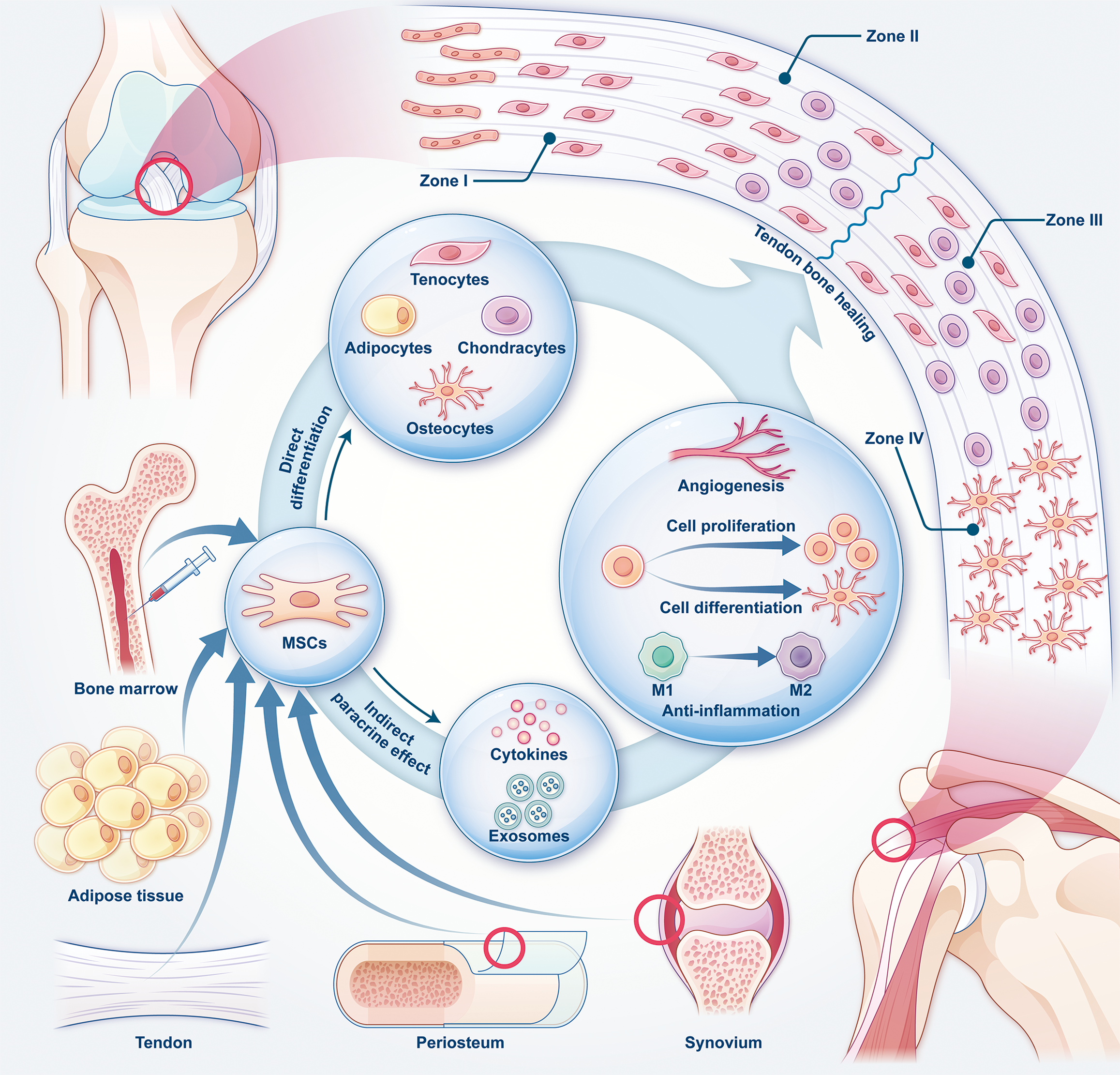
The tendon-bone insertion (TBI) is a specialized tissue that connects tendons or ligaments to bone, providing crucial support and strength for joint movement. TBIs are classified into direct and indirect insertions. Examples of direct insertions include the rotator cuff and anterior cruciate ligament[5]. This structure consists of four zones: Tendon/Ligament (I); unmineralized fibrocartilage (II); mineralized fibrocartilage (III); and bone (IV)[2]. The transitional nature of this structure ensures a gradual shift in mechanical properties from tendon to bone, preventing stress concentration during load transmission[8]. The healing process of TBI occurs in four stages: Inflammation; proliferation; remodeling; and maturation[1]. While TBI has some capacity for self-healing, restoring its natural gradient structure is challenging. This often results in the formation of fibrous scar tissue and a high risk of re-rupture[1].
MSCs are classified into different types based on their source. Bone marrow-derived stem cells (BMSCs) are the most extensively studied and widely used, typically obtained through invasive procedures[2]. Adipose-derived stem cells (ADSCs) are easier to collect in larger quantities from subcutaneous adipose tissue. They have strong adipogenic potential, but their osteogenic and chondrogenic capabilities are comparatively weaker[9]. Synovium-derived stem cells exhibit strong chondrogenic potential but are challenging to extract and expand[2]. Tendon-derived stem cells (TDSCs)/tendon stem progenitor cells (TSPCs) and periosteum-derived stem cells show superior osteogenic, chondrogenic, and adipogenic potential compared with BMSCs although many of their properties are still being explored and validated[10,11].
MSCs promote tendon-bone healing mainly through two mechanisms: Direct differentiation and paracrine effects. In direct differentiation MSCs transform into target cells, strengthening the tendon-bone interface. The paracrine effect involves the secretion of bioactive factors and exosomes that indirectly regulate the healing process[12]. Through exosomes and growth factor release, MSCs stimulate the proliferation and differentiation of endogenous cells, thereby promoting tissue repair[13,14]. Moreover, MSCs modulate inflammation at the TBI site, reducing excessive inflammation and scar formation. They also enhance angiogenesis by releasing exosomes or growth factors, improving blood supply and supporting tissue regeneration[5]. However, their self-renewal ability carries an inherent risk of tumor formation. MSCs may contribute to tumor progression and metastasis by altering cancer cell behavior, regulating immune responses, and promoting angiogenesis[15] (Figure 2).
Despite their therapeutic potential MSCs have several limitations. The most common delivery method, local injection, faces challenges such as cell leakage and low survival rates[2]. MSCs also have a short retention time at the injury site, often requiring multiple injections for optimal results, and there is currently no standardized dosage or administration protocol[7]. Additionally, stem cell therapies carry risks, including carcinogenesis, immune rejection, and ethical concerns regarding the use of embryonic stem cells[6]. Moreover, some stem cell types are difficult to obtain, and the donor’s age can significantly affect MSC functionality[7]. These challenges limit the broader application of MSC therapies and have spurred ongoing efforts to improve their effectiveness.
Mechanical stimulation: MSCs can sense mechanical stimuli through integrin-focal adhesion complexes, mechanosensitive ion channels (such as Piezo1, transient receptor potential channels), and primary cilia. These stimuli activate pathways like Yes-associated protein/transcriptional co-activator with PDZ-binding motif, Wnt/β-catenin, and tran
Cyclic mechanical stretching (CMS) primarily affects MSCs by promoting osteogenesis, a process mediated through the activation of the Smad signaling pathway[17]. In mouse BMSCs CMS induces DNA demethylation by downregulating DNA methyltransferase 3 beta and upregulating Sonic hedgehog, activating the hedgehog pathway. In human BMSCs CMS activates the sirtuin 1-AMP-activated protein kinase pathway[18,19]. Animal models have also confirmed the po
Low-intensity pulsed ultrasound (LIPUS) is a promising preconditioning strategy for MSCs, generating cavitation, and mechanical and thermal effects. As a simple, noninvasive, and safe therapy, LIPUS has been widely used in orthopedic treatments, offering significant benefits for tendon-bone healing[24,25]. Research shows that LIPUS stimulates MSC pro
Extracorporeal shock wave (ESW) therapy applies compressive, tensile, and shear forces through transient pressure waves[29]. ESW has long been shown to promote tendon-bone healing with recent studies uncovering its underlying mechanisms[30]. ESW enhances BMSCs proliferation and osteogenic differentiation as well as osteogenic differentiation in TDSCs and ADSCs although their sensitivity varies: TDSCs > BMSCs > ADSCs[31,32]. Mechanistic studies indicate that ESW primarily influences the miR-138-focal adhesion kinase-ERK1/2 and reactive oxygen species-ERK1/2-bone morphogenetic protein 2 (BMP2)-Smad pathways, ultimately activating RUNX2[32,33].
Compression and fluid shear stress (FSS) can promote MSC osteogenic or chondrogenic differentiation with their effects depending on both intensity and duration. For example, MSC differentiation varies with compression levels. Human BMSCs undergo osteogenesis under 10% dynamic compression but switch to chondrogenesis at 15%[34]. Rapid FSS favors chondrogenesis while slow FSS promotes osteogenesis[35]. Additionally, intermittent FSS is more effective than continuous FSS in promoting osteogenesis, likely due to a resting period that facilitates cytoskeletal remodeling[36]. While these mechanical stimuli can theoretically enhance MSC function, their specific effects on tendon-bone healing require further investigation.
Pharmacological stimulation: Various drugs have been shown to promote BMSCs osteogenic differentiation, presenting significant potential for tendon-bone healing. Secretory leukocyte protease inhibitor, total flavonoids of Rhizoma Drynariae, polyvinylpyrrolidone iodine, baicalein, and icariin all promote MSC osteogenic differentiation, thereby en
Other stimuli: Other stimuli also enhance MSC functionality. Dedifferentiation treatment promotes osteogenic differentiation through the Nanog/nuclear factor of activated T-cells 1/osterix pathway, improving healing[43]. Both Mg2+ and static magnetic fields stimulate MSC proliferation and osteogenic differentiation[44,45]. Hypoxia promotes MSC proliferation and chondrogenic differentiation although its effects on osteogenesis and adipogenesis remain debated[46]. These findings suggest additional strategies for MSC preconditioning (Table 1).
| Ref. | Pretreatment method | Cell type | Effects on stem cells | Mechanism | Animal model |
| Song et al[21], 2017 | Mechanical stimulation | Rabbit BMSCs | Promote proliferation and differentiation | Increase collagen I, collagen III, ALP, OPN, tenascin C, and tenomodulin expression | Rabbit ACLR model |
| Wang et al[20], 2023 | Mechanical stimulation | Mouse BMSCs | Promote chondrogenic differentiation | Stimulate macrophage polarization towards the M2 phenotype and secretion of TGF-β1 | Mouse ACLR model |
| Li et al[17], 2015 | Mechanical stimulation | Rat BMSCs | Inhibit adipogenic differentiation | Activate the TGFβ1/Smad2 signaling pathway | N/A |
| Kusuyama et al[26], 2014 | LIPUS | Mouse MSCs line | Inhibit adipogenic differentiation, promote osteogenic differentiation | Regulate the ROCK-Cot/Tpl2-MEK-ERK signaling pathway and PPARγ2 activity | N/A |
| Chen et al[27], 2023 | LIPUS | hUC-MSCs | Promote chondrogenic differentiation | Inhibit the TNF signaling pathway | Rat cartilage defect model |
| Wang et al[28], 2019 | LIPUS | Rat BMSCs | Promote chondrogenic differentiation | Inhibit autophagy | N/A |
| Zhao et al[29], 2021 | ESW | Human SCB-SPCs | Promote self-renewal | Activate the YAP/TAZ signaling pathway | Rabbit osteochondral defect model |
| Chen et al[31], 2017 | ESW | Rat BMSCs | Promote proliferation and osteogenic differentiation | Increase Col1, OSX, Runx2, and ALP expression | Rat femoral shaft bone defect model |
| Hu et al[32], 2016 | ESW | Human BMSCs, TDSCs, ADSCs | Promote osteogenic differentiation | Inhibit miR-138 to activate the FAK-ERK1/2-RUNX2 signaling pathway | Nude mouse bone induction model |
| Catalano et al[33], 2017 | ESW | Human ADSCs | Promote osteogenic differentiation | Activate the ROS-ERK1/2-BMP2-Smad-RUNX2 signaling pathway | N/A |
| Wu et al[37], 2022 | SLPI | Rat BMSCs | Promote migration and osteogenic differentiation | Upregulate Runx2, ALP, OCN, and OPN gene expression | Rat ACLR model |
| Han et al[38], 2024 | TFRD | Mouse BMSCs | Promote vitality and osteogenic differentiation | Activate ERR1/2-Gga1-TGF-β/MAPK pathway | Rat ACLR model |
| Zhang et al[39], 2017 | PVP-I | Rabbit BMSCs | Promote osteogenic differentiation | Increase the expression of BMP-2 and OPN | Rabbit ACLR model |
| Tian et al[40], 2018 | Baicalein | Rat TDSCs | Promote osteogenic differentiation | Activate the Wnt/β-catenin signaling pathway | Rat calcaneus-Achilles tendon injury model |
| Wang et al[41], 2016 | Icariin | Mouse MSCs | Promote osteogenic differentiation | Activate the Wnt/β-catenin signaling pathway | Mouse calvarial osteolysis model |
| Alipanah-Moghadam et al[42], 2023 | Andrographolide | Rat BMSCs | Increase cell resistance to environmental stress | Induce the expression of HO-1 | N/A |
| Tie et al[43], 2021 | Dedifferentiated | Rabbit BMSCs | Promote osteogenic differentiation | Activate the Nanog/NFATc1/OSX signaling pathway | Rabbit ACLR model |
| Díaz-Tocados et al[44], 2017 | Mg2+ | Rat BMSCs | Promote proliferation and osteogenic differentiation | Activate the Notch1 signaling pathway | Rat femur decellularized scaffold |
| Kim et al[45], 2015 | Static magnetic fields | Human BMSCs | Promote proliferation and osteogenic differentiation | Upregulate ALP, BSP-2, COL1A1, OCN, ON, OPN, OSX, and RUNX2 gene expression | N/A |
Conventional biological scaffolds: Biomaterials can serve as delivery vehicles for MSCs, enhancing their functionality to accelerate tendon-bone healing. For example, 3D-printed poly lactic-co-glycolic acid scaffolds loaded with BMSCs and biomimetic hydroxyapatite gradient scaffolds with human umbilical cord-derived MSCs have shown positive results[47,48]. Calcium silicate nanoparticles-modified natural fish scale biomaterials promote BMSCs and TSPC differentiation by activating the BMP-2/Smad/Runx2 pathway[49]. Additionally, superparamagnetic iron oxide-labeled BMSCs seeded in a biphasic scaffold under a magnetic field improve cell distribution, seeding efficiency, and chondrogenesis through the CDR1as/miR-7/fibroblast growth factor 2 pathway[50].
Hydrogel-based materials: Hydrogel materials are excellent drug carriers due to their prolonged drug retention and high drug-loading efficiency[51]. Hydrogels encapsulating stem cells are widely used in regenerative medicine, reducing cell membrane damage during injection and extending in vivo retention time[52]. A four-layered hydrogel made of UV-crosslinked gelatin/hyaluronic acid, nanoclay, and BMSCs can mimic the natural entheses structure, promoting fibrocartilage regeneration and inhibiting fat infiltration in a rat RCT model[53]. Combining ADSCs with hydrogels, such as fibrin or methacrylated gelatin, or loading ADSCs with platelet-rich plasma in extracellular matrix (ECM) hydrogels has also shown promising repair results[54,55].
Natural biomaterials: Natural biomaterials, such as ECM, cell sheet technology, demineralized bone matrix, and fibrin glue, are increasingly being studied for tendon-bone healing due to their advantages over synthetic materials. ECM provides excellent biocompatibility, bioactivity, and biosafety[56], enhancing MSC proliferation, osteogenic differentiation, and promoting osteoinductive factors in macrophages[57-59]. Cell sheet technology retains cell-to-cell connections and ECM structure, making it highly applicable in regenerative medicine[60]. Cell sheets derived from various sources, including CD34+ cells from the anterior cruciate ligament, periosteal progenitor cells, BMSCs, urine-derived stem cells, ADSCs, TDSCs, and ligament-derived stem cells, have shown promise in tendon-bone healing[61-67]. However, the combination of MSCs with demineralized bone matrix or fibrin glue still requires further validation as some studies in rat RCT models and patients with RCT did not show significant therapeutic benefits[68,69] (Table 2).
| Ref. | Biomaterial | Material type | Cell type | Functions | Model |
| Chen et al[47], 2020 | 3D-printed PLGA scaffolds | Conventional biological scaffolds | Rabbit BMSCs | Support cell growth and differentiation | Rabbit RCT model |
| Yea et al[48], 2020 | Hydroxyapatite-gradient scaffold | Conventional biological scaffolds | hUC-MSCs | Support cell adhesion, migration, and proliferation, promoting osteogenic and chondrogenic differentiation | Rat RCT model |
| Han et al[49], 2023 | CS-FS | Conventional biological scaffolds | Rabbit BMSCs and TSPCs | Enhance cell differentiation and activity, maintaining the phenotype | Rat and rabbit RCT models |
| Zhang et al[50], 2024 | Magnetically seeded biphasic scaffold | Conventional biological scaffolds | SPIO-BMSCs | Increase cell seeding efficiency, promote cell distribution and concentration, and enhance chondrogenic differentiation | Rat RCT model |
| Ji et al[53], 2023 | Cocktail-like gradient gelatin/hyaluronic acid | Hydrogel-based materials | Rat BMSCs | Simulate natural gradient structure, support long-term cell culture and embedding, promote cell growth and differentiation | Rat RCT model |
| Rothrauff et al[54], 2019 | Fibrin or GelMA | Hydrogel-based materials | Rat ADSC | Promote chondrogenic differentiation | Rat RCT model |
| McGoldrick et al[55], 2017 | ECM hydrogel | Hydrogel-based materials | Rat ADSC | Better biocompatibility, enhance repair efficacy | Rat calcaneus-Achilles tendon injury model |
| Shekaran et al[58], 2016 | ECM | Natural biomaterials | Human BMSCs | Promote cell proliferation and osteogenic differentiation | Mouse ectopic mineralization model |
| Deng et al[59], 2021 | ECM | Natural biomaterials | Human BMSCs | Promote macrophage secretion of osteoinductive factors, enhance osteogenic differentiation | Mouse femoral defect model |
| Mifune et al[61], 2013 | Cell sheets | Natural biomaterials | Human ACL-derived CD34+ cell | Increase proprioceptive recovery, graft maturation, and biomechanical strength | Rat ACLR model |
| Chang et al[62], 2012 | Cell sheets | Natural biomaterials | Rabbit PPCs | Maintain cell differentiation capacity, promote fibrocartilage formation | Rabbit ACLR model |
| Tang et al[63], 2020 | Cell sheets combined with acellular scaffolds | Natural biomaterials | Rabbit BMSCs | Promote cell differentiation, enhance new bone and fibrocartilage formation | Rabbit patella-patellar tendon injury model |
| Chen et al[64], 2020 | Cell sheets | Natural biomaterials | Canine USCs | Promote fibrocartilage formation, increase trabecular thickness and biomechanical strength | Canine RCT model |
| Matsumoto et al[65], 2021 | Cell sheets | Natural biomaterials | Human ADSCs | Bone tunnel narrowing, increased biomechanical strength | Rabbit ACLR model |
| Yao et al[66], 2023 | Cell sheets | Natural biomaterials | Rat TDSCs | Enhance bone formation and angiogenesis, regulate macrophage polarization and MMP/TIMP expression | Rat ACLR model |
| Wei et al[67], 2023 | Cell sheets | Natural biomaterials | LDSCs with BMP-2/TGF-β1 gene insertion | Promote osteogenic and tenogenic differentiation, improve biomechanical strength, enhance tissue maturation, inhibit bone tunnel widening | Mouse ACLR model |
Overall, most biomaterials provide stable support for MSCs, enhancing their survival, preventing leakage, and promoting adhesion, proliferation, and differentiation, thereby improving tendon-bone healing quality[70]. Synthetic scaffolds offer high mechanical strength, aiding early tendon-bone repair, but their degradation rate may not align with tissue regeneration, and their degradation products can affect the microenvironment[71]. Hydrogels with their flexibility are ideal for stem cell and drug delivery, allowing control over mechanical properties and drug release with emerging bone-targeted applications[72]. While natural materials offer excellent biocompatibility, they suffer from rapid degradation, poor processability, and lower mechanical strength compared to synthetic scaffolds[71].
Gene modification techniques enhance tendon-bone healing by improving stem cell functions, providing an innovative approach to tissue regeneration with significant clinical potential. For example, lentiviral vectors overexpressing Runx1 and vascular endothelial growth factor (VEGF) A promote osteogenic and chondrogenic differentiation of BMSCs, enhance proliferation, and inhibit miR-205-5p expression, improving healing in rat ACLR and RCT models[73,74]. Adenoviral vectors overexpressing BMP-12, BMP-2, and basic fibroblast growth factor promote tenogenic differentiation, BMSC proliferation, and osteogenic differentiation, enhancing healing in rabbit RCT and ACLR models[75,76]. Additionally, adenoviral overexpression of calcitonin gene-related peptide activates the cAMP/PKA/cAMP response element-binding protein/JUNB pathway, promoting osteogenesis and upregulating Sonic hedgehog expression, improving healing in a mouse ACLR model[77].
With the rapid development of CRISPR-Cas9 gene editing, stem cell research has advanced significantly due to its precision and efficiency[78]. For instance, CRISPR interference to knock down the BMP-2 antagonist Noggin or upregulate Wnt10b and forkhead box c2 enhances osteogenic differentiation of ADSCs and BMSCs, promoting bone regeneration in rat cranial defect models[79,80]. CRISPR-Cas9 overexpression of BMP-2 and VEGF in tonsil-derived MSCs, combined with vitamin D-incorporated poly lactic-co-glycolic acid scaffolds, promotes osteogenesis, angiogenesis, and macrophage M2 polarization, aiding bone regeneration in rat models[81]. Recent studies have shown that injecting CRISPR-engineered BMSCs with SRY-box transcription factor 9 activation and RelA (also known as p65) suppression into the joint cavity promotes cartilage formation and reduces osteoarthritis progression[82]. Furthermore, combining bioinformatics and artificial intelligence can help identify key gene targets to enhance MSC functionality, improving gene editing efficiency and effectiveness. However, using CRISPR/Cas9 to enhance MSC function for tendon-bone healing may pose risks such as off-target effects and immune reactions. These risks can be minimized by designing precise single-guide RNAs and utilizing non-viral delivery systems, such as nanoparticles, to reduce immunogenicity[83]. Currently, CRISPR/Cas9 research in tendon-bone healing remains limited. Targeting key genes or pathways, or using MSC-derived exosomes as nanocarriers for CRISPR/Cas9 delivery, may be more effective strategies for tendon-bone regeneration.
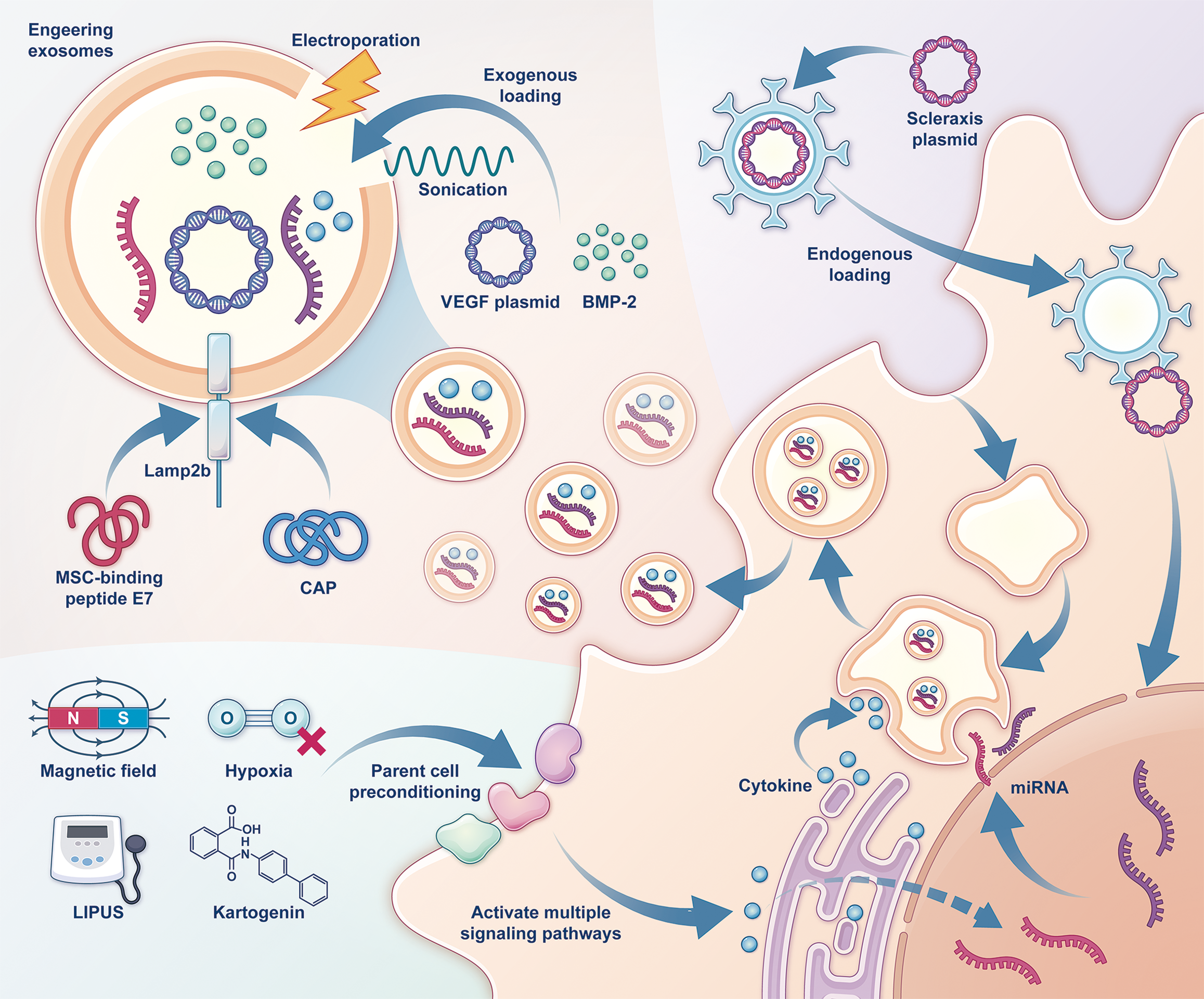
Natural exosomes: MSC-derived exosomes have garnered attention as a promising cell-free therapeutic strategy, with their bioactive substances considered key mediators of stem cell efficacy in various diseases and tendon-bone healing[14]. BMSC-derived exosomes (BMSCs-Exos) and ADSC-derived exosomes promote BMSC proliferation, migration, osteogenic, and chondrogenic differentiation, demonstrating comparable therapeutic effects[84]. BMSCs-Exos also enhance angiogenesis and promote M1 to M2 macrophage polarization via miR-23a-3p targeting interferon regulatory factor 1[85,86]. ADSC-derived exosomes improve the histological characteristics of torn human supraspinatus tendons by enhancing AMP-activated protein kinase signaling and inhibiting Wnt/β-catenin activity[87]. Furthermore, infrapatellar fat pad-derived MSC exosomes show promising healing effects in rat ACLR models[88]. Recent studies also suggest that TSPC-derived exosomes are highly effective in promoting tendon-bone healing[89]. While exosomes have lower immunogenicity than stem cells, challenges such as low yield, poor stability, and limited targeting remain. Currently, research is focused on engineering strategies to improve their therapeutic efficacy[1] (Figure 3).
Parent cell preconditioning: Preconditioning methods not only enhance MSCs but also improve the bioactivity of their derived exosomes. For instance, hypoxia-preconditioned BMSCs-Exos accelerate healing in rat ACLR models by stimulating bone formation and angiogenesis while kartogenin-preconditioned BMSCs-Exos promote collagen maturation and cartilage formation, improving recovery in rat RCT models[90,91]. Magnetically preconditioned BMSCs-Exos using iron oxide nanoparticles and a magnetic field enhance healing in rat ACLR models by boosting miR-21-5p secretion and activating the Smad pathway[92]. LIPUS preconditioning increases miR-140 levels in BMSCs-Exos, promoting chondrogenic differentiation and suppressing adipogenic differentiation, thus improving healing in mouse RCT models[93]. Additionally, lyophilized human umbilical cord stem cell exosomes improve storage and usability while maintaining efficacy[94].
Exosomes delivery platforms: Exosomes with their inherent therapeutic effects can also be engineered as multifunctional drug delivery nanocarriers. Due to their high biocompatibility, exosomes are promising tools for precisely delivering bioactive substances to injured sites, extending therapeutic duration, and supporting mechanistic studies with broad application potential[1,95].
Endogenous loading involves introducing therapeutic molecules into parent cells before exosome generation[1]. For example, scleraxis-overexpressing platelet-derived growth factor receptor alpha (+) BMSCs-Exos secrete more miR-6924-5p, which inhibits osteoclast formation and promotes Achilles tendon healing in mice[96]. Exogenous loading on the other hand involves directly introducing therapeutic molecules into isolated exosomes, a simpler and commonly used method for developing delivery systems[1]. For instance, loading exosomes with BMP-2 or VEGF plasmids promotes bone regeneration with VEGF-loaded exosomes also enhancing angiogenesis[97,98].
A key research focus is engineering exosomes to target specific cells as targeted delivery increases therapeutic concentration and reduces side effects[99]. Lysosome-associated membrane protein 2b, one of the isoforms of lysosome-associated membrane protein 2, is widely used in exosome engineering[99,100]. By fusing cell-targeting peptides, such as human papillomavirus E7 peptide or cartilage affinity peptide, to lysosome-associated membrane protein 2b, exosomes can efficiently deliver cargo to target sites[99]. For example, exosomes targeting synovial fluid-derived MSCs or chondrocytes combined with kartogenin or matrix metalloproteinases 13 small interfering RNA can help alleviate osteoarthritis progression[101,102]. While research on targeted exosomes in tendon-bone healing is still limited, these studies offer valuable insights for future tendon-bone repair strategies, such as developing pH-targeted exosomes to deliver cytokines or microRNA to enhance healing at inflammation sites.
Currently, MSC therapy for tendon-bone healing focuses on improving delivery efficiency, enhancing therapeutic efficacy, and reducing immunogenicity. To improve delivery, combining MSCs with biomaterials such as biological scaffolds and hydrogels helps regulate their release and degradation, ensuring sustained action at the injury site. For enhanced efficacy, strategies like biomaterial support, mechanical stimulation, drug treatments, and gene editing promote MSC functions like proliferation, differentiation, migration, and anti-inflammatory properties, leading to improved healing outcomes. To reduce immunogenicity, MSC-derived exosomes help minimize the immunogenic risks associated with allogeneic MSCs, increasing safety (Table 3).
| Limitations | Strategies | Functions |
| Low delivery efficiency | Biomaterials | Sustained release of MSCs, reduced degradation, preventing cell leakage, and prolonging retention at the injury site |
| Limited direct differentiation potential | Preconditioning/gene modification | Enhancing MSC differentiation towards bone, cartilage, tendon, and other tissues |
| Limited cell functionality | Preconditioning/gene modification | Boosting MSC proliferation, migration, angiogenesis, and immune modulation capabilities |
| Immunogenicity | Exosomes | Acellular therapies that eliminate cellular immunogenicity |
Despite these advances MSC therapy still faces challenges. First, during culture MSCs inevitably undergo phenotypic, functional, and genetic changes that may lead to functional decline and potential tumorigenic risks[15]. Using autologous or allogeneic cells and reducing passage numbers can help minimize these risks[103]. The donor’s age also affects therapeutic efficacy with MSCs from younger donors showing better in vitro proliferation and differentiation potential[104]. Additionally, large-scale MSC expansion for exosome extraction can cause aging-related functional decline. Recent studies suggest replacing fetal bovine serum with human platelet lysate may help address this issue[105]. Furthermore, adhering to good manufacturing practices, regulatory frameworks, and ethical considerations remains a challenge[106].
Future research should combine strategies to overcome these limitations and improve therapeutic outcomes. Investigating new preconditioning methods will provide deeper insights into MSC biology. The use of bioinformatics and artificial intelligence for targeted gene editing could further enhance MSC efficacy. Engineering MSC-derived exosomes for cell-free therapies can help avoid immunogenic risks while improving treatment effectiveness. Exploring new biomaterials could enhance the precise delivery and local retention of MSCs and exosomes. In conclusion, advancing research into MSC optimization strategies will help address the challenges of tendon-bone healing.
| 1. | Qin B, Bao D, Liu Y, Zeng S, Deng K, Liu H, Fu S. Engineered exosomes: a promising strategy for tendon-bone healing. J Adv Res. 2024;64:155-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Xu Y, Zhang WX, Wang LN, Ming YQ, Li YL, Ni GX. Stem cell therapies in tendon-bone healing. World J Stem Cells. 2021;13:753-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 3. | Chen W, Sun Y, Gu X, Cai J, Liu X, Zhang X, Chen J, Hao Y, Chen S. Conditioned medium of human bone marrow-derived stem cells promotes tendon-bone healing of the rotator cuff in a rat model. Biomaterials. 2021;271:120714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 4. | He W, Jiang C, Zhou P, Hu X, Gu X, Zhang S. Role of tendon-derived stem cells in tendon and ligament repair: focus on tissue engineer. Front Bioeng Biotechnol. 2024;12:1357696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Chen Z, Jin M, He H, Dong J, Li J, Nie J, Wang Z, Xu J, Wu F. Mesenchymal stem cells and macrophages and their interactions in tendon-bone healing. J Orthop Translat. 2023;39:63-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 6. | Khan SI, Ahmed N, Ahsan K, Abbasi M, Maugeri R, Chowdhury D, Bonosi L, Brunasso L, Costanzo R, Iacopino DG, Umana GE, Chaurasia B. An Insight into the Prospects and Drawbacks of Stem Cell Therapy for Spinal Cord Injuries: Ongoing Trials and Future Directions. Brain Sci. 2023;13:1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Hao ZC, Wang SZ, Zhang XJ, Lu J. Stem cell therapy: a promising biological strategy for tendon-bone healing after anterior cruciate ligament reconstruction. Cell Prolif. 2016;49:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Spalazzi JP, Gallina J, Fung-Kee-Fung SD, Konofagou EE, Lu HH. Elastographic imaging of strain distribution in the anterior cruciate ligament and at the ligament-bone insertions. J Orthop Res. 2006;24:2001-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Qin Y, Ge G, Yang P, Wang L, Qiao Y, Pan G, Yang H, Bai J, Cui W, Geng D. An Update on Adipose-Derived Stem Cells for Regenerative Medicine: Where Challenge Meets Opportunity. Adv Sci (Weinh). 2023;10:e2207334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 142] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 10. | Tan Q, Lui PP, Rui YF, Wong YM. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng Part A. 2012;18:840-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (19)] |
| 11. | Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 516] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 12. | Ren S, Lin Y, Liu W, Yang L, Zhao M. MSC-Exos: Important active factor of bone regeneration. Front Bioeng Biotechnol. 2023;11:1136453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 13. | Zhu P, Tan H, Gao H, Wang J, Liu Y, Yang D, Wu T. Potential Mechanism and Perspectives of Mesenchymal Stem Cell Therapy for Ischemic Stroke: A Review. Glob Med Genet. 2024;11:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Zou J, Yang W, Cui W, Li C, Ma C, Ji X, Hong J, Qu Z, Chen J, Liu A, Wu H. Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon-bone healing. J Nanobiotechnology. 2023;21:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 202] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 15. | Jiang Y, Zhang P, Zhang X, Lv L, Zhou Y. Advances in mesenchymal stem cell transplantation for the treatment of osteoporosis. Cell Prolif. 2021;54:e12956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 16. | Sun Y, Wan B, Wang R, Zhang B, Luo P, Wang D, Nie JJ, Chen D, Wu X. Mechanical Stimulation on Mesenchymal Stem Cells and Surrounding Microenvironments in Bone Regeneration: Regulations and Applications. Front Cell Dev Biol. 2022;10:808303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 17. | Li R, Liang L, Dou Y, Huang Z, Mo H, Wang Y, Yu B. Mechanical stretch inhibits mesenchymal stem cell adipogenic differentiation through TGFβ1/Smad2 signaling. J Biomech. 2015;48:3665-3671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Wang C, Shan S, Wang C, Wang J, Li J, Hu G, Dai K, Li Q, Zhang X. Mechanical stimulation promote the osteogenic differentiation of bone marrow stromal cells through epigenetic regulation of Sonic Hedgehog. Exp Cell Res. 2017;352:346-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Chen X, Yan J, He F, Zhong D, Yang H, Pei M, Luo ZP. Mechanical stretch induces antioxidant responses and osteogenic differentiation in human mesenchymal stem cells through activation of the AMPK-SIRT1 signaling pathway. Free Radic Biol Med. 2018;126:187-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Wang L, Li S, Xiao H, Zhang T, Liu Y, Hu J, Xu D, Lu H. TGF-β1 derived from macrophages contributes to load-induced tendon-bone healing in the murine rotator cuff repair model by promoting chondrogenesis. Bone Joint Res. 2023;12:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Song F, Jiang D, Wang T, Wang Y, Chen F, Xu G, Kang Y, Zhang Y. Mechanical Loading Improves Tendon-Bone Healing in a Rabbit Anterior Cruciate Ligament Reconstruction Model by Promoting Proliferation and Matrix Formation of Mesenchymal Stem Cells and Tendon Cells. Cell Physiol Biochem. 2017;41:875-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Wang W, Zheng X, Wang H, Zuo B, Chen S, Li J. Mechanical Unloading Promotes Osteoclastic Differentiation and Bone Resorption by Modulating the MSC Secretome to Favor Inflammation. Cell Transplant. 2024;33:9636897241236584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 23. | Shi Y, Li H, Zhang X, Fu Y, Huang Y, Lui PP, Tang T, Dai K. Continuous cyclic mechanical tension inhibited Runx2 expression in mesenchymal stem cells through RhoA-ERK1/2 pathway. J Cell Physiol. 2011;226:2159-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Xia P, Shi Y, Wang X, Li X. Advances in the application of low-intensity pulsed ultrasound to mesenchymal stem cells. Stem Cell Res Ther. 2022;13:214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 25. | Chen C, Zhang T, Liu F, Qu J, Chen Y, Fan S, Chen H, Sun L, Zhao C, Hu J, Lu H. Effect of Low-Intensity Pulsed Ultrasound After Autologous Adipose-Derived Stromal Cell Transplantation for Bone-Tendon Healing in a Rabbit Model. Am J Sports Med. 2019;47:942-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Kusuyama J, Bandow K, Shamoto M, Kakimoto K, Ohnishi T, Matsuguchi T. Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway. J Biol Chem. 2014;289:10330-10344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 27. | Chen Y, Yang H, Wang Z, Zhu R, Cheng L, Cheng Q. Low-intensity pulsed ultrasound promotes mesenchymal stem cell transplantation-based articular cartilage regeneration via inhibiting the TNF signaling pathway. Stem Cell Res Ther. 2023;14:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 28. | Wang X, Lin Q, Zhang T, Wang X, Cheng K, Gao M, Xia P, Li X. Low-intensity pulsed ultrasound promotes chondrogenesis of mesenchymal stem cells via regulation of autophagy. Stem Cell Res Ther. 2019;10:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Zhao Z, Wang Y, Wang Q, Liang J, Hu W, Zhao S, Li P, Zhu H, Li Z. Radial extracorporeal shockwave promotes subchondral bone stem/progenitor cell self-renewal by activating YAP/TAZ and facilitates cartilage repair in vivo. Stem Cell Res Ther. 2021;12:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Wang L, Qin L, Lu HB, Cheung WH, Yang H, Wong WN, Chan KM, Leung KS. Extracorporeal shock wave therapy in treatment of delayed bone-tendon healing. Am J Sports Med. 2008;36:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Chen Y, Xu J, Huang Z, Yu M, Zhang Y, Chen H, Ma Z, Liao H, Hu J. An Innovative Approach for Enhancing Bone Defect Healing Using PLGA Scaffolds Seeded with Extracorporeal-shock-wave-treated Bone Marrow Mesenchymal Stem Cells (BMSCs). Sci Rep. 2017;7:44130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Hu J, Liao H, Ma Z, Chen H, Huang Z, Zhang Y, Yu M, Chen Y, Xu J. Focal Adhesion Kinase Signaling Mediated the Enhancement of Osteogenesis of Human Mesenchymal Stem Cells Induced by Extracorporeal Shockwave. Sci Rep. 2016;6:20875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Catalano MG, Marano F, Rinella L, de Girolamo L, Bosco O, Fortunati N, Berta L, Frairia R. Extracorporeal shockwaves (ESWs) enhance the osteogenic medium-induced differentiation of adipose-derived stem cells into osteoblast-like cells. J Tissue Eng Regen Med. 2017;11:390-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Michalopoulos E, Knight RL, Korossis S, Kearney JN, Fisher J, Ingham E. Development of methods for studying the differentiation of human mesenchymal stem cells under cyclic compressive strain. Tissue Eng Part C Methods. 2012;18:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Lu J, Fan Y, Gong X, Zhou X, Yi C, Zhang Y, Pan J. The Lineage Specification of Mesenchymal Stem Cells Is Directed by the Rate of Fluid Shear Stress. J Cell Physiol. 2016;231:1752-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Liu L, Yu B, Chen J, Tang Z, Zong C, Shen D, Zheng Q, Tong X, Gao C, Wang J. Different effects of intermittent and continuous fluid shear stresses on osteogenic differentiation of human mesenchymal stem cells. Biomech Model Mechanobiol. 2012;11:391-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Wu Y, Shao Y, Xie D, Pan J, Chen H, Yao J, Liang J, Ke H, Cai D, Zeng C. Effect of secretory leucocyte protease inhibitor on early tendon-to-bone healing after anterior cruciate ligament reconstruction in a rat model. Bone Joint Res. 2022;11:503-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 38. | Han L, Wang C, Wang T, Hu Y, Wang H. Total flavonoids of Rhizoma drynariae improves tendon-bone healing for anterior cruciate ligament reconstruction in mice and promotes the osteogenic differentiation of bone mesenchymal stem cells by the ERR1/2-Gga1-TGF-β/MAPK pathway. Environ Toxicol. 2024;39:106-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 39. | Zhang P, Zhi Y, Fang H, Wu Z, Chen T, Jiang J, Chen S. Effects of polyvinylpyrrolidone-iodine on tendon-bone healing in a rabbit extra-articular model. Exp Ther Med. 2017;13:2751-2756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Tian X, Jiang H, Chen Y, Ao X, Chen C, Zhang W, He F, Liao X, Jiang X, Li T, Zhang Z, Zhang X. Baicalein Accelerates Tendon-Bone Healing via Activation of Wnt/β-Catenin Signaling Pathway in Rats. Biomed Res Int. 2018;2018:3849760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Wang J, Tao Y, Ping Z, Zhang W, Hu X, Wang Y, Wang L, Shi J, Wu X, Yang H, Xu Y, Geng D. Icariin attenuates titanium-particle inhibition of bone formation by activating the Wnt/β-catenin signaling pathway in vivo and in vitro. Sci Rep. 2016;6:23827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Alipanah-Moghadam R, Khodaei M, Aghamohammadi V, Malekzadeh V, Afrouz M, Nemati A, Zahedian H. Andrographolide induced heme oxygenase-1 expression in MSC-like cells isolated from rat bone marrow exposed to environmental stress. Biochem Biophys Res Commun. 2023;687:149212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Tie K, Cai J, Qin J, Xiao H, Shangguan Y, Wang H, Chen L. Nanog/NFATc1/Osterix signaling pathway-mediated promotion of bone formation at the tendon-bone interface after ACL reconstruction with De-BMSCs transplantation. Stem Cell Res Ther. 2021;12:576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Díaz-Tocados JM, Herencia C, Martínez-Moreno JM, Montes de Oca A, Rodríguez-Ortiz ME, Vergara N, Blanco A, Steppan S, Almadén Y, Rodríguez M, Muñoz-Castañeda JR. Magnesium Chloride promotes Osteogenesis through Notch signaling activation and expansion of Mesenchymal Stem Cells. Sci Rep. 2017;7:7839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 45. | Kim EC, Leesungbok R, Lee SW, Lee HW, Park SH, Mah SJ, Ahn SJ. Effects of moderate intensity static magnetic fields on human bone marrow-derived mesenchymal stem cells. Bioelectromagnetics. 2015;36:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 46. | Yang Y, Lee EH, Yang Z. Hypoxia-Conditioned Mesenchymal Stem Cells in Tissue Regeneration Application. Tissue Eng Part B Rev. 2022;28:966-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 47. | Chen P, Cui L, Fu SC, Shen L, Zhang W, You T, Ong TY, Liu Y, Yung SH, Jiang C. The 3D-Printed PLGA Scaffolds Loaded with Bone Marrow-Derived Mesenchymal Stem Cells Augment the Healing of Rotator Cuff Repair in the Rabbits. Cell Transplant. 2020;29:963689720973647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Yea JH, Bae TS, Kim BJ, Cho YW, Jo CH. Regeneration of the rotator cuff tendon-to-bone interface using umbilical cord-derived mesenchymal stem cells and gradient extracellular matrix scaffolds from adipose tissue in a rat model. Acta Biomater. 2020;114:104-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 49. | Han F, Li T, Li M, Zhang B, Wang Y, Zhu Y, Wu C. Nano-calcium silicate mineralized fish scale scaffolds for enhancing tendon-bone healing. Bioact Mater. 2023;20:29-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 50. | Zhang C, Jin JL, Zhou CH, Ruan CX, Lei PF, Cai YZ. Magnetic Seeding of SPIO-BMSCs Into a Biphasic Scaffold Can Promote Tendon-Bone Healing After Rotator Cuff Repair. Am J Sports Med. 2024;52:1707-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 51. | Yang C, Teng Y, Geng B, Xiao H, Chen C, Chen R, Yang F, Xia Y. Strategies for promoting tendon-bone healing: Current status and prospects. Front Bioeng Biotechnol. 2023;11:1118468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 52. | Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A. 2012;18:806-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 534] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 53. | Ji W, Han F, Feng X, Shi L, Ma H, Lu Y, Tao R. Cocktail-like gradient gelatin/hyaluronic acid bioimplant for enhancing tendon-bone healing in fatty-infiltrated rotator cuff injury models. Int J Biol Macromol. 2023;244:125421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 54. | Rothrauff BB, Smith CA, Ferrer GA, Novaretti JV, Pauyo T, Chao T, Hirsch D, Beaudry MF, Herbst E, Tuan RS, Debski RE, Musahl V. The effect of adipose-derived stem cells on enthesis healing after repair of acute and chronic massive rotator cuff tears in rats. J Shoulder Elbow Surg. 2019;28:654-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 55. | McGoldrick R, Chattopadhyay A, Crowe C, Chiou G, Hui K, Farnebo S, Davis C, Le Grand A, Jacobs M, Pham H, Chang J. The Tissue-Engineered Tendon-Bone Interface: In Vitro and In Vivo Synergistic Effects of Adipose-Derived Stem Cells, Platelet-Rich Plasma, and Extracellular Matrix Hydrogel. Plast Reconstr Surg. 2017;140:1169-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Gu R, Liu H, Zhu Y, Liu X, Wang S, Liu Y. Is extracellular matrix (ECM) a promising scaffold biomaterial for bone repair? Histol Histopathol. 2021;36:1219-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 57. | Datta N, Holtorf HL, Sikavitsas VI, Jansen JA, Mikos AG. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials. 2005;26:971-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 218] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 58. | Shekaran A, Lam A, Sim E, Jialing L, Jian L, Wen JT, Chan JK, Choolani M, Reuveny S, Birch W, Oh S. Biodegradable ECM-coated PCL microcarriers support scalable human early MSC expansion and in vivo bone formation. Cytotherapy. 2016;18:1332-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 59. | Deng M, Tan J, Dai Q, Luo F, Xu J. Macrophage-Mediated Bone Formation in Scaffolds Modified With MSC-Derived Extracellular Matrix Is Dependent on the Migration Inhibitory Factor Signaling Pathway. Front Cell Dev Biol. 2021;9:714011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | You Q, Lu M, Li Z, Zhou Y, Tu C. Cell Sheet Technology as an Engineering-Based Approach to Bone Regeneration. Int J Nanomedicine. 2022;17:6491-6511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 61. | Mifune Y, Matsumoto T, Takayama K, Terada S, Sekiya N, Kuroda R, Kurosaka M, Fu FH, Huard J. Tendon graft revitalization using adult anterior cruciate ligament (ACL)-derived CD34+ cell sheets for ACL reconstruction. Biomaterials. 2013;34:5476-5487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 62. | Chang CH, Chen CH, Liu HW, Whu SW, Chen SH, Tsai CL, Hsiue GH. Bioengineered periosteal progenitor cell sheets to enhance tendon-bone healing in a bone tunnel. Biomed J. 2012;35:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 63. | Tang Y, Chen C, Liu F, Xie S, Qu J, Li M, Li Z, Li X, Shi Q, Li S, Li X, Hu J, Lu H. Structure and ingredient-based biomimetic scaffolds combining with autologous bone marrow-derived mesenchymal stem cell sheets for bone-tendon healing. Biomaterials. 2020;241:119837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 64. | Chen Y, Xu Y, Li M, Shi Q, Chen C. Application of Autogenous Urine-Derived Stem Cell Sheet Enhances Rotator Cuff Healing in a Canine Model. Am J Sports Med. 2020;48:3454-3466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 65. | Matsumoto T, Sato Y, Kobayashi T, Suzuki K, Kimura A, Soma T, Ito E, Kikuchi T, Kobayashi S, Harato K, Niki Y, Matsumoto M, Nakamura M, Miyamoto T. Adipose-Derived Stem Cell Sheets Improve Early Biomechanical Graft Strength in Rabbits After Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2021;49:3508-3518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 66. | Yao S, Liang Z, Lee YW, Yung PSH, Lui PPY. Bioactive Decellularized Tendon-Derived Stem Cell Sheet for Promoting Graft Healing After Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2023;51:66-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 67. | Wei B, Li Z, Lin Y, Hu X, Xu L, Wang S, Ji M, Lu J. BMP-2/TGF-β1 gene insertion into ligament-derived stem cells sheet promotes tendon-bone healing in a mouse. Biotechnol J. 2023;18:e2200470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 68. | Thangarajah T, Sanghani-Kerai A, Henshaw F, Lambert SM, Pendegrass CJ, Blunn GW. Application of a Demineralized Cortical Bone Matrix and Bone Marrow-Derived Mesenchymal Stem Cells in a Model of Chronic Rotator Cuff Degeneration. Am J Sports Med. 2018;46:98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 69. | Kim YS, Sung CH, Chung SH, Kwak SJ, Koh YG. Does an Injection of Adipose-Derived Mesenchymal Stem Cells Loaded in Fibrin Glue Influence Rotator Cuff Repair Outcomes? A Clinical and Magnetic Resonance Imaging Study. Am J Sports Med. 2017;45:2010-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 70. | Gao C, Peng S, Feng P, Shuai C. Bone biomaterials and interactions with stem cells. Bone Res. 2017;5:17059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 502] [Cited by in RCA: 408] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 71. | Mao Z, Fan B, Wang X, Huang X, Guan J, Sun Z, Xu B, Yang M, Chen Z, Jiang D, Yu J. A Systematic Review of Tissue Engineering Scaffold in Tendon Bone Healing in vivo. Front Bioeng Biotechnol. 2021;9:621483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 72. | Zhang H, Wu S, Chen W, Hu Y, Geng Z, Su J. Bone/cartilage targeted hydrogel: Strategies and applications. Bioact Mater. 2023;23:156-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 78] [Reference Citation Analysis (0)] |
| 73. | Kang K, Geng Q, Cui L, Wu L, Zhang L, Li T, Zhang Q, Gao S. Upregulation of Runt related transcription factor 1 (RUNX1) contributes to tendon-bone healing after anterior cruciate ligament reconstruction using bone mesenchymal stem cells. J Orthop Surg Res. 2022;17:266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 74. | Xu Q, Sun WX, Zhang ZF. High expression of VEGFA in MSCs promotes tendon-bone healing of rotator cuff tear via microRNA-205-5p. Eur Rev Med Pharmacol Sci. 2019;23:4081-4088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 75. | Chen P, Cui L, Chen G, You T, Li W, Zuo J, Wang C, Zhang W, Jiang C. The application of BMP-12-overexpressing mesenchymal stem cells loaded 3D-printed PLGA scaffolds in rabbit rotator cuff repair. Int J Biol Macromol. 2019;138:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 76. | Chen B, Li B, Qi YJ, Ni QB, Pan ZQ, Wang H, Chen LB. Enhancement of tendon-to-bone healing after anterior cruciate ligament reconstruction using bone marrow-derived mesenchymal stem cells genetically modified with bFGF/BMP2. Sci Rep. 2016;6:25940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 77. | Zhao X, Wu G, Zhang J, Yu Z, Wang J. Activation of CGRP receptor-mediated signaling promotes tendon-bone healing. Sci Adv. 2024;10:eadg7380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 78. | Zhou H, Ye P, Xiong W, Duan X, Jing S, He Y, Zeng Z, Wei Y, Ye Q. Genome-scale CRISPR-Cas9 screening in stem cells: theories, applications and challenges. Stem Cell Res Ther. 2024;15:218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 79. | Hsu MN, Yu FJ, Chang YH, Huang KL, Pham NN, Truong VA, Lin MW, Kieu Nguyen NT, Hwang SM, Hu YC. CRISPR interference-mediated noggin knockdown promotes BMP2-induced osteogenesis and calvarial bone healing. Biomaterials. 2020;252:120094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 80. | Hsu MN, Huang KL, Yu FJ, Lai PL, Truong AV, Lin MW, Nguyen NTK, Shen CC, Hwang SM, Chang YH, Hu YC. Coactivation of Endogenous Wnt10b and Foxc2 by CRISPR Activation Enhances BMSC Osteogenesis and Promotes Calvarial Bone Regeneration. Mol Ther. 2020;28:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 81. | Park SY, Lee JK, Lee SH, Kim DS, Jung JW, Kim JH, Baek SW, You S, Hwang DY, Han DK. Multifunctional vitamin D-incorporated PLGA scaffold with BMP/VEGF-overexpressed tonsil-derived MSC via CRISPR/Cas9 for bone tissue regeneration. Mater Today Bio. 2024;28:101254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 82. | Zhao L, Lai Y, Jiao H, Li J, Lu K, Huang J. CRISPR-mediated Sox9 activation and RelA inhibition enhance cell therapy for osteoarthritis. Mol Ther. 2024;32:2549-2562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 83. | Li C, Du Y, Zhang T, Wang H, Hou Z, Zhang Y, Cui W, Chen W. "Genetic scissors" CRISPR/Cas9 genome editing cutting-edge biocarrier technology for bone and cartilage repair. Bioact Mater. 2023;22:254-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 84. | Tan X, Xiao H, Yan A, Li M, Wang L. Effect of Exosomes From Bone Marrow-Derived Mesenchymal Stromal Cells and Adipose-Derived Stromal Cells on Bone-Tendon Healing in a Murine Rotator Cuff Injury Model. Orthop J Sports Med. 2024;12:23259671231210304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 85. | Huang Y, He B, Wang L, Yuan B, Shu H, Zhang F, Sun L. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res Ther. 2020;11:496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 86. | Li Z, Li Q, Tong K, Zhu J, Wang H, Chen B, Chen L. BMSC-derived exosomes promote tendon-bone healing after anterior cruciate ligament reconstruction by regulating M1/M2 macrophage polarization in rats. Stem Cell Res Ther. 2022;13:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 91] [Reference Citation Analysis (38)] |
| 87. | Zhang X, Cai Z, Wu M, Huangfu X, Li J, Liu X. Adipose Stem Cell-Derived Exosomes Recover Impaired Matrix Metabolism of Torn Human Rotator Cuff Tendons by Maintaining Tissue Homeostasis. Am J Sports Med. 2021;49:899-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 88. | Xu J, Ye Z, Han K, Zheng T, Zhang T, Dong S, Jiang J, Yan X, Cai J, Zhao J. Infrapatellar Fat Pad Mesenchymal Stromal Cell-Derived Exosomes Accelerate Tendon-Bone Healing and Intra-articular Graft Remodeling After Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2022;50:662-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 89. | He Y, Lu S, Chen W, Yang L, Li F, Zhou P, Chen Z, Wan R, Zhang Z, Sun Y, Lin J, Chen Y, Luo Z, Xu C, Chen S. Exosomes derived from tendon stem/progenitor cells enhance tendon-bone interface healing after rotator cuff repair in a rat model. Bioact Mater. 2024;40:484-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 90. | Zhang T, Yan S, Song Y, Chen C, Xu D, Lu B, Xu Y. Exosomes secreted by hypoxia-stimulated bone-marrow mesenchymal stem cells promote grafted tendon-bone tunnel healing in rat anterior cruciate ligament reconstruction model. J Orthop Translat. 2022;36:152-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 91. | Cai J, Xu J, Ye Z, Wang L, Zheng T, Zhang T, Li Y, Jiang J, Zhao J. Exosomes Derived From Kartogenin-Preconditioned Mesenchymal Stem Cells Promote Cartilage Formation and Collagen Maturation for Enthesis Regeneration in a Rat Model of Chronic Rotator Cuff Tear. Am J Sports Med. 2023;51:1267-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 92. | Wu XD, Kang L, Tian J, Wu Y, Huang Y, Liu J, Wang H, Qiu G, Wu Z. Exosomes derived from magnetically actuated bone mesenchymal stem cells promote tendon-bone healing through the miR-21-5p/SMAD7 pathway. Mater Today Bio. 2022;15:100319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 93. | Wu B, Zhang T, Chen H, Shi X, Guan C, Hu J, Lu H. Exosomes derived from bone marrow mesenchymal stem cell preconditioned by low-intensity pulsed ultrasound stimulation promote bone-tendon interface fibrocartilage regeneration and ameliorate rotator cuff fatty infiltration. J Orthop Translat. 2024;48:89-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 94. | Lo HL, Lin SY, Ho CJ, Ming-Kung Y, Lu CC. Effect of lyophilized exosomes derived from umbilical cord stem cells on chronic anterior cruciate ligament cell injury. J Orthop Surg Res. 2024;19:554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 95. | Huang J, Xu Y, Wang Y, Su Z, Li T, Wu S, Mao Y, Zhang S, Weng X, Yuan Y. Advances in the Study of Exosomes as Drug Delivery Systems for Bone-Related Diseases. Pharmaceutics. 2023;15:220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 96. | Feng W, Jin Q, Ming-Yu Y, Yang H, Xu T, You-Xing S, Xu-Ting B, Wan C, Yun-Jiao W, Huan W, Ai-Ning Y, Yan L, Hong T, Pan H, Mi-Duo M, Gang H, Mei Z, Xia K, Kang-Lai T. MiR-6924-5p-rich exosomes derived from genetically modified Scleraxis-overexpressing PDGFRα(+) BMMSCs as novel nanotherapeutics for treating osteolysis during tendon-bone healing and improving healing strength. Biomaterials. 2021;279:121242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 97. | Yerneni SS, Adamik J, Weiss LE, Campbell PG. Cell trafficking and regulation of osteoblastogenesis by extracellular vesicle associated bone morphogenetic protein 2. J Extracell Vesicles. 2021;10:e12155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 98. | Zha Y, Li Y, Lin T, Chen J, Zhang S, Wang J. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics. 2021;11:397-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 99. | Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183-3195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 1078] [Article Influence: 215.6] [Reference Citation Analysis (0)] |
| 100. | Qiao L, Hu J, Qiu X, Wang C, Peng J, Zhang C, Zhang M, Lu H, Chen W. LAMP2A, LAMP2B and LAMP2C: similar structures, divergent roles. Autophagy. 2023;19:2837-2852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 101. | Xu X, Liang Y, Li X, Ouyang K, Wang M, Cao T, Li W, Liu J, Xiong J, Li B, Xia J, Wang D, Duan L. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 280] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 102. | Zhang H, Yan W, Wang J, Xie S, Tao WA, Lee CW, Zhang X, Zhang G, Liu Y, Wei D, Hu J, Liu H, Liu F, Nie Y, Chen X, Xu H, Xia J, Wang S. Surface functionalization of exosomes for chondrocyte-targeted siRNA delivery and cartilage regeneration. J Control Release. 2024;369:493-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 103. | Margiana R, Markov A, Zekiy AO, Hamza MU, Al-Dabbagh KA, Al-Zubaidi SH, Hameed NM, Ahmad I, Sivaraman R, Kzar HH, Al-Gazally ME, Mustafa YF, Siahmansouri H. Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Res Ther. 2022;13:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 270] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 104. | Uefuji A, Matsumoto T, Matsushita T, Ueha T, Zhang S, Kurosaka M, Kuroda R. Age-Related Differences in Anterior Cruciate Ligament Remnant Vascular-Derived Cells. Am J Sports Med. 2014;42:1478-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 105. | Zhang Y, Song J, Wang B, Wen Y, Jiang W, Zhang YL, Li ZL, Yu H, Qin SF, Lv LL, Tang TT, Liu BC. Comprehensive Comparison of Extracellular Vesicles Derived from Mesenchymal Stem Cells Cultured with Fetal Bovine Serum and Human Platelet Lysate. ACS Nano. 2025;19:12366-12381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 106. | Tang J, Shen P, Wu X, Chen M, Xu H. Stem cell-derived exosomes: a potential therapeutic strategy for enhancing tendon stem/progenitor cells function in tendon-bone healing. J Orthop Surg Res. 2025;20:658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/