INTRODUCTION
Irritable bowel syndrome (IBS) is a chronic functional disease of the digestive system caused by factors such as genetics, diet structure, gut microbiota, and mild intestinal inflammation[1,2]. The diagnosis of IBS is primarily made based on the symptoms, which typically include recurrent abdominal pain, diarrhea, changes in bowel habits, and abnormal stool characteristics[3-5]. IBS can be categorized into four types based on clinical manifestations: Diarrhea type, constipation type, mixed type, and uncertain type[6]. Various epidemiological surveys have indicated that the global prevalence of IBS ranges from 5% to 25%. In China, the prevalence of IBS is between 5.6% and 11.5%[7]. Diarrheal IBS (IBS-D) is the most common subtype in the country, accounting for 31.5% of all IBS cases[2].
Research on intestinal function in patients with IBS primarily focuses on intestinal inflammation, barrier function, and the composition of intestinal flora. Studies have shown that intestinal dysfunction and imbalance in flora are common in patients with IBS[8-11]. An increasing number of studies have suggested that mesenchymal stem cells (MSCs) possess significant anti-inflammatory and immunomodulatory properties, which could aid in tissue repair[12-14]. MSCs could promote the proliferation and differentiation of T regulatory cells while inhibiting the activity of inflammatory T cells by secreting multiple anti-inflammatory mediators, including tumor necrosis factor (TNF)-stimulating gene 6, prostaglandin E2, indoleamine 2,3-dioxygenase, and transforming growth factor beta, all of which help reduce intestinal mucosal inflammation[15-17]. Additionally, MSCs have been found to inhibit inflammatory factors TNF-α and interleukin-6 (IL-6) and enhance the release of the anti-inflammatory cytokine IL-10[18,19]. Furthermore, they could colonize the intestinal mucosa, repair the damaged tissue, and reshape the diversity and abundance of the damaged intestinal flora[20,21]. For instance, MSCs promote the secretion of prostaglandin E2 by expressing cyclooxygenase, which helps maintain the intestinal barrier function[22]. In addition, MSCs enhance the survival and proliferation of intestinal epithelial cells, upregulate the expression of tight junction-related molecules in intestinal epithelial cells, and support the barrier function[23,24]. Notably, human umbilical cord MSCs (hUC-MSCs) can reshape the composition and diversity of intestinal flora by increasing the levels of Akkermansia, Faecalibaculum, and others, thereby improving colon inflammation and reshaping the T cell immune homeostasis[25]. These observations have been confirmed in several clinical trials focused on inflammatory bowel disease[26].
Using rat models, this study explored the therapeutic effects of hUC-MSCs on IBS-D based on three main aspects: Intestinal inflammation, intestinal barrier function, and intestinal flora. Additionally, it verified the ability of hUC-MSCs to colonize the intestinal tissue.
MATERIALS AND METHODS
Isolation, culture, and fluorescence labeling of hUC-MSCs
The umbilical cords were donated by mothers after providing written informed consent for scientific research purposes. These umbilical cords were cut into 1-2 cm pieces and further shredded into tissue blocks of 1-2 mm3. Then these tissue blocks were cultured in Dulbecco’s Modified Eagle Medium (DMEM)/F12 containing 10% fetal bovine serum (FBS) (ART No. 04-001-1ACS; Biological Industries, CT, United States). The resulting tissue blocks were stored at 37 °C in a 5% CO2 humidified incubator. Once the cells began to migrate, the medium was renewed every 2 days to ensure continuous cell growth. When the cultures reached 80%-90% confluence, a passage culture was performed using 0.25% trypsin-ethylenediamine tetraacetic acid solution. Subsequently, stem cell characterization and functional analyses were performed to assess the phenotype and multidifferentiation potential of MSCs. The MSCs with good growth conditions were saved for later use.
Next, the MSCs were washed twice with phosphate-buffered saline (PBS) for 3 minutes each time after digestion. The cells were suspended in DMEM without any FBS. Chloromethyl dialkylcarbocyanine (CM-Dil; excitation wavelength = 553 nm, emission wavelength = 570 nm) was added to achieve a final concentration of 1 μg/mL, allowing us to detect the cell location. The mixture was first incubated at 37 °C for 5 minutes and then at 4 °C for 15 minutes. Next, the cells were centrifuged at 800 rpm/minute for 5 minutes, after which the supernatant was discarded and the cells were washed twice with PBS. Next, the MSCs were resuspended in a fresh medium and a small sample was inoculated onto a cover glass. They were fixed with Carnoy’s fixative solution after the cells attached to the wall. The cells were rinsed with PBS for 5 minutes and then treated with Hoechst dye solution at room temperature for 15 minutes. After another three rinses with PBS, each lasting 5 minutes, the MSCs were observed under a fluorescence microscope. The labeled cells accounted for over 80% of all cells.
Animal model
The work has been reported in line with the ARRIVE guidelines 2.0. All methods used in this study were carried out in strict accordance with relevant ethical guidelines and regulations to ensure accurate research practices. Sixty specific pathogen-free grade male Sprague-Dawley rats, aged 5-6 weeks, and weighing 180 ± 20 g, were kept in a controlled barrier animal facility. The indoor temperature was maintained between 22 °C and 26 °C, with relative humidity levels of 40%-70%. The rats were housed in groups of three or four per cage, with a 12-hour alternating light and dark cycle. They were provided with a standard, quantitative diet and had free access to drinking water. Bedding materials were changed daily. Animal quality was inspected and the animal quality certificate (No. 422019600000326) was obtained.
The rat model was induced using a combination of acetic acid and binding stress[27,28]. As reported, the rats were anesthetized with ether inhalation on day 1 and subsequently inserted into a silicone tube (8 cm away from the anus) connected to a syringe. After injecting 1 mL of 40 mg/L of acetic acid into the colon, the silicone tube was slowly removed. The anus was then pressed by hand, and the tail was raised for 30 seconds. Following this procedure, the colon was rinsed with 1 mL of 0.01 mol/L of PBS, and the rats were allowed to recover on day 6.
From day 7 to day 9, the rats underwent restraint stress. During this time, they were placed in a special transparent cylindrical cylinder that limited their limb movement but did not compromise their breathing for 30 minutes each day over the course of three days. On day 10, the colorectal dilation method was utilized to confirm the successful establishment of the animal model. Colorectal dilation method included measuring the abdominal wall withdrawal reflex (AWR) score[29], the Bristol fecal score[30], and the fecal water content. A successful establishment of the IBS-D rat model was indicated by an AWR score of 2 or above, a Bristol fecal score of 5 or above, and high fecal water content[31].
Experimental design
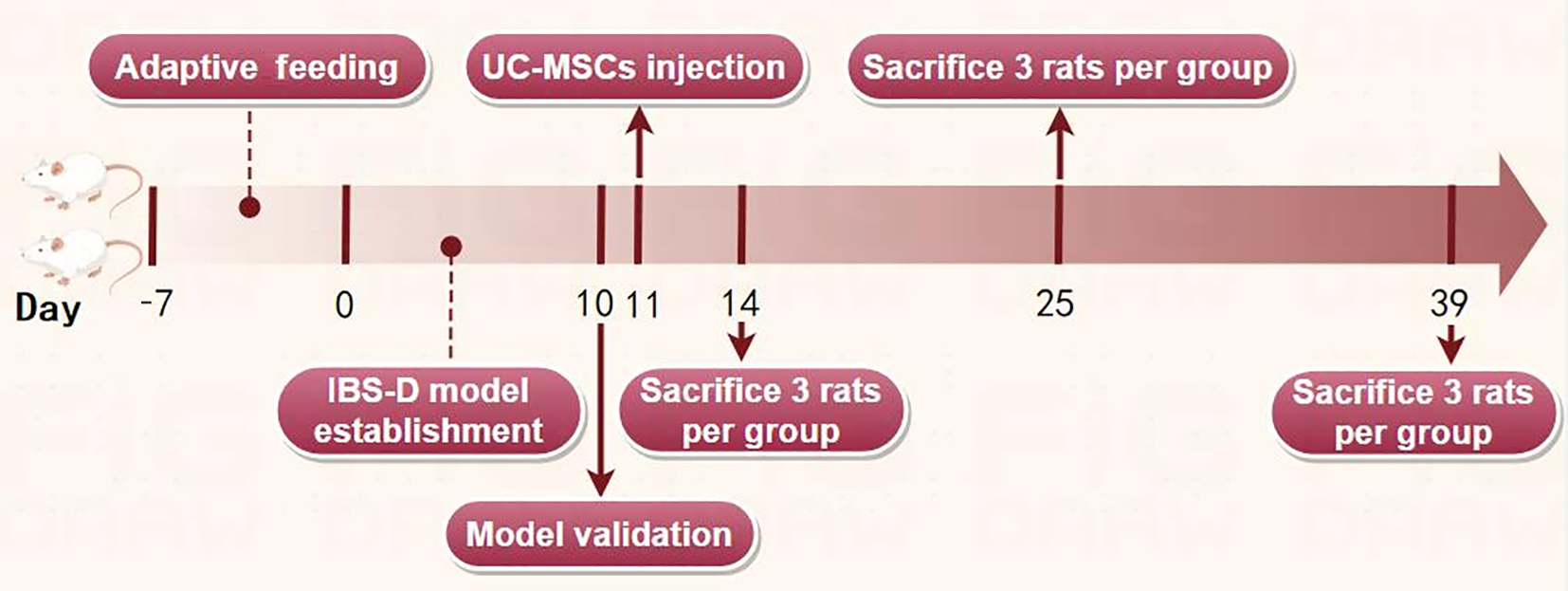
After a 7-day period of adaptive feeding, the aforementioned 60 rats were randomly divided into five groups: Control, model, low-dose, medium-dose, and high-dose, with 12 rats per group. Following the modeling phase, rats in low-dose, medium-dose, and high-dose groups received a single tail-vein injection of CM-Dil-labeled hUC-MSCs, at doses of 1 × 106 cells, 3 × 106 cells, and 6 × 106 cells, respectively, on day 11. The control and model groups were injected with 0.9% sodium chloride solution at the same volume. Three rats from each group were euthanized on days 14, 25, and 39, respectively. When euthanizing the rats, their weights (in grams) were accurately recorded. The rats were anesthetized with an intraperitoneal injection of a 3% pentobarbital sodium solution. After the rats were fully anesthetized (approximately 3-5 minutes), their blood was collected through the abdominal aorta via an abdominal incision. The colons of the rats were also collected. A detailed timeline of the procedure is depicted in Figure 1.
Figure 1 Experimental time point and the corresponding treatment of rats.
IBS-D: Diarrheal irritable bowel syndrome; UC-MSCs: Umbilical cord mesenchymal stem cells.
Rat weight and stool
After the experimental treatment, the rats were weighed daily. The Bristol stool score was used to evaluate the stool characteristics and calculate the fecal water content. The following specific operations were performed: Feces from each group were collected within 2 hours on days 10, 14, 25, and 39, which were then baked at 200 °C for 30 minutes. Changes in stool weight and fecal water content before and after baking were measured. The fecal water content was calculated as follows: Fecal water content = (fecal wet weight - fecal dry weight)/fecal wet weight × 100%. The AWR score was employed to evaluate visceral sensitivity using the method outlined in a previous report[29].
Hematoxylin and eosin staining and hUC-MSC tissue distribution
Three rats from each group were euthanized on days 14, 25, and 39. After dissection, their colon tissues were sliced and stained using hematoxylin and eosin (H&E) staining. The morphology and structure of the colon tissues were examined under a light microscope. Additionally, colon tissue sections were sealed with the fluorescent dye DAPI, allowing for the observation of the distribution of hUC-MSCs in the colon tissue under a fluorescence microscope.
Enzyme-linked immunosorbent assay and western blotting
The serum levels of TNF-α, IL-1β, and IL-6 in each group were detected through enzyme-linked immunosorbent assay. Western blot analysis was conducted to detect the expression levels of the intestinal barrier function-related proteins occludin and zonula occludens-1 (ZO-1). ZO-1 (PAC262Ra01; Cloud-Clone, Wuhan, China), occludin (PAC228Ra01; Cloud-Clone), and GAPDH (PAB932Hu01; Cloud-Clone) were used as the primary antibodies, whereas anti-mouse immunoglobulin G antibody (SAA544Rb19; Cloud-Clone) was employed as the secondary antibody.
Enteric microorganisms
DNA was extracted from 200 mg of stool with the help of the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) and stored at -80 °C before analysis. The sequencing platform Illumina was used to sequence the 16S rRNA of the samples. The primer sequence was as follows: Forward primer: 5’-ACTCCTACGGGAGGCAGCA-3’, reverse primer: 5’-GGACTACHVGGGTWTCTAAT-3’. The original high-throughput sequencing data were managed by conducting the QIIME2 (2019.04) analysis for sequence denoising or operational taxonomic unit (OTU) clustering. The R language was used for analyzing the species composition.
Statistical analyses
Descriptive and analytical statistics were performed using GraphPad Prism version 8. The data are presented as the means ± standard deviations with at least three replicates for each measurement. One-way analysis of variance was used for multiple comparisons, along with t-tests to evaluate significant differences. P < 0.05 was considered statistically significant.
RESULTS
hUC-MSCs improved the body weight of rats
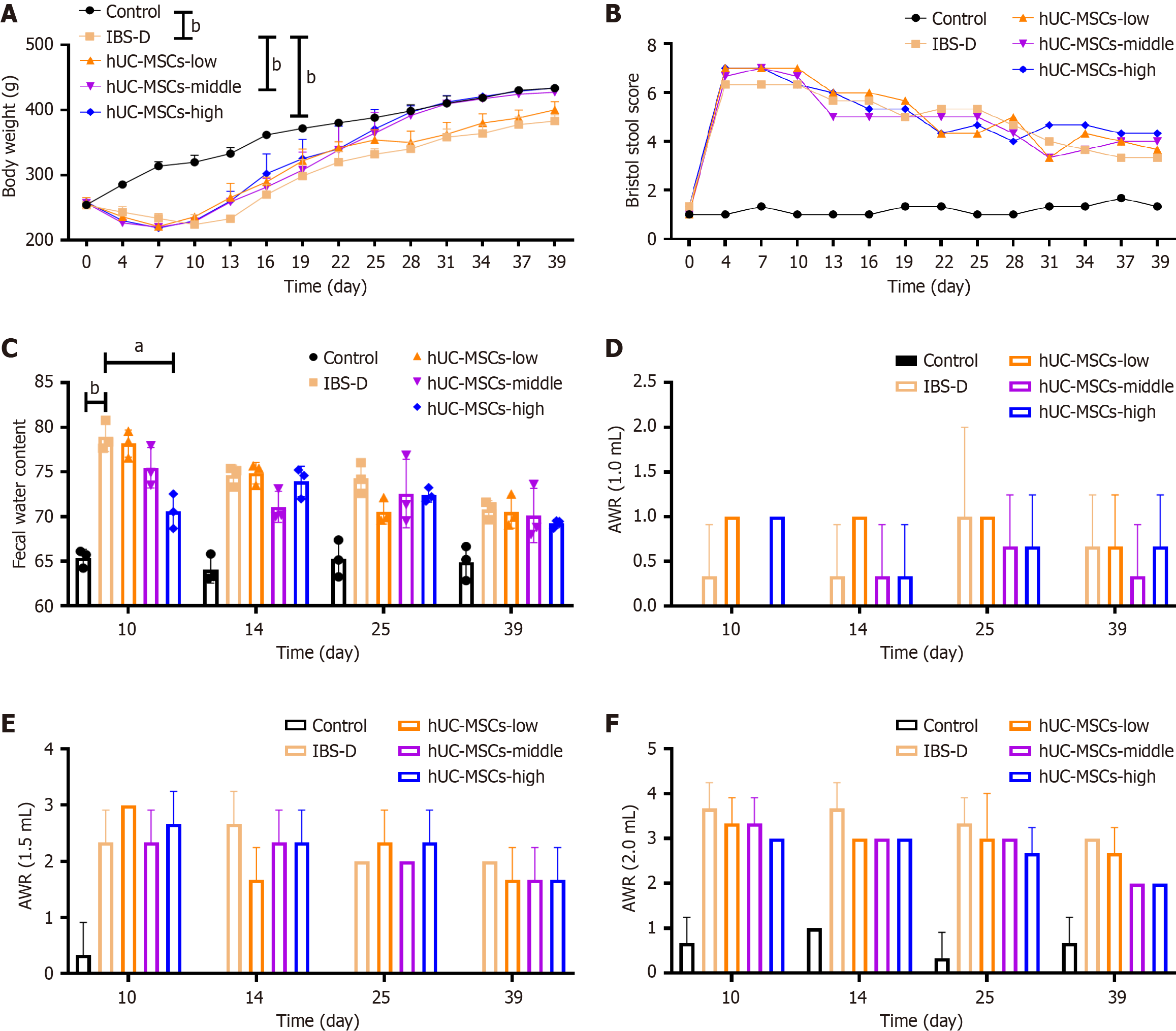
The model group for IBS-D experienced significant weight loss within the first 7 days. By day 28, both the middle and high doses of hUC-MSCs restored the body weight of the rats to the levels comparable to that of the control group (Figure 2A). The Bristol stool score did not show any significant improvement in fecal traits at different doses of hUC-MSCs compared to those of the IBS-D model group (Figure 2B). The fecal water content also did not demonstrate significant changes; however, as the hUC-MSC dose was increased and the treatment duration was prolonged, a noticeable downward trend in the fecal water content compared to that of the IBS-D model group was observed (Figure 2C). The AWR score indicated that hUC-MSCs did not cause any effective improvement in intestinal sensitivity (Figure 2D-F). Nevertheless, under high-intensity stimulation conditions (2.0 mL), the AWR score tended to decrease with higher doses of hUC-MSCs and longer duration of the treatment (Figure 2F).
Figure 2 Body weight, fecal status, and intestinal sensitivity in rats.
A: Body weight of rats; B: Bristol stool score of rats; C: Fecal water content of rats; D: Abdominal withdrawal reflex (AWR) score at 1.0 mL balloon expansion; E: AWR score at 1.5 mL balloon expansion; F: AWR score at 2.0 mL balloon expansion. hUC-MSCs: Human umbilical cord mesenchymal stem cells; IBS-D: Diarrheal irritable bowel syndrome. aP < 0.05, bP < 0.01.
hUC-MSCs reduce inflammation levels and improve intestinal barrier function
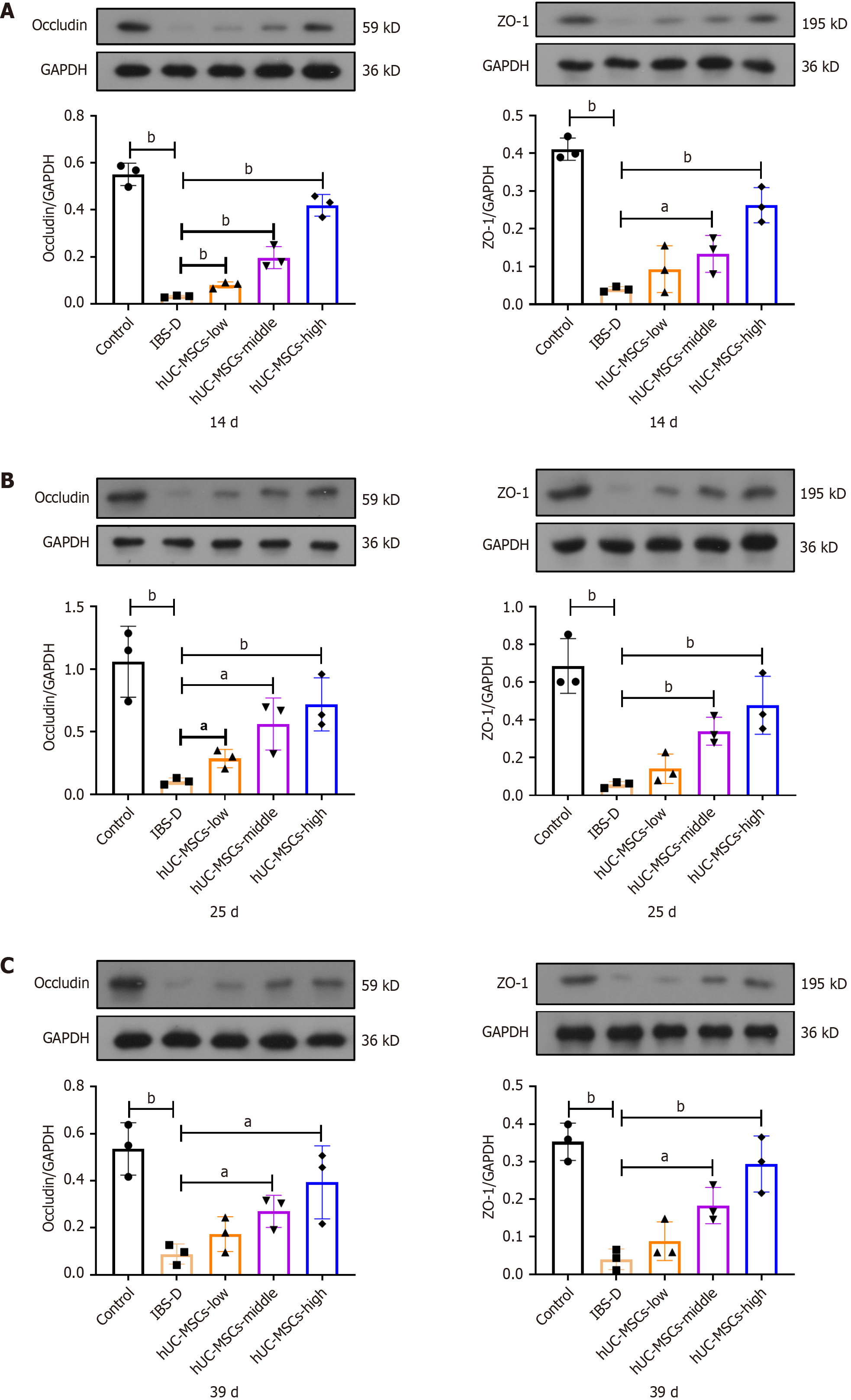
The expression levels of both TNF-α and IL-6 indicate that higher doses of hUC-MSCs are more effective at reducing intestinal inflammation, and this effect is quite significant (Figure 3A and B). Even though there was no notable difference among the various doses of hUC-MSCs, all doses successfully reduced the expression level of IL-1β (Figure 3C). The IBS-D model causes severe damage to intestinal barrier function, making recovery challenging within a certain time frame. By contrast, hUC-MSCs can effectively reverse the inhibition of occludin and ZO-1 expression, thereby significantly restoring intestinal barrier function. Moreover, the degree of recovery noticeably improves on increasing the doses of hUC-MSCs (Figure 4).
Figure 3 Serum level of inflammatory factors in rats.
A: Tumor necrosis factor alpha (TNF-α) level on days 14, 25, and 39 (n = 3); B: Interleukin-6 (IL-6) level on days 14, 25, and 39 (n = 3); C: IL-1β level on days 14, 25, and 39 (n = 3). hUC-MSCs: Human umbilical cord mesenchymal stem cells; IBS-D: Diarrheal irritable bowel syndrome. aP < 0.05, bP < 0.01.
Figure 4 Expression levels of occludin and zonula occludens-1.
A-C: Ratio of occludin and zonula occludens-1 (ZO-1) to glyceraldehyde-3-phosphate dehydrogenase expression on day 14 (A), day 25 (B), and day 39 (C) (n = 3). hUC-MSCs: Human umbilical cord mesenchymal stem cells; IBS-D: Diarrheal irritable bowel syndrome. aP < 0.05, bP < 0.01.
hUC-MSCs colonize colon tissue and relieve intestinal damage
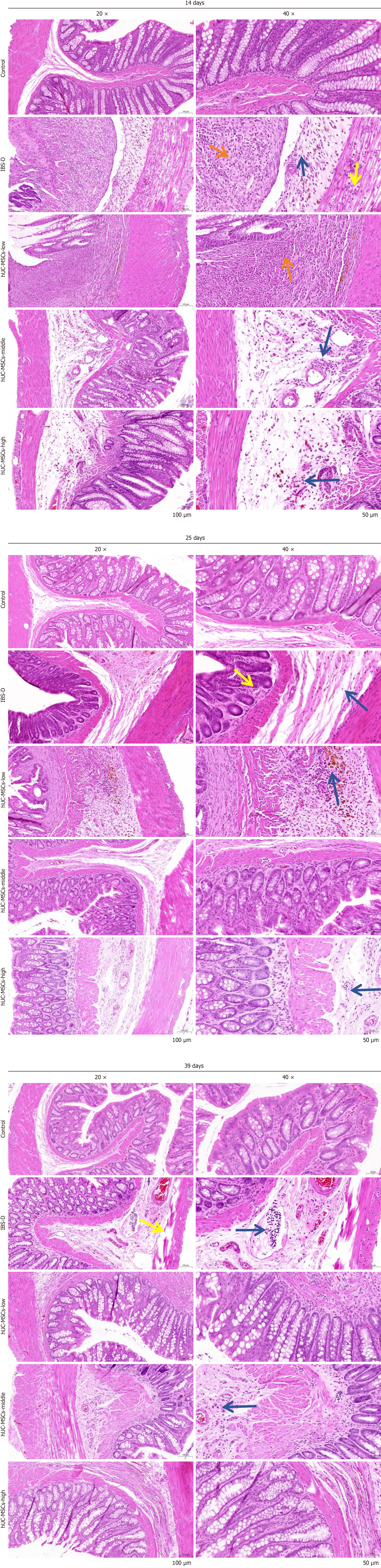
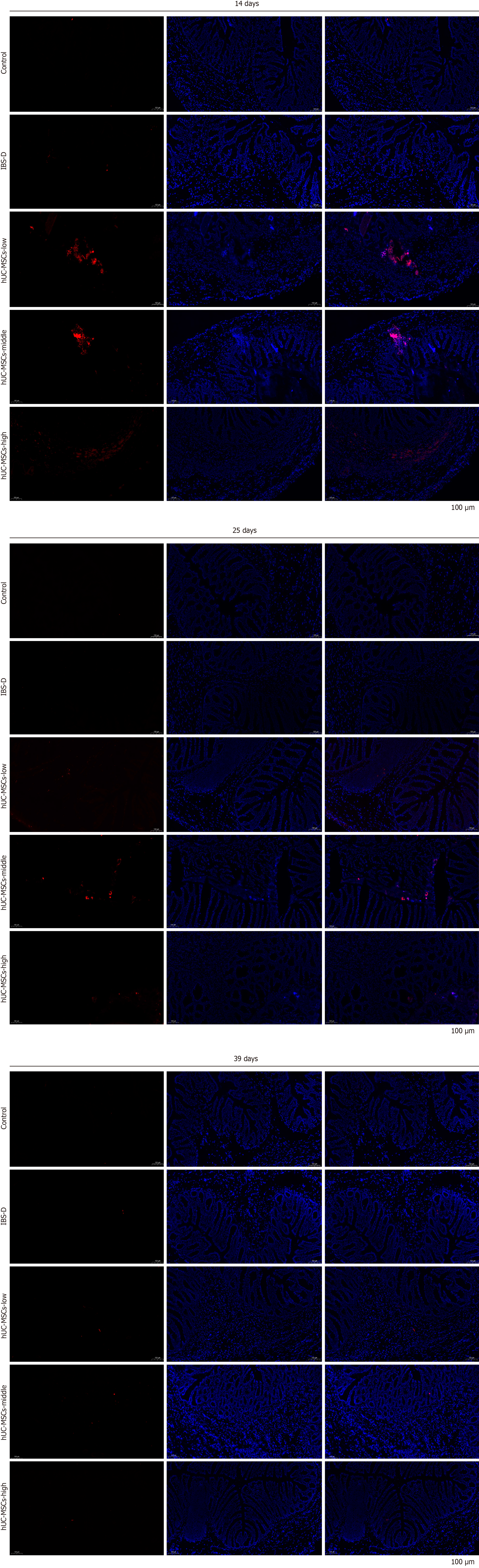
Similarly, H&E staining of colonic mucosal tissue indicated that stem cells could partially alleviate the injury caused in the IBS-D model. This was evidenced by a reduction in connective tissue hyperplasia (Figure 5). The use of a fluorescence labeling tracer for hUC-MSCs revealed the presence of stem cells in the colon tissue; however, their numbers decreased over time and were nearly undetectable by day 39 (Figure 6).
Figure 5 Morphology and structure of colon tissue with hematoxylin and eosin staining.
Morphology and structure of colon tissue of different groups on days 14, 25, and 39. Orange arrows indicate inflammatory cell infiltration; blue arrows indicate that the ulcer has invaded the submucosa and been replaced by proliferating the connective tissue; yellow arrows indicate loose edema of the inner annular muscle and the outer longitudinal muscle. hUC-MSCs: Human umbilical cord mesenchymal stem cells; IBS-D: Diarrheal irritable bowel syndrome.
Figure 6 Distribution of human umbilical cord mesenchymal stem cells in colon tissues.
Distribution of human umbilical cord mesenchymal stem cells (hUC-MSCs) in the colon tissue of different groups on days 14, 25, and 39. IBS-D: Diarrheal irritable bowel syndrome.
hUC-MSCs maintain the abundance and diversity of gut flora
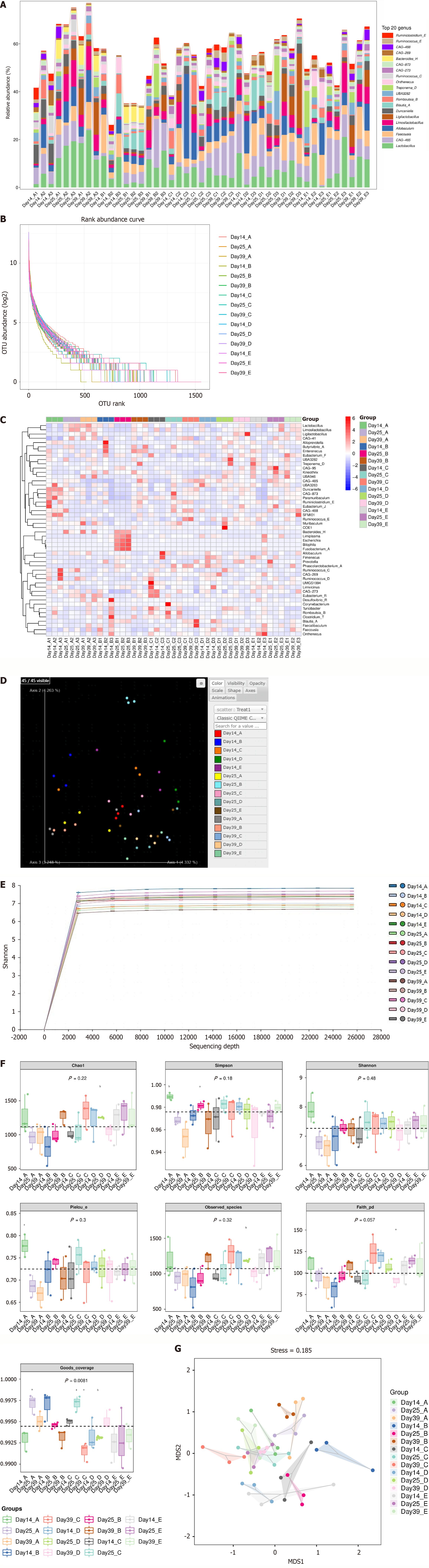
The percentage of generic taxonomic level composition in the IBS-D model group showed a decreasing trend, particularly on day 25 (Figure 7A). A similar result was observed in the abundance grade plot (Figure 7B and Supplementary Figure1). By contrast, the percentage of generic taxonomic levels in the three hUC-MSC groups remained at normal levels, indicating that hUC-MSCs can restore the vitality of intestinal flora and maintain normal levels. On day 25, the percentage of five strains - Bacteroides, Limiplasma, Escherichia, Bilophila, and Fusobacterium - significantly increased in the IBS-D model group but returned to normal levels by day 39. This phenomenon was not observed in the corresponding hUC-MSC group. Lactobacillus showed the opposite trend (Figure 7C and Supplementary Figure 2). Furthermore, principal component analysis revealed that the IBS-D model group on day 25 was positioned at the upper end, far from the other groups. The strain composition of the model groups on days 14 and 25 was primarily concentrated in the upper left corner, while the hUC-MSC group, control group, and day 39 model group were clustered in the lower right corner (Figure 7D). This pattern was consistent with the alpha diversity analysis results (Figure 7E), the beta diversity analysis results (Figure 7F), and non-metric multidimensional scaling results (Figure 7G). The statistical validation of differential taxa was performed with the linear discriminant analysis effect size analysis (Supplementary Figure 3).
Figure 7 Gut microbial abundance and diversity.
A: Histogram of genus-level species composition; B: Abundance grade plot; C: Heat map of genus-level species composition on days 14, 25, and 39 (three samples per group at each time point) for species clustering; D: Three-dimensional ordering diagram of samples by principal component analysis; E: Sparse curve graph of the alpha diversity index; F: Grouped box plot of the alpha diversity index; G: Two-dimensional sorting diagram by non-metric multidimensional scaling. In each picture, A: Control group; B: Diarrheal irritable bowel syndrome group; C: Human umbilical cord mesenchymal stem cell (hUC-MSC)-low group; D: HUC-MSC-middle group; E: HUC-MSC-high group; Day 14_A: Control group on day 14. aP < 0.05, bP < 0.01.
DISCUSSION
IBS is a common functional bowel disorder characterized by recurring abdominal pain, bloating, and abdominal discomfort, which significantly impacts patients’ quality of life[32]. Approximately 5%-17% of the general population worldwide is affected by this condition, although the prevalence varies by region. South America has the highest rates, while Asia has the lowest. The direct costs associated with IBS are estimated to be in the billions of dollars each year[33,34]. The underlying mechanisms of IBS are complex and not yet completely understood. Various factors such as genetics, diet, and gut microbiota have been identified as important risk factors. However, the influence of these factors can vary based on geographic and cultural contexts, that is, their relative significance may differ from one country to another[35]. In China, the most prevalent type of IBS is IBS-D, which accounts for approximately 31.5% of all IBS cases and primarily affects young and middle-aged individuals[2,7,36].
Weight loss is common in patients with IBS-D[37]. Similar outcomes have been noted in rats following modeling. However, both medium and high doses of hUC-MSCs were effective in restoring the weight of the rats over time. The low dose also demonstrated some recovery effects. Increased fecal water content is a common feature of patients with IBS-D, which, together with intestinal barrier dysfunction, leads to frequent diarrhea[38,39]. Previous studies have shown that tight junction proteins ZO-1 and occludin play a critical role in regulating the intestinal barrier function[40-42]. Although hUC-MSCs did not significantly change the Bristol fecal score and the fecal water content, the medium- and high-dose hUC-MSCs markedly improved the expression levels of both occludin and ZO-1. This improvement is significant for enhancing the intestinal physiological function and reducing diarrhea in rats. Visceral hypersensitivity is the main mechanism that causes abdominal pain in patients with IBS-D[43-45]. This condition is characterized by a lowered pain threshold in the colon during mechanical stimulation and heightened sensitivity to pain in both colorectal and somatic regions[46]. We found that the intestinal sensitivity of the rats significantly increased after modeling, while the alleviation of intestinal sensitivity by hUC-MSCs was not significant. We speculated that the mechanism of action of hUC-MSCs did not include this aspect, and there was no evidence suggesting that they could alleviate visceral hypersensitivity.
Intestinal inflammation is a common pathophysiological feature of IBS-D, and it is usually associated with intestinal mucosal tissue damage[47,48]. Our results showed that hUC-MSCs could reduce the expression levels of inflammatory cytokines TNF-α, IL-1β, and IL-6. H&E staining of colonic tissue indicated that the colonic mucosal tissue damage was partially restored following the infusion of hUC-MSCs. Additionally, the CM-Dil tracer experiment also displayed that hUC-MSCs could colonize the colon tissue. Therefore, we hypothesize that hUC-MSCs may facilitate tissue repair and inhibit inflammation by colonizing the injured site in the colon and utilizing their unique repair and paracrine functions. This conclusion was consistent with the results of previous studies[14,49-52].
In recent years, research on the intestinal flora has gained significant attention worldwide, with scientists linking it to various conditions, including cancer, obesity, type 2 diabetes, and cardiovascular disease[53-56]. Of course, gut flora also plays a crucial role in the pathogenesis of functional gastrointestinal diseases, particularly IBS[57-60]. Our study results indicated a decrease in the relative abundance of intestinal flora in the model group, which is particularly noticeable on day 25. Previous studies have also indicated that the diversity of intestinal flora reduced in patients with general intestinal diseases[57,58], which aligns with the results from day 14 (Supplementary Figure 1). In general, hUC-MSCs appeared to enhance the diversity and relative abundance of intestinal flora in rat models to some extent. Lactobacillus has been demonstrated to be crucial for maintaining the intestinal barrier function[61,62]. Similar to previous studies[63,64], our study also showed that Lactobacillus populations decreased in the model group on day 25 but recovered by day 39 in the hUC-MSC groups (Figure 7C, Supplementary Figures 2A and 3). We considered that hUC-MSCs contributed to the recovery of Lactobacillus populations, just as they could significantly reverse the decrease in the expressions of ZO-1 and occludin caused by the modeling process. Furthermore, the levels of Escherichia, Bacteroides, and Fusobacterium were significantly upregulated compared to that in the control group on day 25; however, the normal levels returned by day 39 in the hUC-MSC group (Figure 7C; Supplementary Figures 2B, 2D, 2F and 3). Generally, Fusobacterium was more common in patients with inflammatory bowel disease. Even colorectal adenoma and cancer[65-67], as well Escherichia[68], Bacteroides[69,70], and Bilophila[71], are more common in patients with IBS compared with healthy individuals. These findings strongly suggest that hUC-MSCs could relieve intestinal damage and inflammation by improving the number of beneficial bacteria and reducing that of harmful bacteria. Of course, the rat’s own recovery cannot be ignored, except for the role of hUC-MSCs. In the present study, both principal component analysis and non-metric multidimensional scaling analyses indicated that the samples from the day 25 model group had great difference with those of the other groups and relatively similar to the model and hUC-MSC groups on day 14.
This study demonstrated that hUC-MSCs can reduce the expression of inflammatory factors, enhance the population of intestinal barrier proteins, and regulate intestinal flora by colonizing the colon tissue and hence can be used to treat IBS-D. However, the study has some limitations as well. Due to the small sample size, the changes in relevant indicators were not significant both pre- and post-treatment. Additionally, there was a lack of clinical case verification for the treatment. We believe that increasing the number of animal samples in future studies will provide stronger experimental data to support the initiation of clinical trials for IBS-D. Furthermore, the study results suggested that hUC-MSCs could serve as potential cellular therapies for the treatment of IBS-D.