INTRODUCTION
Bone and joint diseases constitute a complex group of disorders characterized by degeneration of joint structures and impaired function[1]. These conditions exhibit diverse clinical manifestations and involve multiple pathogenic mechanisms. They are primarily defined by structural joint damage and functional disability, encompassing a range of pathological types, with osteoarthritis (OA), rheumatoid arthritis (RA), and gouty arthritis serving as representative examples[2,3]. These diseases are commonly associated with varying degrees of articular cartilage damage[4,5]. Given that chondrocytes are highly differentiated cells with limited proliferative capacity, and constitute only 5%-10% of the total cartilage mass, their ability to self-repair is significantly constrained. Furthermore, their localization within the extracellular matrix restricts their regenerative potential[6], making effective tissue repair after injury particularly challenging[7].
Currently, treatment strategies for bone and joint diseases can be broadly categorized into three approaches: Non-pharmacological, pharmacological, and surgical interventions[8]. Non-pharmacological therapies, including exercise programs, weight management, and physical therapy, are typically recommended for early-stage patients and may help delay disease progression[9]. Pharmacological treatments, such as non-steroidal anti-inflammatory drugs, are primarily aimed at alleviating pain, improving joint function, and enhancing quality of life[10]. For individuals with advanced disease and significant functional impairment, surgical options - such as arthroscopic debridement, microfracture surgery, or total joint replacement - are often considered[11]. However, these conventional treatments still face considerable limitations in terms of postoperative recovery, cartilage regeneration, and long-term therapeutic outcomes[12]. Consequently, the effective repair of articular cartilage defects has emerged as a critical clinical challenge requiring urgent global attention.
In recent years, stem cell therapy has gained prominence as a key area of research in regenerative medicine, showing significant potential for the repair of articular cartilage injuries[13]. Clinical studies have demonstrated that mesenchymal stem cells (MSCs), whether derived from autologous or allogeneic sources, exhibit favorable safety profiles in the treatment of OA[14]. These cells not only alleviate clinical symptoms but also show promising capacity in promoting cartilage regeneration. Under specific microenvironmental conditions, MSCs can differentiate into functional chondrocytes and secrete a variety of bioactive factors that regulate chondrocyte proliferation and apoptosis, thereby contributing to the maintenance of cartilage homeostasis and the facilitation of tissue repair[15].
Recent studies have demonstrated that the therapeutic effects of MSCs are primarily mediated through their paracrine signaling mechanisms. In comparison to direct stem cell transplantation, MSC-derived exosomes (MSC-Exos) offer enhanced biosafety, reduced immunogenicity, and no risk of tumorigenesis, positioning them as a promising focus in the development of “cell-free” therapeutic strategies. Importantly, while MSC-Exos exhibit considerable potential in the treatment of osteoarticular diseases, several challenges - including rapid in vivo clearance, limited targeting efficiency, and uncontrolled release - remain significant barriers to their clinical translation. To overcome these limitations, various delivery carrier systems have recently been developed, such as hydrogels, nanospheres, self-assembled nanoparticles, and three-dimensional (3D)-printed scaffolds[16]. These delivery platforms not only extend the half-life of exosomes and improve their retention and bioactivity at target sites, but also allow for precise control over exosome release kinetics[17]. As a result, the development of efficient, safe, and controllable MSC-Exos delivery systems has become a critical area for advancing the clinical application of exosome-based therapies.
OSTEOARTICULAR DISEASES
OA is one of the most common clinical conditions in orthopedic practice, with an incidence rate that continues to rise, thereby imposing significant burdens on healthcare systems and patients’ quality of life[18]. Based on its etiological and pathological features, it can be categorized into types such as degenerative OA, immune-mediated RA, and metabolic-associated gouty arthritis, among others. Despite their distinct origins, most joint disorders ultimately result in varying degrees of damage to cartilage and subchondral bone structures, converging on a common pathological pathway primarily characterized by chronic inflammation, impaired tissue remodeling, and limited regenerative capacity[19].
Articular cartilage is especially susceptible to damage in these pathological conditions. Its primary cellular constituents, chondrocytes, are highly terminally differentiated cells that account for only a small fraction of the total cartilage tissue volume (approximately 5%-10%). Embedded densely within an avascular and aneural extracellular matrix, these cells face significant barriers to effective regeneration after injury[20]. As a result, articular cartilage demonstrates markedly limited regenerative capacity following structural damage, presenting a major clinical challenge in the treatment of osteoarticular disorders[21].
OA
OA is the most prevalent joint disorder worldwide, primarily characterized by progressive degeneration of articular cartilage, subchondral bone sclerosis, mild synovial inflammation, and osteophyte formation[22]. Currently, an estimated 528-595 million people globally are affected by OA, with the incidence showing a significant age-related increase, particularly among women and individuals with obesity. Major risk factors include aging, excessive body weight, uneven mechanical loading, and metabolic dysregulation[23].
OA most commonly affects weight-bearing joints, particularly the knees, hips, and distal interphalangeal joints of the hands. The main clinical manifestations include chronic pain, joint stiffness, limited range of motion, and joint deformities. As the condition progresses, it can substantially impair activities of daily living and work capacity, leading to a significant decline in patients’ quality of life[24]. At the pathological mechanism level, OA is characterized by a chronic degenerative process driven by the synergistic effects of biomechanical disturbances and imbalances in the joint microenvironment, involving coordinated changes across multiple joint tissue structures[25]. As a specialized avascular and aneural connective tissue lacking a lymphatic system, articular cartilage depends on the passive diffusion of nutrients from synovial fluid to support its metabolic functions, which results in a delayed response to injury and a limited capacity for self-repair.
In the early stages of pathology, chondrocytes initiate repair mechanisms, characterized by cell clustering, proliferation, and enhanced expression of growth factors, all of which are intended to preserve matrix homeostasis[26]. However, this reparative response is often transient and eventually gives way to metabolic dysregulation within the extracellular matrix. At this stage, degradation-related enzymes, such as matrix metalloproteinases, remain persistently overexpressed. Simultaneously, an elevated rate of chondrocyte apoptosis combined with inadequate synthesis of new matrix components undermines the biomechanical integrity of cartilage tissue[27]. This cascade of events disrupts the dynamic balance between matrix synthesis and degradation, resulting in progressive structural and functional deterioration of cartilage and ultimately leading to an irreversible degenerative process[28,29].
RA
RA is a chronic, systemic, autoimmune-mediated inflammatory disease. Currently, approximately 0.5% to 1.0% of the global population suffers from this condition, with women being particularly at risk, exhibiting a prevalence rate approximately 2 times to 3 times higher than that observed in men[30]. Common symptoms include pain affecting multiple joints and morning stiffness. If not treated promptly, localized areas of necrosis, adhesion of granulation tissue, and fibrous tissue hyperplasia may develop on the joint surface, ultimately leading to progressive joint ankylosis, destruction, deformity, and functional impairment[31]. Without adequate control, RA can result in irreversible joint dysfunction and a significant decline in quality of life[32].
The core pathological process of RA primarily takes place within the synovial membrane and the synovial fluid microenvironment[33]. Persistent activation of synovial macrophages leads to the release of large amounts of proinflammatory cytokines, such as tumor necrosis factor-α, interleukin (IL)-1, and IL-6. These inflammatory mediators not only sustain localized inflammatory responses but also interact synergistically with fibroblast-like synoviocytes (FLS) to promote osteoclast differentiation and activation, thereby contributing to accelerated bone erosion[34].
In addition, activated FLS can secrete large amounts of degrading enzymes, such as matrix metalloproteinases, which compromise the structural integrity of articular cartilage and further accelerate the degradation process at the synovium-cartilage-bone interface[35]. FLS can also amplify bone destruction indirectly by activating the nuclear factor-kappa B signaling pathway and enhancing intercellular interactions with T cells and osteoclasts[36]. Moreover, RA-associated FLS exhibit invasive and migratory properties, enabling them to migrate from the initial site of inflammation to other synovial tissues, thereby leading to the simultaneous involvement of multiple joints[37].
Gouty arthritis
Gouty arthritis is a metabolic joint disorder characterized by the deposition of monosodium urate crystals in joints and surrounding tissues due to elevated serum uric acid levels, which trigger acute and intense inflammatory responses. As of 2020, approximately 55 million individuals were affected globally, with an age-standardized prevalence rate of 659 cases per 100000 people[38]. The incidence shows considerable geographical variation, influenced by dietary patterns, obesity prevalence, and metabolic syndrome, with particularly high susceptibility observed in male and obese populations.
Gouty arthritis typically presents with acute episodes characterized by the sudden onset of severe pain, erythema, swelling, and warmth in one or more joints. If inadequately managed, it can progress to chronic and destructive arthropathy. The prevalence of gout is markedly higher in males than in females and shows an increasing trend with advancing age[39]. Elevated serum urate levels represent the primary risk factor for gout development; however, genetic predisposition, medication use, hypertension, type 2 diabetes, impaired renal function, and alcohol consumption are also recognized as significant contributing factors[40,41]. From a molecular mechanistic perspective, monosodium urate crystals can be phagocytosed by local joint macrophages and activate the nucleotide-binding oligomerization domain-, leucine-rich repeat-, and pyrin domain- containing receptor 3 inflammasome, initiating a pro-inflammatory signaling cascade that culminates in IL-1β secretion and neutrophil chemotaxis. Beyond causing structural joint damage, gout is strongly associated with cardiovascular, metabolic, and renal complications, significantly increasing all-cause mortality and the overall disease burden[42]. Recurrent attacks not only impair physical function and limit daily activities but also result in work absenteeism and reduced labor productivity, leading to considerable economic consequences[43]. Without appropriate treatment, gout may develop into a chronic condition marked by tophus formation, persistent joint pain, articular erosion, and ultimately irreversible functional impairment[44].
Although OA, RA, and gouty arthritis differ in their etiologies and underlying pathological mechanisms, they all have the potential to cause irreversible damage to articular cartilage and surrounding bone tissues, sharing common pathological features such as chronic inflammation, structural deterioration, and impaired regenerative capacity. These pathological changes ultimately result in progressive joint dysfunction, which constitutes the primary pathological basis for functional impairment and reduced quality of life.
GENERAL TREATMENT OF BONE AND JOINT DISEASES
Current mainstream therapeutic approaches primarily focus on inflammation control and symptom alleviation but lack effective strategies to reverse structural degeneration of articular tissues. As a result, the restoration of damaged cartilage and the promotion of localized tissue regeneration have become critical challenges that require innovative breakthroughs in the field of joint disease treatment. The management of osteoarticular disorders mainly involves three modalities: Non-pharmacological interventions, pharmacological therapy, and surgical procedures. These interventions can be flexibly combined according to disease progression, patient characteristics, and the severity of functional impairment. However, existing treatment options remain largely symptom-oriented and inflammation-focused, showing significant limitations in terms of reversing joint structural damage or promoting cartilage regeneration.
Non-pharmacological therapies
Non-pharmacological interventions are widely recommended as the first-line treatment for patients in the early stages of the disease and primarily include exercise therapy, weight management, physical therapy, and the use of assistive devices[45]. These approaches help improve joint mobility, delay disease progression, and reduce reliance on medications[46]. However, their effectiveness heavily depends on long-term patient adherence, they show limited efficacy in mid-to-late-stage patients with structural damage, and they lack the capacity for definitive tissue repair.
Pharmacological therapy
Pharmacotherapy currently represents the most prevalent therapeutic approach and is primarily categorized into the following classes: Non-steroidal anti-inflammatory drugs, analgesics, glucocorticoids, and disease-modifying antirheumatic drugs. Although these medications can effectively alleviate symptoms, they fundamentally function as symptomatic treatments and are unable to halt or reverse the degenerative processes affecting cartilage and bone structures[47]. Prolonged administration may also lead to adverse effects such as gastrointestinal disturbances, hepatorenal toxicity, and immunosuppression[48]. Furthermore, a subset of RA patients demonstrates inadequate response or develops resistance to disease-modifying antirheumatic drugs and biologic agents, significantly limiting the broad applicability of these pharmacotherapies[49].
Surgical intervention
For patients with moderate-to-severe or end-stage osteoarticular diseases who do not respond to conservative treatment, surgical intervention becomes necessary. Common procedures include arthroscopic debridement, osteotomy, and arthroplasty. These surgical approaches demonstrate notable efficacy in alleviating pain and restoring joint mobility, with total joint replacement showing particularly effective outcomes in end-stage OA. However, such interventions involve significant surgical trauma, prolonged postoperative recovery periods, and considerable financial costs, along with potential complications such as prosthetic loosening, infection, and deep vein thrombosis[50]. Importantly, surgical procedures do not possess the ability to regenerate cartilage, and their long-term therapeutic efficacy remains uncertain[51].
A comprehensive analysis indicates that while current therapeutic approaches can partially alleviate symptoms and delay disease progression, they are unable to effectively restore the structural and functional integrity of joints. Particularly in the early stages of the disease, the absence of sensitive and specific diagnostic biomarkers impedes timely intervention. In advanced stages, once cartilage degeneration has occurred, the intrinsic regenerative capacity is severely compromised, limiting treatment goals to “symptom management” rather than “disease modification”.
Against this backdrop, cell-free regenerative therapies based on MSC-Exos are emerging as a promising strategy in the fields of precision medicine and tissue engineering for OA. Their advantages - including low immunogenicity, enhanced tissue permeability, targeted anti-inflammatory effects, and chondroprotective properties - position them as a potentially groundbreaking therapeutic approach. This discussion will focus on the mechanistic insights, delivery strategies, and translational challenges associated with MSC-Exos in the management of articular diseases.
BIOLOGICAL CHARACTERISTICS AND MECHANISM OF ACTION OF EXOSOMES DERIVED FROM STEM CELLS
In recent years, MSCs have shown significant promise in the treatment of osteoarticular diseases due to their multipotent differentiation capacity and tissue regeneration potential[52]. However, their direct clinical application remains challenging due to limitations such as low post-transplantation survival rates, the risk of ectopic differentiation, and immune rejection[53]. In contrast, MSC-Exos, as a cell-free therapeutic strategy, have gained increasing attention in the field of regenerative medicine[54,55]. These nanovesicles (30-150 nm in diameter) exhibit structural stability[56], excellent biocompatibility, and minimal immunogenicity. MSC-Exos contain a variety of bioactive molecules, including microRNAs, long non-coding RNAs, mRNAs, proteins, and lipids, which can be internalized by target cells through endocytosis[57], thereby mediating intercellular signaling and functional regulation[58].
In the context of osteoarticular diseases, MSC-Exos exert therapeutic effects through three primary mechanisms: First, they significantly reduce inflammatory responses by promoting macrophage polarization toward an anti-inflammatory phenotype and modulating the activity of synovial fibroblast-like cells, thereby regulating the articular immune microenvironment[59]. Second, MSC-Exos upregulate the expression of key chondrogenic markers such as SOX9, collagen type II alpha 1, and cartilage oligomeric matrix protein, which enhances chondrocyte proliferation and extracellular matrix synthesis, while simultaneously suppressing catabolic proteins including collagen type X alpha 1, runt-related transcription factor 2, and matrix metalloproteinase 13 to inhibit cartilage degeneration[60]. Third, they regulate subchondral bone remodeling by modulating the receptor activator for nuclear factor kappa B ligand-receptor activator of nuclear factor kappa B-tumor necrosis factor receptor-associated factor 6 signaling pathway, thereby balancing osteoclastic and osteogenic activities and maintaining joint biomechanical stability[61].
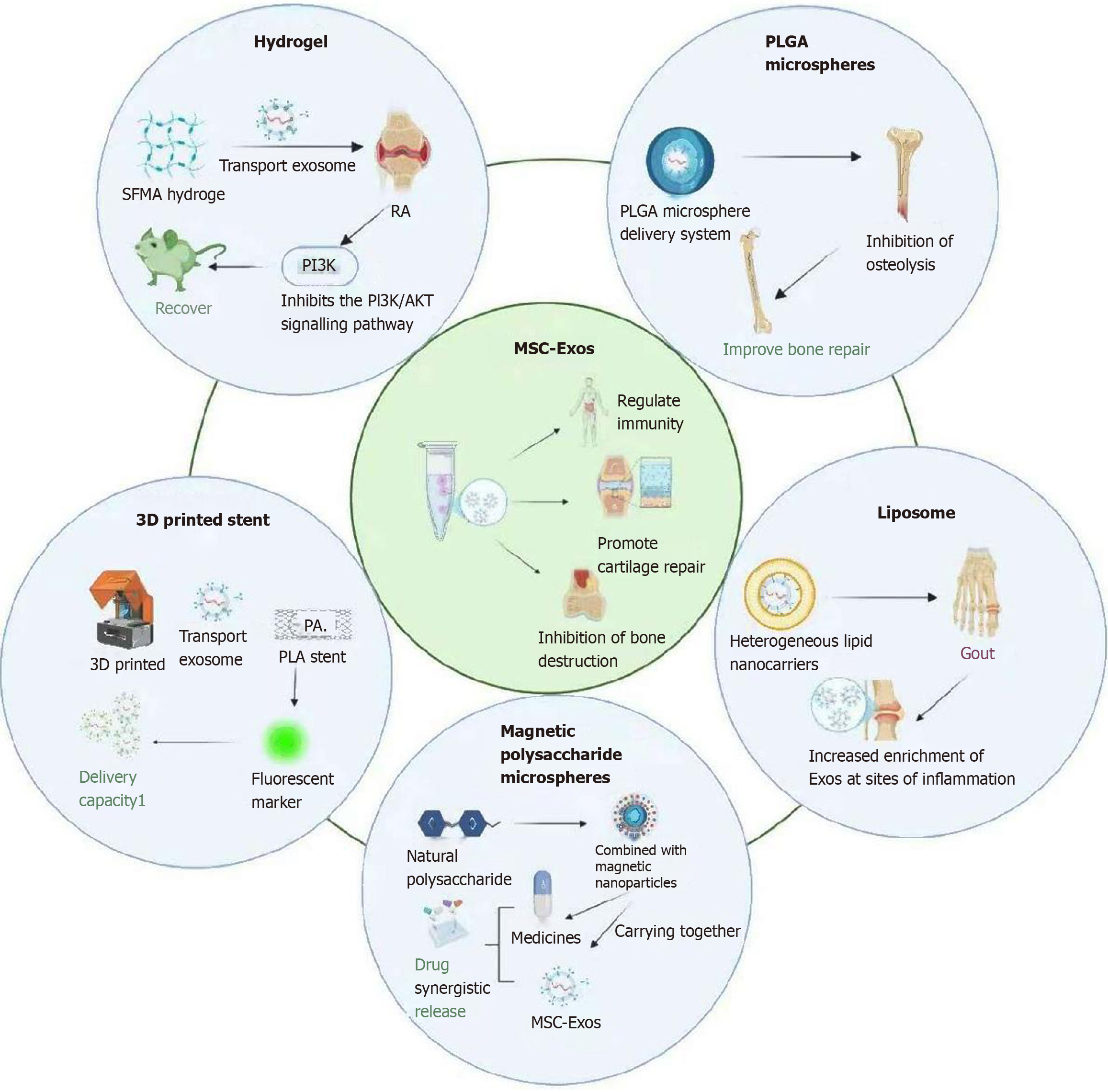
Although MSC-Exos offer multiple biological advantages in the treatment of osteoarticular diseases, they also have inherent limitations. For example, their short in vivo retention time, low targeted delivery efficiency, and lack of controlled-release mechanisms collectively limit their sustained clinical efficacy[62]. Moreover, the current absence of standardized preparation protocols and quality assessment systems for exosomes, combined with their complex bioactive composition and poorly defined mechanisms of action, further complicates their clinical translation[63,64]. To address these challenges, researchers have increasingly focused on integrating MSC-Exos with various “intelligent delivery systems” in recent years. By improving their stability, targeting capability, and controlled release properties, this strategy enables more efficient and precise therapeutic outcomes[65]. Particularly in conditions characterized by limited cartilage regenerative capacity, such as OA, MSC-Exos-based carrier platforms are regarded as a promising approach for overcoming current treatment limitations and promoting both structural restoration and functional regeneration (Figure 1).
Figure 1 Different carrier technologies for delivering stem cell-derived exosomes.
SFMA: Silk fibroin glycidyl methacrylate; RA: Rheumatoid arthritis; PI3K: Phosphatidylinositol 3-kinase; AKT: Protein kinase B; PLGA: Poly(lactic-co-glycolic acid); MSC-Exos: Mesenchymal stem cell-derived exosomes; 3D: Three-dimensional; PA: Phosphatidic acid; PLA: Polylactic acid.
DIFFERENT CARRIER TECHNOLOGIES
Hydrogel delivery systems
Hydrogels are hydrophilic 3D polymer networks characterized by excellent biocompatibility, injectability, and tunable degradation rates[66]. Naturally derived hydrogels, such as hyaluronic acid and gelatin-chitosan composites, exhibit structural similarity to the extracellular matrix of articular cartilage. This biomimetic property allows for effective simulation of the cartilage microenvironment, thereby enhancing localized exosome retention and achieving sustained-release effects[17].
In a study[67], researchers developed a light-responsive silk fibroin glycidyl methacrylate (SFMA) hydrogel to deliver exosomes derived from olfactory mucosa MSCs (Exos@SFMA) for the treatment of synovial inflammation in a murine model of RA. The SFMA hydrogel enables rapid in situ gelation within the joint cavity via photocrosslinking, forming a stable scaffold that supports sustained exosome release. In vivo results demonstrate that Exos@SFMA significantly upregulates programmed death-ligand 1 expression, inhibits the phosphatidylinositol 3-kinase/protein kinase B signaling pathway, and suppresses follicular helper T cell differentiation, thereby modulating the immune microenvironment and effectively attenuating RA disease progression.
Further investigation revealed that a precursor solution containing 100 μg/mL over expression-MSC-Exos underwent photo-triggered gelation within approximately 200 seconds under 365 nm irradiation, rapidly forming an Exos@SFMA hydrogel. This rapid in situ gelation enables precise spatial control over exosome delivery and ensures localized retention at the target site, thereby significantly enhancing therapeutic efficacy and the potential for clinical translation.
Zeng et al[68] reported a thermosensitive self-healing hydrogel designed for the co-delivery of MSC-Exos and icariin in the treatment of OA models. This system exhibits excellent tissue adhesion, self-healing capability, and thermosensitive responsiveness. It rapidly forms a stable gel at body temperature, enabling synchronized and sustained release of both exosomes and the drug. In OA models, the synergistic effect of exosomes and icariin significantly enhances chondrocyte proliferation and migration, suppresses matrix metalloproteinase expression, and improves cartilage structural degeneration, thereby demonstrating substantial therapeutic potential.
Hydrogels, as ideal carriers for exosome delivery, exhibit significant therapeutic potential in the treatment of RA and OA due to their excellent biocompatibility, controllable release profiles, and stimuli-responsive properties. By integrating multifunctional hydrogel systems with MSC-Exos, these platforms not only extend the retention time of exosomes at the target site but also enhance their immunomodulatory and tissue regenerative capabilities. The versatility and adaptability of hydrogel platforms further offer broad opportunities for the development of personalized treatment strategies. Looking ahead, the multimodal integration of hydrogels with complementary delivery technologies is anticipated to further improve their clinical translational value.
Poly(lactic-co-glycolic acid) microsphere delivery system
Poly(lactic-co-glycolic acid) (PLGA) is a linear polyester copolymer synthesized through the ring-opening polymerization of lactic acid and glycolic acid. It demonstrates excellent biodegradability and biocompatibility, undergoing gradual hydrolysis in vivo into carbon dioxide and water without leaving long-term residual effects, which makes it widely used in controlled-release platforms such as microspheres and nanoparticles[69]. The degradation rate of PLGA can be precisely regulated by adjusting the lactide-to-glycolide ratio, making it an ideal material for drug delivery systems[70].
In a study[71], researchers developed exosome-loaded PLGA nanospheres (PLGA-Exos) to enable sustained exosome release while preserving their bioactivity. To evaluate in vivo efficacy, a polyethylene glycol particle-induced mouse calvarial osteolysis model was established. On the second day post-modeling, in vivo imaging system detection revealed fluorescence signals from locally injected DIR-labeled free exosomes and PLGA-Exos. Results showed that PLGA-Exos exhibited approximately 40% higher fluorescence intensity on day 7 than the free exosomes group, attributable to sustained release, effectively inhibiting periprosthetic osteolysis and promoting bone repair. Further analysis indicated that PLGA-Exos gradually degraded within bone tissue while maintaining exosomal integrity and activity, demonstrating favorable safety profiles and therapeutic stability. Compared with conventional single-dose exosome administration, this delivery system achieves prolonged local therapeutic concentrations, reduces dosing frequency, and offers significant advantages for the treatment of chronic osteolytic diseases.
For chronic osteoarticular diseases, PLGA microsphere carriers offer several advantages: Significantly prolonging the local retention time of MSC exosomes to prevent rapid clearance; preserving the structural integrity of exosomes and reducing degradation by bodily fluids; enhancing localized therapeutic concentrations through gradient release; and being particularly well-suited for chronic degenerative conditions that require precise and repeated administration.
The aforementioned studies indicate that the PLGA microsphere system provides an effective carrier platform for improving the therapeutic stability and targeting precision of MSC exosomes, representing a key strategy for addressing the clinical translation bottlenecks of exosome-based therapies. However, it should be noted that certain biodegradable polymers, such as PLGA, can generate acidic degradation by-products, which may trigger local chronic inflammatory responses. This risk highlights the need to further optimize material composition and degradation kinetics in the future design and application of such scaffolds, in order to achieve a balanced trade-off between regenerative efficacy and long-term biosafety.
3D printed polylactic acid bioactive scaffolds
Polylactic acid (PLA) is a biodegradable polymer widely used in tissue engineering, with its degradation rate being precisely adjustable through modifications in the lactic acid monomer ratio and polymerization structure. With excellent mechanical properties and biocompatibility, PLA offers distinct advantages in various applications related to cartilage and bone tissue regeneration[72]. PLA scaffolds exhibit structural stability and precise moldability, making them particularly suitable for the fabrication of customized bone defect models using 3D printing technology. The 3D printing technique imparts highly tunable porosity, morphological compatibility, and drug-loading capacity to PLA scaffolds, thereby creating an optimal microenvironment for cell adhesion, proliferation, and exosome release[73].
Building upon this foundation, the surface modification or loading of MSC-Exos onto PLA scaffolds enables the development of an intelligent delivery platform with dual functionalities: Immunomodulation and osteogenic promotion. Zhang et al[16] developed a 3D-printed cartilage scaffold incorporating MSC-Exos, and PKH26 fluorescent labeling experiments confirmed effective uptake of the released exosomes by target cells, demonstrating robust bioactive delivery capacity. In vitro studies revealed that this scaffold significantly upregulates osteogenesis-related gene expression, enhances calcium salt deposition and mineralization capacity, and exhibits pronounced pro-osteogenic effects.
In an animal model of OA, the PLA-Exo scaffold significantly accelerated cartilage repair, promoted neocartilage formation, and achieved superior repair quality compared to standalone exosome administration or conventional scaffolds. This approach effectively integrates the regenerative potential of MSC-Exos with the fabrication advantages of 3D printing technology, demonstrating promising therapeutic potential for OA treatment. However, long-term implantation of 3D-printed PLA scaffolds still poses potential risks of immune rejection and chronic inflammation, presenting significant challenges to their clinical translation.
Magnetic polysaccharide microspheres
Magnetic polysaccharide microspheres (MPMs) are composite microspheres fabricated using microfluidic electrospray technology, based on methacrylic anhydride-functionalized natural polysaccharides combined with Fe3O4@MgSiO3 magnetic nanoparticles[74]. Compared to conventional synthetic polymers, these carriers exhibit enhanced biocompatibility and tissue affinity derived from natural polysaccharides, allowing for stable loading of drugs or exosomes. Moreover, the embedded magnetic particles enable precise locomotion and controlled release under external magnetic fields, thereby improving targeting accuracy and therapeutic efficacy[75]. The localized magnetic control capability provides it with superior release kinetics and therapeutic persistence compared to single-vector systems, highlighting its strong potential as a dynamically controllable and synergistic therapeutic microcarrier platform for osteoarticular diseases.
The MPM developed by Yang et al[76] enables simultaneous loading of MSC-Exos and the anti-inflammatory drug diclofenac sodium. In a rat model of OA, this system not only achieves stable capture and sustained release of exosomes but also facilitates synergistic drug release, significantly ameliorating joint inflammation and cartilage degeneration. To further evaluate its in vivo performance, the researchers established a surgically induced OA rat model and monitored exosome retention after intra-articular injection. Results showed that MPM-loaded exosomes remained detectable within the joint cavity for up to two weeks, whereas free exosomes were cleared within seven days. By leveraging magnetically guided targeting and synergistic release capabilities, MPM outperforms single-carrier systems in both release durability and therapeutic efficacy, highlighting its potential as a dynamically controllable delivery platform for osteoarticular diseases. However, further investigation is needed to determine whether the Fe3O4@MgSiO3 nanoparticles in the system accumulate in joint tissues over time - a critical factor for assessing long-term safety and clinical translatability.
Lipid nanocarrier systems
Liposomes are biocompatible carriers composed of one or more amphiphilic phospholipids, distinguished by their characteristic bilayer structure[77]. This unique architecture allows for the encapsulation of hydrophilic drugs within the aqueous core as well as the incorporation of hydrophobic agents into the lipid bilayer. As a result, liposomes are capable of simultaneously delivering both hydrophilic and hydrophobic therapeutics, making them an efficient drug delivery system[78]. Their enhanced drug-loading capacity and sustained-release properties help prevent premature drug degradation in vivo, thereby improving drug stability and bioavailability.
Xu et al[79] developed a hybrid liposomal nanocarrier platform by fusing exosomes with liposomes for the treatment of inflammatory joint diseases, such as gouty arthritis. This platform improves the accumulation and stability of exosomes at inflammatory sites while extending their local retention time. Through modulation of surface charge and encapsulation efficiency, the system exhibited enhanced anti-inflammatory effects and tissue protective capabilities in animal models.
Liposomes, as advanced nano-carriers, have the distinct capability of simultaneously encapsulating both hydrophilic and hydrophobic drugs, thereby effectively improving drug stability and bioavailability. The hybrid nano-carriers generated by fusing exosomes with liposomes not only enhance the targeted localization and retention of exosomes at inflammatory sites but also significantly boost therapeutic efficacy. This strategy offers new insights into the precise treatment of inflammatory joint diseases, particularly gouty arthritis, and demonstrates considerable application potential and promising prospects for clinical translation.
CONCLUSION
MSC-Exos represent an advanced cell-free therapeutic strategy with significant therapeutic potential for a range of osteoarticular disorders, including OA, RA, and gouty arthritis, due to their excellent biocompatibility, low immunogenicity, and multifunctional regulatory mechanisms. By modulating the immune microenvironment, promoting cartilage repair, and inhibiting bone destruction, MSC-Exos provide a transformative complement to conventional therapeutic approaches. However, the breadth and depth of their clinical application are still limited by challenges in vivo delivery, such as a short half-life, insufficient targeting specificity, and uncontrolled release kinetics.
First, exosomes derived from different stem cell sources exhibit significant variations in yield, composition, and functional activity. The current lack of a standardized large-scale production and quality control system directly compromises the consistency and accessibility of clinical-grade exosome formulations. Second, the degradation kinetics of delivery carriers differ between animal models and humans - for example, PLGA microspheres display distinct release profiles and metabolic behaviors under varying physiological conditions, which may affect the therapeutic efficacy and safety of exosomes. Furthermore, the absence of standardized methodologies for evaluating in vivo exosome release kinetics and bioactivity hampers cross-study comparability and reproducibility. Concurrently, the translation of therapeutic efficacy across species poses considerable challenges: Significant differences exist between rodents and humans in joint biomechanical loading, immune system responses, and rates of disease progression. These factors may contribute to discrepancies in exosome efficacy observed in human applications relative to animal models, thereby limiting the reliability of direct extrapolation to clinical settings.
In the future, advancing the clinical translation of MSC-Exos and their delivery systems will require breakthroughs in three key areas: First, establishing stable and scalable methods for exosome preparation and purification, along with developing quality control standards that meet regulatory requirements; second, systematically investigating the degradation kinetics and long-term biosafety of delivery vehicles in human physiological environments; third, constructing a standardized platform to evaluate release kinetics and functional activity, thereby enhancing reproducibility across laboratories and disease models. By integrating cutting-edge technologies such as high-throughput screening, stimuli-responsive materials, bioprinting, and genetic engineering, more precise, controllable, and personalized delivery systems are expected to emerge. These advancements will ultimately facilitate the successful translation of MSC-Exos from benchtop research to clinical applications in the treatment of osteoarticular diseases.
Meanwhile, the advancement of personalized treatment strategies requires not only conceptual formulation but also practical implementation. For instance, carrier selection can be tailored to the severity of joint damage: Injectable hydrogels or microspheres may be sufficient for localized repair in mild-to-moderate lesions, whereas 3D scaffold-based composite systems may be more appropriate for severe cartilage defects. Concurrently, a patient’s immune status should serve as a key determinant in optimizing exosome delivery systems - individuals with hyperactivated immune responses may benefit from carriers equipped with immunomodulatory properties to suppress excessive inflammation. Moreover, metabolic conditions such as diabetes and hyperuricemia can influence exosome degradation and release kinetics, underscoring the need for personalized adjustments in dosage and administration frequency. Integrating clinical stratification, carrier selection, and individualized dose optimization will ultimately advance MSC-Exos delivery systems toward precision medicine and enhanced translational potential.
ACKNOWLEDGEMENTS
The authors acknowledge the support and help from Hunan Province Sports Medicine Clinical Medicine Research Center and Hunan Provincial Engineering Research Center for Precision Diagnosis and Treatment and Rehabilitation of Joint and Sports Medicine Synergy of Traditional Chinese Medicine and Western Medicine.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Science Project of Hunan Provincial Healthy Commission, 20230844.
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade C
Novelty: Grade B, Grade D
Creativity or Innovation: Grade C, Grade C
Scientific Significance: Grade B, Grade D
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Guo DM, MD, PhD, China; Xu TC, MD, PhD, Chairman, Consultant, Director, Head, Principal Investigator, Professor, China S-Editor: Wang JJ L-Editor: A P-Editor: Zhang YL