Published online Nov 26, 2025. doi: 10.4252/wjsc.v17.i11.113201
Revised: September 17, 2025
Accepted: October 31, 2025
Published online: November 26, 2025
Processing time: 99 Days and 15.2 Hours
One of the most prevalent long-term effects of diabetes is diabetic kidney disease (DKD), which is linked to problems with the metabolism of amino acids, fats, and carbohydrates. The fundamental pathogenic mechanisms of DKD cannot be adequately treated by current clinical medicines; they can only slow the illness’s progression to end-stage renal disease. We will concentrate on integrating human umbilical cord mesenchymal stem cell (hUC-MSC)-related mechanisms and potential applications into the therapy of DKD, as this work shows that hUC-MSCs can be used to treat metabolic liver obesity associated with diabetes. Future studies on the connection between hUC-MSCs and related illnesses ought to be promoted.
Core Tip: This letter to the editor focuses on investigating how human umbilical cord mesenchymal stem cells (hUC-MSCs) contribute to diabetic kidney damage. At the same time, explore the similarities and differences of hUC-MSCs in treating metabolic dysfunction-associated steatotic liver disease and diabetic kidney disease. There are benefits of using hUC-MSCs to treat metabolic disorders. Future studies on the relationship between hUC-MSCs and the management of metabolic disorders ought to be promoted.
- Citation: Liu Y, Shan XQ, Li YJ, Gao WL, Zhao L. Role of human umbilical cord mesenchymal stem cells in diabetic kidney disease. World J Stem Cells 2025; 17(11): 113201
- URL: https://www.wjgnet.com/1948-0210/full/v17/i11/113201.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i11.113201
We were intrigued by the paper “Human umbilical cord mesenchymal stem cells ameliorate liver metabolism in diabetic rats with metabolic-associated fatty liver disease” published by Zhou et al[1]. According to the study, diabetic rats with metabolic dysfunction-associated steatotic liver disease (MASLD) may benefit greatly from human umbilical cord mesenchymal stem cells (hUC-MSCs) therapy in terms of liver damage and anomalies in glucose and lipid metabolism[1]. With their numerous anti-inflammatory, anti-oxidative, islet-regeneration, and metabolic-regulating properties, hUC-MSCs have emerged as a highly effective therapeutic approach for the treatment of diabetes and its consequences. One of the most dangerous and prevalent side effects of diabetes is diabetic kidney disease (DKD), which is also a leading contributor to end-stage renal disease. The present clinical treatment is linked to numerous metabolic problems and is not optimal. The findings in this research offer a more comprehensive outlook and potential for stem cell treatment of DKD. Thus, the goal of this letter is to add to the mechanism of DKD treatment using hUC-MSCs.
This paper examined how hUC-MSCs affected the metabolism of the liver in diabetic rats suffering from MASLD. Three groups were created using diabetic rats as a model: The control group, the diabetes group, and the diabetes plus hUC-MSCs therapy group. According to the experimental findings, diabetic rats’ fasting blood glucose and serum triglyceride levels dropped, while liver damage and fat degeneration improved. HUC-MSCs are comparatively effective for MASLD, according to the study. Significant metabolic differences between the hUC-MSC treatment group and the diabetes group were revealed by the results of metabolomic analysis, indicating that hUC-MSC regulates liver metabolism. These major metabolic differences can also be used to properly diagnose diabetes associated with MASLD and to predict and impact the therapeutic effect of hUC-MSC in diabetes with MASLD. The metabolic regulatory treatment of MASLD is given a new paradigm by this study, which also offers suggestions for potential clinical treatments.
This article’s hUC-MSCs can enhance MASLD’s lipid and glucose metabolism, which also applies to DKD. DKD can develop as a result of hyperglycemia and lipotoxicity, which can cause oxidative stress, an inflammatory response, and apoptosis. These conditions can also directly harm glomeruli, podocytes, and renal tubules. Given that DKD and MASLD share a route of insulin resistance and lipotoxic damage, clinical evidence reported by Targher et al[2] indicates that individuals with MASLD have a markedly elevated chance of acquiring DKD. In addition, fundamental research has demonstrated that hUC-MSCs enhance insulin sensitivity and intrahepatic lipid metabolism while lowering aberrant lipids through the activation of the liver’s hepatocyte nuclear factor 4 alpha/carboxylesterase 2 pathway. Because of its systemic nature, this action can directly lessen kidney damage[3]. Therefore, improving glucose and lipid metabolism in patients with MASLD can also effectively reduce the risk of DKD. In addition, hUC-MSCs can also reduce lipid deposition in the kidneys of DKD rats[4]. They can also reduce the release of pro-inflammatory cytokines by improving blood glucose levels in DKD mouse models, thereby reducing renal fibrosis and other pathological changes[5].
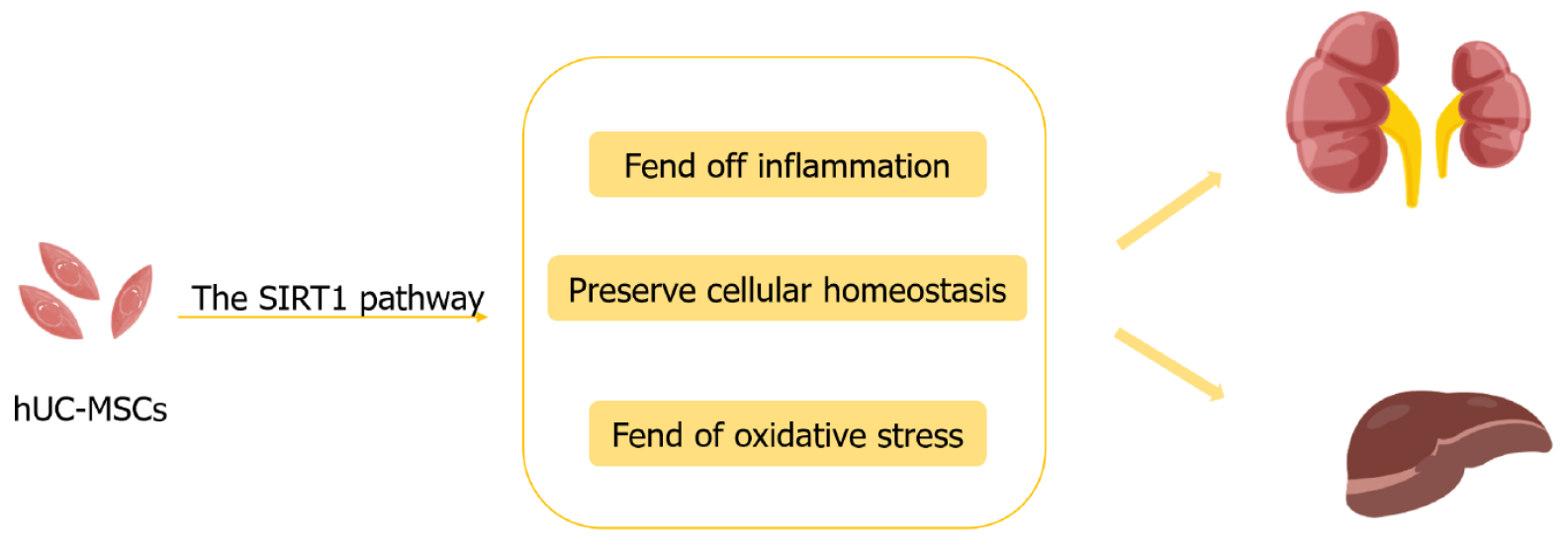
Furthermore, the sirtuin 1 (SIRT1) pathway is shared by the liver and kidney in preserving cellular homeostasis and fending off inflammation and oxidative stress (Figure 1). The kidney and liver share the SIRT1 pathway, which helps to maintain cell homeostasis in the face of inflammation and oxidative damage. The SIRT1 pathway, which improves mitochondrial function, increases autophagy, suppresses inflammation, and controls cell death in both hepatocytes and kidney cells, can be activated in the liver and kidney by hUC-MSCs therapy[6,7]. Naturally, the SIRT1 pathway protects the kidneys and liver, but tissue specificity affects how active it is. In addition to the previously described hUC-MSCs therapy, which enhances glucose and lipid metabolism, this is still another popular treatment approach that targets metabolic impairment in several organs. However, the kidney exhibits distinct organ specificity in contrast to the liver studies discussed in this article. For instance, podocytes are an essential part of the glomerular filtration barrier and are in charge of stopping protein leakage. One of the main processes causing reduced renal function in DKD is capsular cell damage and death. HUC-MSCs can preserve podocytes through exosomes[8], which is crucial for slowing the course of DKD.
Furthermore, certain decreases in blood creatinine, urea nitrogen, and urine protein levels, together with an increase in glomerular filtration rate or creatinine clearance, demonstrate that hUC-MSC therapy can preserve and enhance kidney function[9,10]. Additionally, it can lessen the severity of renal tubular injury, glomerulosclerosis, and renal fibrosis[11,12], and it may offer a means to repair glomerular sclerosis and lower inflammation in DKD animals[13]. According to the aforementioned article, hUC-MSCs have a variety of therapeutic uses and could one day be used to treat further diabetic problems as well as other metabolic disorders.
Transplanting hUC-MSCs into mice is known as allogeneic transplantation, and it can cause immunological depletion, short-term adverse effects, and other problems. In the meantime, syngeneic transplantation of mouse umbilical cord mesenchymal stem cells might exhibit more profound symptoms of recovery[14]; nevertheless, there is currently very little research on rat umbilical cord mesenchymal stem cells targeting MASLD or other disease models, and there is a lack of actual experimental data to support the theoretical assumptions described above. More study is currently being done on hUC-MSCs because of their robust proliferative ability, low immunological response, and low danger of contamination.
According to the article, using hUC-MSCs to treat MASLD and DKD requires addressing distinct pathological centers and damage mechanisms in the liver and kidneys, as well as variations in treatment outcomes and evaluation standards at the conclusion of the course of treatment. As a result, as the paper points out, there are several obstacles to study on hUC-MSCs in the treatment of DKD and MASLD, including the instability of mixed cell injections, a dearth of long-term clinical data, and unidentified possible adverse effects. However, as the article notes, hUC-MSCs have the ability to treat both MASLD and diabetes, and ongoing research initiatives continue to hold out hope for the treatment of DKD using hUC-MSCs. To continue verifying efficacy and evaluating the treatment’s long-term benefits and possible adverse effects, we must carry out comprehensive mechanistic research and clinical trials in a variety of animal models in the future.
| 1. | Zhou KB, Nie L, Wang ML, Xiao DH, Zhang HY, Yang X, Liao DF, Yang XF. Human umbilical cord mesenchymal stem cells ameliorate liver metabolism in diabetic rats with metabolic-associated fatty liver disease. World J Stem Cells. 2025;17:105266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (5)] |
| 2. | Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18:599-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 586] [Article Influence: 117.2] [Reference Citation Analysis (0)] |
| 3. | Li B, Cheng Y, Yu S, Zang L, Yin Y, Liu J, Zhang L, Mu Y. Human Umbilical Cord-Derived Mesenchymal Stem Cell Therapy Ameliorates Nonalcoholic Fatty Liver Disease in Obese Type 2 Diabetic Mice. Stem Cells Int. 2019;2019:8628027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Zhang K, Zheng S, Wu J, He J, Ouyang Y, Ao C, Lang R, Jiang Y, Yang Y, Xiao H, Li Y, Li M, Wang H, Li C, Wu D. Human umbilical cord mesenchymal stem cell-derived exosomes ameliorate renal fibrosis in diabetic nephropathy by targeting Hedgehog/SMO signaling. FASEB J. 2024;38:e23599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 5. | Huang LL, Hou YY, Yang J, Liao XN, Ma JS, Wang WC, Quan YX, Jiang HY, Bai YH. Mitigation of Ferroptosis in Diabetic Kidney Disease Through Mesenchymal Stem Cell Intervention via the Smad2/3/METTL3/S1PR1 Axis. FASEB J. 2025;39:e70714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Song J, Wang L, Wang L, Guo X, He Q, Cui C, Hu H, Zang N, Yang M, Yan F, Liang K, Wang C, Liu F, Sun Y, Sun Z, Lai H, Hou X, Chen L. Mesenchymal stromal cells ameliorate mitochondrial dysfunction in α cells and hyperglucagonemia in type 2 diabetes via SIRT1/FoxO3a signaling. Stem Cells Transl Med. 2024;13:776-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Yi S, Cong Q, Zhu Y, Xu Q. Mechanisms of Action of Mesenchymal Stem Cells in Metabolic-Associated Fatty Liver Disease. Stem Cells Int. 2023;2023:3919002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | Wang Y, Liu J, Wang H, Lv S, Liu Q, Li S, Yang X, Liu G. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Diabetic Kidney Disease Through the NLRP3 Signaling Pathway. Stem Cells. 2023;41:368-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 9. | Yue Y, Yeh JN, Chiang JY, Sung PH, Chen YL, Liu F, Yip HK. Intrarenal arterial administration of human umbilical cord-derived mesenchymal stem cells effectively preserved the residual renal function of diabetic kidney disease in rat. Stem Cell Res Ther. 2022;13:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 10. | Xu N, Liu J, Li X. Therapeutic role of mesenchymal stem cells (MSCs) in diabetic kidney disease (DKD). Endocr J. 2022;69:1159-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Qin J, Peng ZZ, Li Q, Wen R, Tao LJ. Renal Fibrosis and Mitochondrial Damage. Chin Med J (Engl). 2018;131:2769-2772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Gao Z, Zhang C, Peng F, Chen Q, Zhao Y, Chen L, Wang X, Chen X. Hypoxic mesenchymal stem cell-derived extracellular vesicles ameliorate renal fibrosis after ischemia-reperfusion injure by restoring CPT1A mediated fatty acid oxidation. Stem Cell Res Ther. 2022;13:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Hsiao PJ, Kao WY, Sung LC, Lin CY, Tsou LL, Kao YH, Chou CL, Lee KT. The Role of Mesenchymal Stem Cells in Treating Diabetic Kidney Disease: Immunomodulatory Effects and Kidney Regeneration. Int J Med Sci. 2025;22:1720-1735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Moinuddin FM, Yolcu YU, Wahood W, Siddiqui AM, Chen BK, Alvi MA, Goyal A, Nesbitt JJ, Windebank AJ, Yeh JC, Petrucci K, Bydon M. Early and sustained improvements in motor function in rats after infusion of allogeneic umbilical cord-derived mesenchymal stem cells following spinal cord injury. Spinal Cord. 2021;59:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/