Published online May 26, 2024. doi: 10.4252/wjsc.v16.i5.551
Revised: January 29, 2024
Accepted: April 1, 2024
Published online: May 26, 2024
Processing time: 145 Days and 10.2 Hours
Embryonic stem cells (ESCs) serve as a crucial ex vivo model, representing epiblast cells derived from the inner cell mass of blastocyst-stage embryos. ESCs exhibit a unique combination of self-renewal potency, unlimited proliferation, and pluripotency. The latter is evident by the ability of the isolated cells to differentiate spontaneously into multiple cell lineages, representing the three primary embryonic germ layers. Multiple regulatory networks guide ESCs, directing their self-renewal and lineage-specific differentiation. Apoptosis, or programmed cell death, emerges as a key event involved in sculpting and forming various organs and structures ensuring proper embryonic development. How
To investigate the regulatory impact of apoptosis on the early differentiation of ESCs into cardiac cells, using mouse ESC (mESC) models - mESC-B-cell lym
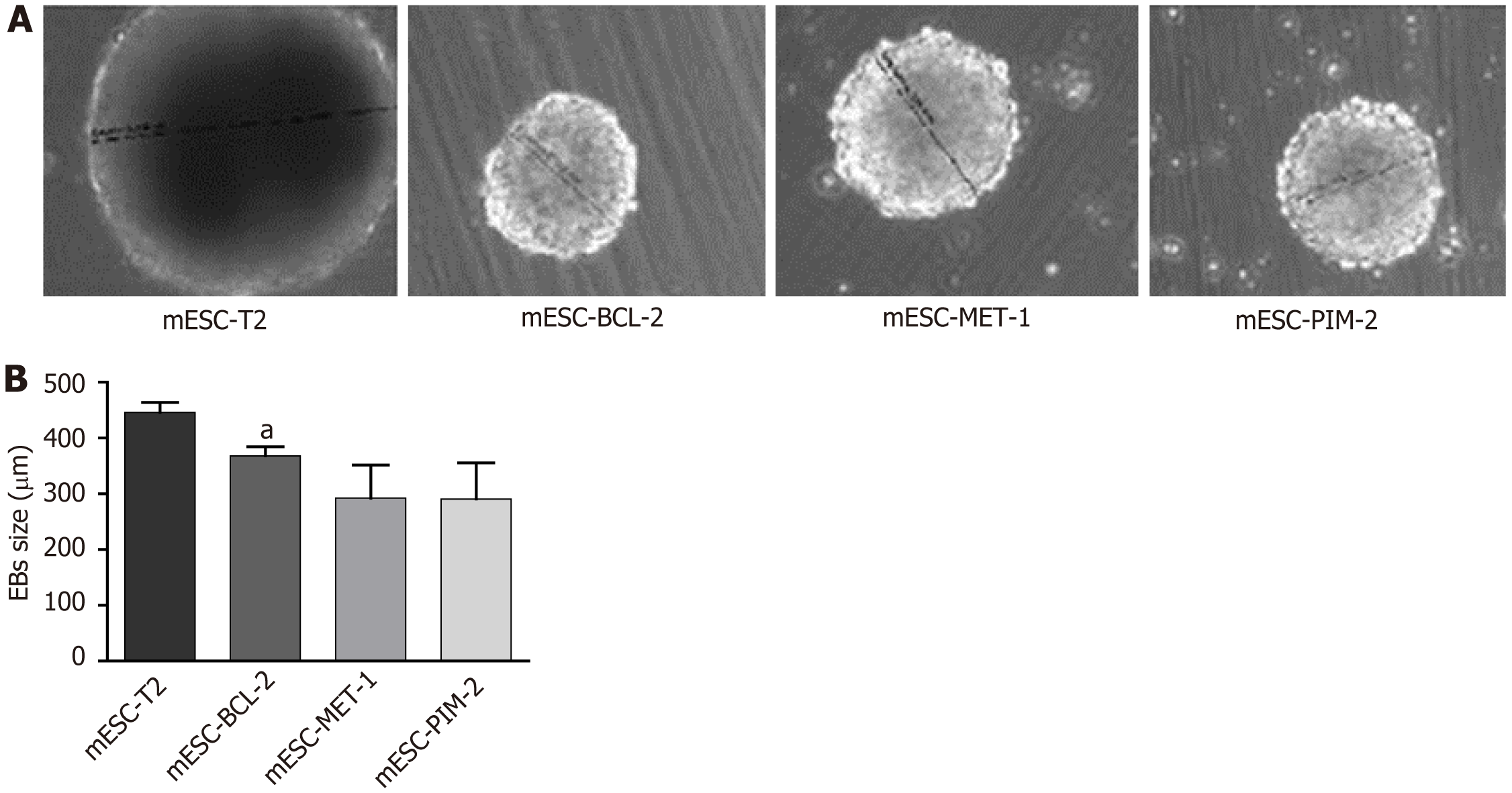
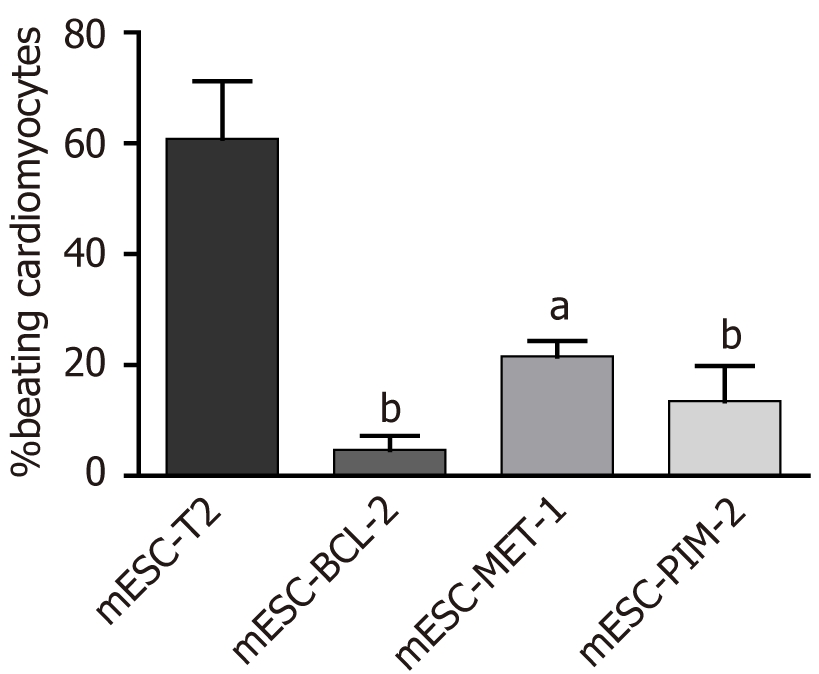
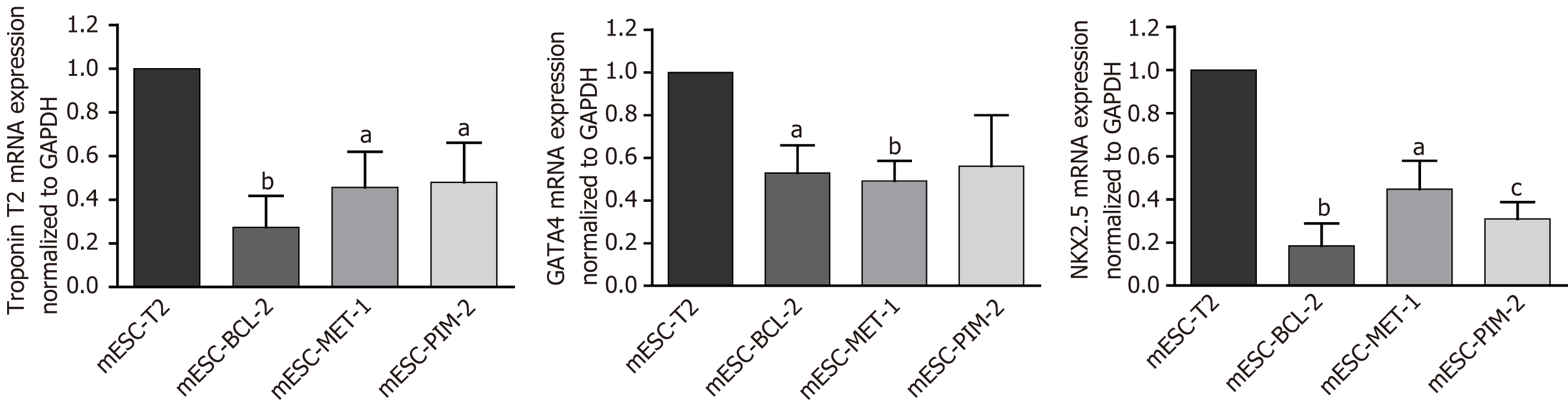
mESC-T2 (wild-type), mESC-BCL-2, mESC-PIM-2, and mESC-MET-1 have been used to assess the effect of potentiated apoptotic signals on cardiac differentiation. The hanging drop method was adopted to generate embryoid bodies (EBs) and induce terminal differentiation of mESCs. The size of the generated EBs was measured in each condition compared to the wild type. At the functional level, the percentage of cardiac differentiation was measured by calculating the number of beating cardiomyocytes in the manipulated mESCs compared to the control. At the molecular level, quantitative reverse transcription-polymerase chain reaction was used to assess the mRNA expression of three cardiac markers: Troponin T, GATA4, and NKX2.5. Additionally, troponin T protein expression was evaluated through immunofluorescence and western blot assays.
Our findings showed that the upregulation of Bcl-2, Pim-2, and Met-1 genes led to a reduction in the size of the EBs derived from the manipulated mESCs, in comparison with their wild-type counterpart. Additionally, a decrease in the count of beating cardiomyocytes among differentiated cells was observed. Furthermore, the mRNA expression of three cardiac markers - troponin T, GATA4, and NKX2.5 - was diminished in mESCs overexpressing the three anti-apoptotic genes compared to the control cell line. Moreover, the overexpression of the anti-apoptotic genes resulted in a reduction in troponin T protein expression.
Our findings revealed that the upregulation of Bcl-2, Pim-2, and Met-1 genes altered cardiac differentiation, providing insight into the intricate interplay between apoptosis and ESC fate determination.
Core Tip: Embryonic stem cells (ESCs) exhibit unique characteristics of self-renewal and pluripotency, allowing for their spontaneous differentiation into multiple cell lineages. Apoptotic signaling presents one of the crucial networks that influence embryonic development. In this study, the upregulation of anti-apoptotic genes B-cell lymphoma 2, Pim-2, and metallothionein-1 in mouse ESCs altered cardiac differentiation. This alteration was evidenced by a decreased size of embryoid bodies, a reduced number of beating cardiomyocytes, and an attenuated expression of cardiac markers. The study emphasizes the critical role of apoptosis in cardiac differentiation, providing insights into the regulatory impact of apoptosis on early differentiation pathways.
- Citation: Yehya A, Azar J, Al-Fares M, Boeuf H, Abou-Kheir W, Zeineddine D, Hadadeh O. Cardiac differentiation is modulated by anti-apoptotic signals in murine embryonic stem cells. World J Stem Cells 2024; 16(5): 551-559
- URL: https://www.wjgnet.com/1948-0210/full/v16/i5/551.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i5.551
Embryonic stem cells (ESCs) are the ex vivo equivalent of epiblast cells of blastocyst-stage embryos[1,2]. ESCs exhibit unique self-renewal potency, allowing them to proliferate extensively in a primitive undifferentiated state in vitro. In addition, these cells possess a pluripotent ability, enabling them to differentiate spontaneously into multiple cell lineages, representing the three embryonic germ layers[1,2]. The indefinite self-renewal capacity and pluripotency both define the generic features of ESCs and propose them as a unique cell model in early mammalian developmental research and cellular therapy.
Several regulatory networks constrain ESCs and direct their self-renewal machinery and lineage-specific differentiation during embryonic development. Apoptosis, or programmed cell death (PCD), is a crucial event that ensures proper embr
Taking advantage of isolated mouse ESCs (mESCs), this model properly simulates the early stages of cell lineage specification and spontaneous apoptosis in vitro. Maintaining mESCs in the naïve undifferentiated state in vitro requires the presence of leukemia inhibitory factor (LIF). The latter stabilizes mESCs and enables them to proliferate as aggregates without significant loss of viability. Withdrawal of LIF triggers mESC differentiation and colonization into three-dimensional (3D) aggregates called embryoid bodies (EBs), which harbor a variety of cell types including cardiac, neural, and adipose cells. Similar to what is evidenced in vivo, a significant proportion of cells die by apoptosis under this condition[1]. Hence, the mESC model can potentially help decipher the molecular mechanisms that initiate and execute apoptosis during ESC differentiation.
Apoptosis is naturally activated during development in response to multiple physiological cues and is triggered mainly via two multi-factorial pathways: The intrinsic or mitochondrial pathway and the extrinsic or death receptor pathway[8]. Mitochondrial apoptosis is delicately regulated by an interactive balance between the members of the B-cell lymphoma 2 (BCL-2) protein family, represented by anti-apoptotic factors (e.g., BCL-2, BCL-xL, and MCL-1) and their pro-apoptotic counterparts (e.g., BID, BAD, PUMA, BAX, and BAK)[9,10]. This interaction is critically influenced by several factors that can ultimately affect the life-or-death commitment of cells. In fact, several kinases phosphorylate BCL-2 family members at a number of sites and modulate their function. PIM-2 kinase, for example, phosphorylates the pro-apoptotic BCL-2 homology domain 3 (BH3)-only protein, BAD, preventing its interaction with BCL-xL and thereby suppressing apoptosis[11,12]. Moreover, metallothioneins (METs), known to act as metal-chelating agents, affect indirectly the intrinsic pathway and prompt a p53-mediated apoptotic activation[13]. Zinc ions play a crucial role in maintaining the conformation and affinity of p53 to DNA[14]. METs can bind to zinc, and this can reduce the transcriptional activity of p53 and thus inactivate the pro-apoptotic BH3-only protein, PUMA, a downstream target of p53. Subsequently, the pro-apoptotic effectors BAK and BAX do not localize to the mitochondria, inhibiting the release of cytochrome c and preventing the induction of PCD[15].
To understand the potential effect of apoptosis on early ESC differentiation, we used three mESC models previously generated by Duval et al[16]: mESC-T2 (wild-type), mESC-BCL-2 (overexpressing Bcl-2 gene), mESC-PIM-2 (overexpressing Pim-2 gene), and mESC-MET-1 (overexpressing Met-1 gene). We examined the effect of the anti-apoptotic gene overexpression on ESC commitment into mesodermal lineages, specifically cardiac differentiation. Our findings suggest that the overexpression of the anti-apoptotic genes influences the cardiac differentiation fate of mESCs.
mESC-T2 (wild-type), mESC-BCl-2 (overexpressing Bcl-2 gene), mESC-MET-1 (overexpressing Met-1 gene), and mESC-PIM-2 (overexpressing Pim-2 gene) were generously provided by Duval et al[16]. Cells were grown feeder-free on 0.1% gelatin-coated dishes and were maintained in Dulbecco’s Modified Eagle’s Medium - high glucose (Sigma-Aldrich) supplemented with 10% fetal bovine serum (Sigma-Aldrich), 1 × non-essential amino acids (Sigma-Aldrich), 1 mmol/L sodium pyruvate (Sigma-Aldrich), 1 × penicillin-streptomycin (Biowest), 5 μg/mL plasmocin prophylactic (InvivoGen), 300 mg/mL G418 (Sigma-Aldrich), and 100 μM β-mercaptoethanol (Sigma-Aldrich). The mESCs were maintained at a pluripotent undifferentiated stage by the addition of 10 ng/mL mouse LIF. Cells were routinely passaged, and the medium was changed every other day. Cells were kept at 37 °C in a humidified incubator containing 5% CO2 and 95% air.
mESC colonies were enzymatically dissociated with 0.1% EDTA/Trypsin (Sigma-Aldrich). Cells were then counted and suspended as “hanging drops” at a density of 1 × 103 cells in 20 μL of culture medium on the inner surface of petri dish lids containing 10 mL phosphate-buffered saline (PBS; Sigma-Aldrich). Cells were left for 2 d in the absence of LIF to ensure spontaneous aggregation and formation of EBs. On day 3, the formed EBs were transferred and maintained for 3 d in suspension in petri dishes containing 10 mL of culture medium. On day 7, EBs were plated on a gelatin-coated six-well plate to induce terminal differentiation. The medium was changed every other day.
Cardiomyocytes were identified after 1-4 d of EBs being plated on gelatin-coated plates, with their spontaneous contraction indicating synchronized beating. The number of beating EBs in all conditions was counted and represented as a percentage of control.
Total RNA was extracted from the four cell lines using RNeasy Micro Kit (Qiagen) according to the manufacturer’s instructions. cDNA synthesis was performed using the Super Script III First-Strand Synthesis System (Invitrogen). Polymerase chain reaction (PCR) was carried out using the Platinum Taq Polymerase (Invitrogen). Quantitative reverse transcription-PCR (qRT-PCR) was performed in duplicates on Bio-Rad CFX Connect using the SYBR green PCR master mix. All samples were normalized to the housekeeping gene Gapdh and gene expression relative to control was calculated using the ΔΔCt method. Primer sequences are listed in Supplementary Table 1.
Total protein was extracted from the four cell lines with RIPA lysis buffer and then mixed with β-mercaptoethanol and 2× Laemmli Sample Buffer [65.8 mmol/L Tris-HCl, pH 6.8, 2.1% SDS, 26.3% (w/v) glycerol, and 0.01% bromophenol blue; Bio-Rad]. Proteins were separated by electrophoresis on a 10% SDS-polyacrylamide gel and transferred onto poly
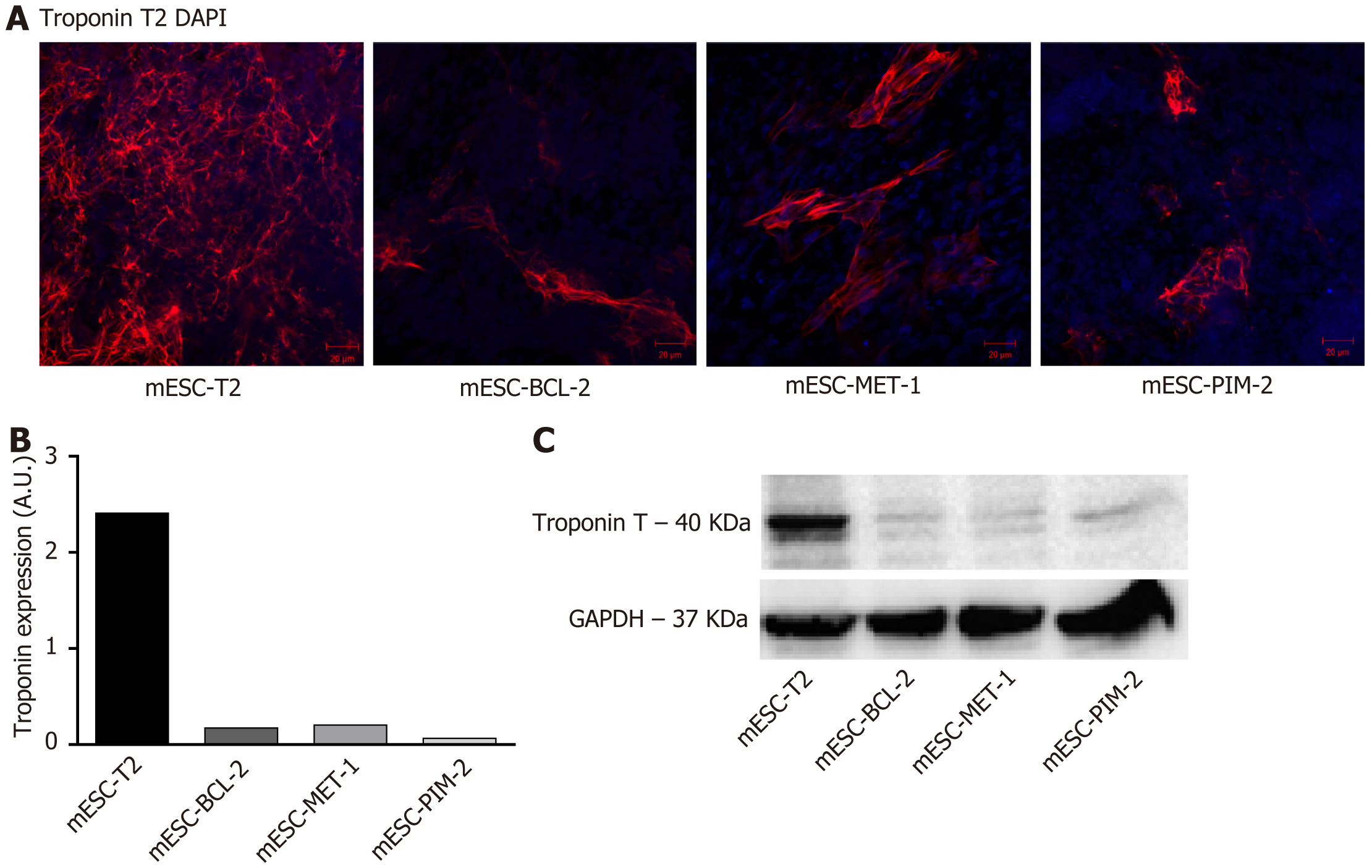
Indirect immunofluorescence (IF) staining was performed on EBs to characterize the expression of the cardiac marker Troponin. Cells were washed with PBS and fixed with 4% paraformaldehyde (Sigma-Aldrich). Cells were then permeabilized with 0.2% Triton X-100 and blocked for 1 h at room temperature with a blocking buffer containing PBS with 0.1% bovine serum albumin (BSA; Amresco), 0.2% Triton X-100, 0.05% Tween-20, and 10% normal goat serum (ThermoFisher). Subsequently, cells were incubated overnight at 4 °C with the specific primary anti-TNNT2 antibody (CT3, DSHB, 1/50) diluted in PBS containing 1% BSA. After washing with PBS containing 0.1% Tween-20, the secondary antibody Goat Anti-Mouse IgG H&L (Alexa Fluor® 568; ab175473, Abcam; 1/200) was added for 1 h at 37 °C. The nuclei were stained with anti-fade reagent Fluoro-gel with 4’,6-diamidino-2-phenylindole di-hydrochloride (Abcam). Signals were detected using a Carl Zeiss LSM 710 Laser scanning confocal microscope, and images were acquired and analyzed using the Carl Zeiss ZEN 2012 image software.
Data were analyzed using GraphPad Prism 7 (version 7.0; GraphPad Software Inc., La Jolla, CA, United States). All results are shown as the mean ± standard error of mean. A Student’s t-test was employed in this study. In all statistical tests, the mean of the conditioned groups was compared to the mean of the control group. A P value < 0.05 was considered statistically significant, with levels of significance represented as follows: aP < 0.05; bP < 0.01; cP < 0.001.
To investigate the effect of overexpressing the Bcl-2, Pim-2, and Met-1 genes on the ability of mESCs to form EBs, we attempted to differentiate the mESC lines using the hanging-drop EB formation method in LIF-free medium. Subsequently, we monitored the sizes of the EBs formed after 7 d. Our results showed that the overexpression of the anti-apoptotic genes decreased the sizes of the generated EBs in mESC-BCL-2, mESC-PIM-2, and mESC-MET-1 compared to the wild-type counterpart, mESC-T2 (Figure 1). These results suggest that the genetic manipulation of mESC-BCL-2, mESC-PIM-2, and mESC-MET-1 triggers defects in the efficiency of EB formation and the development of mESCs in vitro.
Cardiomyogenic differentiation is among the earliest lineages to arise, both in vivo during embryo formation and in vitro during ESC differentiation[17]. To assess the effect of overexpressing the Bcl-2, Pim-2, and Met-1 genes on cardiac differentiation, we analyzed the spontaneous beating areas that represent ultimately differentiated cardiomyocytes within the EB-derived colonies (Video). The beating of cardiomyocytes was not detected until 13 d after the induction of differentiation. The percentage of beating was calculated in EB-derived colonies on day 15 of differentiation (Figure 2). On day 15, mESC-T2 colonies showed a high percentage of beating, while weak beatings were observed in a small number of EB colonies formed by mESC-BCL-2, mESC-PIM-2, and mESC-MET-1. Moreover, the regions of spontaneously active cardiomyocytes were scarcer and smaller in the EBs overexpressing the Bcl-2 gene compared to other conditions. These results indicate that the overexpression of Bcl-2, Pim-2, and Met-1 genes significantly suppresses cardiac differentiation in mESCs.
To further support the observation of the impact of Bcl-2, Pim-2, and Met-1 overexpression on cardiac differentiation, mESC cultures were examined for the differential mRNA expression of cardiac troponin T, GATA4, and NKX2.5 after 14 d of differentiation. The results revealed that the expression of the three myocardial-specific marker genes was signi
The protein expression of troponin T was further evaluated using indirect IF staining and western blot. mESC-T2, mESC-BCL-2, mESC-MET-1, and mESC-PIM-2 cells were collected on day 14 of differentiation and prepared for IF and western blot analyses. Using anti-troponin T antibodies, the IF staining showed that troponin T expression was more significant in the wild-type mESC-T2 compared to mESC-BCL-2, mESC-PIM-2, and mESC-MET-1 (Figure 4A and B). Similarly, the immunoblots clearly indicated a significant suppression in troponin T expression in the conditioned mESCs compared to the wild type (Figure 4C).
PCD plays a crucial role in early embryonic development, ensuring proper organ formation[18-20]. Apoptosis invol
The objective of this study was to investigate the impact of anti-apoptotic signals on the commitment of ESCs toward mesodermal lineages, particularly in the context of cardiac differentiation. To achieve this goal, we utilized three types of mESCs genetically modified to overexpress three anti-apoptotic genes, namely, Bcl-2, Met-1, and Pim-2. BCL-2 plays a prominent and well-established role in promoting development during early mammalian embryogenesis. Functioning as a guardian protein, it ensures the proper execution of the cell death program and contributes to shaping the developing embryo[21]. Additionally, experiments have identified various markers with anti-apoptotic potential expressed in the early stages of differentiation that include MET-1 and PIM-2 proteins[16,22]. The latter elicits their indirect pro-survival effect mainly by influencing the activity of BCL-2 family members.
Our results revealed that the overexpression of the three anti-apoptotic genes led to a decrease in the size of EBs at day 7 of differentiation, potentially reflecting an induced defect in embryonic development and differentiation processes in vitro. We then assessed the effect of the overexpression of Bcl-2, Met-1, and Pim-2 genes on cardiac differentiation. Based on our results, these genes appear to modulate the differentiation of EBs into cardiomyocytes. From a functional perspective, the overexpression of these genes causes a decrease in the number of beating cardiomyocytes at day 15 of differentiation. In addition, our study demonstrated that the overexpression of anti-apoptotic genes reduced the mRNA expression level of three cardiac markers - troponin T, GATA4, and NKX2.5. Troponin is a regulatory protein of the thin filament or actin of striated muscle. The troponin complex is a part of a structure called the sarcomere, which is the basic unit of muscle contraction. The Tnnt2 gene encodes the cardiac troponin or troponin T found only in the heart muscle[23]. The Gata4 gene encodes a member of the GATA family of zinc-finger transcription factors that regulate genes involved in embryogenesis and myocardial differentiation and function[24]. Additionally, the Nkx2.5 gene encodes a homeobox-containing transcription factor with a role in heart formation and development[25]. Our qRT-PCR experiments showed that the overexpression of anti-apoptotic genes causes a decrease in the mRNA expression level of these markers at day 14 of differentiation. Moreover, both immunolabeling and western blot experiments indicated that the overexpression of anti-apoptotic genes can lead to a decrease in the expression of troponin T protein in EBs at day 14 of differentiation. Collectively, the anti-apoptotic genes Bcl-2, Met-1, and Pim-2 displayed a comparable influence on mESCs’ cardiac fate determination. These findings suggest that apoptosis is important for the cardiac specification of ESCs. Furthermore, our preliminary results suggest a role for apoptosis in early mesodermal development. Brachyury is an early mesodermal marker; it is expressed in the early stages of mesodermal differentiation[26]. Cells derived from mESC-T2 and mESC-MET-1 were prepared on day 6 of differentiation to detect the expression of Brachyury. qRT-PCR analysis showed that the stable expression of the Met-1 gene significantly decreased the mRNA expression of Brachyury (Supplementary Figure 1).
The prominent roles of anti-apoptotic BCL-2 family members are well-established during early mammalian embryogenesis[21,27]. A recent study involving human induced pluripotent stem cells demonstrated that the elimination of Bcl-2 resulted in a reduction in the size of spontaneously contracting cells[28]. Conversely, an investigation involving human ESCs (hESCs) found that overexpressing Bcl-2 significantly enhanced the survival of hESCs without compromising their ability to transform into cardiomyocytes[29]. The discrepancy between our findings and prior studies may be attributed to the fluctuation of Bcl-2 expression during the process of cardiomyocyte differentiation[30]. Additionally, an important signaling pathway in ESCs is the PIM signaling pathway. PIM2 is highly expressed in ESCs, but not in somatic cells, and it functions as a crucial stemness regulator. The expression of PIM kinases can be activated by the LIF signaling pathway and downstream JAK-STAT, which are critical to ESC pluripotency[31]. A study by Sun et al[32] showed that the kno
In summary, our findings indicate that the overexpression of Bcl-2, Pim-2, and Met-1 anti-apoptotic genes affects the cardiac differentiation of mESCs, revealing a central role for these genes in cardiomyocyte differentiation. Additional experiments are necessary to delineate the specific stages of signaling pathways that intersect with both the apoptotic pathway and the cardiac differentiation pathway. Conducting these experiments is crucial for enhancing our under
We thank Aya Abou Hammoud for transporting the cell lines utilized in this research study from France to Lebanon. We extend our gratitude to all members of Dr. Abou-Kheir Laboratory and the staff of the core facilities in the DTS Building at the American University of Beirut for their technical help and support.
| 1. | Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5956] [Cited by in RCA: 5493] [Article Influence: 122.1] [Reference Citation Analysis (0)] |
| 2. | Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634-7638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3879] [Cited by in RCA: 3622] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 3. | Brill A, Torchinsky A, Carp H, Toder V. The role of apoptosis in normal and abnormal embryonic development. J Assist Reprod Genet. 1999;16:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 140] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Hardy K. Apoptosis in the human embryo. Rev Reprod. 1999;4:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 198] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Fabian D, Koppel J, Maddox-Hyttel P. Apoptotic processes during mammalian preimplantation development. Theriogenology. 2005;64:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Hardy K, Spanos S, Becker D, Iannelli P, Winston RM, Stark J. From cell death to embryo arrest: mathematical models of human preimplantation embryo development. Proc Natl Acad Sci USA. 2001;98:1655-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Wang ES, Reyes NA, Melton C, Huskey NE, Momcilovic O, Goga A, Blelloch R, Oakes SA. Fas-Activated Mitochondrial Apoptosis Culls Stalled Embryonic Stem Cells to Promote Differentiation. Curr Biol. 2015;25:3110-3118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Voss AK, Strasser A. The essentials of developmental apoptosis. F1000Res. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 9. | Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20:175-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 1527] [Article Influence: 218.1] [Reference Citation Analysis (0)] |
| 10. | Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1913] [Cited by in RCA: 2441] [Article Influence: 203.4] [Reference Citation Analysis (0)] |
| 11. | Fox CJ, Hammerman PS, Cinalli RM, Master SR, Chodosh LA, Thompson CB. The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev. 2003;17:1841-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 276] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Yan B, Zemskova M, Holder S, Chin V, Kraft A, Koskinen PJ, Lilly M. The PIM-2 kinase phosphorylates BAD on serine 112 and reverses BAD-induced cell death. J Biol Chem. 2003;278:45358-45367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 223] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | Dziegiel P, Pula B, Kobierzycki C, Stasiolek M, Podhorska-Okolow M. Metallothioneins: Structure and Functions. In: Metallothioneins in Normal and Cancer Cells. Cham: Springer International Publishing, 2016: 3-20. |
| 14. | Ha JH, Prela O, Carpizo DR, Loh SN. p53 and Zinc: A Malleable Relationship. Front Mol Biosci. 2022;9:895887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Dutsch-Wicherek M, Sikora J, Tomaszewska R. The possible biological role of metallothionein in apoptosis. Front Biosci. 2008;13:4029-4038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Duval D, Trouillas M, Thibault C, Dembelé D, Diemunsch F, Reinhardt B, Mertz AL, Dierich A, Boeuf H. Apoptosis and differentiation commitment: novel insights revealed by gene profiling studies in mouse embryonic stem cells. Cell Death Differ. 2006;13:564-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Auda-Boucher G, Bernard B, Fontaine-Pérus J, Rouaud T, Mericksay M, Gardahaut MF. Staging of the commitment of murine cardiac cell progenitors. Dev Biol. 2000;225:214-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Zakeri Z, Lockshin RA. Cell death during development. J Immunol Methods. 2002;265:3-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Zakeri Z, Penaloza CG, Smith K, Ye Y, Lockshin RA. What cell death does in development. Int J Dev Biol. 2015;59:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Wanner E, Thoppil H, Riabowol K. Senescence and Apoptosis: Architects of Mammalian Development. Front Cell Dev Biol. 2020;8:620089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Opferman JT, Kothari A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ. 2018;25:37-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 266] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 22. | Duval D, Malaisé M, Reinhardt B, Kedinger C, Boeuf H. A p38 inhibitor allows to dissociate differentiation and apoptotic processes triggered upon LIF withdrawal in mouse embryonic stem cells. Cell Death Differ. 2004;11:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Wei B, Jin JP. TNNT1, TNNT2, and TNNT3: Isoform genes, regulation, and structure-function relationships. Gene. 2016;582:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 24. | Oka T, Xu J, Molkentin JD. Re-employment of developmental transcription factors in adult heart disease. Semin Cell Dev Biol. 2007;18:117-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993;119:419-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 497] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 26. | Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1300] [Cited by in RCA: 1269] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 27. | Trouillas M, Saucourt C, Duval D, Gauthereau X, Thibault C, Dembele D, Feraud O, Menager J, Rallu M, Pradier L, Boeuf H. Bcl2, a transcriptional target of p38alpha, is critical for neuronal commitment of mouse embryonic stem cells. Cell Death Differ. 2008;15:1450-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Vervliet T, Duelen R, Pradhan A, La Rovere R, Roderick HL, Sampaolesi M. Cardiomyocyte differentiation from human induced pluripotent stem cells is delayed following knockout of Bcl-2. J Cell Sci. 2023;136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Ardehali R, Inlay MA, Ali SR, Tang C, Drukker M, Weissman IL. Overexpression of BCL2 enhances survival of human embryonic stem cells during stress and obviates the requirement for serum factors. Proc Natl Acad Sci USA. 2011;108:3282-3287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Ghiasi P, Hosseinkhani S, Ansari H, Aghdami N, Baharvand H. Comparison of BAX and Bcl-2 Expression During Human Embryonic Stem Cell Differentiation into Cardiomyocytes and Doxorubicin-induced Apoptosis. BMMJ. 2016;. |
| 31. | Mary Photini S, Chaiwangyen W, Weber M, Al-Kawlani B, Favaro RR, Jeschke U, Schleussner E, Morales-Prieto DM, Markert UR. PIM kinases 1, 2 and 3 in intracellular LIF signaling, proliferation and apoptosis in trophoblastic cells. Exp Cell Res. 2017;359:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Sun H, Cao J, Zhao L, Zhu S, Chen S, Li Y, Zhao B, Zhao T. PIM2 regulates stemness through phosphorylation of 4E-BP1. Sci Bull (Beijing). 2017;62:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Lu J, Baccei A, Lummertz da Rocha E, Guillermier C, McManus S, Finney LA, Zhang C, Steinhauser ML, Li H, Lerou PH. Single-cell RNA sequencing reveals metallothionein heterogeneity during hESC differentiation to definitive endoderm. Stem Cell Res. 2018;28:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0